Abstract
Whole-cell currents in cultured hippocampal neurons were recorded to investigate the effects of SO-3, a new O-superfamily conopeptide derived from Conus striatus, on voltage-sensitive channels.
SO-3 had no effect on voltage-sensitive sodium currents, delayed rectifier potassium currents, and transient outward potassium currents.
Similar to the selective N-type calcium channel blocker ω-conotoxin MVIIA (MVIIA), SO-3 could concentration-dependently inhibit the high voltage-activated (HVA) calcium currents (ICa).
MVIIA(3 μM), 10 μM nimodipine, and 0.5 μM ω-agatoxin IVA (Aga) could selectively block the N-, L-, and P/Q-type ICa, which contributed ∼32, ∼38, and ∼21% of the HVA currents in hippocampal neurons, respectively. About 31% of the total HVA currents were inhibited by 3 μM SO-3. SO-3 (3 μM) and 3 μM MVIIA inhibited the overlapping components of HVA currents, whereas no overlapping component was inhibited by 3 μM SO-3 and 10 μM nimodipine, or by 3 μM SO-3 and 0.5 μM Aga. Also, 3 μM SO-3 had no effect on R-type currents.
SO-3 had less inhibitory effects on non-N-type HVA currents than MVIIA at higher concentrations (30 and 100 μM).
The inhibitory effects of SO-3 and MVIIA on HVA currents were almost fully reversible. However, the recovery from block by MVIIA was more rapid than recovery from block by SO-3.
It is concluded that SO-3 is a new ω-conotoxin selectively targeting N-type voltage-sensitive calcium channels. Considering the significance of N-type calcium channels for pain transduction, SO-3 may have therapeutic potential as a novel analgesic agent.
Keywords: SO-3, ω-conotoxins, voltage-sensitive calcium channels, voltage-sensitive potassium channels, voltage-sensitive sodium channels, N-type calcium channel blockers, pain, hippocampal neurons, patch-clamp techniques
Introduction
Conopeptides are small, structured peptide toxins secreted by the venomous marine snails of the genus Conus for prey capture, defense, and competitor deterrence. It is now clear that in the venoms of living species in the genus Conus there are about 50,000 different conotoxins, most of which are 12–30 amino acids in length and contain two to four disulfide bridges, and can selectively target a specific voltage-gated ion channel, ligand-gated ion channel, or G-protein-coupled receptor. These potential pharmaceutical benefits have led to the use of certain conotoxins as tools for ion channel research as well as for the treatment and diagnosis of neurological diseases (Nielsen et al., 2000; Olivera & Cruz, 2001; Terlau & Olivera, 2004).
Conotoxins are defined to a number of structural classes according to the characteristic arrangement of Cys-residues. The O-superfamily, which contains four-loop, 6-Cys peptides, comprises ω-conotoxins (voltage-sensitive calcium channel (VSCC) blockers), κ-conotoxins (voltage-sensitive potassium channel (VSPC) blockers), μO-conotoxins (voltage-sensitive sodium channel (VSSC) blockers), and δ-conotoxins (inhibitors of the fast inactivation of VSSCs). These four classes of conotoxins are unusually hydrophobic peptides with the same cysteine framework C–C–CC–C–C, giving these peptides a similar inhibitory cysteine knot (ICK) motif (Table 1) (Nielsen et al., 2000; Olivera & Cruz, 2001; Terlau & Olivera, 2004).
Table 1.
Selected O-superfamily conopeptides targeting different voltage-sensitive ion channels
| Peptide | Conus Specie | Sequencea | Targeted ion channelb | Reference | |
|---|---|---|---|---|---|
| ω-GVIA | C. geographus | CKSOGSSCSOTSYNCCR-SCNOYTKRCY* (4) | VSCCs, N-type | Olivera et al. (1984) | |
| ω-MVIIA | C. magus | CKGKGAKCSRLMYDCCTGSC–RSGKC* (5) | VSCCs, N-type | Olivera & Cruz (2001) | |
| ω-MVIIC | C. magus | CKGKGAPCRKTMYDCCSGSCGRR-GKC* (6) | VSCCs, P/Q/N-type | Hillyard et al. (1992) | |
| ω-CVIA | C. catus | CKSTGASCRRTSYDCCTGSC–RSGRC* (4) | VSCCs, N-type | Lewis et al. (2000) | |
| ω-CVIB | C. catus | CKGKGASCRKTMYDCCRGSC–RSGRC* (6) | VSCCs, N/P/Q-type | Lewis et al. (2000) | |
| ω-CVIC | C. catus | CKGKGQSCSKLMYDCCTGSCSRR-GKC* (5) | VSCCs, N/P/Q-type | Lewis et al. (2000) | |
| ω-CVID | C. catus | CKSKGAKCSKLMYDCCSGSCSGTVGRC* (4) | VSCCs, N-type | Lewis et al. (2000) | |
| ω-SVIA | C. striatus | CRSSGSPCGVTS-ICC-GRC–YRGKCT* (4) | poor activity on VSCCs | Ramilo et al. (1992) | |
| ω-SVIB | C. striatus | CKLKGQSCRKTSYDCCSGSCG-RSGKC* (5) | VSCCs, P/Q-type | Ramilo et al. (1992) | |
| SO-3c | C. striatus | CKAAGKPCSRIAYNCCTGSC–RSGKC* (5) | ? | ||
| δ-SVIE | C. striatus | DGCSSGGTFCGIHOGLCCSEFCFLWCITFID | VSSCs | Bulaj et al. (2001) | |
| μO-MrVIA | C. marmoreus | ACRKKWEYCIVPIIGFIYCCPGLICGPFVCV | VSSCs | McIntosh et al. (1995) | |
| μO-MrVIB | C. marmoreus | ACSKKWEYCIVPILGFVYCCPGLICGPFVCV | VSSCs | McIntosh et al. (1995) | |
| κ-PVIIA | C. purpurascens | CRIONQKCFQHLDDCCSRKCNRFNKCV | VSPCs | Shon et al. (1998) |
All ω-conotoxins are C-terminally amidated (*), each net positive charge is indicated in the braces after the sequence of the corresponding ω-conotoxin. The disulfide bridges motif of ω-conotoxins and its four loops (Δ) are also displayed above the sequences.
Targeted ion channels are determined from radioligand binding and/or electrophysiology experiments.
The individual peptides in one conotoxin family may selectively target a diverse set of different molecular isoforms within the same ion channel family. For example, ω-conotoxin MVIIA and MVIIC, both derived from Conus magus, have high identity in sequence, but quite different selectivity: MVIIA is highly selective for N-type VSCCs, whereas MVIIC is more selective for the P/Q-type (Nielsen et al., 1999a). Among the ω-conotoxins CVIA–CVID, all identified from C. catus, CVIA and CVID are selective towards the N-type VSCCs, while CVIB and CVIC show no difference between N- and P/Q-type VSCCs (Lewis et al., 2000; Nielsen et al., 2000; Adams et al., 2003). On the other hand, peptides derived from different cone snail species, although their conopeptide sequences vary largely except the cysteine framework, may act on the same ion channel subtype, as both ω-conotoxin MVIIA and GVIA can block the same N-type VSCCs (Olivera & Cruz, 2001; Terlau & Olivera, 2004) (Table 1).
The venoms of each species always contain a unique array of more than 100 peptides, which are used by the genus Conus for divergent biotic purposes. For this reason, corresponding venom components with divergent pharmacological properties in one particular Conus species are expected. In searching for new conotoxins with some therapeutic potential, more than 25 species of Conus from the South China Sea have been collected. Also, by gene screening, we identified a new conopeptide termed SO-3 from a fish-eating snail, C. striatus (Lu et al., 1999). SO-3 contains 25 amino-acid residues and the same cysteine framework as O-superfamily conotoxins. Previously, three O-superfamily conopeptides, including two ω-conotoxins (SVIA and SVIB) and one δ-conotoxin (SVIE), have been isolated from the venom of C. striatus biochemically (Table 1) (Ramilo et al., 1992; Bulaj et al., 2001). SO-3 shows 56% sequence identity with SVIB, a P/Q-type VSCCs blocker, and 72% with MVIIA, an N-type VSCC blocker. In addition, it has the same positive charges as SVIB and MVIIA (Table 1). Further bioactivity evaluation on the synthetic SO-3 showed that SO-3 is a potent analgesic agent with effects similar to MVIIA (Dai et al., 2003). However, the ion channel target of SO-3 is still unclear. Here, we observed the effects of SO-3 on voltage-sensitive ion currents in primary cultured hippocampal neurons. The results indicated that SO-3 is a selective N-type VSCC blocker.
