Abstract
Antagonizing glutamatergic neurotransmission by blockade of AMPA-type glutamate receptors (GluR) is a promising pharmacological strategy for neuroprotection in neurodegenerative diseases and acute treatment of stroke.
We investigated the interaction of the adamantane derivative IEM-1460 with human wild-type and mutant AMPA-type GluR channels. Different recombinant homooligomeric human AMPA-type GluR channels and a rat nondesensitizing mutant GluR (GluR2 L504Y) channel were expressed in HEK293 cells and investigated using the patch-clamp technique in combination with ultrafast agonist application.
When IEM-1460 was coapplied with glutamate, an open channel block mechanism was observed at slow desensitizing GluR2 flip (⩾0.1 mM IEM-1460) and nondesensitizing GluR2 L504Y channels (⩾1 μM IEM-1460).
A competitive block of AMPA-type channels was observed with IC50 values for the dose block curves of 0.1 mM IEM-1460 at human unmutated and 10 μM IEM-1460 at mutant GluR channels.
Nondesensitizing GluR2 L504Y channels were used to further characterize the block mechanism. After equilibration with the agonist, a current decay upon coapplication of glutamate and IEM-1460 was observed. The recovery from block was independent of the glutamate and IEM-1460 concentration. The extent of current inhibition as well as the time constant of current decay upon addition of the blocker to the test solution were dependent on agonist concentration; this strongly points to an additional competitive-like block mechanism of IEM-1460 at human AMPA-type GluR channels.
The data were interpreted in the frame of a molecular scheme with two binding sites of IEM-1460 at the receptor, one at the unliganded resting and the other at the fully liganded open state of the channels.
Keywords: Patch clamp, ultrafast application, human AMPA-type receptors, block mechanism
Introduction
Glutamate is the major excitatory neurotransmitter of the central nervous system, which is toxic under certain pathological conditions. Its main targets are ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), NMDA and kainate-type glutamate receptor (GluR) channels. Excessive release of glutamate is thought to participate in a pathophysiological cascade, which leads to neuronal death in different acute neurological disorders like ischemic stroke and traumatic brain damage or in chronic neurodegenerative disorders like amyotrophic lateral sclerosis (ALS) or Parkinson's disease (Shaw, 1994; Williams et al., 1997; Dingledine et al., 1999). Excitatory synaptic transmission is mediated mainly via the fast gating AMPA-type GluR channels and these channels are thought to play a crucial role in chronic neurodegeneration. For example, glutamate-induced motor neuron death can be prevented by blockade of AMPA-type GluR channels but not NMDA receptor antagonists (Vandenberghe et al., 2000; Urushitani et al., 2001). For that reason, targeting of human AMPA-type GluR channels is an interesting therapeutical principle in chronic neurodegenerative disorders but despite proven neuroprotective properties in cell culture assays, none of the AMPA-type GluR channel antagonists has so far passed clinical testing (Dingledine et al., 1999).
Adamantane derivatives such as IEM-1460 block AMPA- and NMDA-type GluR channels. IEM-1460 was especially used to identify Ca2+-permeable AMPA-type receptors in the central nervous system, because it interacts specifically with these channels. The action of IEM-1460 at AMPA-type GluR channels was interpreted as an open channel block (Magazanik et al., 1997). In the present study, we investigated the effect of IEM-1460 on different recombinantly expressed wild-type and nondesensitizing mutant GluR channels and could show that this drug blocks exclusively Ca2+-permeable GluR channels, that is, GluR channels that do not contain GluR2 R subunits, which renders GluR channels Ca2+-impermeable. Additionally, from the data of the study, which contains also experiments with the nondesensitising GluR2 L504Y channels (Rosenmund et al., 1998; Stern-Bach et al., 1998), it is concluded that IEM-1460 works via a combined competitive and open channel block mechanism.
Methods
Transient expression of human recombinant GluR channels
Transformed human embryonic kidney (HEK) 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum (FCS), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in a 5% CO2/95% air incubator. They were plated on 12 mm glass coverslips coated with poly-L-lysine. Cells were suspended in a buffer used for transfection (in mM: 50 K2HPO4, 20 K-Acetate, pH 7.35). For transient transfection, cDNAs of human or rat GluR channels (GluR1 flop, GluR2 flip GQ, GluR2 flip GN, GluR2 flip GR, nondesensitizing GluR2flipGQ (L504Y), subcloned into expression vectors) were added to the suspension (25 μg ml−1). For cotransfection of GluR1 flop and GluR2 flip GR, different ratios of cDNAs were used (Schlesinger et al., 2005). A reporter cDNA encoding green fluorescent protein (GFP, 10 μg ml−1) was added to visually identify transfected cells. For transfection an electroporation device by EquiBio (U.K.) was used. Transfected cells were replated on glass coverslips in DMEM containing 10% FCS and incubated for at least 15 h. As was performed in previous studies (Krampfl et al., 2001; 2002; Grosskreutz et al., 2003; Schlesinger et al., 2005), the different human GluR channels were abbreviated by indicating the respective subunit at the first position (GluR1, 2 or 3), followed by determination of the flip/flop splice variant, the R/G and the Q/R/N editing site.
