Abstract
Besides its antimuscarinic effects, propiverine may possess an additional mode of action. We compared the effects of propiverine, three of its metabolites (M-5, M-6, M-14) and atropine in human, pig and mouse urinary bladder preparations in order to elucidate the nature of a possible additional mode of action.
Like the parent compound, M-5, M-6 and M-14 reduced to variable degrees the contractions elicited by electric field stimulation (EFS) of isolated, urothelium-denuded detrusor strips. In mouse the atropine-resistant and therefore the nonadrenergic, noncholinergic component of contractile response to EFS was reduced by M-5, M-14 and propiverine, but was hardly affected by M-6.
Atropine, propiverine and M-6 significantly shifted the cumulative concentration–response curves for carbachol (CCh) to higher concentrations. Atropine and M-6 did not affect the maximum tension induced by CCh. Propiverine, M-5 and M-14 reduced the maximum CCh effect, suggesting at least one additional mode of action. This pattern of response was observed in all the three species, albeit with some differences in sensitivity to the various agents.
In freshly isolated human detrusor smooth muscle cells, propiverine and M-14 inhibited the nifedipine-sensitive L-type calcium current (ICa) in a concentration-dependent manner. In contrast, the effects of M-5 and M-6 on ICa were insignificant in the concentration range examined.
The investigated responses to propiverine and its metabolites suggest that impairment of maximum CCh-induced contractions is due to strong effect on ICa and that this may be associated with the presence of the aliphatic side chain.
Keywords: Propiverine and metabolites; electric field stimulation; concentration–response curves for carbachol; human, porcine and mouse detrusor; L-type calcium current
Introduction
Activation of muscarinic (M) receptors plays a major role in the control of urinary bladder contractility (Andersson, 1993). Although M2 receptors are the predominant subtype found in detrusor muscle (Yamanishi et al., 2001), M3 receptors appear to mediate urinary bladder contractile responses in many species (Yamanishi et al., 2000; Chess-Williams et al., 2001; Choppin & Eglen, 2001; Fetscher et al., 2002). Detrusor contraction in response to muscarinic receptor (M receptor) stimulation requires Ca2+ entry via nifedipine-sensitive L-type calcium channels (Schneider et al., 2004a; Wegener et al., 2004).
Antimuscarinic drugs are used for the treatment of the overactive bladder syndrome (OAB; Sellers et al., 2001; Andersson et al., 2002; Andersson & Yoshida 2003; Ouslander, 2004). In addition to its antimuscarinic effect, propiverine appears to have additional spasmolytic effects (Andersson et al., 1999; Wuest et al., 2002). Thus, propiverine inhibits contractile responses elicited by electric field stimulation (EFS) as well as acetylcholine (ACh)-induced contractions in human detrusor strips (Wada et al., 1995). The drug potently reduces KCl-induced contractions in guinea-pig (Haruno, 1991; Tokuno et al., 1993), as well as KCl- and CaCl2-induced contractions in human bladder strips (Wada et al., 1995). In rabbit, propiverine also impairs intracellular Ca2+ homeostasis in addition to its antagonistic muscarinic effects (Madersbacher & Mürtz, 2001).
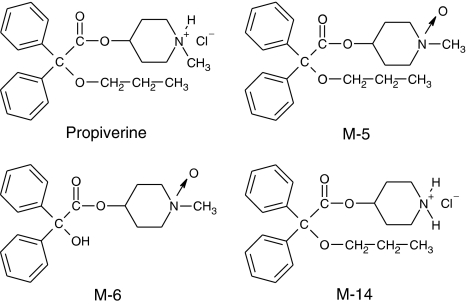
Propiverine is rapidly absorbed after oral administration and is subject to extensive first-pass metabolism, giving rise to several active metabolites, for example, M-5, M-6 and M-14 (for chemical structures, see Figure 1). At 24 h after oral application, these compounds can be recovered among other derivatives from the human urine (in percent of the original dose): propiverine, 2–3%; M-5, 20%; M-6, 5%; M-14, 1% (Haustein & Hüller, 1988). Siepmann et al. (1998) reported that after 5 days of treatment with multiple dosing of propiverine the following maximum serum concentrations were detected (median; range): propiverine (155 (96–240) ng ml−1) corresponding to 0.38 (0.24–0.59) μM, and its N-oxide M-5 (645 (385–955) ng ml−1) corresponding to 1.68 (1.00–2.49) μM. The metabolites M-6 and M-14 were not detected in serum but in urine only.
Figure 1.
Chemical structures of propiverine and its metabolites M-5, M-6 and M-14.
The pharmacological activities of these metabolites have not yet been studied in detail (Andersson et al., 1999). In order to estimate their contribution to the therapeutic action, we have chosen the three main metabolites in man (M-5, M-6 and M-14) for investigation of their effects on detrusor muscle and used atropine and the congener propiverine for comparative purposes. Pig detrusor was studied because of its similarity to human detrusor. Mouse detrusor was studied because of its high nonadrenergic–noncholinergic (NANC) component of contraction (Wuest et al., 2002). Furthermore, we investigated the effects of these compounds on contractions in human, pig and mouse urinary bladder preparations and on the L-type calcium current (ICa) in human and porcine detrusor smooth muscle cells (DMSC) to estimate additional mechanisms of propiverine and possibly also of its metabolites.
Methods
Materials
Human urinary bladder tissue was obtained from 10 male and 11 female patients (age range: 58–80 years) undergoing radical cystectomy for bladder cancer. All patients had given informed written consent in accordance with the regulations of the local ethical committee. Samples from tumour-free parts of the bladder wall were taken. After removal of the serosa and mucosa, four to eight muscle strips (10–15 mm long, 4–5 mm wide) were dissected from each sample. The remaining tissue was stored overnight at 4°C and used for isolation of detrusor smooth muscle cells (DSMC).
Muscle strips from pig and mouse bladder were prepared as described previously (see Wuest et al., 2002). Urinary bladders of female pigs were obtained from a local abattoir transported to the laboratory in transport buffer at 4°C. Serosa and mucosa were removed from a 2 × 2 cm tissue piece of the anterior wall, out of which four to eight longitudinal muscle strips (7–10 mm long, 2–4 mm wide) were dissected.