Methods
Cell culture
Primary cultures of hippocampal cells were established using a modification of the previous procedure (Banker & Cowan, 1979). Wistar rats at postnatal day 1 were deeply anesthetized with ether and decapitated, in accordance with the guideline of the Beijing Institutes for Biological Sciences Animal Research Advisory Committee. The hippocampi were dissected in ice-cold Ca2+- and Mg2+-free PBS, incubated at 37°C with 0.125% trypsin (type XI; Sigma Co., Ltd, St Louis, MO, U.S.A.) for 20 min, and then triturated to dissociate cells. After being washed, the single cell suspension was diluted with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10% horse serum (HS), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. Cells were plated in 35 mm plastic culture dishes (Nunc, Denmark) pre-coated with poly-L-lysine (75 μg ml−1) at a density of 5.0 × 105 cells per dish, and cultured at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. After 24-h incubation, the culture medium was changed to 2 ml of DMEM containing 10% FBS, 2 mM glutamine, 1% N2, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. The proliferation of non-neuronal cells was controlled by treating with 3 μM cytosine arabinofuranoside (c-Ara) for 48 h at the second day in culture. Subsequently, half of the medium was replaced twice a week. All experiments were performed on cells aging 10–12 days in vitro. c-Ara was purchased from Sigma Co., Ltd, and other reagents including DMEM, FBS, HS, and N2 were obtained from GIBCO Life Technologies (Rockville, MD, U.S.A.).
Recording solutions
The extracellular solution for whole-cell recordings contained (in mM): NaCl 140, KCl 5, MgCl2 1, CaCl2 2, Dextrose 10, HEPES 10. The pH for extracellular solution was adjusted to 7.4 with NaOH and the osmolarity was adjusted to 320 mOsm with sucrose. For K+ current recording, the extracellular solution was supplemented with 1 μM tetrodotoxin (TTX) to block Na+ channels, and 0.4 mM CdCl2 to block all Ca2+ channels and Ca2+-activated K+ channels. For Na+ current recording, the same extracellular solution was used, except TTX was replaced with 5 mM tetraethylammonium chloride (TEA-Cl) and 2 mM 4-aminopyridine (4-AP) to block the delayed rectified K+ channels and transient outward K+ channels, respectively, For Ca2+ current recording, 1 μM TTX, 5 mM TEA-Cl, and 2 mM 4-AP were added to the extracellular solution to block Na+ channels and K+ channels. The intracellular pipette solution used for K+ current recording contained (in mM): KCl 140, HEPES 10, EGTA 10, and ATP-Na2 2, and the pH was adjusted to 7.2 by KOH. For Na+ and Ca2+ current recording, the solution contained (in mM): CsCl 140, HEPES 10, EGTA 10, TEA-Cl 5, and ATP-Na2, 2, and the pH was adjusted to 7.2 by CsOH. The osmolarity of the pipette solutions was adjusted to 320 mOsm. TEA-Cl, 4-AP, TTX, CdCl2, and ATP-Na2 were purchased from Sigma Co., Ltd.
Electrophysiology recordings
Patch pipettes (with resistances of 2–4 MΩ after being filled with intracellular solutions) were prepared using a two-stage microelectrode puller (PC-10; Narishige, Tokyo, Japan) and fire-polished using a MF-830 microforge (Narishige) just before recording. All experiments were carried out under room temperature (20–22°C). Voltage-clamp recordings were performed in the whole-cell patch-clamp configuration according to standard patch-clamp methods (Hamill et al., 1981) using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA, U.S.A.). Data were sampled at 5 kHz and filtered at 2 kHz (−3 dB, four-pole Bessel filter) on-line by using the pCLAMP 8.2 software (Axon Instruments, Foster City, CA, U.S.A.). Series resistance was compensated (>80%) in all experiments, and leak subtraction was carried out using a P/−4 protocol online. For all experiments, as soon as the whole-cell configuration had been acquired, current was allowed to stabilize for approximately 5 min before the experimental voltage command was administered. Only those recordings with stable holding currents and access resistance were accepted. Cells showing current rundown at a rate of currents more than 1% min−1 in the absence of treatments were discarded. All calcium currents (ICa) were normalized for differences in cell sizes by transformation to current density (pA pF−1; dividing each current (Ica) measure by the whole-cell membrane capacitance for that cell).
Drugs and drug application
The synthesis, folding, and purification of SO-3 were performed in Beijing Institute of Biotechnology, China as described in Dai et al. (2003). Using the designated conditions, the folding peptide of SO-3 was obtained in greater than 98% purity after two steps of HPLC purification. The final peptide content in lyophilized powder was approximately 79%. Using MALDI-TOF, the molecular mass (single isotope) of SO-3 was 2560.1 Da, in agreement with the calculated molecular mass of 2561.1 Da. Nimodipine, ω-conotoxin MVIIA (MVIIA), and ω-agatoxin IVA (Aga) were purchased from Sigma Co., Ltd. Nimodipine was stored in opaque containers at −20°C as a 10 mM stock solution in 100% ethanol. SO-3, MVIIA, and Aga were dissolved in extracelllular solutions to make 1 mM stock solutions that were aliquot and stored at −20°C. Each stock solution was diluted to the test concentration with extracelular solution. Bovine serum albumin (BSA (1 mg ml−1); Sigma) was present in all final extracelular solutions to prevent the nonspecific binding of peptides. To control for vehicle effects, 0.1% (v v−1) ethanol was present in the extracelular solutions throughout the experiments when nimodipine would be applied, and experiments were performed under restricted light conditions. During the experiments, cells were rinsed twice with extracellular solution, then continuously perfused with the test concentration of drug(s) by using a gravity-fed bath perfusion system. The flow rate was approximately 5 × 10−3 ml s−1. The distance of the flow pipette from the cell was approximately 150 μm and the pipette tip diameter was about 300 μm. The total volume of the extracellular solution was kept in 1.5 ml during the experiments.
Data analysis
The concentration–response curves for the effects of SO-3 and MVIIA on the high voltage-activated (HVA) ICa were fitted according to the logistic equation
where C is the concentration of SO-3 or MVIIA, E(C) is the effect evoked by SO-3 or MVIIA at concentration C, Emax denotes the maximal possible effect, EC50 is the concentration at which half-maximal effect is obtained. nH is the Hill coefficient. Origin 6.0 (OriginLab, Northampton, MA, U.S.A.) software was used to perform nonlinear fit of data. SPSS 12.0 software (SPSS Inc., Chicago, IL, U.S.A.) was used for statistical analysis. All data are expressed as mean±s.e.m., and P<0.05 indicates statistically significant.
Results
No effect of SO-3 on voltage-sensitive sodium currents (INa)
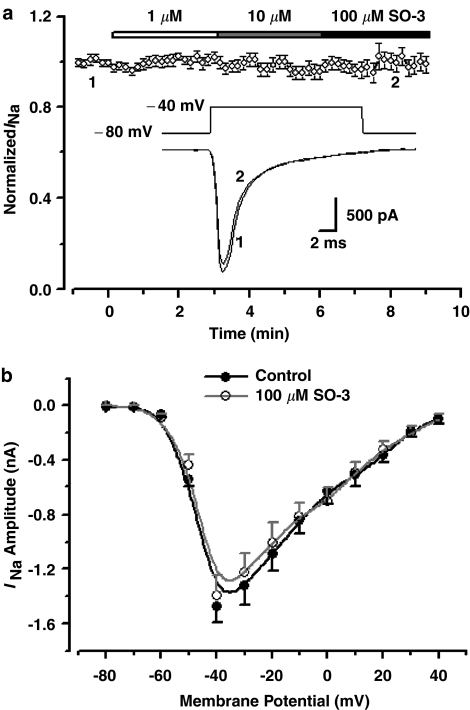
Hippocampal neurons were clamped at a holding potential (Vh) of −80 mV, and depolarized to −40 mV at 10 s intervals to activate inward Na+ currents which were blocked by 1 μM TTX completely and reversibly (data were not shown). After a 3-min application of 1, 10, and even 100 μM SO-3, the INa amplitudes were not affected. The current–voltage (I–V) curves remained unaffected after application of 100 μM SO-3. These observations indicated that SO-3 had no effect on INa (Figure 1).
Figure 1.
No effect of SO-3 on voltage-sensitive INa in primary cultured hippocampal neurons. (a) The time course of the effects of 1, 10 and 100 μM SO-3 on peak INa. Currents were evoked by 20 ms depolarizing voltage step commands from a Vh of −80 to −40 mV at 10 s intervals. Peak INa amplitudes were normalized to the values in the absence of SO-3 and plotted against exposure time. Data are shown as mean±s.e.m., n=13. Drugs were applied to the cell as indicated by the horizontal bar. Inset: Pulse paradigm and the representative current traces at the time point 1 (black) and 2 (gray). (b) I–V relationship of peak INa before and after 2-min exposure to 100 μM SO-3. Each point represents the mean of eight cells. INa's were evoked by a series of 20 ms depolarizing steps from a Vh of −80 to +40 mV, with 10 mV increments and 2 s intervals.