Patch-clamp experiments
Patch-clamp measurements were performed on outside-out excised patches or small cells lifted from the bottom for rapid application experiments using standard methods (Hamill et al., 1981). Patch pipettes were pulled from borosilicate glass tubes in two stages with a horizontal DMZ pipette puller (Zeitz Instruments, Augsburg, Germany). They were coated with Sylgard and fire-polished. Pipette series resistance was 7–10 MΩ when filled with intracellular solution containing (in mM): 140 KCl, 11 EGTA, 10 HEPES, 10 glucose, 2 MgCl2. The osmolarity was adjusted to 340 mos ml−1 with mannitol. HEK293 cells were superfused with an extracellular solution containing (in mM): 162 NaCl, 5.3 KCl, 2 CaCl2, 0.67 NaH2PO4, 0.22 KH2PO4, 15 HEPES, 5.6 glucose. The pH of both solutions was adjusted to 7.3. Sodium-L-glutamate (monohydrate) was obtained from Merck (U.S.A.). The adamantane derivative IEM-1460 was obtained from SIGMA (Deisenhofen, Germany) and stored as stock solutions. The final concentrations were freshly prepared for each experiment.
Data were recorded with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, U.S.A.). Membrane currents were sampled with 20 kHz using a Digidata 1200 Interface and the pCLAMP6 software suit on a PC (Axon Instruments, Union City, CA, U.S.A.). For further analysis data were filtered at 5 kHz. The holding potential was kept at −60 mV or +60 mV. For statistical analysis, the unpaired Student's t-test was used. Differences were considered significant at the P<0.05 level. All data were given as mean±s.e.m. (n, number of experiments).
A piezo-driven, double-barrelled ultrafast perfusion system was used for application of the neurotransmitter glutamate to excised outside-out membrane patches or small cells (Franke et al., 1987). With our system, the time for solution exchange was regularly <100 μs (Krampfl et al., 2002; Grosskreutz et al., 2003; Schlesinger et al., 2004) and at best ∼40 μs (20–80% rise time). It was estimated by measurements of liquid junction currents with a 10-fold difference in ionic strength at the tip of leaky patch pipettes at the end of the experiments. The time for complete exchange of the background solution took around 10 s. To minimize unspecific run down effects of the ion currents, the interval between successive glutamate pulses was 30 s. For the quantitative evaluation, 4–12 current traces were averaged for each experiment.
Results
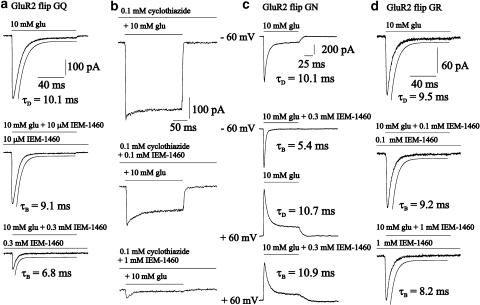
In the experiments of Figure 1, outside-out patches containing human GluR2 flip GQ, GluR2 flip GN and GluR2 flip GR channels were preincubated for at least 30 s with different concentrations of IEM-1460 via the background flow of the fast application system prior to the application of 100 ms pulses of 10 mM glutamate+blocker. Figure 1a shows original current traces of such experiments. When IEM-1460 was coapplied with 10 mM glutamate to outside-out patches containing GluR2 flip GQ channels, the peak current amplitude decreased significantly from −396.4 pA at control to −327.3 and −98.2 pA at 10 μM or 0.3 mM IEM-1460, respectively. In contrast, we observed only a slight reduction of the peak current amplitude from –136.7 to –126.1 pA at GluR2 flip GR channels in presence of 1 mM IEM-1460 (Figure 1d). The time constant of current decay decreased with increasing IEM-1460 concentrations added to GluR2 flip GQ or GN channels (Figure 1a and c), which is typical for open channel block mechanisms (Bufler et al., 1996a, 1996b). The current decay could be fitted with a single exponential as is shown by the straight line. Open channel block mechanisms are characterized by a increase of the rate constant of current decay in fast application experiments (Bufler et al., 1996b) and relieve of the block at positive membrane potentials. In contrast to GluR2 channels, which are not edited at the Q/R site, N-edited channels are impermeable for calcium ions but not rectifying at positive potentials (Burnashev et al., 1992). Therefore, it was possible to test the potential dependence of the block of human GluR2 flip GN channels by IEM-1460. The time constant of current decay was 5.4 ms (mean 5.6±0.5 ms (n=3)) at a holding potential of −60 mV after coapplication of 10 mM glutamate and 0.3 mM IEM-1460, and decreased to 10.9 ms (mean 11.3±0.4 ms (n=4)) after switching the holding potential to +60 mV (Figure 1c), an effect which was described for the open channel block by local anesthetics at nicotinic acetylcholine receptor channels (Colquhoun & Sheridan, 1982) and also for IEM-1460 at AMPA-type channels (Magazanik et al., 1997). When outside-out patches containing human GluR2 flip GQ channels were preincubated with 0.1 mM cyclothiazide, the peak current amplitude was also blocked by IEM-1460 (Figure 1b). The decrease of the peak current amplitude in presence of 0.1 and 1 mM IEM-1460 summarized to 0.49±0.04% (n=3) and 0.19±0.06% (n=4), respectively. The mean blocking concentration of IEM-1460 was 0.1 mM, quite close to the IC50 values without cyclothiazide.