Male C57Bl6 (Charles River) mice weighing 20–30 g were killed by cervical dislocation. The urinary bladder was removed at the bladder neck. After cutting off the dome of the bladder, the remaining muscle ring was opened longitudinally and cut into strips after removal of the mucosa (two strips per bladder for electric field and four strips for carbachol (CCh) stimulation).
Detrusor muscle contraction experiments
Muscle strips were mounted in 25-ml (human and pig) or 5-ml organ baths (mouse) containing carbogen-gassed Tyrode's solution maintained at 37°C. Tension generated was measured with an isometric force transducer (GM 2, Föhr Medical Instruments, Seeheim/Ober Beerbach, Germany), amplified and recorded with a data and recording system (Chart 4.0™, ADInstruments, Sydney, Australia). Resting load was set to 10 mN for human and pig and 5 mN for mouse preparations, and was readjusted after 30 min. During the equilibration period of 60 min, the bath solution was changed once.
In EFS-experiments, pig and mouse strips were challenged twice with CCh (1 μM in pig and 1 or 10 μM in mouse for 15 min each) with a 15 min washout period between exposures. After 20 min of stabilization, muscle strips were subjected to EFS. Human detrusor strips were not initially exposed to CCh because of the marked desensitization. In preliminary experiments, the mean amplitude of the first EFS-induced contractions was only about one-quarter of the amplitude obtained without prior CCh challenge, and contraction amplitudes almost doubled in the course in time-matched control (TMC) experiments (unpublished results). The parameters for EFS (stimulator, Föhr Medical Instruments, Seeheim/Ober Beerbach, Germany) were: pulse duration 1 ms at 30 Hz with 90 mA. Stimuli trains lasted 2 s (mouse) or 5 s (pig, man). The compounds under investigation were added in cumulatively increasing concentrations with 30 min between increments. To estimate the non-neuronally mediated portion of muscle contraction under our stimulation conditions, nerve conduction was completely blocked by adding the neurotoxin tetrodotoxin (TTX, 1 μM) at the end of each experiment. Average values for the EFS-induced muscle contraction amplitudes were obtained from the last five contractions before the next concentration increase. The magnitude of drug effect is given in percent inhibition of the electrically evoked contraction amplitude before any substance addition (=100%).
The antimuscarinic actions of the compounds were examined in separate experiments by assessing their effects on cumulative concentration–response curves (CRC) for CCh. The first CRC was obtained at the end of the equilibration period of 60 min. After a washout period of 1 h, the test drug was added; a second CRC for CCh in the presence of the test compound was started after one further hour. TMC experiments were run without any drug added. Peak increase in force of contraction induced by the individual CCh concentrations was expressed as percent of the maximum effect observed during the first CRC.
Isolation of DSMC
Mucosa and serosa-free tissue pieces of human or pig urinary bladder were cut into small pieces, washed three times in chilled, nominally Ca2+-free Tyrode's solution. Tissue pieces were then transferred into Ca2+-free Tyrode's solution containing 279 U ml−1 collagenase type I (Worthington) for pig or 266 U ml−1 for man plus 4.6 mg ml−1 protease type XXIV (Sigma-Aldrich) and gently stirred for 45 min. After 10 min the Ca2+ concentration was raised to 0.2 mM. Stirring was continued in Tyrode's solution (0.2 mM Ca2+) with collagenase only until long, narrow and spindle-shaped DSMC were seen (after 10–20 min). Cells were released from the tissue mass by gentle trituration. The cell suspension was centrifuged, the pellet resuspended and stored until use in Ca2+ (0.5 mM) containing Tyrode's solution at room temperature. The cells remained viable for up to 8 h.
Whole-cell recording of ICa
ICa was measured at room temperature with standard voltage-clamp technique (Axopatch 200, Axon Instruments, Foster City, CA, U.S.A.), ISO 2 software was used for data acquisition and analysis (MFK, Niedernhausen, Germany). Heat-polished pipettes were pulled from borosilicate filamented glass (Hilgenberg, Malsfeld, Germany). Tip resistances were 2–6 MΩ, seal resistances were about 1 GΩ. Cell capacitance (CM) was calculated from steady-state current during depolarizing ramp pulses (1 V s−1) from −40 to −35 mV. ICa were measured from a holding potential of −60 at +10 mV test potential. For further isolation of ICa from contaminating currents, Na+ was replaced with tetraethylammonium ions and K+ was replaced with Cs+ to block K+ currents. Current–voltage relations were obtained by a series of test pulses between −50 and +50 mV. The experiments were performed with the following Na+-free superfusion solution (in mM): tetraethylammonium chloride 120, CsCl 10, HEPES 10, BaCl2 3.8, MgCl2 1 and glucose 20, pH 7.4 (adjusted with CsOH). The pipette solution (pH 7.2) included (in mM): cesium methanesulphonate 90, CsCl 20, HEPES 10, Mg-ATP 4, Tris-GTP 0.4, EGTA 10 and CaCl2 3, with calculated free Ca2+ concentration of ∼60 nmol l−1 (computer program EQCAL, Bio soft, Cambridge, U.K.). Current amplitudes were determined as the difference between peak inward current and current at the end of the depolarising step or as nifedipine-sensitive peak inward current.
A system for rapid solution changes allowed application of substances in the close vicinity of the cells (Cell Micro Controls, Virginia Beach, VA, U.S.A.; ALA Scientific Instruments, Long Island, NY, U.S.A.).
