Abstract
Prednisolone, a potent anti-inflammatory drug, has proved ineffective in treating acute respiratory distress syndrome (ARDS). ARDS is associated with superoxide (O2•−) generation, which negates nitric oxide (NO). NO also downregulates NADPH oxidase and inhibits O2•− formation. A possible reason for the lack of effect of prednisolone may due to an inhibition of eNOS expression. In order to test this proposal, the effect of prednisolone on O2•− formation and the expression of gp91phox (catalytic subunit of NADPH oxidase) and eNOS in pig pulmonary artery (PA) segments and PA endothelial cells (PAECs) and PA vascular smooth muscle cells (PAVSMCs) was investigated.
PA segments and cells were incubated with prednisolone and tumour necrosis factor-alpha (TNF-α) for 16 h. O2•− formation was measured spectrophometrically and gp91phox and eNOS expression by Western blotting. The role of the NO–cGMP axis was studied using morpholinosydnonimine hydrochloride, the diethylamine/NO complex (DETA-NONOate), the guanylyl cyclase inhibitor, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one (ODQ) and the stable cGMP analogues, 8-bromo cGMP and 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP). NO release was studied using a fluorescence assay and O2•−–NO interactions with a nitrite/nitrate assay.
Prednisolone elicited significant increase in O2•− formation in intact PA segments and PAECs, but not PAVSMCs, in a concentration-dependent manner. In endothelium-denuded segments, prednisolone slightly enhanced O2•− release. TNF-α further increased prednisolone-enhanced O2•− formation in intact PA segments and PAECs. NADPH oxidase inhibitor, apocynin, inhibited O2•− formation. Increased O2•− release and gp91phox expression in PAECs elicited by prednisolone was blocked by SIN-1 (3-morpholinosydnonimine hydrochloride), DETA-NONOate, 8-pCPT-cGMP and 8-bromo cGMP. The effects of SIN-1 on gp91phox expression were reversed by ODQ. Finally, eNOS protein expression was significantly reduced by prednisolone.
Prednisolone increases O2•− in porcine PAECs through a downregulation of endogenous eNOS expression. Since the NO–cGMP axis inhibits gp91phox expression, the resultant decrease in endogenous NO formation then augments NADPH oxidase activity, which in turn results in increased O2•− formation. Since O2•− promotes inflammation, this mechanism may explain why prednisolone is ineffective in treating ARDS. Therapeutically, the coadministration of an NO donor may render prednisolone more effective in treating ARDS.
Keywords: Prednisolone, superoxide, NADPH oxidase, eNOS, pig pulmonary artery
Introduction
Acute respiratory distress syndrome (ARDS) is a condition characterised by a time-dependent worsening of intrapulmonary inflammation and hypertension (Weinacker & Vaszar, 2001). Therapeutically, ARDS is a difficult and sometimes intractable condition to treat. Despite the central aetiological role of inflammation, anti-inflammatory drugs, including NSAIDs and steroids, have proved surprisingly ineffective in treating ARDS (Jantz & Shan, 1999; Stuart-Smith & Jeremy, 2001). In particular, prednisolone, a potent anti-inflammatory drug, has no beneficial effect in treating early phase of ARDS (Bone et al., 1987; Luce et al., 1988; Meduri & Chrousos, 1998). Understanding why the acute administration of prednisolone fails to influence the progress of ARDS, despite its proven efficacy in other inflammatory conditions, may lead to improved therapy and to a fundamental understanding as to why ARDS may differ mechanistically from other acute inflammatory conditions.
The possible reason for this lack of therapeutic benefit may lie in the inhibitory effects of prednisolone on the expression of factors that not only promote inflammation but also reduce or limit inflammation (Newton, 2000). For example, prednisolone may inhibit the expression of nitric oxide (NO) synthase, thereby reducing NO formation. Some studies have shown that inhalational NO ameliorates ARDS (Klinger, 2002), although this approach elicits severe side effects possible due to the locally high levels of NO elicited by the means of delivery (Weinberger et al., 2001). By contrast, a recent clinical trial, in which the NOS inhibitor, 546C88, was studied for safety and efficacy in 797 patients with septic shock, it was found that the inhibitor caused a significant increase in mortality (Lopez et al., 2004). This latter trial would seem to indicate that the protective effect of endogenous NOS, in particular eNOS, may outweigh the other negative effects of NO.
Of the many factors that promote ARDS, oxidative stress (OS) plays a central role in the aetiology of ARDS (Chabot et al., 1998). Principal among the reactive oxygen species (ROS) generated by OS is superoxide (O2•−), which reacts with NO to produce peroxynitrite (ONOO−), promoting not only vasoconstriction but also the adhesion of leucocytes and platelets (Stuart-Smith & Jeremy, 2001). LPS, cytokines and eicosanoids rapidly upregulate the expression of both gp91phox and endothelial NOS expression in pulmonary arterial tissue and endothelial cells (Muzaffar et al., 2003). It was further demonstrated that the inhibition of eNOS or removal of the endothelium markedly increases O2•− formation, indicating a protective role for the simultaneous upregulation of eNOS (Muzaffar et al., 2003; Muzaffar et al., 2004a). It was also demonstrated that SIN-1 (3-morpholinosydnonimine hydrochloride)- and NO-donating aspirin inhibit cytokine-induced NADPH oxidase expression (Muzaffar et al., 2004a). It is reasonable to suggest, therefore, that prednisolone, through an inhibitory effect on eNOS expression, may actually augment O2•− formation and increase NADPH oxidase expression.