Figure 1.
Original traces of currents elicited by 100 ms pulses of 10 mM glutamate (glu) or 10 mM glutamate+the respective IEM-1460 concentration after preincubation of outside-out patches from HEK293 cells transfected with human GluR2 flip GQ (a), GluR2 flip GR channels (d) and of currents elicited by 200 ms pulses of 10 mM glutamate and 0.1 mM cyclothiazide with or without IEM-1460 of human GluR2 flip GQ channels (b). The straight lines (a) show the time course of desensitisation fitted by a single exponential. The middle right column (c) shows original traces of currents elicited by 100 ms pulses of 10 mM glutamate or 10 mM glutamate+the respective IEM-1460 concentration from HEK293 cells transfected with human GluR2 flip GN at different holding potentials as indicated. Values of τD and τB are indicated. The holding potential was −60 mV when not stated otherwise.
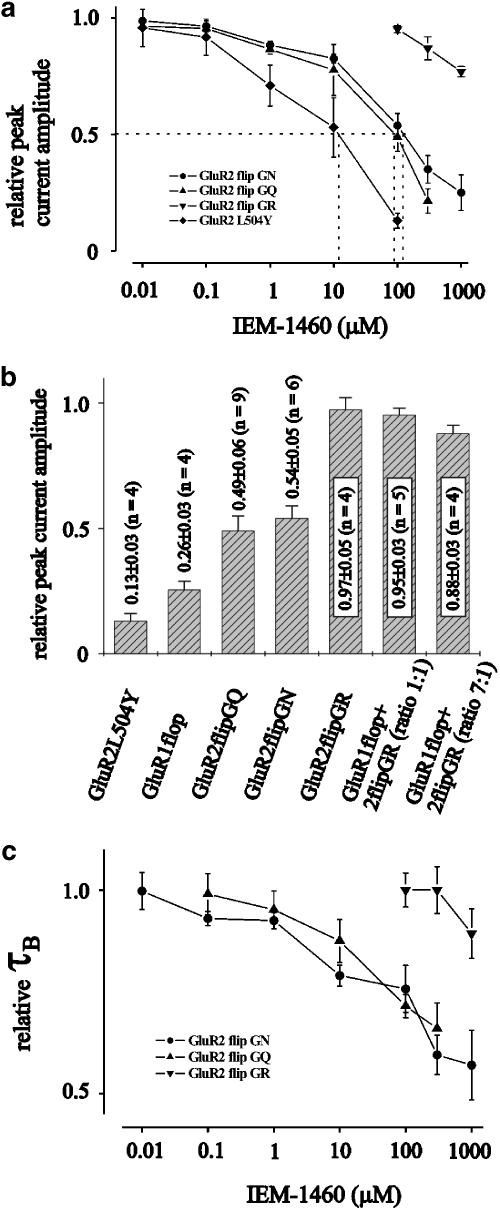
The diagram in Figure 2a shows the dose–response curves of different experiments like that of Figure 1. The data were normalized to the peak current amplitude observed after application of 10 mM glutamate. A significant block effect was observed at 1 μM IEM-1460 when outside-out patches containing human GluR2 flip GQ or GluR2 flip GN channels were used. Relative peak current amplitudes were reduced to values of 0.21±0.05 (n=5) and 0.25±0.08 (n=5) at GluR2 flip GQ or GluR2 flip GN channels in presence of 0.3 or 1 mM IEM-1460. The IC50 of IEM-1460 (see dotted lines in Figure 2a) was 95 and 103 μM, respectively. When outside-out patches containing human GluR2 flip GR channels were used, only a slight relative block effect approximating 0.77±0.02 (n=4) compared to the control experiments was observed at 1 mM IEM-1460. Figure 2b shows the relative peak current amplitude when 0.1 mM IEM-1460 was applied to different GluR channels. As also shown in Figure 2a, GluR2 flip GQ or GluR2 flip GN channels were blocked by ∼50% by 0.1 mM IEM-1460. The same blocker concentration was nearly twice as effective at GluR1 flop channels while nearly no block effect could be observed when Ca2+-impermeable human GluR2 flip GR channels were used. Additionally, IEM-1460 was tested at heteromeric human GluR1 flop/GluR2 flip GR channels. We found that the presence of a small amount of human GluR2 flip GR cDNA is sufficient to prevent the channels from block by IEM-1460 (see the two right bars in Figure 2b with the ratios of GluR1 flop and GluR2 flip GR cDNA as indicated). At a cDNA ratio of GluR1 flop : GluR2 flip GR channels of 7 : 1, the relative peak current amplitude reached a value of 0.88 of control. Human GluR2 flip channels desensitize relatively slowly with time constants of desensitization, τD, in range of 10 ms in presence of a saturating glutamate concentration (Grosskreutz et al., 2003; Schlesinger et al., 2005; Figure 1a, c and d). When 0.3 or 1 mM IEM-1460 were added to the 10 mM glutamate containing test solution, the relative decrease of the desensitization was 0.57±0.09 (n=5) for the GluR2 flip GQ and 0.66±0.06 (n=6) for the GluR2 flip GN (Figure 2c). However, when τD of the repective human GluR channels was <5 ms (as is the case at GluR1 flop channels (Krampfl et al., 2001; Grosskreutz et al., 2003; Schlesinger et al., 2005), we observed no significant acceleration of current decay in presence of 10 mM glutamate+IEM-1460 (not shown).