Drugs
The composition of the solutions was (in mM): transport buffer: NaCl 149, KCl 2.7, CaCl2 1.8, NaH2PO4 0.1, Na2HPO4 0.7, glucose 5.6, pH 7.4 adjusted with NaOH; Tyrode solution: NaCl 127, KCl 5.4, MgCl2 1.05, CaCl2 1.8, NaH2PO4 0.4, Na2CO3 22, glucose 5.6, pH 7.4 when gassed with 95% O2 and 5% CO2. All chemicals were of analytical grade and purchased from SIGMA-ALDRICH (Taufkirchen, Germany). CCh, atropine sulphate and TTX acetate were also purchased from SIGMA-ALDRICH. Propiverine-HCl (2,2-diphenyl-2-propoxy-acetic acid [1-methyl-piperid-4-yl]-ester-hydrochloride), M-5 (2,2-diphenyl-2-propoxy-acetic acid [1-methyl-piperid-4-yl]-ester-N-oxide-trans), M-6 (2,2-diphenyl-2-hydroxy-acetic acid [1-methyl-piperid-4-yl]-ester-N-oxide-trans) and M-14 (2,2-diphenyl-2-propoxy-acetic acid [piperid-4-yl]-ester) were provided by APOGEPHA Arzneimittel GmbH, Dresden, Germany. Drugs were made up as a 0.1 M stock solution in Milli-Q-water (CCh) or dimethyl sulphoxide (DMSO; all anticholinergics), and further diluted with Milli-Q-water. The highest concentration of DMSO 1% (v v−1) did not affect the amplitude or time course of detrusor contractions.
Data analysis
All data are expressed as the mean±s.e.m. Individual IC50's (molar drug concentration producing 50% inhibition of maximum contractile response to EFS) were determined by nonlinear regression analysis for each individual experiment using GraphPad Prism® 3.02 (GraphPad Software Inc., San Diego, U.S.A.). Mean IC50 values±s.e.m. from n experiments are expressed as −log IC50 [M] (in Table 1). Differences between drugs were tested by Student's t-test, or one-way ANOVA with an additional Bonferroni's multiple comparison test, and were considered significant for P<0.05.
Table 1.
Inhibitory effects of drugs on EFS-induced contractions in detrusor strips
| Compound | n | Human | n | Pig | n | Mouse |
|---|---|---|---|---|---|---|
| Propiverine NS | 5 | 5.44±0.56 | 8 | 4.73±0.12 | 8 | 5.09±0.09 |
| M-5NS | 6 | 4.14±0.11 | 6 | 3.90±0.15 | 8 | 3.89±0.11 |
| M-6 | 7 | 7.05±0.15** | 13 | 5.38±0.34 | 8 | 4.69±0.31 |
| M-14NS | 6 | 4.40±0.29 | 16 | 4.24±0.23 | 8 | 4.69±0.11 |
| AtropineNS | 5 | 7.60±0.38 | 18 | 7.82±0.08 | 15 | 7.85±0.10 |
Data expressed as −log IC50 values (means±s.e.m.; IC50 in M). n, number of experiments.
NS – no statistical significant differences between the three species.
P<0.01 for −log IC50 value in man vs pig and mouse.
Cumulative CRC were analysed by nonlinear regression of each individual experiment using GraphPad Prism® 3.02 (GraphPad Software Inc., San Diego, U.S.A.), mean EC50 values for CCh (molar concentration producing 50% of the maximum contraction response) were calculated for CRC before and after test drug addition. pA2 values for the antagonistic effect of M-6 on CRC for CCh in human detrusor were estimated from Schild plot analysis (Arunlakshana & Schild, 1959).
Results
Effects on electrically induced contractions
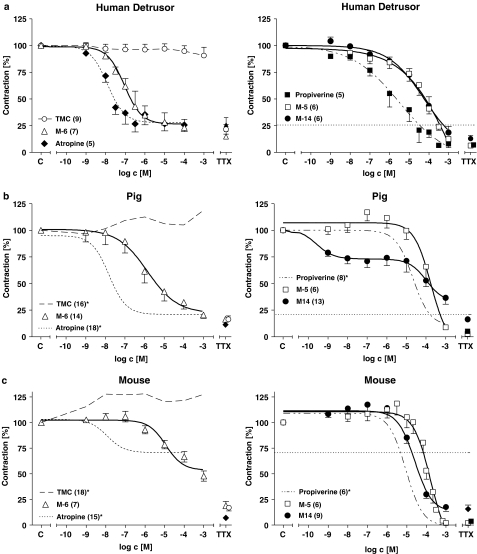
The drug effects on EFS-elicited tension and the TMC in human, pig and mouse detrusor are summarized in Figure 2. In TMC with human detrusor, force development remained constant within a period of 4 h (Figure 2a). In mouse force increased by about 25% during the first hour and remained stable thereafter (Figure 2c), and a similar, though less pronounced, time course was observed for TMC experiments in pig detrusor (Figure 2b).
Figure 2.
Average CRCs for the effects of M-5, M-6 and M-14 in comparison to those of propiverine and atropine on EFS-evoked contractions in detrusor muscle from man (a), pig (b) and mouse (c). Force is expressed in percent of the pre-drug control value (=100%). TMC, time-matched control without any drug added. The horizontal dashed lines in the right side of the figure represent the contraction amplitude in the presence of the highest atropine concentration. The percent of direct smooth muscle contractions in the presence of 1 μM TTX is plotted for each experimental group on the far right of each diagram. Mean values±s.e.m. from n experiments. *Labelled data in pig and mouse were taken from previous work and are included for comparative purposes (Wuest et al., 2002).
In human detrusor, atropine reduced EFS-induced force of contraction with high potency (for −log IC50 value, see Table 1). In the presence of the highest atropine concentration, contraction amplitude still amounted to 26±6% of control (n=5; P<0.001 vs TMC). TTX (1 μM) could not further reduce electrically evoked contractions (26±7%, n=5, Figure 2a). This TTX-resistant amplitude was similar as in TMC (22±5%, n=6, NS). The CRC for propiverine was less steep, required higher concentrations and reached 8±3% of control (n=5; P<0.001 vs TMC). There were distinct differences between the effects of the metabolites. M-5 and M-14 were less potent than propiverine (see Table 1), but depressed force to a similar degree (i.e. M-5 to 12±3% of control (n=6; P<0.001 vs TMC) and M-14 to 18±6% of control (n=6; P<0.001 vs TMC). The CRC for M-6 was distinct from that of M-5 or M-14. The slope was as steep as with atropine though M-6 was slightly less potent (Table 1). This difference was not statistically significant. M-6 decreased force to 23±8% of control (n=7; P<0.001 vs TMC).