In order to test this proposal, the effect of prednisolone on O2•− formation and the expression of eNOS and gp91phox was studied in pig isolated pulmonary arteries (PAs) and cultured vascular smooth muscle cells (PAVSMCs) arterial and endothelial cells (PAECs) derived from these arteries.
Methods
Dissection and incubation of pulmonary arteries
Lungs were obtained from White Landrace male pigs of body weight ranging from 20 to 35 kg. All animals were given humane care in compliance with the rules and regulations of Bristol University and the U.K. Home Office. Pigs were anaesthetised with an intravenous injection of ketamine hydrochloride (10 mg kg−1; Ketaset Injection, Fort Dodge Animal Health, Southampton, U.K.) and inhaled halothane (1–2% in oxygen), exsanguinated and lungs removed. PAs (3–4 mm diameter) were dissected out and placed in Dulbecco's minimum essential medium (DMEM) with Glutamax-1 (GibcoBRL, Paisley, Scotland) and cut into 2 mm2 (Muzaffar et al., 2003, 2004a, 2004b).
PAVSMCs and PAECs were prepared as described previously (Muzaffar et al., 2003, 2004a, 2004b). PAECs were grown in endothelial cell growth medium (PromoCell, Heidelberg, Germany) at 37°C in a 95% air–5% CO2 incubator. PAVSMCs were maintained in DMEM (containing 10% foetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin) at 37°C in a 95% air–5% CO2 incubator. Subconfluent cultures of pulmonary VSMCs were growth arrested by washing in sterile phosphate-buffered saline (PBS, GibcoBRL) and incubating in serum-free DMEM supplemented 100 U ml−1 penicillin and 100 μg ml−1 streptomycin for 48 h.
Effect of prednisolone on O2•− formation
PAVSMCs, PAECs or PA segments (±endothelium) were incubated with the prednisolone (±tumour necrosis factor-alpha (TNF-α), 10 ng ml−1) for 16 h at 37°C in a 95% air–5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Germany). Following incubation, segments or cells were washed with DMEM three times and equilibrated in DMEM without phenol red for 10 min at 37°C in a 95% air–5% CO2 incubator. In all, 20 μM horseradish cytochrome c (Sigma Chemical Co., Poole, Dorset, U.K.) with or without 500 U ml−1 copper–zinc superoxide dismutase (SOD; Sigma Chemical Co.) was added and incubated at 37°C in a 95% air–5% CO2 incubator for an hour. The reaction medium was removed and reduction of cytochrome c determined at 550 nm in an Anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, U.K.) and converted to nmol of O2•−, using ΔE550 nm=21.1 mM−1 cm−1 as the extinction coefficient. The reduction of cytochrome c that was inhibitable with SOD reflected actual O2•− release. Segments were blotted, dried and weighed, data being expressed as nmol of O2•− mg tissue−1 h−1. Cells were rinsed in PBS, lysed with 0.1% (v v−1) Triton X-100 and total protein content measured using BCA-protein assay kit (Pierce, Rockford, IL, U.S.A.). The results were expressed as μmol of O2•− mg protein−1 h−1.
Effect of inhibitors of O2•−-generating enzymes on prednisolone-induced O2•−release
In order to determine the source of the O2•− elicited by prednisolone, PAECs were preincubated with apocynin (1 μM; Sigma Chemical Co.; an inhibitor of NADPH oxidase (Stolk et al., 1994), diphenyleneiodonium chloride (DPI; 10 μM; Sigma Chemical Co.; another NADPH oxidase inhibitor (Griendling et al., 1994)), allopurinol (100 μM; Sigma Chemical Co.; a xanthine oxidase inhibitor (Greene & Paller, 1992)), rotenone (10 μM; Sigma Chemical Co.; an electron transfer chain inhibitor (Meier et al., 1989)) and aspirin (100 μM; Sigma Chemical Co.; a cyclooxygenase inhibitor (Tate et al., 1984)).
Effect of SIN-1, DETA-NONOate, 8-bromo-cGMP, 8-pCPT-cGMP and ODQ on prednisolone-induced O2•−release
To investigate the role of NO in mediating the effect of prednisolone on O2•− formation in this experimental setting, PAECs were incubated with the NO donors, SIN-1 (100 nM) or diethylamine/NO complex (DETA-NONOate; 100–500 μM) with prednisolone for 16 h. In order to determine whether the effects of NO on O2•− formation was mediated by the cyclic GMP, PAECs were incubated with the stable and permeable analogues of cGMP: 8-bromo-cGMP or 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP), at both 10 and 100 μM, or with the guanylyl cyclase inhibitor, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one (ODQ; 1 μM, over a 16-h incubation with prednisolone (±SIN-1 or DETA-NONOate). PAECs were preincubated with ODQ for at least an hour before commencing the 16-h time course. The production of O2•− was measured by ferricytochrome c assay, as described above. In all studies, possible toxic effects of drugs were routinely assessed by checking cell density (index of cell death) before and after incubations and no alterations were observed (data not shown). All data are adjusted for protein and as such any cell loss would be compensated for.