Figure 2.
Dose inhibition curves of different experiments of the type shown in Figure 1. (a) Inhibition of the relative peak current amplitudes elicited by 100 ms pulses of 10 mM glutamate after preincubation of outside-out patches from HEK293 cells containing GluR channels as indicated with different concentrations of IEM-1460. Each point is the average value of at least five independent experiments. (b) Average block of currents elicited by 100 ms pulses of 10 mM glutamate after preincubation of outside-out patches from HEK293 cells by 0.1 mM IEM-1460. Each point is the average of at least five independent experiments. (c) Relative decrease of τB of currents elicited by 100 ms pulses of 10 mM glutamate after preincubation of outside-out patches from HEK293 cells containing GluR2 as indicated with different concentrations of IEM-1460. Each point is the average value of at least five independent experiments.
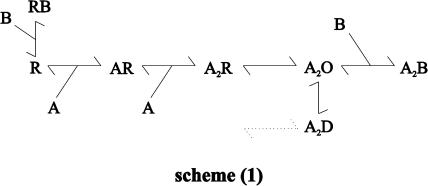
From the experiments of Figures 1 and 2 it was suggested that IEM-1460 elicited both, a competitive (reduction of the peak current amplitude in preincubation experiments) (Figures 1, 2a and b) and open channel block (decrease of current decay in presence of IEM-1460+glutamate, voltage dependence of the time constant of current decay; Figures 1 and 2c) at AMPA-type GluR channels. The principal kinetic features of AMPA-type channels can be predicted by a simple kinetic scheme with two binding sites (the suggested two other glutamate binding sites do not add significantly to the respective current amplitude elicited by glutamate; Rosenmund et al., 1998) and two desensitized states of the receptor (Krampfl et al., 2002). Figure 3 shows such a simplified scheme. In this model, R means the receptor, A the agonist and D the desensitized state. The different states of the receptor are connected via binding and unbinding rate constants and the isomerization rates α and β. Assuming an open channel block model, the blocker binds to the open state of the receptor (A2O) via the binding and unbinding rate constants b+1 and b−1. For a competitive block, the blocking molecule must bind to the unligand state (R) via b+2 and b−2. In such a model, it is predicted that the time course of current decay decreases when the concentration-dependent binding rate constant of the blocker (reciprocal value of τB × [B]) is faster than the desensitization rate constant due the transition from the A2O to the A2B state of the channel (as shown in Bufler et al. (1996a, 1996b) for physostigmin, procaine and D-tubocurarine). This was the case at human GluR2 flip GQ and GluR2 flip GN channels (Figures 1 and 2c). In case of competitive block (transition from the R- to the RB-state of the channels), agonists compete with the antagonist for the same binding site at a receptor (Colquhoun & Sheridan, 1982) and the amount of block depend on the blocker and agonist concentration (Figures 1, 2a, 4 and 5). The dose-dependent decrease of the peak current amplitude in presence of IEM-1460 with an IC50 value of 0.1 mM cannot be declared by a pure open channel block mechanism.
Figure 3.
Kinetic scheme for an open channel and competitive block of AMPA-type GluR channels by IEM-1460. In this scheme, R stands for the resting state of the receptor, A for the agonist, B for the blocker and D for the desensitized state of the receptor. The different states of the receptor are connected via the rate constants as indicated.
Figure 4.
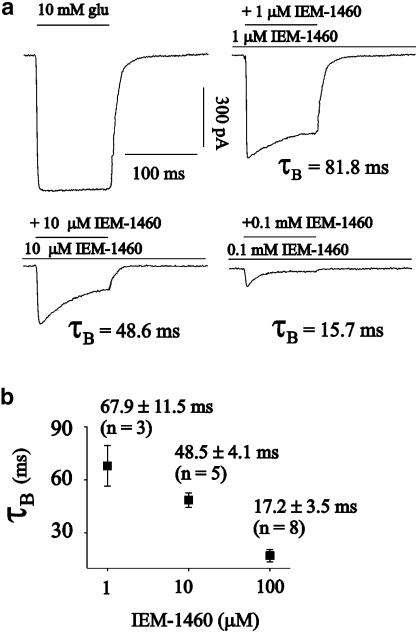
Dose-inhibition experiments with IEM-1460 at nondesensitizing GluR2L504Y channels. (a) Original traces of currents elicited by 100 ms pulses of 10 mM glutamate or 10 mM glutamate+the respective IEM-1460 concentration after preincubation of outside-out patches from HEK293 cells transfected with GluR2 L504Y channels with different concentrations of IEM-1460 as indicated (the τB values of the respective experiments are indicated). (b) The average values of τB were plotted vs the respective IEM-1460 concentration (see Figure 2a for the values of the relative steady-state current amplitudes). The holding potential was −60 mV at all experiments.