In pig detrusor, the CRC for atropine was similar to that in human detrusor (Figure 2b; Table 1). Contraction in the presence of maximum atropine concentration was 20±2% of control (n=18, NS vs human detrusor). Propiverine was slightly less potent (difference did not reach statistical significance) and the CRC was steeper than in human detrusor. However, like in human detrusor, propiverine reduced force similiarly to 10±1% of control (n=8). The CRC for M-5 lay in a similar concentration range as for human detrusor. M-14 reduced contraction amplitude by about 25% already at very low concentrations (1 nM), the remainder of the response occurred within the same concentration range as in human strips. At the highest M-14 concentration (1 mM), force was reduced to 29±5% of control (n=16). Compared with atropine, the −log IC50 value of M-6 was more than 2 log units lower than for atropine and force was depressed to the same extent (to 19±3% of control; n=14).
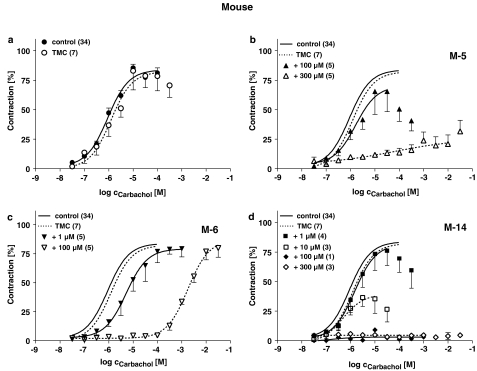
In mouse detrusor, the effects of the compounds on EFS-induced contractions produced yet a different pattern of responses (Figure 2c). In this species, TTX (1 μM) reduced TMC contractions to 17±3% of control. The percent contraction in the presence of the highest atropine concentration was 70±3% of control (n=15). TTX (1 μM) further reduced contractions to 7±1% of control, indicating a large NANC component of contractions of about 60% of predrug control (see Wuest et al., 2002). Propiverine completely suppressed force of contraction with a steep CRC in a similar concentration range as for pig detrusor. M-5 also suppressed force completely with about 1 log unit lower potency than propiverine. M-14 was slightly less potent and also somewhat less efficacious (force reduction to 18±4% of control; n=8). With M-6, higher concentrations than with propiverine were required for half maximum force reduction, 1 mM of M-6 reduced force of contraction to 48±5% of control (n=8).
Impairment of the NANC component (i.e. the difference contraction in the presence of atropine and TTX) in mouse detrusor by the three investigated metabolites suggests that besides their antimuscarinic effects one or more additional mechanisms of action may occur. Therefore, the influence of the compounds on CRCs for CCh was also studied.
Cumulative CRC for CCh
Increasing concentrations of CCh (30 nM–100 μM, 5 min exposure time each) elicited strong force of contraction in all the three investigated species. The mean −log EC50 (M) from all first CRCs were: man 6.15±0.07 (n=52); pig 5.76±0.08 (n=51) and mouse 5.69±0.09 (n=34). The effects of M-5, M-6 and M-14 on the cumulative CRCs for CCh in human, pig and mouse detrusor are summarized in Figures 3, 4 and 5, the −log EC50 values and maximum effects of CCh are listed in Tables 2, 3 and 4.
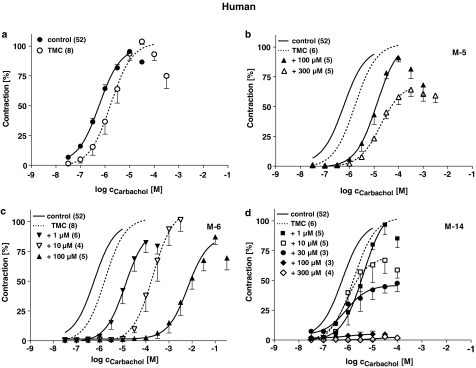
Figure 3.
Effects of M-5 (b), M-6 (c) and M-14 (d) on cumulative CRC for CCh in human detrusor muscle strips in comparison to TMC experiments without any drugs added (a). Data are shown as means±s.e.m. from n experiments. Responses to CCh are expressed in percent of the maximum effect in the first CRC. Note that the continuous and dashed lines have been fitted to the mean values without considering the decline of force at very high concentrations. Control data in (a) are averaged values from the first CRC's in all experiments (n=46, continuous line) and are also together with the TMC data (dashed line) depicted in (b), (c) and (d) without data points.
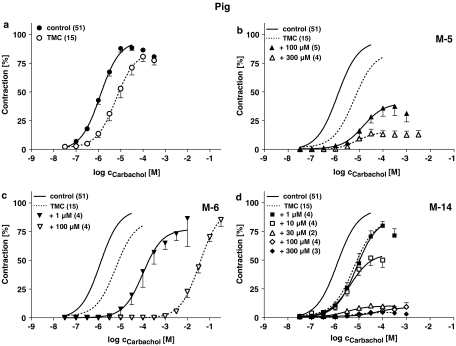
Figure 4.
Effects of M-5 (b), M-6 (c) and M-14 (d) on cumulative CRC for CCh in pig detrusor strips in comparison to TMC experiments without any drugs added (a). Data are shown as means±s.e.m. from n experiments. Responses to CCh are expressed in percent of the maximum effect in the first CRC. Lay-out like in the Figure 4.
Figure 5.
Effects of M-5 (b), M-6 (c) and M-14 (d) on cumulative CRC for CCh in mouse detrusor strips in comparison to TMC experiments without any drugs added (a). Data are shown as means±s.e.m. from n experiments. Responses to CCh are expressed in percent of the maximum effect in the first CRC. Lay-out like in Figure 4.
Table 2.