Effect of prednisolone on gp91phox and eNOS expression
Following 16-h incubations with prednisolone (±various activators and inhibitors), as described above, PAECs were washed 3 × with PBS and lysed with Tris buffer (50 mM, pH 7.4) containing 1% (v v−1) SDS, EDTA (10 mM), PMSF (1 mM), pepstatin (0.05 mM) and leupeptin (0.2 mM). Extracts were boiled at a 1 : 1 ratio with loading buffer (50 mM Tris (pH 6.8); 4% (w v−1) sodium dodecyl sulphate; 10% (v v−1) glycerol; 4% (v v−1) 2-mercaptoethanol; 2 mg ml−1 bromophenol blue). Samples of equal protein (20 μg) were loaded onto 10% Tris-glycine sodium dodecyl sulphate gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed with either mouse anti-gp91phox (1 : 500 dilution; Transduction Laboratories, U.K.) (Yu et al., 1998) or anti-eNOS (1 : 2500 dilution; Transduction Laboratories). The endogenous mouse gp91phox shows a 58 kDa band, instead of a 91 kDa observed in human. Both mouse and human deglycosylated gp91phox are 54 kDa. This difference is due to less glycosylation sites in the mouse sequence (Bjorgvinsdottir et al., 1996). In initial Western blots, both human and porcine neutrophil lysates ran as a smear starting from 90 to 50 kDa. For the representative blot, much less quantity of neutrophil lysates was loaded for cleaner detection. The blots were then incubated with goat anti-mouse antibody conjugated to horseradish peroxidase (1 : 2000 dilution) and developed by enhanced chemiluminescence (Amersham International, Little Chalfont, Bucks, U.K.). Rainbow markers (14–220 kDa; Amersham) were used for molecular weight determination. Membranes were reprobed with anti-GAPDH monoclonal antibody (Chemicon International, CA, U.S.A.) as an internal control for equal protein loading.
Determination of NO release from PAECs
The NO released by PAECs into the supernatants was measured by a fluorescent dye, 4,5-diaminofluorescein (DAF-2; Sigma Chemical Co.) (Nakatsubo et al., 1998; Leikert et al., 2001; Rathel et al., 2003). Following incubations with prednisolone and TNF-α, for 16 h, cells were washed with PBS and then preincubated with L-arginine (100 μM in PBS, 5 min, 37°C). In some experiments, L-NAME (1 mM) was added 5 min before the addition of L-arginine. Subsequently, DAF-2 (1 μM) was added and cells were incubated in the dark at 37°C for 30 min. Then, the fluorescence of the supernatants was measured at room temperature using a spectrofluorimeter (Fluorolite 1000, Dynatech Laboratories) with excitation wavelength set at 494 nm and emission wavelength at 520 nm. The bandwidth was 10 nm for both excitation and emission. The sensitivity was programmed on high.
Nitrite measurements
NO generated by PAECs in response to prednisolone with or without SIN-1 (100 nM) was measured as nitrite concentration using an assay kit that is based on the Griess reaction (Nims et al., 1996). Cells were cultured and exposed to prednisolone as described above in multiwell plates. At the end of the incubation, supernatants were removed and centrifuged to rid of cell debris. Nitrate in the clarified culture supernatants was converted to nitrite by nitrate reductase. The nitrite was then reacted with sulphanilamide and N-(1-naphthyl)ethylenediamine and detected chromogenically by measuring optical density at 540 nm using spectrophotometer. A standard curve was calibrated using sodium nitrite at concentration from 0 to 25 μM and the concentration of nitrite calculated by linear regression.
Data analysis
Data are expressed as mean±s.e.m. and n indicates the number of animals used. Student's unpaired t-test or one-way factorial ANOVA was used to determine the difference in the data. A P-value of <0.05 was considered statistically significant. Multiple group comparisons were made using one-way ANOVA.
Results
Effect of prednisolone on O2•− release from pulmonary arteries
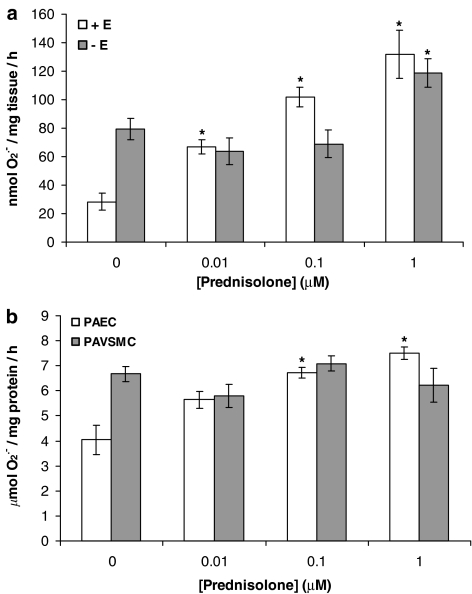
Prednisolone elicited a statistically significant, concentration-dependent, increase in the formation of O2•− in intact pig PA segments (with endothelium; Figure 1a) and in PAECs (Figure 1b) following a 16-h incubation. In endothelium-denuded segments, however, prednisolone slightly enhanced O2•− release only at 1 μM (Figure 1a) and had no significant effect on O2•− release from PAVSMCs at any concentration (Figure 1b).
Figure 1.
Effect of prednisolone (0.01–1 μM) on O2•− formation by (a) whole pig PA segments (with [+E] or without [−E] endothelium) and (b) cultured PAECs and cultured PAVSMCs following a 16-h incubation. Data=mean±s.e.m.; n=6. *P<0.05, significantly increased compared to control (0).