Figure 5.
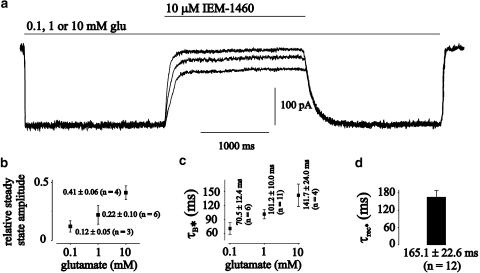
Dependence of the inhibition of mutant GluR2 L504Y channels on glutamate concentration. (a) Original current traces elicited by preincubation with 0.1, 1 or 10 mM glutamate and additional 3-s pulses of 10 μM IEM-1460+0.1, 1 or 10 mM to GluR2 L504Y channels. Amplitudes adjusted to the values at 10 mM glutamate for overlay. The holding potential was −60 mV at all experiments. (b) Dependence of the relative steady-state current amplitude of currents elicited by 0.1, 1 or 10 mM glutamate after application of 10 μM IEM-1460 on the respective glutamate concentration. (c) Dependence of τB* of currents elicited by 0.1, 1 or 10 mM glutamate after application of IEM-1460 on the respective glutamate concentration. (d) Value of τrec* after the end of 3-s pulses of 10 μM IEM-1460.
The affinity of glutamate to different AMPA-type channels was between 0.1 and 1 mM (Dingledine et al., 1999; Krampfl et al., 2002) and therefore near that of the IC50 of IEM-1460 at human GluR2 flip GQ or GluR2 flip GN channels (Figure 2). To better understand the mode of action of IEM-1460 at AMPA-type channels, further experiments were performed with nondesensitizing mutant GluR2 L504Y channels (Rosenmund et al., 1998; Stern-Bach et al., 1998; Mansour et al., 2001). The upper current trace of Figure 4a shows a typical nondesensitizing current upon application of a saturating concentration of 10 mM glutamate. When increasing IEM-1460 concentrations were added to the test (containing 10 mM glutamate) and background solution, we observed a decrease of the peak current amplitude starting at a low concentration of 0.1 μM IEM-1460 with a ∼10-fold lower IC50 of 15 μM compared to human GluR2 flip GQ or GluR2 flip GN channels (Figures 2a and 4) and a current decay in presence of the blocker, which could be fitted with a single exponential (Figure 4). When glutamate without IEM-1460 was applied, no desensitization of the channels was observed as described for these mutant channels (Rosenmund et al., 1998). At 1 μM IEM-1460, a slow component of current decay with τB=81.8 ms occurred, which decreased to τB=15.7 ms at 0.1 mM (Figure 4a). The average values are shown in the diagram of Figure 4b. The observation of the current decay in presence of IEM-1460 is compatible with an open channel block mechanism, that is, a transition from the A2O to the A2B state in the kinetic scheme (Figure 3). However, as was concluded from the experiments with unmutated human GluR channels of Figures 1 and 2, the strong block of IEM-1460 in the preincubation experiments (Figure 4) with the nondesensitizing GluR2 L504Y mutant points to an additional competitive block effect.
Adamantane derivatives like IEM-1460 were introduced as AMPA-channel blocking compounds in the paper of Magazanik et al. (1997). They performed electrophysiological experiments at oocytes transfected with cDNA of GluR1, GluR2 and GluR3 subunits with intracellular recording techniques. Currents were elicited by the agonist kainate, which elicits a nondesensitizing current response at AMPA-type channels. IEM-1460 was applied during the current response and they analyzed the decrease of the amplitude. In the experiments of Figures 1, 2 and 4, the glutamate concentration was held constant and the blocker concentration was varied. Reverse experiments are shown in Figure 5. We were interested, how the block of nondesensitizing mutant GluR2 L504Y channels by IEM-1460 is influenced by different glutamate concentrations. In case of competitive block, it is suggested that the onset of block becomes slower and the maximal block effect smaller with increasing agonist concentrations because of the competition of the agonist and the blocker at the unliganded R-state of the receptor. In the experiment of Figure 5a, 0.1, 1 or 10 mM glutamate was applied over a period of 12 s. A current amplitude of –210.3 pA was elicited by 1 or 10 mM glutamate, corresponding to the maximal open probability of the channels, a current amplitude of –185.3 pA at 0.1 mM glutamate. Amplitudes were normalized to the maximum current amplitude in the figure for reasons of graphical clarity. After 3 s, a concentration step was performed into a solution containing 10 μM IEM-1460 (this concentration is in the range of the IC50 at GluR2 L504Y channels, Figure 2a)+the respective glutamate concentration. At 0.1 mM glutamate, the current decayed nearly to the baseline with a time constant, τB*, of 65.2 ms upon application of 10 μM IEM-1460 (for mean values see Figure 5b and c). At higher glutamate concentrations, the time constant of current decay became significantly slower and the steady-state current amplitude increased. For example, when 10 mM glutamate was applied, τB* was 160.4 ms (mean values see Figure 5c) and the relative steady-state current amplitude was 0.35 (mean values see Figure 5b). When the steady-state current amplitude had reached an equilibrium (this was the case after ∼500 ms for all glutamate concentrations tested), a concentration step back to a glutamate containing solution without IEM-1460 was performed. The time course of recovery from block could be fitted with a single exponential, τrec*, at all glutamate concentrations tested (165.1±22.6 ms (n=12), see Figure 5d), reflecting unbinding of IEM-1460 from the receptor. When glutamate was removed from the receptor, the current decayed monoexponentially to the baseline with a time constant, τdeact, of 15.2 ms (17.7±2.9 (n=10)), which is likely to reflect unbinding of glutamate, deactivation, from GluR2 L504Y channels.