Effects of propiverine metabolites on the CRC for CCh in human detrusor
| First CRC- | Second CRC | ||||
|---|---|---|---|---|---|
| n | −log EC50 | −log EC50 | Δ−log EC50a | Effmax | |
| TMC | 8 | 6.13±0.25 | 5.64±0.20 | 0.50±0.11 | 108±3 |
| M-5 | |||||
| 100 μM | 5 | 5.99±0.19 | 4.95±0.16 | 0.54±0.18** | 92±4** |
| 300 μM | 5 | 6.37±0.15 | 4.68±0.09 | 1.19±0.13*** | 66±6*** |
| M-6 | |||||
| 1 μM | 5 | 6.08±0.17 | 4.78±0.19 | 0.74±0.19** | 89±5* |
| 10 μM | 4 | 6.30±0.20 | 3.60±0.20 | 2.19±0.26*** | 110±9 |
| 100 μM | 5 | 6.07±0.18 | 2.19±0.27 | 3.37±0.31*** | 81±7* |
| M-14 | |||||
| 1 μM | 5 | 5.52±0.22 | 5.34±0.19 | −0.11±0.06 | 99±8* |
| 10 μM | 5 | 6.43±0.23 | 5.71±0.14 | 0.22±0.14 | 78±10** |
| 30 μM | 3 | 6.69±0.19 | 5.94±0.18 | 0.25±0.09 | 48±4*** |
| 100 μM | 3 | 6.84±0.14 | Not calculated | Not calculated | 5±3*** |
| 300 μM | 4 | 6.01±0.26 | Not calculated | Not calculated | 5±3*** |
−log EC50 (M) and Effmax (% of the maximum response in the first CRC) was determined from curve fitting to the experimental data obtained in each individual muscle strip. Data as means±s.e.m., n=number of detrusor strips. Δ−log EC50 are the differences between first and second CRC (i.e. the shift of CRC to higher CCh concentrations) and were corrected for the mean shift for TMC (with exception for the TMC itself).
P<0.05;
P<0.01;
P<0.001.
Tested for difference from zero.
Table 3.
Effects of propiverine metabolites on the CRC for CCh in pig detrusor
| n | First CRC- | Second CRC | |||
|---|---|---|---|---|---|
| −log EC50 | −log EC50 | Δ−log EC50a | Effmax | ||
| TMC | 15 | 5.64±0.19 | 5.00±0.13 | 0.63±0.11 | 90±3 |
| M-5 | |||||
| 100 μM | 5 | 5.91±0.17 | 4.94±0.26 | 0.34±0.21 | 40±8*** |
| 300 μM | 4 | 5.99±0.14 | 5.02±0.16 | 0.34±0.08 | 14±4*** |
| M-6 | |||||
| 1 μM | 5 | 5.69±0.22 | 3.82±0.30 | 1.24±0.13*** | 85±13 |
| 100 μM | 4 | 5.97±0.10 | 1.44±0.06 | 3.89±0.15*** | 83±5 |
| M-14 | |||||
| 1 μM | 4 | 5.86±0.12 | 5.03±0.20 | 0.20±0.25 | 81±4 |
| 10 μM | 4 | 6.11±0.06 | 5.34±0.11 | 0.18±0.08 | 54±7*** |
| 30 μM | 2 | 5.99 | 5.44 | −0.09 | 10*** |
| 100 μM | 4 | 5.15±0.39 | 4.64±0.43 | −0.24±0.26 | 8±4*** |
| 300 μM | 3 | 6.06±0.14 | 5.08±0.30 | 0.35±0.21 | 5±1*** |
−log EC50 (M) and Effmax (% of the maximum response in the first CRC) was determined from curve fitting to the experimental data obtained in each individual muscle strip. Data as means±s.e.m., n=number of detrusor strips. Δ−log EC50 are the differences between first and second CRC (i.e. the shift of CRC to higher CCh concentrations) and were corrected for the mean shift for TMC (with exception for the TMC itself).
P<0.05;
P<0.01;
P<0.001.
Tested for difference from zero.
Table 4.
Effects of propiverine metabolites on the CRC for CCh in mouse detrusor
| First CRC- | Second CRC | ||||
|---|---|---|---|---|---|
| n | −log EC50 | −log EC50 | Δ−log EC50a | Effmax | |
| TMC | 7 | 5.42±0.23 | 5.71±0.35 | −0.29±0.20 | 97±7 |
| M-5 | |||||
| 100 μM | 5 | 5.75±0.18 | 5.52±0.20 | 0.52±0.18 | 73±16 |
| 300 μM | 5 | 6.07±0.21 | 5.54±0.61 | 0.82±0.75 | 25±7*** |
| M-6 | |||||
| 1 μM | 5 | 5.41±0.22 | 4.81±0.19 | 0.89±0.12*** | 90±6 |
| 100 μM | 5 | 6.07±0.18 | 2.19±0.27 | 3.91±0.24*** | 106±14 |
| M-14 | |||||
| 1 μM | 4 | 5.20±0.22 | 5.55±0.20 | −0.06±0.24 | 88±8 |
| 10 μM | 3 | 5.84±0.03 | 6.06±0.24 | 0.07±0.25 | 40±9** |
| 100 μM | 1 | 5.82 | Not calculated | Not calculated | 9 |
| 300 μM | 3 | 6.10±0.31 | Not calculated | Not calculated | 7±2*** |
−log EC50 (M) and Effmax (% of the maximum response in the first CRC) were determined from curve fitting to the experimental data obtained in each individual muscle strip. Data as means±s.e.m., n=number of detrusor strips. Δ−log EC50's are the differences between first and second CRCs (i.e. the shift of CRC to higher CCh concentrations) and were corrected for the mean shift for TMC (with exception for the TMC itself).
P<0.05;
P<0.01;
P<0.001.
Tested for difference from zero.
In human detrusor, the second CRC for CCh in TMC experiments was slightly shifted to the right by about one half log unit, in pig detrusor the rightward shift was somewhat larger and in mouse detrusor both CRCs were almost superimposed. Only in pig detrusor the maximum effect (Effmax) was slightly reduced to about 90% of the Effmax in the first CRC.
The metabolites profoundly affected CCh-induced contractions in a concentration-dependent manner. While M-6 only shifted the CRCs to the right and did not affect maximum CCh responses, M-5 additionally reduced Effmax. In contrast M-14 did not shift the CRC, but suppressed Effmax almost completely. The response patterns to the individual metabolites were comparable in all three investigated species. Differences were only detected for M-5, which was more effective in reducing Effmax in pig and mouse compared to human detrusor, but shifted the CRC for CCh to a larger extent human strips.
The pA2 value calculated from Schild regression analysis for M-6 was 6.93±0.14 (slope 1.34±0.09; R2=0.99) in human detrusor. The respective pA2 value for atropine was 8.91±0.38 (R2=0.94; data were taken from Wuest et al., 2005), suggesting that M-6 has a lower affinity for M receptors of human detrusor than atropine.