No effect of SO-3 on delayed rectifier potassium currents (IK (DR)) and transient outward potassium currents (IK (A))
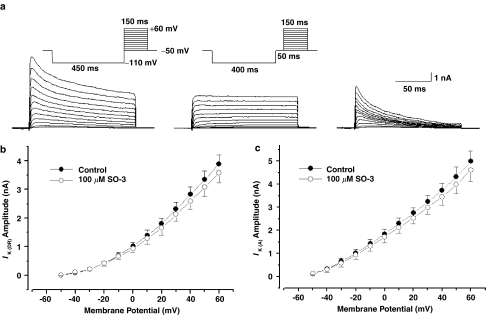
IK (DR) and IK (A) were isolated using the electrophysiological and pharmacological methods (Yang et al., 2001). The experimental pulse protocols and subtraction procedure are shown in Figure 2a. Currents at the end of the depolarizing pulse (148 ms) were referred to as IK (DR). The peak currents of the subtracted traces were referred to as IK (A). As Figure 2b and c showed, no significant effect on IK (DR) and IK (A) was observed after exposure to 100 μM SO-3.
Figure 2.
No effect of SO-3 on voltage-sensitive potassium currents. (a) The example family traces of outward potassium currents (IK) (left), IK (DR) (middle) and IK (A) (right). The upper inset shows the stimulus waveforms of IK and IK (DR), respectively. IK were evoked by 150 ms depolarizing pulses from −50 to +60 mV in 10 mV steps following a hyperpolarizing prepulse of 450 ms to −110 mV. IK (DR) were elicited using a similar protocol, except that for a 50 ms interval at −50 mV was inserted after the hyperpolarizing prepulse of 400 ms to −110 mV. IK (A) were isolated by subtracting the trace of IK (DR) from IK. (b, c) I–V curves of IK (DR) and IK (A), both n=9. Currents were recorded before (black) and after (gray) 2-min exposure to 100 μM SO-3.
Concentration-dependent inhibition of SO-3 on HVA ICa
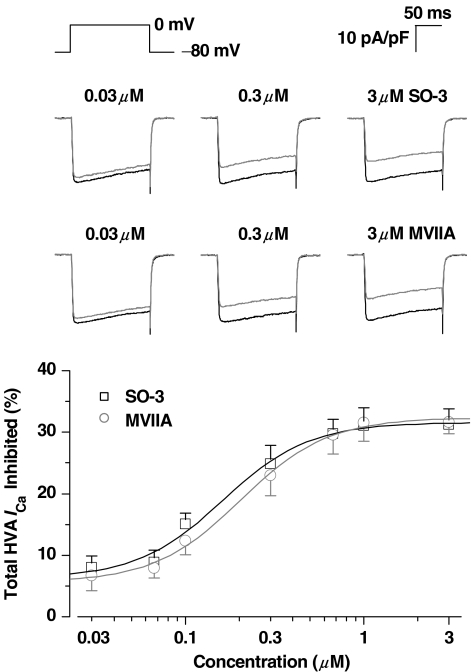
HVA ICa were evoked by 150 ms depolarizing voltage step commands from a Vh of −80 mV in primary cultured hippocampal neurons. Under the present experiments procedure, the inward ICa reached maximum amplitude when depolarizing to 0 mV. Figure 3 indicated a concentration-dependent inhibition of SO-3 on HVA ICa, and these inhibitory effects were similar to that of MVIIA, a determined N-type calcium channel blocker. In most cases, the inhibitory effects of a given concentration of peptides occurred within 10–20 s application and reached a stable maximum after 30–50 s. For both SO-3 and MVIIA, there was a plateau of inhibition at 1–3 μM. The calculated EC50's for SO-3 and MVIIA were 0.16 and 0.20 μM, respectively.
Figure 3.
Concentration-dependent inhibition of SO-3 on HVA ICa. Currents were evoked by depolarizing step pulse from −80 to 0 mV with 150 ms duration. Upper: The representative current traces before (black) and after (gray) 2-min exposure to 0.03, 0.3, and 3 μM SO-3 or MVIIA. Lower: Semilog concentration–response curves for the percentage inhibition of peak HVA ICa by SO-3 or MVIIA, which were fitted with the logistic equation 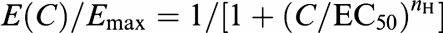 (see Methods). Data are shown as mean±s.e.m. For SO-3 groups n=6–15, and for MVIIA n=5–8. There is no significant difference of the inhibition rates between SO-3 and MVIIA under each concentration (unpaired Student's two-tailed t-test, P>0.05).The EC50, Emax and nH values for SO-3 were 0.16 μM, 31.58% and 1.80, respectively; for MVIIA, they were 0.20 μM, 32.37% and 1.78, respectively.
(see Methods). Data are shown as mean±s.e.m. For SO-3 groups n=6–15, and for MVIIA n=5–8. There is no significant difference of the inhibition rates between SO-3 and MVIIA under each concentration (unpaired Student's two-tailed t-test, P>0.05).The EC50, Emax and nH values for SO-3 were 0.16 μM, 31.58% and 1.80, respectively; for MVIIA, they were 0.20 μM, 32.37% and 1.78, respectively.
Inhibitory effects of SO-3 on I–V relationships of ICa
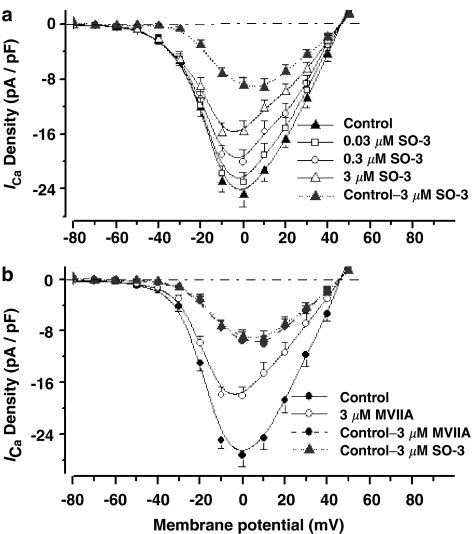
To identify whether the inhibition of ICa caused by SO-3 in cultured hippocampal neurons is voltage-dependent, the I–V relationships in the presence or absence of SO-3 were studied (Figure 4). These results confirmed the concentration-dependent inhibition on ICa after 2-min exposure to 0.03, 0.3, and 3 μM SO-3. The inhibitory effects of SO-3 on ICa were obvious at the potentials between –20 and +40 mV, and no obvious ICa was inhibited below the potential of –30 mV. These results indicated that SO-3 had inhibitory effects on HVA ICa, other than on low voltage-activated (LVA), that is, T-type currents. I–V relationships also showed no significant change of the threshold of activation of ICa and the reversal potential after application of SO-3. In order to illustrate the effects of SO-3 more clearly, the I–V curve of the 3 μM SO-3-sensitive currents was obtained by subtracting the currents in the presence of 3 μM SO-3 from the currents in the absence of 3 μM SO-3. The I–V curve of the subtracted 3 μM SO-3-sensitive currents was very similar to the subtracted 3 μM MVIIA-sensitive currents, indicated that, at the concentration of 3 μM, SO-3 and MVIIA had similar effects on I–V relationships of ICa.
Figure 4.
Inhibitory effects of SO-3 on I–V relationship of ICa. ICa were evoked by 150 ms depolarizing step commands from −80 to +50 mV with 10 mV increments and 10 s intervals. (a) I–V curves of peak ICa density before (Control) and after 2-min exposure to 0.03, 0.3, and 3 μM SO-3; the I–V curve of 3 μM SO-3-sensitive current density (Control – 3 μM SO-3, obtained by subtracting the current density in the presence of 3 μM SO-3 from those of Control; gray dotted line) was also plotted. Data are shown as mean±s.e.m., n=9 for each group. (b) I–V curves of ICa density before (Control) and after 2-min exposure to 3 μM MVIIA (n=7). The 3 μM MVIIA-sensitive current density (Control – 3 μM MVIIA; gray dash line) was also subtracted, and the I–V curve of 3 μM MVIIA-sensitive current density was similar to that of 3 μM SO-3-sensitive current density (from the same data in (a)).