The results of the blockade of recombinant AMPA-type channels by IEM-1460 are summarized as follows: (1) IEM-1460 blocks the peak current amplitudes through different recombinant human GluR channels with an IC50 between 10 μM and 0.1 mM (Figures 1, 2 and 4a). As recently shown, the affinity of IEM-1460 to R-edited GluR2 channels was much lower with an IC50of ≫1 mM (Figure 2c, Magazanik et al., 1997). The low affinity of IEM-1460 to human homomeric edited GluR2 channels is passed on heteromeric channels transfected with a low amount of cDNA of GluR2flip GR subunits (Figure 2b). (2) The block was more effective at human nonmutated GluR1 than GluR2 channels (Figure 2b). (3) The time constant of current decay in presence of glutamate+IEM-1460 decreased with increasing blocker concentrations when τD of the respective channels was >5 ms (Figures 1 and 2d), and was voltage dependent (Figure 1). (4) The time course and amount of block was dependent on the glutamate concentration whereas the recovery from block was concentration independent in case of the nondesensitizing GluR2 L504Y channels (Figure 5).
Conclusion
Despite there is a broad variety of drugs that modulate and block AMPA-type GluR in vitro, none of the AMPA-blockers that underwent clinical testing so far were appropriate for a therapeutical use in patients (Doble, 1999). In our study, we tested the recently described GluR antagonist IEM-1460 for receptor interactions at the molecular level. IEM-1460 binds at Ca2+-permeable AMPA-type GluR channels and not at Ca2+-impermeable channels (Magazanik et al., 1997; Tikhonov et al., 2000). This means that currents through AMPA-type channels are blocked by IEM-1460 except the respective channels contain a Q/R edited GluR2 subunit, which renders the respective channels Ca2+-impermeable. The IC50 of IEM-1460, measured at oocytes transfected with GluR1 or three subunits after application of 0.1 mM kainate, was ∼2 μM (Magazanik et al., 1997).
In the present study, we tested IEM-1460 at different human recombinant GluR channels and nondesensitizing mutant GluR2 L504Y subunits using the patch-clamp technique in combination with fast solution exchange techniques. The kinetic properties of these channels are well known from recently published studies (Stern-Bach et al., 1998; Krampfl et al., 2002; Grosskreutz et al., 2003; Schlesinger et al., 2005) and these receptor channels were therefore well suitable for in vitro pharmacological studies. Fast solution exchange methods in combination with the patch-clamp technique allow the physiological analysis of postsynaptic mechanism on a molecular level. Human Ca2+-permeable AMPA-type channels are by a factor of ∼50 less sensitive to IEM-1460 (Figure 2a) than recombinant rodent Ca2+-permeable channels expressed in oocytes (Magazanik et al., 1997). Similarly to this study, it was shown that the IC50 is shifted at least by a factor of 1000 to the right when homomeric Ca2+-impermeable GluR2 flip GR channels were tested (Figures 1 and 2). The sensitivity of human GluR2 flip GN channels to IEM-1460 was the same as that of human GluR2 flip GQ channels, GluR1 channels were double as effectively blocked than GluR2 flip GQ or GluR2 flip GN channels (Figure 2b) and the mutant GluR2 L504Y channels were most sensitive to IEM-1460 with an IC50 of 15 μM (Figure 2a and b). As was shown in previous studies (Magazanik et al., 1997; Buldakova et al., 1999), IEM-1460 was nearly ineffective at GluR2 flip GR channels that have a very low Ca2+-permeability. This specific effect of IEM-1460 was clearly confirmed by the results of our study (Figures 1 and 2) and it holds also true when low amounts of cDNA of GluR2 flip GR subunits are used for coexpression at HEK293 cells (Figure 2b). The IC50 of unmutated human AMPA-type channels (except GluR2 flip GR channels) was in the range of low sensitivity rat hippocampal neurons (Magazanik et al., 1997). Beside the different experimental design of the studies, species differences might play a role for the different affinity of IEM-1460.