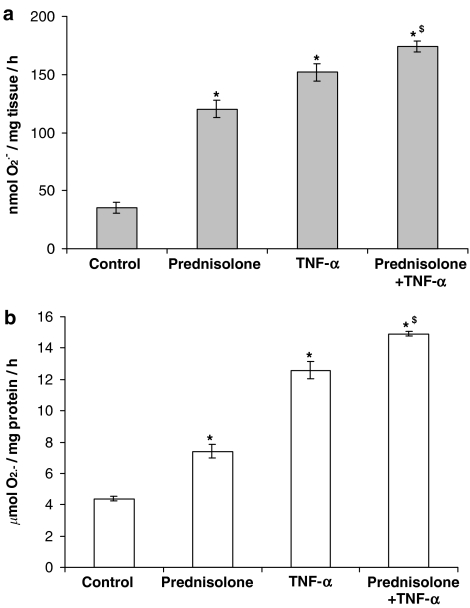
The increase in the formation of O2•− promoted by prednisolone (1 μM) was similar to (but not greater than) that elicited with TNF-α (10 ng ml−1) in intact PA segments and PAECs (Figure 2). The combination of TNF-α and prednisolone elicited a further statistically significant enhancement of O2•− formation in intact PA segments and PAECs (Figure 2a and b).
Figure 2.
Effect of TNF-α (10 ng ml−1) on prednisolone-enhanced O2•− formation by (a) endothelium-intact whole pig PA segments and (b) cultured PAECs following a 16-h incubation. Data=mean±s.e.m.; n=6. *P<0.05, significantly increased compared to control. $P<0.05, significantly increased compared to prednisolone alone.
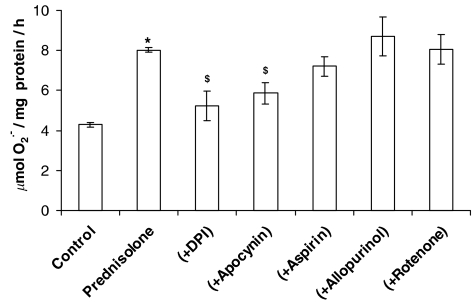
Effect of inhibitors on prednisolone-enhanced O2•− release from PAECs
O2•− formation measured after the 16-h incubation of PAECs with prednisolone was inhibited by apocynin and DPI (both NADPH oxidase inhibitors), but not allopurinol (a xanthine oxidase inhibitor), aspirin (a cyclooxygenase inhibitor) or rotenone (mitochondrial electron transport chain inhibitor) (Figure 3). This indicates that the effect of prednisolone is mediated principally by NADPH oxidase.
Figure 3.
Effect of DPI (10 μM), apocynin (1 μM), aspirin (100 μM), allopurinol (100 μM) or rotenone (10 μM) on prednisolone (1 μM)-stimulated O2•− formation by PAECs following a 16-h incubation. Data=mean±s.e.m.; n=6. *P<0.05, comparing prednisolone-induced levels to control in the absence of inhibitors. $P<0.05, significantly inhibited compared to prednisolone-treated cells.
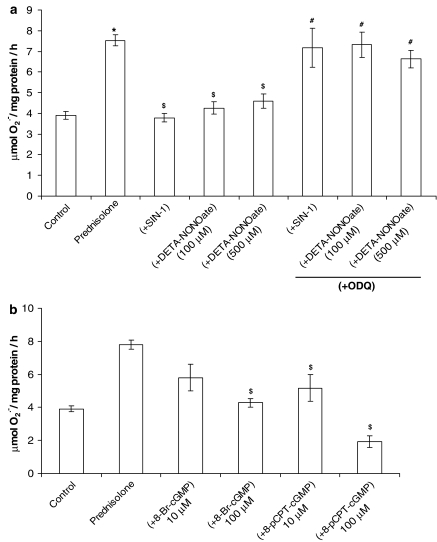
The stimulatory effect of prednisolone on O2•− release from PAECs was blocked by coincubation of prednisolone with the NO donors, SIN-1 and DETA-NONOate (Figure 4a). 8-Bromo-cGMP and 8-pCPT-cGMP (both permeable stable analogues of cGMP) mimicked the effects of SIN-1 and DETA-NONOate on prednisolone-stimulated O2•− formation (Figure 4b) and ODQ reversed effect over the same time course (Figure 4a). These data indicate that endogenous NO plays a role in mediating the effect of prednisolone on NADPH oxidase and O2•− formation through a cGMP-dependent mechanism and not through quenching of O2•−. We have previously demonstrated that SIN-1 and endogenous NO derived from eNOS downregulates NADPH oxidase expression in PA (Muzaffar et al., 2004a).
Figure 4.
(a) Effect of SIN-1 (100 nM) and DETA-NONOate (100–500 μM)+ODQ (1 μM) on prednisolone (1 μM)-stimulated O2•− formation by PAECs over a 16-h incubation. (b) Effect of 8-bromo-cGMP (8-Br-cGMP) and 8-pCPT-cGMP on prednisolone (1 μM)-stimulated O2•− formation by PAECs following a 16-h incubation. Please note that the drugs were present throughout the incubation phase, cells being thoroughly washed, prior to the measurement of superoxide. Data=mean±s.e.m.; n=6. *P<0.05, comparing prednisolone-induced levels to control. $P<0.05, significantly inhibited compared to prednisolone-treated cells. #P<0.05, significantly enhanced compared to prednisolone+SIN-1/DETA-NONOate-treated cells.