Since detrusor contractions depend on the extracellular Ca2+ concentration, the reduction of the maximum CCh responses with propiverine, M-5 and M-14 could be due to a block of Ca2+ entry. In the following experiments, we therefore examined whether inhibition of ICa's could be responsible for the observed effects.
Effects on ICa
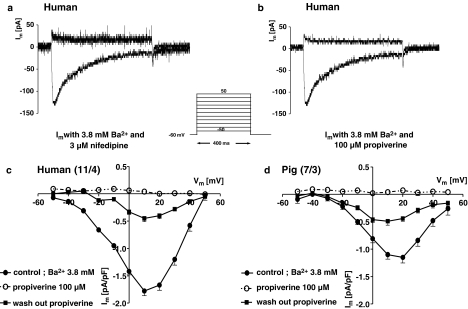
Inward currents in freshly isolated DSMC were studied with the conventional whole-cell patch-clamp technique. Human and pig DSMC were of similar size, resulting from their membrane capacitances which were 43±2 (n=31) and 47±2 pF (n=20), respectively. Current traces in human DSMC for the test pulse of +10 mV from a holding potential of −60 mV showed a rapidly activating, slowly inactivating inward current, that was completely suppressed by the dihydropyridine nifedipine (Figure 6a). This current was also blocked by 100 μM propiverine (Figure 6b). The current–voltage relation was characteristic for L-type calcium channels with maximum at +10 mV (−1.39±0.13 pA pF−1, n=37 for human DSMC) and an apparent reversal potential arround +50 mV (Figure 6c). ICa at +10 mV was smaller in porcine compared to human DSMC: −0.75±0.08 pA pF−1 (n=20). With 100 μM propiverine the block was complete throughout the potential range of the I–V curve, and was only partially reversible during washout. Similar results were obtained in pig DSMC (Figure 6d). Block of ICa by propiverine was concentration-dependent (Figure 7a) in a similar manner as the reduction of the CCh-induced maximum contractile force (Figure 7c).
Figure 6.
Current traces for the ICa in human DSMC in the presence of 3 μM nifedipine (a) and 100 μM propiverine (b). Current–voltage relations (I–V) for human (c) and porcine cells (d). Data of the I–V curves are shown as means±s.e.m. in pA pF−1 from n investigated cells. Effect of 100 μM propiverine on the I–V curves and washout are shown.
Figure 7.
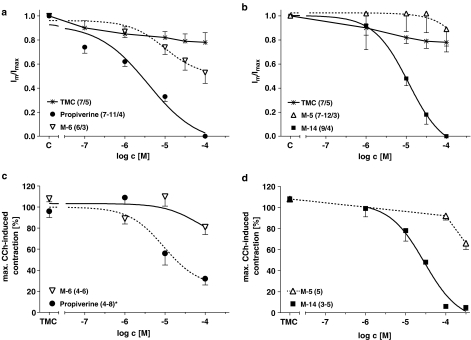
Cumulative CRCs for the effects of propiverine, M-6 (a) and M-5, M-14 (b) on the maximum ICa at +10 mV in comparison to TMC experiments without any drug added. Effects of propiverine (*labelled data were taken from previous work and are included for comparative purposes, Wuest et al., 2005) and M-6 (c) and M-5 and M-14 (d) on the maximum CCh-induced contraction during the first CRC. All data are shown as means±s.e.m. from n measured cells from x different patients or n investigated detrusor muscle strips.
The effects of the metabolites M-5, M-6 and M-14 on ICa in human DSMC are also shown in Figure 7. While M-14 like propiverine almost completely inhibited ICa, M-6 had only small effects and M-5 showed no block at all in the investigated concentration range (Figure 7a and c). The respective −log IC50 values were: for propiverine 5.49±0.08 (n=7–11 cells from four patients), for M-6 4.97±0.11 (n=6/3) and for M-14 5.19±0.07 (n=9/4).
The observed pattern of responses of ICa to block by propiverine and its metabolites are similar to their effects on the maximum CCh-induced contraction (Figure 7b and d). While propiverine and M-14 strongly reduced Effmax, M-6 did not affect maxiumum CCh response. A remarkable but smaller effect of M-5 was only seen at higher concentrations.
Discussion
The main findings of our study were: (i) Propiverine and the metabolites M-5, M-6 and M-14 reduced electrically evoked contractions in human, pig and mouse detrusor, albeit to different extents. (ii) M-6 shifted the CRC for CCh to the right, M-5 additionally reduced the maximum CCh-induced contractions like propiverine, and M-14 only impaired the maximum response without a rightward shift of the CRC. (iii) Propiverine and M-14 strongly inhibited ICa in human DSMC, whereas M-5 and M-6 had only marginal effects. (iv) Our findings suggest that suppression of the CCh maximum effect may be related to a direct block of ICa.
Effects on EFS-induced contractions
EFS excites intramural nerve endings, leading to release of ACh and other neurotransmitters involved in stimulation of detrusor smooth muscle contraction (for a review, see Andersson, 1993). With the selected EFS parameters, detrusor contractions were not only neurogenic but also myogenic (TTX-resistant). Block of the cholinergic contraction by atropine (Bayliss et al., 1999) was reported to leave a small NANC component in man and pig, but a sizeable one in mouse detrusor (Sibley, 1984; Bayliss et al., 1999). Under the same experimental conditions as used for this study we have recently detected an NANC component of contraction of about 60% in mouse, but only 7% in pig detrusor (Wuest et al., 2002). In the present study, human preparations did not exhibit NANC contractions. This could be due to the selected stimulation parameters. Pessina et al. (2001) showed that with rising stimulation frequencies from 1 to 15 Hz contraction amplitudes were enhanced in all species. Despite the long stimulus duration (i.e. 1 ms), we found a similar frequency dependence of EFS-induced contraction amplitudes in mouse and pig as recently reported for rat, guinea-pig, monkey and human detrusor (pulse duration 0.05 ms, Pessina et al., 2001). These authors described a negligible NANC component in man and monkey (like our findings for man and pig) and a large NANC component in rat and guinea-pig (like our findings in mouse). Therefore, mouse detrusor muscle appears to be a suitable model for studying additional spasmolytic effects, which are considered not to be mediated via the M receptor pathway. M-5 and M-14 did in fact suppress EFS-evoked contractions in the mouse, suggesting that these two metabolites possess additional effects like the parent compound propiverine. However, such an effect was not observed with M-6, which marginally affected the atropine-resistant (NANC) contractions. These observations are in line with the proposal, that an unchanged aliphatic side chain in the propiverine derivatives is associated with one or more additional actions (Siegmund et al., 1990), whereas after change to the hydroxylic group only antimuscarinic action is retained (Haruno et al., 1989; Haruno, 1991, see below).