Magazanik et al. (1997) investigated IEM-1460 at recombinant GluR channels expressed in oocytes with the two-electrode voltage-clamp technique and at AMPA-type GluR channels expressed from freshly dissociated hippocampal neurones with the whole-cell patch-clamp technique. When IEM-1460 was applied to the nondesensitizing kainate-activated ion current, they found a reversible block of the current amplitude (similar to the experiments at nondesensitizing GluR2 L504Y channels shown in Figure 5). To elucidate the molecular mechanism of IEM-1460 at AMPA-type channels, we performed two different types of experiments with IEM-1460 at different human GluR channels and the mutant GluR2 L504Y channel. First, different concentrations of IEM-1460 were held constant throughout an experiment and 10 mM glutamate was applied pulsewise (Figures 1, 2 and 4). Under these conditions, two effects were observed: The peak current amplitude of GluR channel currents of human GluR1, GluR2 flip GQ, GluR2 flip GN and GluR2 L504Y channels (Figures 1, 2a, b and 4), and the time constant of current decay of the relatively slowly or nondesensitizing GluR2 flip or GluR2 L504Y channels (Figures 1, 2d and 4) decreased with increasing concentrations of IEM-1460. The second type of experiments was performed only at GluR2 L504Y channels. Different concentrations of glutamate were applied continuously and 10 μM IEM-1460 (which elicited a ∼50% blockade at these channels) (Figures 2a and 4) pulsewise. A concentration-dependent increase of the steady-state current amplitude and τB* was observed, whereas the value of τrec* was independent on the glutamate concentration (Figure 5). Magazanik et al. (1997) postulated an open channel block mechanism of IEM-1460 from experiments with kainate-induced nondesensitizing currents. From our experiments with ultra-fast application of test-solutions, some hints for an open channel block mechanism of IEM-1460 were found: The time constant of current decay, τB, decreased in presence of IEM-1460 by ∼50% when slowly desensitizing human GluR2 flip channels were investigated (Figures 1 and 2d) and a distinct current decay, fitted with a single exponential, occurred in presence of IEM-1460 at nondesensitizing GluR2 L504Y channels (Figure 4), similar to the open channel block by different drugs at nicotinic acetylcholine receptor channels and GABAA receptor channels (Bufler et al., 1996b; Krampfl et al., 2001; Mohammadi et al., 2001a, 2001b). Additionally, the value of τB was voltage dependent (Figure 1c) as it is a characteristic feature of open channel block mechanisms (Colquhoun & Sheridan, 1982). However, some other features of the block of GluR channels by IEM-1460 cannot be explained by an open channel block mechanism. Especially the marked decay of the peak current amplitude after preincubation with IEM-1460 (Figures 1, 2a, b and 4) and the glutamate dependency of the blockade in Figure 5 are not compatible with an exclusive open channel block mechanism. At competitive block, antagonists compete with the agonist for binding at the unliganded receptor and, when the channels are pre-equilibrated with the blocker (in the experiments of Figures 1 and 4, preincubation was at least 30 s before glutamate+blocker was applied), there should be an equilibrium between the resting (R) and blocked (RB) state (Figure 3). As a result of the lower values of the binding rate constants (b+1 and b+2 in Figure 3) at lower glutamate concentrations, the probability of a transition from the R- to the RB-state of the receptor increases at constant blocker concentrations. Consequently, assuming a scheme as suggested in Figure 3, the time constant of current decay decreased and the amount of block increased with decreasing glutamate concentrations at a constant blocker concentration at nondesensitizing GluR channels (Figure 5). The effects of IEM-1460 at GluR channels can therefore be explained by a combination of both, open channel and competitive block. This is in contrast to experiments with the structurally related compounds argiotoxin and philanthotoxin, which show an isolated noncompetitive block at recombinant GluR channels (Brackley et al., 1993). If IEM-1460 binds, beside the occupation of the open state of the receptor, only to the unliganded state of the receptor or also to lower liganded receptor states (AR in Figure 3) cannot be decided from the experiments shown here.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (DFG, Bu938/5-2). We thank Dr C. Rosenmund for providing us with the cDNA clones of GluR L504Y subunits and helpful discussions, U. Jensen for expert help with cDNA preparation and maintenance of cell cultures, and A. Niesel and J. Kilian for technical support.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- ALS
amyotrophic lateral sclerosis
- DMEM
Dulbecco's modified Eagle's medium
- GluR
glutamate receptor
- HEK
human embryonic kidney
- FCS
fetal calf serum
- GFP
green fluorescent protein
References
- BRACKLEY P.T., BELL D.R., CHOI S.K., NAKANISHI K., USHERWOOD P.N. Selective antagonism of native and cloned kainite and NMDA receptors by polyamine-containing toxins. J. Pharmacol. Exp. Ther. 1993;266:1573–1580. [PubMed] [Google Scholar]