Effect of prednisolone on gp91phox and eNOS protein expression in PAECs
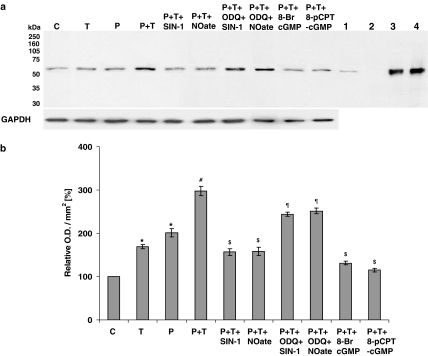
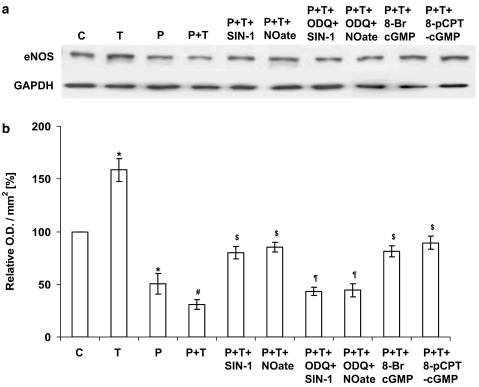
Following a 16-h incubation of PAECs, gp91phox (active catalytic subunit of NADPH oxidase) protein expression, as assessed by Western blotting, was significantly enhanced by 1 μM prednisolone (Figure 5), whereas eNOS protein expression was significantly reduced by 1 μM prednisolone (Figure 6). TNF-α further enhanced prednisolone-induced gp91phox expression (Figure 5) and decreased eNOS protein expression (Figure 6) in PAEC lysates. The effects were inhibited by SIN-1 and DETA-NONOate and guanylyl cyclase activators, 8-bromo-cGMP and 8-pCPT-cGMP. ODQ blocked the effects of SIN-1 and DETA-NONOate on gp91phox (Figure 5) and eNOS (Figure 6) protein expression. These data indicate that an upregulation of NADPH oxidase mediates prednisolone-stimulated O2•− formation and the downregulation of eNOS by prednisolone, and therefore a decrease in endogenous NO may contribute to the upregulation of NADPH oxidase.
Figure 5.
Western analysis of NADPH oxidase in PAECs using a monoclonal antibody directed against the gp91phox subunit of mouse macrophage NADPH oxidase. Cells were either not treated or stimulated overnight with either prednisolone (P; 1 μM) or TNF-α (T; 10 ng ml−1) alone or with combination of the two (±SIN-1 (100 nM); DETA-NONOate (NOate; 500 μM); SIN-1+ODQ (1 μM); DETA-NONOate+ODQ; 8-bromo-cGMP (100 μM) and 8-pCPT-cGMP (100 μM)). Where applied, PAECs were preincubated with ODQ for at least an hour before SIN-1 or DETA-NONOate was added. Cellular lysates of stimulated mouse macrophages (lane 1), pig neutrophils (lane 3) and human neutrophils (lane 4) were used as positive controls. Whole-cell lysate of PAVSMCs (lane 2) was used as an internal control and GAPDH expression as a loading control. Panel a shows the representative blot and panel b the results of the densitometric analyses of six blots (expressed as relative optical density (OD) mm−2). *P<0.05, significantly increased compared to control. #P<0.05, significantly increased compared to prednisolone/TNF-α-treated cells only. $P<0.05, significantly inhibited compared to prednisolone and TNF-α-treated cells. ¶P<0.05, significantly enhanced compared to cells treated with SIN-1 or DETA-NONOate alone in the presence of prednisolone and TNF-α.
Figure 6.
Endothelial NOS protein expression in PAEC as measured by Western blotting. Cells were either not treated or stimulated overnight with either prednisolone (P; 1 μM) or TNF-α (T; 10 ng ml−1) alone or with combination of the two (±SIN-1 (100 nM); DETA-NONOate (NOate; 500 μM); SIN-1+ODQ (1 μM); DETA-NONOate+ODQ; 8-bromo-cGMP (100 μM) and 8-pCPT-cGMP (100 μM)). Where applied, PAECs were preincubated with ODQ for at least an hour before SIN-1 or DETA-NONOate was added. Panel a shows the representative blot and panel b the results of the densitometric analyses of six blots (expressed as relative optical density (OD) mm−2). GAPDH expression was used as a loading control. *P<0.05, significantly different from control. #P<0.05, significantly decreased compared to prednisolone/TNF-α-treated cells only. $P<0.05, significantly increased compared to prednisolone and TNF-α-treated cells. ¶P<0.05, significantly inhibited compared to cells treated with SIN-1 or DETA-NONOate alone in the presence of prednisolone and TNF-α.
Effect of prednisolone on NO formation
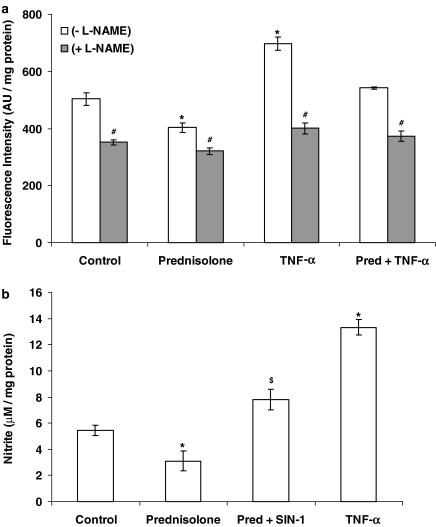
NO levels measured as change in DAF-2 fluorescence intensity and nitrite concentration in culture supernatants of prednisolone-treated PAECs were consistent with the downregulation of eNOS protein expression measured by immunoblotting (Figure 7). TNF-α, on the other hand, augmented DAF-2 fluorescence intensity and nitrite formation following 16-h incubation (Figure 7). The presence of SIN-1 over the 16-h incubation with prednisolone resulted in increased nitrite production from PAECs (Figure 7b).
Figure 7.