In man, pig and mouse, the order of potency was: M-6> propiverine>M-5. Propiverine and M-5 differ in structure only at the nitrogen. We speculate that the oxidation of the tertiary amine may have a negative influence on the drug's potency to reduce EFS-evoked contractions. It can be noted that the change from the tertiary amine to the secondary amine structure has very little effect on the potency and the overall impairment of EFS-evoked contractions.
Effects on CRC for CCh
In TMC experiments the second CRC for CCh was shifted to higher concentrations, which is probably due to desensitization of the M receptors as previously reported for pig and mouse detrusor (Wuest et al., 2002) and confirmed herein for human samples. Only in pig the maximum CCh-stimulated contraction during the second CRC was somewhat smaller, suggesting a larger desensitization of the CCh response in this species.
Like atropine, M-6 merely shifted the CCh CRCs to the right without any effect on the maximum response, suggesting that this compound behaves as a competitive antagonist in this system. Similar results have been obtained with M-6 in guinea-pig ileum and detrusor (Haruno et al., 1989; Haruno, 1991). The respective pA2 values for M-6 and atropine reported for ileum (6.53 and 9.15; Siegmund et al., 1990) are in good agreement with the one reported here, that is, 6.93 for the effect of M-6 and 8.91 for atropine.
M-5, on the other hand, exhibited a mixed action like propiverine (Yono et al., 1999; Wuest et al., 2002) by shifting the CRC, and in addition reducing Effmax, which is in line with the effects of a unsurmountable antagonist. M-14 only reduced Effmax without any shift of the CRC, and hence is expected to have the most prominent ‘additional' actions. Evidence in guinea-pig detrusor suggests that the ‘additional' effects could be related to depolarization dependent on Ca2+ influx, since propiverine (⩾10 μM) and the N-oxide M-5 (⩾100 μM) reduced KCl-induced contractions, whereas M-6 had no effects (Haruno et al., 1989).
Effects on ICa
Propiverine is considered to act with at least one additional mode of action, reducing detrusor contractility (Wada et al., 1995; Madersbacher & Mürtz, 2001). It has been reported to block ICa in rat and guinea-pig DSMC (Tokuno et al., 1993). Our data confirm the blocking effect on ICa in man and pig, which was as large as the effect of nifedipine. In accordance with the literature, we found larger current density in human than in porcine DSMC (Kajioka et al., 2002).
The differences in effects of the metabolites M-5, M-6, and M-14 on ICa are in agreement with the extent of impairment of the maximum CCh response during detrusor contraction. Like propiverine, M-14 almost completely blocked ICa and also strongly reduced Effmax. However, M-6, which merely shifted the CRCs like a competitive antagonist, had only minor effects on ICa, which may even be due to the large variability. Furthermore, M-6 only marginally reduced the atropine-resistant component of contraction during EFS in mouse detrusor. This may be another evidence for competitive muscarinic antagonism of M-6. Lack of ICa block by M-5 could be due to the reduced potency of this metabolite (see above).
The amount of block of ICa by M-14 was comparable with that of propiverine and nifedipine. M-14 did not affect the potency of CCh. This response pattern is similar to the effects of nifedipine on CRC for CCh (Schneider et al., 2004b). We conclude that the spasmolytic effect of M-14 is mainly due to block of calcium influx via L-type calcium channels and not due to antagonism at M receptors. Siegmund et al. (1990) suggested that the aliphatic side chain is responsible for the nonspecific properties in addition to antagonism at M receptors. We would extend this conclusion by suggesting that the aliphatic side chain is necessary for the blocking effect on ICa.
In summary, we have shown different effects of propiverine's main metabolites in the human system, that is, M-5, M-6 and M-14, on contractility of detrusor muscle in man, pig and mouse and on ICa in human and porcine DSMC. Changes in the molecular structure of propiverine lead to a change in the antagonistic action on M receptors and to a change in additional block of ICa. The unsurmountable effect on CCh-induced contractions seems to involve a direct block of ICa. We conclude that the main metabolites of propiverine (M-5, M-6 and M-14) may well contribute to its clinical effect.
Acknowledgments
We thank Ms S. Kirsch and Ms A. Weiss for their excellent technical assistance.