Selectivity of SO-3 on VSCC subtype
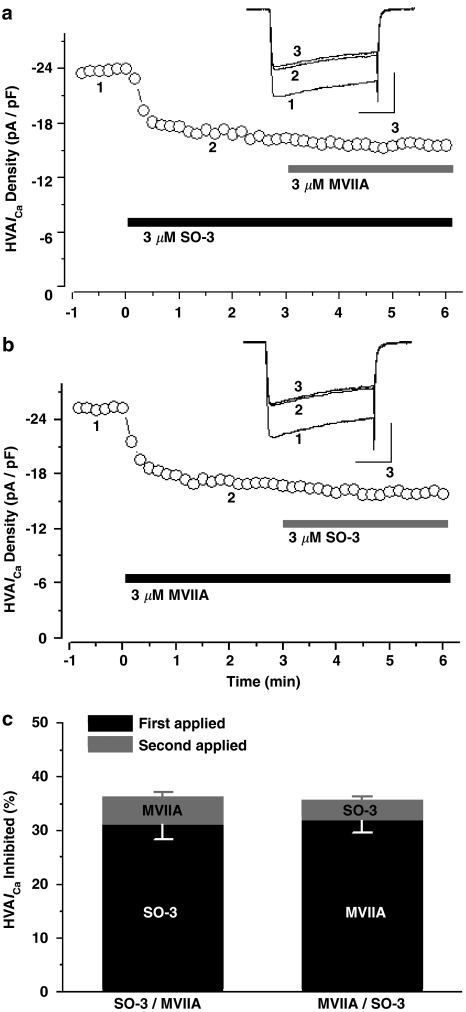
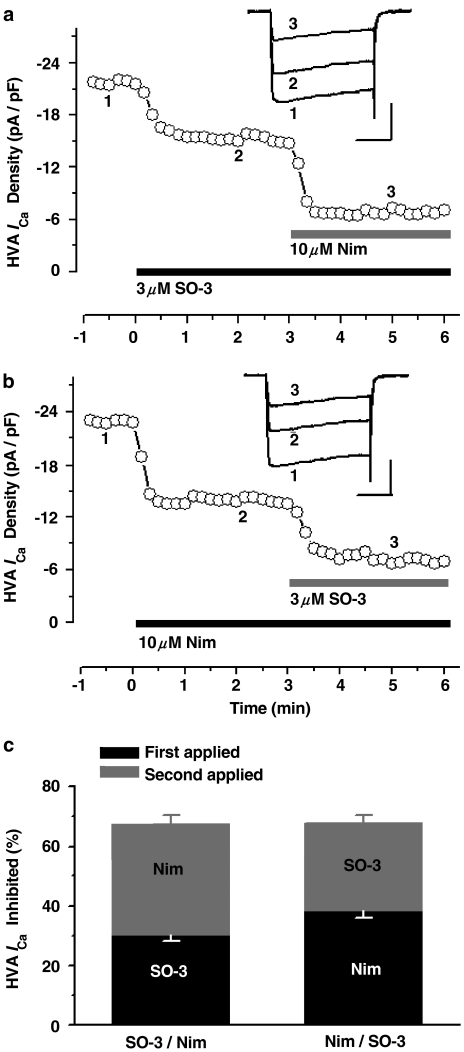
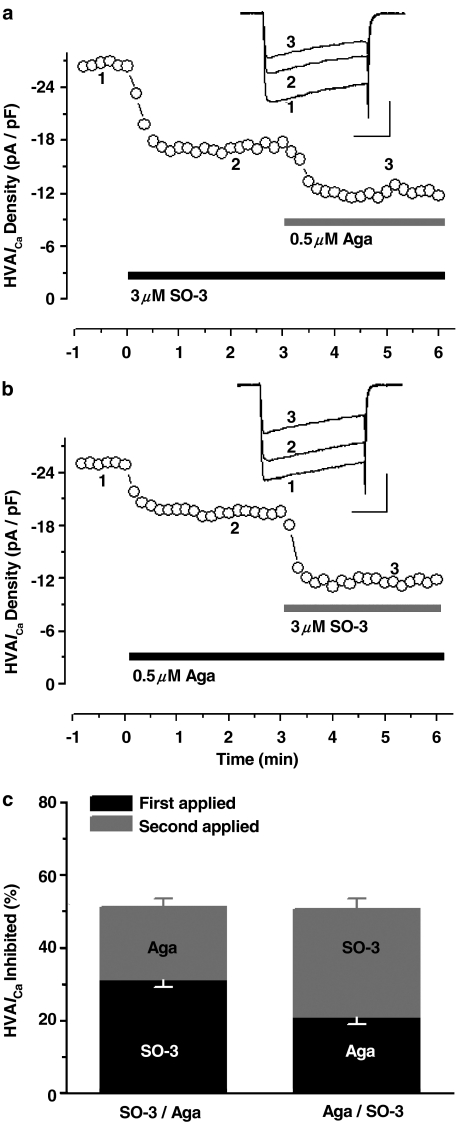
Previous experiments have proved the inhibitory effects of SO-3 on HVA ICa; here, we further examined whether HVA ICa inhibited by SO-3 is VSCC-subtype selective. At least four distinct types of HVA VSCCs (L-, N-,P/Q-, and R-type) are expressed in cultured hippocampal neurons and sensitive to different blockers (Currie & Fox, 1997; Nakashima et al., 1998; Newcomb et al., 1998; Blalock et al., 1999; Scamps et al., 2000). In our studies, SO-3 and one of three distinct VSCC blockers (MVIIA, nimodipine or Aga) were additionally applied to cultured hippocampal neurons in three separate experiments with drug presentation order reversed (Figures 5, 6 and 7). According to previous reports (Nakashima et al., 1998; Blalock et al., 1999; Scamps et al., 2000), 10 μM nimodipine, 3 μM MVIIA, and 0.5 μM Aga were used to define L-, N-, and P/Q-type currents in these experiments, respectively. SO-3 (3 μM) was also used. Typically, SO-3, MVIIA, and Aga required 30–50 s exposure to give stable inhibition, whereas nimodipine acted more rapidly (∼20–30 s).
Figure 5.
SO-3 and MVIIA inhibited overlapping components of the HVA ICa. (a, b) The time course showing the inhibitory effects of SO-3 (3 μM) and MVIIA (3 μM) on peak HVA ICa, respectively. Drugs were applied to the cell as indicated by the horizontal bars; the order of drug application was switched from SO-3 first (a) to MVIIA first (b). Currents were evoked by 150 ms depolarizing voltage step commands from −80 to 0 mV at 10 s intervals. Upper inset shows the current traces at the time points (1, 2 and 3). Scale bars: 10 pA pF−1, 50 ms. The inhibition of ICa amplitude was similar whether first applied SO-3 or MVIIA, and no further inhibition was observed after additional application of another drug in each group. (c) The peak HVA ICa inhibited by SO-3 and MVIIA were converted to a fraction of the total currents. Data are shown as mean±s.e.m., for both SO-3 applied first and MVIIA applied first, n=8. Two-way ANOVA on repeated measures (RM), P=0.872 for drug type, P<0.001 for presentation order, P=0.478 for interaction.
Figure 6.
SO-3 and nimodipine (Nim) inhibited nonoverlapping components of HVA ICa. (a, b) shows the time course of the inhibitory effects of SO-3 (3 μM) and Nim (10 μM) on peak HVA ICa, with switching the order of drug application from SO-3 first (a) to Nim first (b). The elicited current protocol was the same as that in Figure 5. The upper inset shows the example recordings made at the time points 1, 2, and 3. Scale bars: 10 pA pF−1, 50 ms. (c) shows that the calculated fraction of HVA ICa inhibited by SO-3 and Nim was unchanged by switching the order of drug application from SO-3 first to Nim first, the percentage inhibition of Nim (∼38%) was greater than that of SO-3 (∼30%). Data are shown as mean±s.e.m., for both SO-3 applied first and Nim applied first, n=8. Two-way ANOVA on RM, P=0.008 for drug type, P=0.682 for presentation order, P=0.974 for interaction.
Figure 7.
SO-3 and ω-agatoxin (Aga) inhibited nonoverlapping components of HVA ICa. The time course (a, b) and the histograms (c) show that the fraction of HVA ICa inhibited by SO-3 (3 μM) and Aga (0.5 μM) was unchanged by switching the order of drug application from SO-3 first to Aga first; the percentage inhibition of SO-3 (∼31%) was greater than that of Aga (∼21%). The experiment protocol was as same as that in Figure 5 and 6. Two-way ANOVA on RM, P=0.001 for drug type, P=0.544 for presentation order, P=0.923 for interaction. Data are shown as mean±s.e.m., for both SO-3 applied first and Aga applied first, n=7. Scale bars: 10 pA pF−1, 50 ms.