(a) Effect of prednisolone (1 μM) and TNF-α (10 ng ml−1) on DAF-2 fluorescence intensity corresponding to the NO production from the PAEC in the presence or absence of L-NAME (1 mM). (b) Effect of prednisolone (1 μM) in the presence or absence of SIN-1 (100 nM) and TNF-α (10 ng ml−1) on nitrite production by PAEC following a16-h incubation. Data=mean±s.e.m.; n=6. *P<0.05, significantly altered compared to control. #P<0.05, significantly reduced compared to corresponding L-NAME absent values. $P<0.05, significantly increased compared to prednisolone alone.
Discussion
The present study demonstrates that prednisolone augments O2•− formation in porcine intact isolated PA (i.e. with endothelium) and in isolated PAECs, but not isolated PAVSMCs, and a decrease in NO formation in isolated PAECs. The mechanisms underlying this effect involve differential effects on the expression/activity of NADPH oxidase and eNOS and the relative levels of O2•− and NO (as nitrites). The decrease in endothelial NO formation, in turn, may elicit vasoconstriction in the VSMC component of the pulmonary arteries. Although EDHF is deemed more important in mediating relaxation of pulmonary microvessels, NO still plays a significant role in promoting relaxation of the pulmonary vasculature (Stuart-Smith & Jeremy, 2001). Reduced endothelial NO bioavailability may also render the vessels susceptible to further inflammation, since NO inhibits adhesion molecule expression and other inflammatory events (Jeremy et al., 1999).
Firstly, increased O2•− formation after the 16-h incubation of both intact PA segments (with endothelium) and PAECs with prednisolone was inhibited by apocynin and DPI (both NADPH oxidase inhibitors). By contrast, rotenone and allopurinol had no effect at concentrations that inhibit O2•− formation in PAECs through mitochondrial respiration and xanthine oxidase, respectively (Muzaffar et al., 2005a). Aspirin, too, had no effect at a concentration that we have shown to inhibit prostanoid formation in pulmonary arterial cells (Muzaffar et al., 2004b). Furthermore, following a 16-h incubation of PAECs with prednisolone, the expression of gp91phox (active catalytic centre of NADPH oxidase) was also significantly enhanced by 1 μM prednisolone. Taken together, these data indicate that the increase in O2•− formation elicited by prednisolone is mediated by an augmentation of NADPH oxidase protein expression. By contrast, prednisolone had no effect on O2•− formation in PAVSMCs, indicating that the effect of prednisolone is confined to PAECs and is mediated by a system(s) other than a direct downregulation of gp91phox expression. This contrasts with a previous report on human VSMCs in which prednisolone was found to inhibit O2•− formation and p22phox expression (Marumo et al., 1998). This may indicate variations of responses between species and vascular beds. Furthermore, Marumo et al. (1998) did not study equivalent ECs, in which we found the most emphatic changes.
Augmented O2•− formation by prednisolone in PAECs was blocked by coincubation of the steroid over the 16-h preincubation phase with the NO donors, SIN-1 and DETA NONOate. Since the cells were washed free of drugs after the 16-h incubation phase and prior to the assessment of O2•− release, these effects could not be ascribed to direct chemical reactions of O2•− with NO derived from SIN-1 or DETA NONOate (i.e. a ‘quenching' effect). The effects over 16 h of the NO donors was also mimicked by the stable cGMP analogues, 8-bromo-cGMP and 8-pCPT-cGMP, but blocked by the guanylyl cyclase inhibitor, ODQ. These data clearly demonstrate that the effect of prednisolone is mediated through an inhibitory effect on the NO–cGMP–PKG axis. In the present study, prednisolone also had a marked inhibitory effect on eNOS expression in PAECs. In previous studies, we have demonstrated that SIN-1 inhibits the expression of gp91phox (Muzaffar et al., 2004a), and that NO derived from eNOS inhibits the expression of gp91phox in the PA via a guanylyl cyclase-dependent mechanism (Muzaffar et al., 2004a). Another study has also demonstrated that glucocorticoids, in particular cortisol, downregulates eNOS expression in bovine cultured aortic endothelial cells (Rogers et al., 2002). It is suggested, therefore, that prednisolone, by inhibiting eNOS expression, reduces NO levels in the PA and since NO blocks gp91phox expression, this would explain the effect of prednisolone on gp91phox expression and enhanced O2•− formation. Also, since NO reacts readily with O2•− to form reactive nitrogen species (RNS), effectively removing O2•−, the reduction of eNOS expression would also increase O2•− via removal of this direct chemical mechanism. Indeed, we have previously shown increased nitrotyrosine levels (index of RNS) in endothelial cells in intact pulmonary arteries incubated under identical conditions (Muzaffar et al., 2003).
It appears that endogenous integrity of the NO formation is important in the aetiology of ARDS since inhalational NO has proved beneficial in treating the condition (Klinger, 2002). However, the benefits of inhaled NO in treating ARDS have proved ambivalent (Folkerts et al., 2001; Weinberger et al., 2001; Klinger, 2002; Wang et al., 2003). Adverse effects include a life-threatening ‘rebound' increase in pulmonary vascular resistance, as well as methemoglobinaemia and cellular apoptosis (Weinberger et al., 2001). These observations indicate, therefore, that an upregulation of eNOS may in fact be deleterious when accompanied by an increase in O2•− formation. By contrast, in a recent multicentre clinical trial, in which the NOS inhibitor, 546C88, was studied for safety and efficacy in 797 patients with septic shock, it was found that the inhibitor caused a significant increase in mortality (Lopez et al., 2004). This latter trial would seem to indicate that the protective effect of endogenous NOS, in particular eNOS, may outweigh the other negative effects of NO and the metabolites derived from its reaction with O2•−. However, inhalational NO may still be deleterious due the large intrapulmonary levels of NO elicited by this treatment.