Abbreviations
- ACh
acetylcholine
- CCh
carbamoylcholine chloride (carbachol)
- CRC
concentration–response curve
- DSMC
detrusor smooth muscle cells
- EFS
electric field stimulation
- ICa
L-type calcium current
- M receptors
muscarinic receptors
- NANC
nonadrenergic–noncholinergic
- OAB
overactive bladder syndrome
- TMC
time-matched control
- TTX
tetrodotoxin
References
- ANDERSSON K.-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ANDERSSON K.-E., APPELL R., AWAD S., CHAPPLE C., DRUTZ H., FINKEBEINER A., FOURCROY J., HAAB F., WEIN A.Pharmacological treatment of urinary incontinence Incontinence, Second International Consultation on Incontinence 2002U.K.: Plybridge Distributors Ltd.481–511.ed. Abrams, P., Cardozo, L., Khoury, S. & Wein, A. pp [Google Scholar]
- ANDERSSON K.-E., APPELL R., CARDOZO L.D., CHAPPLE C., DRUTZ H.P., FINKEBEINER A.E., HAAB F., VELA NAVARRETE R. The pharmacological treatment of urinary incontinence. BJU Int. 1999;84:923–947. doi: 10.1046/j.1464-410x.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E., YOSHIDA M. Antimuscarinics and the overactive detrusor – Which is the main mechanism of action. Eur. Urol. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLISS M., WU C., NEWGREEN D., MUNDY A.R., FRY C.H. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- CHESS-WILLIAMS R., CHAPPLE C.R., YAMANISHI T., YASUDA K., SELLERS D.J. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J. Auton. Pharmacol. 2001;21:243–248. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M. Pharmacological characterization of muscarinic receptors in mouse isolated urinary bladder smooth muscle. Br. J. Pharmacol. 2001;133:1035–1040. doi: 10.1038/sj.bjp.0704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FETSCHER C., FLEICHMAN M., SCHMIDT M., KREGE S., MICHEL M.C. M3 muscarinic receptors mediate contraction of human urinary bladder. Br. J. Pharmacol. 2002;136:641–644. doi: 10.1038/sj.bjp.0704781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARUNO A. Anticholinergic effects of propiverine hydrochloride (P-4) and its metabolites in isolated guinea pig ileum and in anesthetized dogs. Öyö Yakuri/Pharmacometrics. 1991;41:461–466. [Google Scholar]
- HARUNO A., YAMASAKI Y., MIYOSHI K., MIYAKE H., TSUCHIYA K., KOSAKA M., NAGAI M., IRIKI M. Effects of propiverine hydrochloride and its metabolites on isolated guinea-pig urinary bladder. Folia Pharmacol. Japan. 1989;94:145–150. doi: 10.1254/fpj.94.145. [DOI] [PubMed] [Google Scholar]
- HAUSTEIN K.-O., HÜLLER G. On the pharmacokinetics and metabolism of propiverine in man. Eur. J. Drug Metab. Pharmacokinet. 1988;13:81–90. doi: 10.1007/BF03191308. [DOI] [PubMed] [Google Scholar]
- KAJIOKA S., NAKAYAMA S., MCMURRAY G., ABE K., BRADING A.F. Ca2+ channel properties in smooth muscle cells of the urinary bladder from pig and human. Eur. J. Pharmacol. 2002;443:19–29. doi: 10.1016/s0014-2999(02)01593-5. [DOI] [PubMed] [Google Scholar]
- MADERSBACHER H., MÜRTZ G. Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic) World J. Urol. 2001;19:324–335. doi: 10.1007/s003450100223. [DOI] [PubMed] [Google Scholar]
- OUSLANDER J.G. Management of overactive bladder. N. Engl. J. Med. 2004;350:786–799. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- PESSINA F., MARAZOVA K., KALFIN R., SGARAGLI G., MANGANELLI A., MILENOV K. Mechanical response to electric field stimulation of rat, guinea-pig, monkey and human detrusor muscle: a comparative study. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:543–550. doi: 10.1007/s002100100407. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER T., FETSCHER C., KREGE S., MICHEL M.C. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J. Pharmacol. Exp. Therap. 2004a;309:1148–1153. doi: 10.1124/jpet.103.063735. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER T., HEIN P., MICHEL M.C. Signal transduction underlying carbachol-induced contraction of rat urinary bladder. I. Phospholipases and Ca2+ sources. J. Pharmacol. Exp. Therap. 2004b;308:47–53. doi: 10.1124/jpet.103.058248. [DOI] [PubMed] [Google Scholar]
- SELLERS D.J., CHAPPLE C.R., CHESS-WILLIAMS R. Potential therapeutic targets for treatment of the overactive bladder. World J. Urol. 2001;19:307–311. doi: 10.1007/pl00007102. [DOI] [PubMed] [Google Scholar]
- SIBLEY G.N. A comparison of sponteneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGMUND W., NIGUSSIE M., TALAHUN K., AITENFISSU H., FRANKE G., WENGLER A. Anticholinergic properties of propiverine and its metabolites. Pharmazie. 1990;45:67–68. [PubMed] [Google Scholar]
- SIEPMANN M., NOKHODIAN A., THÜMMLER D., KIRCH W. Pharmacokinetics and safety of propiverine in patients with fatty liver desease. Eur. J. Clin. Pharmacol. 1998;54:767–771. [Google Scholar]
- TOKUNO H., CHOWDHURY J.U., TOMITA T. Inhibitory effects of propiverine on rat and guinea-pig urinary bladder muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:659–662. doi: 10.1007/BF00167244. [DOI] [PubMed] [Google Scholar]
- WADA Y., YOSHIDA M., KITANI K., KIKUKAWA H., ICHINOSE A., TAKAHASHI W., GOTOH S., INADOME A., MACHIDA J., UEDA S. Comparison of the effects of anticholinergic drugs on human isolated urinary bladder. Arch. Int. Pharmacodyn. 1995;330:76–79. [PubMed] [Google Scholar]
- WEGENER J.W., SCHULLA V., LEE T.S., KOLLER A., FEIL S., FEIL R., KLEPPISCH T., KLUGBAUER N., MOOSMANG S., WELLING A., HOFMANN F. An essential role of Cav1.2 L-type calcium channel for urinary bladder function. FASEB J. 2004;18:1159–1161. doi: 10.1096/fj.04-1516fje. [DOI] [PubMed] [Google Scholar]
- WUEST M., AVERBECK B., REIF S., BRÄTER M., RAVENS U. Different responses to drugs against overactive bladder in detrusor muscle of pig, guinea pig and mouse. Eur. J. Pharmacol. 2002;454:59–69. doi: 10.1016/s0014-2999(02)02478-0. [DOI] [PubMed] [Google Scholar]
- WUEST M., MORGENSTERN K., GRAF E.M., BRAETER M., HAKENBERG O.W., WIRTH M.P., RAVENS U.Cholinergic and purinergic responses in isolated human detrusor in relation to age J. Urol. 2005. in press [DOI] [PubMed]
- YAMANISHI T., CHAPPLE C.R., CHESS-WILLIAMS R. Which muscarinic receptor is important in the bladder. World J. Urol. 2001;19:299–306. doi: 10.1007/s003450100226. [DOI] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., CHESS-WILLIAMS R. The role of M2 muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br. J. Pharmacol. 2000;131:1482–1488. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONO M., YOSHIDA M., WADA Y., KIKUKAWA H., TAKAHASHI W., INADOME A., SESHITA H., UEDA S. Pharmacological effects of tolterodine on human isolated urinary bladder. Eur. J. Pharmacol. 1999;368:223–230. doi: 10.1016/s0014-2999(99)00036-9. [DOI] [PubMed] [Google Scholar]