As Figure 5 showed, after application of 3 μM SO-3 or MVIIA, the inhibition amounts of HVA ICa were similar (∼31 and ∼32%, respectively), and no further inhibition was observed after additional application of the other drug. The remained HVA ICa could be blocked almost completely by 0.4 mM CdCl2 within 20 s (data were not shown). These results suggested that SO-3 and MVIIA inhibited the overlapping components of HVA ICa in cultured hippocampal neurons (two-way ANOVA on repeated measures, P<0.001 for presentation order), and there was no significant difference between the effects of SO-3 and MVIIA (P=0.872 for drug type). Therefore, these two drugs may share a common site of inhibition in this preparation. Unlike the experiments described above, sequential addition of SO-3 and nimodipine, and SO-3 and Aga, both produced additive inhibition on HVA ICa; 3 μM SO-3-, 10 μM nimodipine-, and 0.5 μM Aga-sensitive currents contributed ∼31, ∼38, and ∼21% of the total HVA ICa, respectively, regardless of the presentation order of these blockers (for the presentation order, for SO-3/nimodipine pair, P=0.682; for SO-3/Aga pair, P=0.544), and there were significant differences between the effects of SO-3 and nimodipine (P=0.008), and between SO-3 and Aga (P=0.001) (Figures 6 and 7). These results indicated that no overlapping component of HVA ICa was inhibited by SO-3 and nimodipine, or by SO-3 and Aga, and suggested that SO-3 did not inhibit L- and P/Q-type currents at the concentration tested.
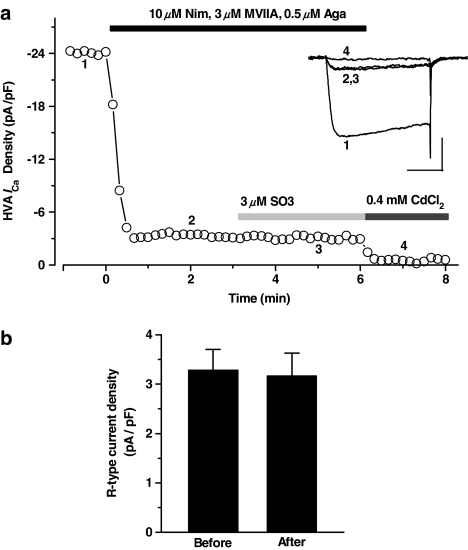
The effects of SO-3 on R-type HVA ICa were further examined. L-, N-, and P/Q-type currents were blocked by 10 μM nimodipine, 3 μM MVIIA, and 0.5 μM Aga, and the isolated R-type currents contributed ∼12% of the total HVA ICa. After exposure to 3 μM SO-3, the R-type currents remained unaffected (Figure 8). These results also suggested that SO-3 did not inhibit R-type currents at the concentration tested.
Figure 8.
No effect of SO-3 on R-type HVA ICa. (a) R-type currents were isolated after application of 10 μM Nim, 3 μM MVIIA and 0.5 μM Aga and contributed ∼12% of the total HVA ICa. After additional application of 3 μM SO-3, no obvious R-type current was further inhibited, but these currents were almost completely blocked by 0.4 mM CdCl2. The inset shows the current traces at each time points. Currents were evoked by 150 ms depolarizing voltage step commands from −80 to 0 mV at 10 s intervals. Scale bars: 10 pA pF−1, 50 ms. (b) The histograms for R-type current density before and after application of 3 μM SO-3 (paired Student's t-test, P>0.05). Data are shown as mean±s.e.m., n=7.
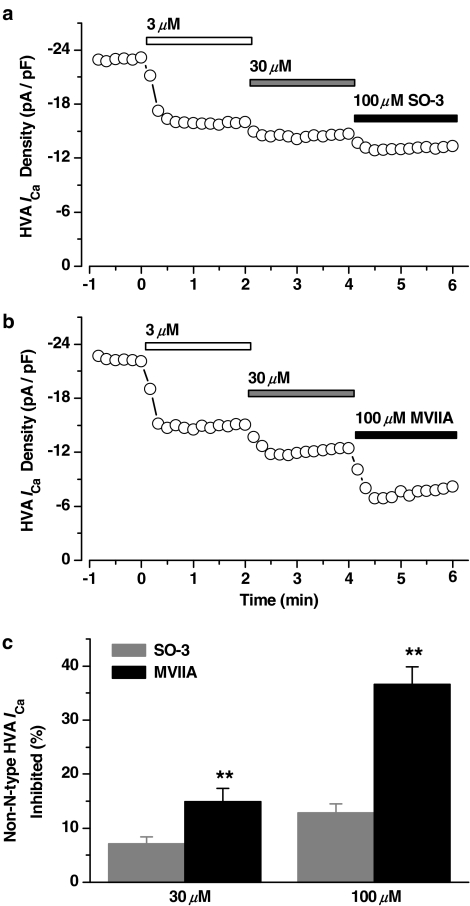
Several previous studies have demonstrated that ω-conotoxin GVIA and MVIIA can block the heterologously expressed L-, P/Q-, and R-type VSCCs at high concentrations (Williams et al., 1992b; Zhang et al., 1993; Feng et al., 2003). We also observed the effects of the higher concentrations of SO-3 on non-N-type HVA ICa in cultured hippocampal neurons. As Figure 9 showed, 3 μM SO-3 and MVIIA were first applied to block the N-type ICa, the remaining HVA ICa's were both further inhibited by additional application of 30 and 100 μM SO-3 and MVIIA. About 7 and 13% of the total HVA ICa were further inhibited by 30 and 100 μM SO-3, respectively, and more fractions of HVA ICa (∼15 and ∼37%, respectively) were further inhibited by 30 and 100 μM of MVIIA (unpaired Student's t-test, P<0.01). These results indicated that SO-3 had less inhibitory effects on non-N-type VSCCs than MVIIA at high concentrations.
Figure 9.
Higher concentrations of SO-3 and MVIIA inhibited non-N-type HVA ICa in cultured hippocampal neurons. (a, b) The time course showing the inhibitory effects of 3, 30, and 100 μM SO-3 (a) and MVIIA (b) on peak HVA ICa, respectively. Currents were evoked by 150 ms depolarizing voltage step commands from −80 to 0 mV at 10 s intervals. (c) The non-N-type HVA ICa inhibited by 30 and 100 μM SO-3 and MVIIA were converted to a fraction of the total currents. Data are shown as mean±s.e.m. For SO-3 groups, n=8, and for MVIIA, n=6. **P<0.01; statistically significant difference from the effect of the same concentration of SO-3 (unpaired Student's t-test).
Reversibility of the inhibitory effects of SO-3 on HVA ICa
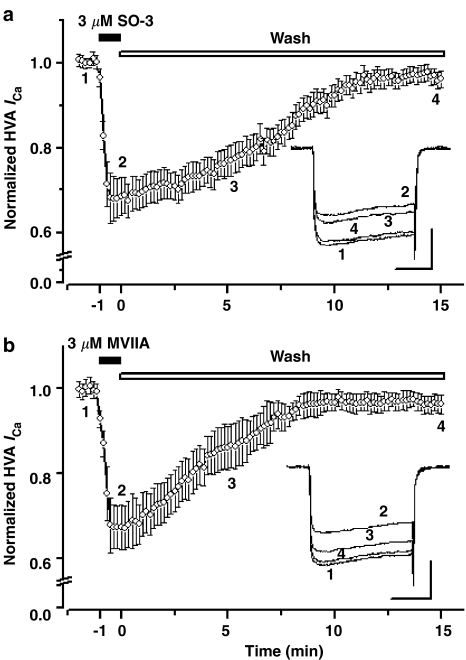
The different degree of recovery from the block effects of several ω-conotoxins, including GVIA, MVIIA, and CVID, on VSCCs was reported previously (Williams et al., 1992a; Kristipati et al., 1994; Lin et al., 1997; Stocker et al., 1997; Feng et al., 2001; 2003; Liang & Elmslie, 2002; Mould et al., 2004). Under the present experiments procedure, the inhibitory effects of SO-3 and MVIIA on HVA ICa were both almost fully reversible. However, the recovery from block by MVIIA was more rapid than recovery from block by SO-3. The half-time for recovery for SO-3 and MVIIA were 7.52±0.56 min and 4.14±0.45 min, respectively (unpaired Student's t-test, P<0.01) (Figure 10).
Figure 10.
Reversibility of the inhibitory effects of SO-3 on HVA ICa. Currents were evoked by 150 ms depolarizing voltage step commands from −80 to 0 mV at 10 s intervals and were normalized to the values before application of 3 μM SO-3 (upper) and 3 μM MVIIA (lower). Both SO-3 and MVIIA were applied to the cells for 60 s. The inhibitory effects of SO-3 and MVIIA on HVA ICa were almost completely reversed after washing ∼12 min (SO-3) and ∼10 min (MVIIA), and the recovery from block by MVIIA was more rapid than recovery from block by SO-3. Data are shown as mean±s.e.m., both n=7. The inset shows the sample current traces at each time points. Scale bars: 10 pA pF−1, 50 ms.
Discussion
Voltage-sensitive ion channel currents were well characterized to date in primary cultured hippocampal neurons, which gives facilities for the identification of the distinct ion channel target of SO-3. The main finding in this study is that SO-3, a new conopeptide which has the same disulfide bond motif as the O-superfamily conotoxins, selectively inhibits N-type voltage-sensitive ICa and has no effect on voltage-sensitive sodium and potassium currents.