With regard to corticosteriods and ARDS, circulating cortisol is markedly elevated in patients with ARDS (Bernard et al., 1987; Vermes et al., 1995). Glucocorticoid receptor affinity in lungs is also increased in experimental ARDS (Liu et al., 1993) and glucocorticoid administration diminishes eNOS activity, in vivo (Middelveld et al., 1999). It has also been demonstrated that endotoxin and cytokines augment the expression of eNOS in PAECs, which may constitute a protective mechanism in ARDS (Muzaffar et al., 2003). It is not unreasonable to suggest, therefore, that increased circulating cortisol in ARDS may contribute to the progression of the syndrome via down regulation of lung eNOS and/or prevention of eNOS upregulation.
In the present study, TNF-α and prednisolone together augmented the formation of O2•−, which may be of pathophysiological importance, since TNF-α plays an axiomatic role in mediating the progress of ARDS (Chabot et al., 1998; Muzaffar et al., 2003). This observation consolidates, therefore, that the administration of prednisolone may worsen ARDS through augmentation of OS. The mechanisms underlying this interaction appear paradoxical since TNF-α upregulates eNOS in pulmonary PAECs (Muzaffar et al., 2003) and in this study we found that prednisolone down-regulates the enzyme. However, TNF-α also directly upregulates gp91phox expression and markedly augments the formation of O2•− (Muzaffar et al., 2004b). Since the present study demonstrates that the prednisolone also augments gp91phox expression, it is likely that the interaction between TNF-α and prednisolone involves the potentiation of gp91phox expression that overrides effects of NO derived from eNOS. This may be of therapeutic importance since exogenous NO may be unable to counteract the pro-inflammatory impact of O2•− in ARDS. One intriguing possibility that may explain this mechanism is that O2•− itself can augment both gp91phox expression and O2•− formation by isolated PAECs (Muzaffar et al., 2005b).
To summarise, it appears that the O2•− generated by the simultaneous down regulation of eNOS coupled with the upregulation of gp91phox is the underlying mechanism by which the prednisolone elicits an increase in O2•− formation in PAECs. There are clinical implications to this study. Prednisolone, a potent anti-inflammatory drug, is surprisingly ineffective in treating ARDS (Bone et al., 1987; Luce et al., 1988; Meduri & Chrousos, 1998), an aggressive acute inflammatory condition. However, ARDS is now firmly associated with the generation of O2•−, which elicits a number of important proinflammatory events, including activation of leucocytes and platelets, the expression of adhesion molecules and vasoconstriction, all of which augment ARDS (Chabot et al., 1998). Thus, the augmentation of O2•− formation may explain why prednisolone is ineffective in treating acute ARDS. Furthermore, the reduction of NO elicited by prednisolone would render the vasculature even more susceptible to inflammation since NO inhibits all the events promoted by O2•−. The overall implication of this study is that prednisolone may be rendered more effective in treating ARDS if NO was administered simultaneously with the glucocorticoid. It is notable, however, that despite its lack of effect on early phases of ARDS, prednisolone has been shown to be effective in preventing longer term remodelling and fibrosis in patients who survive ARDS (Meduri & Chrousos, 1998). It would be intriguing, therefore, to study the role of eNOS in mediating maladaptive lung repair and fibrosis.
Abbreviations
- ARDS
acute respiratory distress syndrome
- O2•−
superoxide
- ONOO−
peroxynitrite
- OS
oxidative stress
- PAECs
pulmonary artery endothelial cells
- PAVSMCs
pulmonary artery vascular smooth muscle cells
References
- BERNARD G.R., LUCE J.M., SPRUNG C.L., RINALDO J.E., TATE R.M., SIBBALD W.J., KARIMAN K., HIHGGINS S., BRADLEY R., METZ C.A. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N. Engl. J. Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- BJORGVINSDOTTIR H., ZHEN L., DINAUER M.C. Cloning of murine gp91phox cDNA and functional expression in a human X-linked chronic granulomatous disease cell line. Blood. 1996;87:2005–2010. [PubMed] [Google Scholar]
- BONE R.C., FISCHER J.C.J., CLEMMER T.P., SLOTMAN G.J., METZ C.A. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92:1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- CHABOT F., MITCHELL J.A., GUTTERIDGE J.M.C., EVANS A.M. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11:745–757. [PubMed] [Google Scholar]
- FOLKERTS G., KLOEK J., MUIJSERS R.B., NIJKAMP F.P. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- GREENE E.L., PALLER M.S. Xanthine oxidase produces O2•− in posthypoxic injury of renal epithelial cells. Am. J. Physiol. 1992;263:F251–F255. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., MINIERI C.A., OLLERENSHAW J.D., ALEXANDER R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- JANTZ M.A., SHAN S.A. Corticosteroids in acute respiratory failure. Am. J. Respir. Crit. Care Med. 1999;160:1079–1100. doi: 10.1164/ajrccm.160.4.9901075. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., ROWE D., EMSLEY A.M., NEWBY A.C. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc. Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- KLINGER J.R. Inhaled nitric oxide in ARDS. Crit. Care Clin. 2002;18:45–68. doi: 10.1016/s0749-0704(03)00064-2. [DOI] [PubMed] [Google Scholar]