- BUFLER J., FRANKE C., PARNAS H., DUDEL J. Open channel block by physostigmine and procaine in embryonic-like nicotinic receptors of mouse muscle. Eur. J. Neurosci. 1996a;8:677–687. doi: 10.1111/j.1460-9568.1996.tb01253.x. [DOI] [PubMed] [Google Scholar]
- BUFLER J., WILHELM R., PARNAS H., FRANKE C., DUDEL J. Open channel and competitive block of the embryonic form of the nicotinic receptor of mouse myotubes by (+)-tubocurarine. J. Physiol. 1996b;495:83–95. doi: 10.1113/jphysiol.1996.sp021575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULDAKOVA S.L., VOROBJEV V.S., SHARONOVA I.N., SAMOILOVA M.V., MAGAZANIK L.G. Characterization of AMPA receptor populations in rat brain cells by the use of subunit-specific open channel blocking drug, IEM-1460. Brain Res. 1999;846:52–58. doi: 10.1016/s0006-8993(99)01970-8. [DOI] [PubMed] [Google Scholar]
- BURNASHEV N., MONYER H., SEEBURG P.H., SAKMANN B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN D., SHERIDAN R.E. The effect of tubocurarine competition on the kinetics of agonist action on the nicotinic receptor. Br. J. Pharmacol. 1982;75:77–86. doi: 10.1111/j.1476-5381.1982.tb08759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The Glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- DOBLE A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol. Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- FRANKE C., HATT H., DUDEL J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci. Lett. 1987;77:199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- GROSSKREUTZ J., ZOERNER A., SCHLESINGER F., KRAMPFL K., DENGLER R., BUFLER J. Kinetic properties of human AMPA-type glutamate receptors expressed in HEK293 cells. Eur. J. Neurosci. 2003;17:1173–1178. doi: 10.1046/j.1460-9568.2003.02531.x. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- KRAMPFL K., SCHLESINGER F., WOLFES H., DENGLER R., BUFLER J. Functional diversity of recombinant human AMPA type glutamate receptors: possible implications for selective vulnerability of motor neurons. J. Neurol. Sci. 2001;191:19–23. doi: 10.1016/s0022-510x(01)00626-8. [DOI] [PubMed] [Google Scholar]
- KRAMPFL K., SCHLESINGER F., ZOERNER A., KAPPLER M., DENGLER R., BUFLER J. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur. J. Neurosci. 2002;15:51–62. doi: 10.1046/j.0953-816x.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- MAGAZANIK L.G., BULDAKOVA S.L., SAMOILOVA M.V., GMIRO V.E., MELLOR I.R., USHERWOOD P.N. Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J. Physiol. 1997;505:655–663. doi: 10.1111/j.1469-7793.1997.655ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOUR M., NAGARAJAN N., NEHRING R.B., CLEMENTS J.D., ROSENMUND C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- MOHAMMADI B., HAESELER G., LEUWER M., DENGLER R., KRAMPFL K., BUFLER J. Structural requirements of phenol derivatives for direct activation of chloride currents via GABA(A) receptors. Eur. J. Pharmacol. 2001a;421:85–91. doi: 10.1016/s0014-2999(01)01033-0. [DOI] [PubMed] [Google Scholar]
- MOHAMMADI B., KRAMPFL K., MOSCHREF H., DENGLER R., BUFLER J. Interaction of the neuroprotective drug riluzole with GABA(A) and glycine receptor channels. Eur. J. Pharmacol. 2001b;415:135–140. doi: 10.1016/s0014-2999(01)00847-0. [DOI] [PubMed] [Google Scholar]
- ROSENMUND C., STERN-BACH Y., STEVENS C.F. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER F., MEYWIRTH J., KRAMPFL K., GROSSKREUTZ J., PETRI S., MAUTH C., JUST L., BADER A., BUFLER J. Ligand-gated channels in early mesencephalic neuronal precursors: immunocytochemical and electrophysiological analysis. Eur. J. Neurosci. 2004;19:2371–2376. doi: 10.1111/j.0953-816X.2004.03343.x. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER F., TAMMENA D., KRAMPFL K., BUFLER J. Desensitization and resensitization are independently regulated in human recombinant GluR subunit coassemblies. Synapse. 2005;55:176–182. doi: 10.1002/syn.20110. [DOI] [PubMed] [Google Scholar]
- SHAW P.J. Excitotoxicity and motor neurone disease: a review of the evidence. J. Neurol. Sci. 1994;124:6–13. doi: 10.1016/0022-510x(94)90170-8. [DOI] [PubMed] [Google Scholar]
- STERN-BACH Y., RUSSO S., NEUMANN M., ROSENMUND C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- TIKHONOV D.B., MAGAZANIK L.G., MELLOR I.R., USHERWOOD P.N. Possible influence of intramolecular hydrogen bonds on the three-dimensional structure of polyamine amides and their interaction with ionotropic glutamate receptors. Receptors Channels. 2000;7:227–236. [PubMed] [Google Scholar]
- URUSHITANI M., NAKAMIZO T., INOUE R., SAWADA H., KIHARA T., HONDA K., AKAIKE A., SIMOHAMA S. N-methyl-D-aspartate receptor-mediated mitochondrial Ca(2+) overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca(2+) influx. J. Neurosci. Res. 2001;63:377–387. doi: 10.1002/1097-4547(20010301)63:5<377::AID-JNR1032>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- VANDENBERGHE W., ROBBERECHT W., BRORSON J.R. AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J. Neurosci. 2000;20:123–132. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS T.L., DAY N.C., INCE P.G., KAMBOJ R.K., SHAW P.J. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]