Ion channels target of SO-3
In our experiments, INa, IK (DR), IK (A) and ICa, in primary cultured hippocampal neurons were isolated using the electrophysiological and pharmacological methods. Our results indicated that SO-3 had shown no effect on INa, IK (DR), and IK (A), even applied at a concentration of 100 μM (Figures 1 and 2). However, a concentration-dependent inhibition of SO-3 on HVA ICa was observed (Figure 3), and the I–V relationship also showed the inhibitory effects of SO-3 on HVA ICa (Figure 4). We confirm that SO-3 selectively targets VSCCs, other than VSSCs or VSPCs. The ω-, κ-, μO-, and δ-conotoxins, which all belong to the O-superfamily conotoxins, target the calcium, potassium, and sodium channels, respectively (Olivera & Cruz, 2001; Terlau & Olivera, 2004). Our studies indicate that SO-3 is a new ω-conotoxin.
To date, several ω-conotoxins have been identified from fish-, worm-, or mollusk-hunting Conus species. Although ω-conotoxins contain the same cysteine framework as κ-, μO-, and δ-conotoxins and belong to the same O-conotoxin superfamily, they have some structural characteristics different from κ-, μO-, and δ-conotoxins. All ω-conotoxins are C-terminally post-translationally modified and amidated. Besides the conserved cysteine framework, there are three conserved residues Gly5, Tyr13, and Ser19 (numbering of MVIIA) throughout this set of peptides except SVIA (Table 1). It is known that Tyr13 residue is important for binding to the calcium channels (Kim et al., 1995; Lew et al., 1997; Nielsen et al., 1999b). Apart from these characteristics, all ω-conotoxins possess 4–6 positive charges due to the high content of basic amino-acid residues, which are also known to play an important role in the blocking of calcium channels (Lew et al., 1997; Nielsen et al., 2000). Among these three conotoxins derived from C. striatus, SVIA is the smallest ω-conotoxin without the Tyr13, Ser19 residues, and has relatively poor activity on most calcium channels found in mammalian systems (Ramilo et al., 1992), whereas SO-3 has primary structural characteristics similar to SVIB and other derived ω-conotoxins, which may contribute to its selectivity on VSCCs.
VSCCs subtype selectivity of SO-3
As reported before, MVIIA (3 μM), nimodipine (10 μM) and Aga (0.5 μM) can selectively block the N-, L-, and P/Q-type ICa in hippocampal neurons, respectively (Currie & Fox, 1997; Nakashima et al., 1998; Blalock et al., 1999; Scamps et al., 2000). In the present studies, 3 μM MVIIA-, 10 μM nimodipine-, 0.5 μM Aga-sensitive currents in cultured hippocampal neurons contributed ∼32, ∼38, and ∼21% of HVA ICa, respectively, which were consistent with the previous studies (Nakashima et al., 1998; Blalock et al., 1999; Scamps et al., 2000). SO-3 (3 μM)-sensitive currents contributed ∼31% of HVA ICa, which was very similar to the percentage of MVIIA-sensitive currents. SO-3 and MVIIA inhibited the overlapping components of HVA ICa in cultured hippocampal neurons, and no overlapping component of HVA ICa was inhibited by SO-3 and nimodipine, or by SO-3 and Aga (Figures 5, 6 and 7). These results suggest that SO-3 shares a common site of inhibition with MVIIA, but not with nimodipine and Aga. Our research also demonstrated that 3 μM SO-3 had no effect on R-type HVA ICa (Figure 8). These results indicate that SO-3 selectively blocks N-type VSCCs and does not inhibit L-, P/Q- and R-type currents at this concentration.
Both ω-conotoxins SVIB and SO-3 were isolated from C. striatus. SVIB is selective for the P/Q-type VSCCs (Ramilo et al., 1992), whereas SO-3 is shown to selectively target the N-type VSCCs in the present study. SO-3 demonstrates a 56% sequence identity to SVIB and a higher (72%) sequence identity to MVIIA. However, the identity in primary sequences is insufficient to define VSCC selectivity. Sequence hypervariability has been observed between functionally homologous ω-conotoxins. ω-Conotoxins with high sequence identity may also demonstrate different selectivities (Table 1). Therefore, studies of secondary and tertiary structures of SO-3 and other ω-conotoxins might help much to understand the subtype selectivity in blocking VSCCs. The three-dimensional solution structure of SO-3 was determined by 1H NMR and showed that it contains a short antiparallel β-sheet involving residues 6–9, 19–21, and 24–25 similar to MVIIA, and the disulfide bridge pattern of SO-3 was also identical to the cystine framework that found in MVIIA and other ω-conotoxins (Yan et al., 2003). These results indicate that the identity of SO-3 and MVIIA in three-dimensional structure contribute to their selectively targeting the same N-type VSCCs. Additionally, the previous structure–activity analysis of ω-conotoxins also indicates that loops 1 and 3 have little effect on selectivity, whereas loops 2 and 4 are critical determinants in controlling the selectivity of ω-conotoxins for N- or P/Q-, R-type VSCCs (Nielsen et al., 1999a; 2000; Lewis et al., 2000). Hence, the assumption that SO-3 and SVIB target different VSCC subtypes due to the difference in their three-dimensional structure requires further studies.
Reversibility of the inhibitory effects of SO-3 on HVA ICa
Previously, some researchers have reported and discussed the reversibility of the blocking effects of several ω-conotoxins on the transiently expressed N-type VSCCs and these results are not consistent with each other. Taken together, the blocking effect of GVIA is poorly reversible, while that of both MVIIA and CVID are readily reversible (Williams et al., 1992a; Kristipati et al., 1994; Lin et al., 1997; Stocker et al., 1997; Feng et al., 2001; 2003; Liang & Elmslie, 2002; Mould et al., 2004). Since ω-conotoxin GVIA dissociates very slowly from VSCCs, it may be difficult to control in a clinical setting, and is therefore not an ideal drug candidate. In our study, the inhibitory effects of SO-3 and MVIIA on HVA ICa in primary cultured hippocampal neurons were both almost completely reversed, and the recovery from block by MVIIA was more rapid than recovery from block by SO-3 (Figure 10). The recovery from block by ω-conotoxins is partly dependent on the divalent cations in extracellular solution (Liang & Elmslie, 2002), the Vh (Stocker et al., 1997; Feng et al., 2003), and the α2δ auxiliary subunit of VSCCs (Mould et al., 2004). Apart from these, the amino-acid residue of ω-conotoxins at position 10 has a significant impact on the extent of the reversibility of these toxins (Mould et al., 2004). SO-3 and MVIIA, both with Arg at position 10 (Table 1), can reversibly block the VSCCs, whereas GVIA demonstrates different reversibility because of the different residue (Hyp) at position 10 (Table 1). The different extent of recovery from the block by SO-3 and MVIIA may due to the different sequence of the amino-acid residues near this position or other unknown mechanisms.
Potential therapeutic implications
N-type calcium channels located in pre-synaptic nerve terminals regulate the influx of calcium which is necessary for neurotransmitter release in both the central and peripheral nervous systems (Jarvis & Zamponi, 2001; Zamponi, 2003). Selective blockers of N-type VSCCs may have potential neuroprotective application in ischemic brain injury by increasing blood flow, reducing glutamate release, and reducing postsynaptic calcium influx (Pringle et al., 1996; Bowersox et al., 1997). However, considerable research in the last decade has focused on the therapeutic potential of the N-type VSCCs blockers as a new class of analgesic agents (Vanegasa & Schaible, 2000; Scott et al., 2002). N-type calcium channels are highly present at the pre-synaptic terminals of nociceptive neurons in dorsal horn of the spinal cord where they regulate the release of the key pro-nociceptive neurotransmitters such as glutamate, substance P, neurokinin A, and/or calcitonin gene-related peptide (Jarvis & Zamponi, 2001; Adams et al., 2003). The crucial role of the N-type channels in nociception is also supported by the evidence that mice lacking the N-type channel gene have higher pain thresholds compared to wild type (Hatakeyama et al., 2001; Kim et al., 2001; Saegusa et al., 2002).
Recently, the pain-relieving effects of several ω-conotoxins have been characterized. MVIIA (also known as SNX-111, Ziconotide or Prialt) is under phase III clinical trial in humans with severe intractable pain (Staats et al., 2004; Doggrell, 2004). Another peptide, CVID (also known as AM336), is more selective towards the N-type VSCC than MVIIA, may have superior clinical utility and lower side-effects relative to MVIIA for the alleviation of persistent pain states (Scott et al., 2002; Smith et al., 2002). Our previous studies also showed that SO-3 displays an analgesic potency similar to MVIIA in a range of acute and chronic pain models in rodents, yet has less adverse effects compared with identical dosages of MVIIA injected intrathecally (Dai et al., 2003).