- LEIKERT J.F., RATHEL T.R., MULLER C., VOLLMAR A.M., DIRSCH V.M. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001;506:131–134. doi: 10.1016/s0014-5793(01)02901-5. [DOI] [PubMed] [Google Scholar]
- LIU L.Y., SUN B., TIAN Y., LU B.Z., WANG J. Changes of pulmonary glucocorticoid receptor and phospholipase A2 in sheep with acute lung injury after high dose endotoxin infusion. Am. Rev. Respir. Dis. 1993;148:878–881. doi: 10.1164/ajrccm/148.4_Pt_1.878. [DOI] [PubMed] [Google Scholar]
- LOPEZ A., LORENTE J.A., STEINGRUB J., BAKKER J., MCLUCKIE A., WILLATTS S., BROCKWAY M., ANZUETO A., HOLZAPFEL L., BREEN D., SILVERMAN M.S., TAKALA J., DONALDSON J., ARNESON C., GROVE G., GROSSMAN S., GROVER R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit. Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- LUCE J.M., MONTGOMERY A.B., MARKS J.D., TURNER C.A., METZ C.A., MURRAY J.F. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with sepsis. Am. Rev. Respir. Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- MARUMO T., SCHINI-KERTH V.B., BRANDES R.P., BUSSE R. Glucocorticoids inhibit superoxide anion production and p22 phox mRNA expression in human aortic smooth muscle cells. Hypertension. 1998;32:1083–1088. doi: 10.1161/01.hyp.32.6.1083. [DOI] [PubMed] [Google Scholar]
- MEDURI G.U., CHROUSOS G.P. Duration of glucocorticoid treatment and outcome in sepsis: is the right drug used the wrong way. Chest. 1998;114:355–360. doi: 10.1378/chest.114.2.355-a. [DOI] [PubMed] [Google Scholar]
- MEIER B., RADEKE H.H., SELLE S., YOUNES M., SIES H., RESCH K., HABERMEHL G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem. J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDELVELD R.J., WANECEK M., BERGMAN D., WEITZBERG E., ALVING K. Effect of cortisol-synthesis inhibition on endotoxin-induced porcine acute lung injury, shock, and nitric oxide production. Shock. 1999;12:382–390. doi: 10.1097/00024382-199911000-00007. [DOI] [PubMed] [Google Scholar]
- MUZAFFAR S., JEREMY J.Y., ANGELINI G.D., STUART-SMITH K., SHUKLA N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., ANGELINI G.D., JEREMY J.Y. Nitroaspirins and morpholinosydnonimine, but not aspirin, inhibit the formation of superoxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation. 2004a;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., ANGELINI G.D., JEREMY J.Y. Hypoxia simultaneously induces the expression of gp91phox and endothelial nitric oxide synthase in the pulmonary artery. Thorax. 2005a;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., ANGELINI G.D., JEREMY J.Y.Superoxide promotes gp91phox expression in pulmonary artery endothelial cells Br. J. Pharmacol. 2005b(in press) [DOI] [PMC free article] [PubMed]
- MUZAFFAR S., SHUKLA N., LOBO C., ANGELINI G.D., JEREMY J.Y. Iloprost inhibits superoxide formation and gp91phox expression induced by the thromboxane A2 analogue U46619, 8-isoprostane F2a, prostaglandin F2a, cytokines and endotoxin in the pig pulmonary artery. Br. J. Pharmacol. 2004b;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATSUBO N., KOJIMA H., KIKUCHI K., NAGOSHI H., HIRATA Y., MAEDA D., IMAI Y., IRIMURA T., NAGANO T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- NEWTON R. Molecular mechanisms of glucocorticoid action: what is important. Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMS R.W., COOK J.C., KRISHNA M.C., CHRISTODOULOU D., POORE C.M., MILES A.M., GRISHAM M.B., WINK D.A. Colorimetric assays for nitric oxide and nitrogen oxide species formed from nitric oxide stock solutions and donor compounds. Methods Enzymol. 1996;268:93–105. doi: 10.1016/s0076-6879(96)68012-4. [DOI] [PubMed] [Google Scholar]
- RATHEL T.R., LEIKERT J.J., VOLLMAR A.M., DIRSCH V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proc. Online. 2003;5:136–142. doi: 10.1251/bpo55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS K.M., BONAR C.A., ESTRELLA J.L., YANG S. Inhibitory effect of glucocorticoid on coronary artery endothelial function. Am. J. Physiol. 2002;283:H1922–H1928. doi: 10.1152/ajpheart.00364.2002. [DOI] [PubMed] [Google Scholar]
- STOLK J., HILTERMANN T.J., DIJKMAN J.H. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell. Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- STUART-SMITH K., JEREMY J.Y. Microvessel damage in acute respiratory distress syndrome: the answer may not be NO. Br. J. Anaesth. 2001;87:272–279. doi: 10.1093/bja/87.2.272. [DOI] [PubMed] [Google Scholar]
- TATE R.M., MORRIS H.G., SCHROEDER W.R., REPINE J.E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J. Clin. Invest. 1984;74:608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERMES I., BEISHUIZEN A., HAMPSINK R.M., HAANEN C. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J. Clin. Endocrinol. Metab. 1995;80:1238–1242. doi: 10.1210/jcem.80.4.7714094. [DOI] [PubMed] [Google Scholar]
- WANG T., EL KEBIR D., BLAISE G. Inhaled nitric oxide in 2003: a review of its mechanisms of action. Can. J. Anaesth. 2003;50:839–846. doi: 10.1007/BF03019384. [DOI] [PubMed] [Google Scholar]
- WEINACKER A.B., VASZAR L.T. Acute respiratory distress syndrome: physiology and new management strategies. Annu. Rev. Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- WEINBERGER B., LASKIN D.L., HECK D.E., LASKIN J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001;59:5–16. doi: 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- YU L., QUINN M.T., CROSS A.R., DINAUER M.C. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]