The neurotransmitter release from most central synapses is mainly controlled by P/Q- and R-type calcium channels (Meir et al., 1999), and, P/Q-type VSCCs exists primarily in neuromuscular junction, selective P/Q-type VSCCs blockers (such as MVIIC and SVIB) are likely to be lethal at relatively low dose and are considered unuseful in pain treatment (Bowersox et al., 1995; Katz et al., 1997). Consequently, ω-conotoxin more selective for N-type VSCCs than P/Q and other type VSCCs can largely minimize the side-effects in analgesic therapy. As mentioned previously, SO-3 does not inhibit L-, P/Q-, and R-type currents at the concentrations (1–3 μM) where its inhibition on N-type currents is maximum (Figures 5, 6, 7 and 8), and it has less effects on non-N-type HVA currents than MVIIA at higher concentrations (30 and 100 μM) (Figure 9), which may contribute to the different side-effects of SO-3 and MVIIA observed in previous animal experiments. These characteristics also indicate that SO-3 may have more advantages in clinical field as an analgesic candidate.
Acknowledgments
This work was supported by grants from the National High-Tech Research and Development Program (863) of China (2001AA624150) and partly supported by grants from the National Natural Science Foundation of China (30100240). We thank Drs Qiuyun Dai and Xiaowei Zhou (Beijing Institute of Biotechnology, Beijing, China) for synthesis and identification of SO-3. Thanks are also due to Professor Shuangqing Peng (Beijing Institute of Pharmacology and Toxicology, Beijing, China) for helpful discussions and comments on the manuscript.
Abbreviations
- Aga
ω-agatoxin IVA
- CVID
ω-conotoxin CVID
- EGTA
ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HVA
high voltage-activated
- ICa
calcium currents
- IK (A)
transient outward potassium currents
- IK (DR)
delayed rectifier potassium currents
- INa
sodium currents
- I–V
current–voltage
- MVIIA
ω-conotoxin MVIIA
- TEA-Cl
tetraethylammonium chloride
- TTX
tetrodotoxin
- Vh
holding potential
- VSCCs
voltage-sensitive calcium channels
- VSPCs
voltage-sensitive potassium channels
- VSSCs
voltage-sensitive sodium channels
References
- ADAMS D.J., SMITH A.B., SCHROEDER C.I., YASUDA T., LEWIS R.J. ω-Conotoxin CVID inhibits a pharmacologically distinct voltage-sensitive calcium channel associated with transmitter release from preganglionic nerve terminals. J. Biol. Chem. 2003;278:4057–4062. doi: 10.1074/jbc.M209969200. [DOI] [PubMed] [Google Scholar]
- BANKER G.A., COWAN W.M. Further observations on hippocampal neurons in dispersed cell culture. J. Comp. Neurol. 1979;187:469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- BLALOCK E.M., PORTER N.M., LANDFIELD P.W. Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures. J. Neurosci. 1999;19:8674–8684. doi: 10.1523/JNEUROSCI.19-19-08674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWERSOX S.S., MILJANICH G.P., SUGIURA Y., LI C., NADASDI L., HOFFMAN B.B., RAMACHANDRAN J., KO C.P. Differential blockade of voltage-sensitive calcium channels at the mouse neuromuscular junction by novel ω-conopeptides and ω-agatoxin-IVA. J. Pharmacol. Exp. Ther. 1995;273:248–256. [PubMed] [Google Scholar]
- BOWERSOX S.S., SINGH T., LUTHER R.R. Selective blockade of N-type voltage-sensitive calcium channels protects against brain injury after transient focal cerebral ischemia in rats. Brain Res. 1997;747:343–347. doi: 10.1016/s0006-8993(96)01325-x. [DOI] [PubMed] [Google Scholar]
- BULAJ G., DELACRUZ R., AZIMI-ZONOOZ A., WEST P., WATKINS M., YOSHIKAMI D., OLIVERA B.M. Conotoxin structure/function through a cladistic analysis. Biochemistry. 2001;40:13201–13208. doi: 10.1021/bi010683a. [DOI] [PubMed] [Google Scholar]
- CURRIE K.P.M., FOX A.P. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J. Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAI Q., LIU F., ZHOU Y., LU B., YU F., HUANG P. The synthesis of SO-3, a conopeptide with high analgesic activity derived from Conus striatus. J. Nat. Prod. 2003;66:1276–1279. doi: 10.1021/np030099y. [DOI] [PubMed] [Google Scholar]
- DOGGRELL S.A. Intrathecal ziconotide for refractory pain. Expert Opin. Investig. Drugs. 2004;13:875–877. doi: 10.1517/13543784.13.7.875. [DOI] [PubMed] [Google Scholar]
- FENG Z.P., DOERING C.J., WINKFEIN R.J., BEEDLE A.M., SPAFFORD J.D., ZAMPONI G.W. Determinants of inhibition of transiently expressed voltage-gated calcium channels by ω-conotoxins GVIA and MVIIA. J. Biol. Chem. 2003;278:20171–20178. doi: 10.1074/jbc.M300581200. [DOI] [PubMed] [Google Scholar]
- FENG Z.P., HAMID J., DOERING C., BOSEY G.M., SNUTCH T.P., ZAMPONI G.W. Residue Gly1326 of the N-type calcium channel α1B subunit controls reversibility of ω-conotoxin GVIA and MVIIA block. J. Biol. Chem. 2001;276:15728–15735. doi: 10.1074/jbc.M100406200. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HATAKEYAMA S., WAKAMORI M., INO M., MIYAMOTO N., TAKAHASHI E., YOSHINAGA T., SAWADA K., IMOTO K., TANAKA I., YOSHIZAWA T., NISHIZAWA Y., MORI Y., NIIDOME T., SHOJI S. Differential nociceptive responses in mice lacking the α1B subunit of N-type Ca2+ channels. Neuroreport. 2001;12:2423–2427. doi: 10.1097/00001756-200108080-00027. [DOI] [PubMed] [Google Scholar]
- HILLYARD D.R., MONJE V.D., MINTZ I.M., BEAN B.P., NADASDI L., RAMACHANDRAN J., MILJANICH G., AZIMI-ZOONOOZ A., MCINTOSH J.M., CRUZ L.J., IMPERIAL J.S., OLIVERA B.M. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- JARVIS E.S., ZAMPONI G.W. Interactions between presynaptic Ca2+ channels, cytoplasmic messengers and proteins of the synaptic vesicle release complex. Trends Pharmacol. Sci. 2001;22:519–525. doi: 10.1016/s0165-6147(00)01800-9. [DOI] [PubMed] [Google Scholar]
- KATZ E., PROTTI D.A., FERRO P.A., ROSATO S., UCHITEL O.D. Effects of Ca2+ channel blocker neurotoxins on transmitter release and presynaptic currents at the mouse neuromuscular junction. Br. J. Pharmacol. 1997;121:1531–1540. doi: 10.1038/sj.bjp.0701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM C., JUN K., LEE T., KIM S.S., MCENERY M.W., CHIN H., KIM H.L., PARK J.M., KIM D.K., JUNG S.J., KIM J., SHIN H.S. Altered nociceptive response in mice deficient in the α1B subunit of the voltage-dependent calcium channel. Mol. Cell Neurosci. 2001;18:235–245. doi: 10.1006/mcne.2001.1013. [DOI] [PubMed] [Google Scholar]
- KIM J.I., TAKAHASHI M., OHTAKE A., WAKAMIYA A., SATO K. Tyr13 is essential for the activity of ω-conotoxin mviia and gvia, specific N-type calcium channel blockers. Biochem. Biophys. Res. Commun. 1995;206:449–454. doi: 10.1006/bbrc.1995.1063. [DOI] [PubMed] [Google Scholar]
- KRISTIPATI R., NADASDI L., TARCZY-HORNOCH K., LAU K., MILJANICH G.P., RAMACHANDRAN J., BELL J.R. Characterization of the binding of ω-Conopeptides to different classes of non-L-type neuronal calcium channels. Mol. Cell Neurosci. 1994;5:219–228. doi: 10.1006/mcne.1994.1026. [DOI] [PubMed] [Google Scholar]
- LEW M.J., FLINN J.P., PALLAGHY P.K., MURPHY R., WHORLOW S.L., WRIGHT C.E., NORTON R.S., ANGUS J.A. Structure–function relationships of ω-conotoxin GVIA. Synthesis, structure, calcium channel binding, and functional assay of alanine-substituted analogs. J. Biol. Chem. 1997;272:12014–12023. doi: 10.1074/jbc.272.18.12014. [DOI] [PubMed] [Google Scholar]
- LEWIS R.J., NIELSEN K.J., CRAIK D.J., LOUGHNAN M.L., ADAMS D.A., SHARPE I.A., LUCHIAN T., ADAMS D.J., BOND T., THOMAS L., JONES A., MATHESON J.L., DRINKWATER R., ANDREWS P.R., ALEWOOD P.F. Novel ω-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J. Biol. Chem. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- LIANG H., ELMSLIE K.S. Rapid and reversible block of N-type calcium channels (CaV 2.2) by ω-conotoxin GVIA in the absence of divalent cations. J. Neurosci. 2002;22:8884–8890. doi: 10.1523/JNEUROSCI.22-20-08884.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN Z., HAUS S., EDGERTON J., LIPSCOMBE D. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron. 1997;18:153–166. doi: 10.1016/s0896-6273(01)80054-4. [DOI] [PubMed] [Google Scholar]
- LU B.S., YU F., ZHAO D., HUANG P.T., HUANG C.F. Conopeptides from Conus striatus and Conus textile by cDNA cloning. Peptides. 1999;20:1139–1144. doi: 10.1016/s0196-9781(99)00116-3. [DOI] [PubMed] [Google Scholar]
- MCINTOSH J.M., HASSON A., SPIRA M.E., GRAY W.R., LI W., MARSH M., HILLYARD D.R., OLIVERA B.M. A new family of conotoxins that blocks voltage-gated sodium channels. J. Biol. Chem. 1995;270:16796–16802. doi: 10.1074/jbc.270.28.16796. [DOI] [PubMed] [Google Scholar]
- MEIR A., GINSBURG S., BUTKEVICH A., KACHALSKY S.G., KAISERMAN I., AHDUT R., DEMIRGOREN S., RAHAMIMOFF R. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol. Rev. 1999;79:1019–1088. doi: 10.1152/physrev.1999.79.3.1019. [DOI] [PubMed] [Google Scholar]
- MOULD J., YASUDA T., SCHROEDER C.I., BEEDLE A.M., DOERING C.J., ZAMPONI G.W., ADAMS D.J., LEWIS R.J. The α2δ auxiliary subunit reduces affinity of ω-conotoxins for recombinant N-type (Cav2.2) calcium channels. J. Biol. Chem. 2004;279:34705–34714. doi: 10.1074/jbc.M310848200. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA Y.M., TODOROVIC S.M., COVEY D.F., LINGLE C.J. The anesthetic steroid (+)-3α-hydroxy-5α-androstane-17β-carbonitrile blocks N-, Q-, and R-type, but not L- and P-type, high voltage-activated Ca2+ current in hippocampal and dorsal root ganglion neurons of the rat. Mol. Pharmacol. 1998;54:559–568. doi: 10.1124/mol.54.3.559. [DOI] [PubMed] [Google Scholar]
- NEWCOMB R., SZOKE B., PALMA A., WANG G., CHEN X.H., HOPKINS W., CONG R., MILLER J., URGE L., TARCZY-HORNOCH K., LOO J.A., DOOLEY D.J., NADASDI L., TSIEN R.W., LEMOS J., MILJANICH G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- NIELSEN K.J., ADAMS D., THOMAS L., BOND T., ALEWOOD P.F., CRAIK D.J., LEWIS R.J. Structure–activity relationships of ω-conotoxins MVIIA, MVIIC and 1, 4 loop splice hybrids at N and P/Q-type calcium channels. J. Mol. Biol. 1999a;289:1405–1421. doi: 10.1006/jmbi.1999.2817. [DOI] [PubMed] [Google Scholar]
- NIELSEN K.J., ADAMS D.A., ALEWOOD P.F., LEWIS R.J., THOMAS L., SCHROEDER T., CRAIK D.J. Effects of chirality at Tyr13 on the structure–activity relationships of ω-conotoxins from conus magus. Biochemistry. 1999b;38:6741–6751. doi: 10.1021/bi982980u. [DOI] [PubMed] [Google Scholar]
- NIELSEN K.J., SCHROEDER T., LEWIS R. Structure–activity relationships of ω-conotoxins at N-type voltage-sensitive calcium channels. J. Mol. Recognit. 2000;13:55–70. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<55::AID-JMR488>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., CRUZ L.J. Conotoxins, in retrospect. Toxicon. 2001;39:7–14. doi: 10.1016/s0041-0101(00)00157-4. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., MCINTOSH J.M., CRUZ L.J., LUQUE F.A., GRAY W.R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984;23:5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- PRINGLE A.K., BENHAM C.D., SIM L., KENNED Y.J., IANNOTTI F., SUNDSTROM L.E. Selective N-type calcium channel antagonist omega conotoxin MVIIA is neuroprotective against hypoxic neurodegeneration in organotypic hippocampal-slice cultures. Stroke. 1996;27:2124–2130. doi: 10.1161/01.str.27.11.2124. [DOI] [PubMed] [Google Scholar]
- RAMILO C.A., ZAFARALLA G.C., NADASDI L., HAMMERLAND L.G., YOSHIKAMI D., GRAY W.R., KRISTIPATI R., RAMACHANDRAN J., MILJANICH G., OLIVERA B.M. Novel α- and ω-conotoxins from Conus striatus venom. Biochemistry. 1992;31:9919–9926. doi: 10.1021/bi00156a009. [DOI] [PubMed] [Google Scholar]
- SAEGUSA H., MATSUDA Y., TANABE T. Effects of ablation of N- and R-type Ca2+ channels on pain transmission. Neurosci. Res. 2002;43:1–7. doi: 10.1016/s0168-0102(02)00017-2. [DOI] [PubMed] [Google Scholar]
- SCAMPS F., VIGUES S., RESTITUITO S., CAMPO B., ROIG A., CHARNET P., VALMIER J. Sarco-endoplasmic ATPase blocker 2, 5-di (tert-butyl)-1, 4-benzohydroquinone inhibits N-, P-, and Q- but not T-, L-, or R-type calcium currents in central and peripheral neurons. Mol. Pharmacol. 2000;58:18–26. doi: 10.1124/mol.58.1.18. [DOI] [PubMed] [Google Scholar]
- SCOTT D.A., WRIGHT C.E., ANGUS J.A. Actions of intrathecal ω-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur. J. Pharmacol. 2002;451:279–286. doi: 10.1016/s0014-2999(02)02247-1. [DOI] [PubMed] [Google Scholar]
- SHON K., STOCKER M., TERLAU H., STUHMER W., JACOBSEN R., WALKER C., GRILLEY M., WATKINS M., HILLYARD D.R., GRAY W.R., OLIVERA B.M. Conotoxin PVIIA: a peptide inhibiting the shaker K+ channel. J. Biol. Chem. 1998;273:33–38. doi: 10.1074/jbc.273.1.33. [DOI] [PubMed] [Google Scholar]
- SMITH M.T., CABOT P.J., ROSS F.B., ROBERTSON A.D., LEWIS R.J. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- STAATS P.S., YEARWOOD T., CHARAPATA S.G., PRESLEY R.W., WALLACE M.S., BYAS-SMITH M., FISHER R., BRYCE D.A., MANGIERI E.A., LUTHER R.R., MAYO M., MCGUIRE D., ELLIS D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- STOCKER J.W., NADASDI L., ALDRICH R.W., TSIEN R.W. Preferential interaction of ω-conotoxins with inactivated N-type ca2+ channels. J. Neurosci. 1997;17:3002–3013. doi: 10.1523/JNEUROSCI.17-09-03002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERLAU H., OLIVERA B.M. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- VANEGASA H., SCHAIBLE H.G. Effects of antagonists to high-threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain. 2000;8:9–18. doi: 10.1016/s0304-3959(99)00241-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M.E., BRUST P.F., FELDMAN D.H., PATTHI S., SIMERSON S., MAROUFI A., MCCUE A.F., VELICELEBI G., ELLIS S.B., HARPOLD M.M. Structure and functional expression of an ω-conotoxin-sensitive human N-type calcium channel. Science. 1992a;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M.E., FELDMAN D.H., MCCUE A.F., BRENNER R., VELICELEBI G., ELLIS S.B., HARPOLD M.M. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron. 1992b;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- YAN Y.B., TU G.Z., LUO X.C., DAI Q.Y., HUANG P.T., ZHANG R.Q. Three-dimensional solution structure of ω-conotoxin SO3 determined by 1HNMR. Chin. Sci. Bull. 2003;48:1097–1102. [Google Scholar]
- YANG F., FENG L., ZHENG F., JOHNSON S.W., DU J., SHEN L., WU C.P., LU B. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat. Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- ZAMPONI G.W. Regulation of presynaptic calcium channels by synaptic proteins. J. Pharmacol. Sci. 2003;92:79–83. doi: 10.1254/jphs.92.79. [DOI] [PubMed] [Google Scholar]
- ZHANG J.F., RANDALL A.D., ELLINOR P.T., HORNE W.A., SATHER W.A., TANABE T., SCHWARZ T.L., TSIEN R.W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]