Abstract
The neurobiological mechanism underlying the negative motivational component of withdrawal from acute opiate dependence is far from understood.
Our objectives were to determine whether the glutamatergic system is involved in the motivational component of morphine withdrawal in acutely dependent rats and such an involvement is associated with dopaminergic neurotransmission.
We examined the effects of various kinds of glutamate receptor antagonists on conditioned place aversion (CPA) induced by naloxone-precipitated withdrawal from a single morphine exposure 24 h before. Furthermore, the influence of pretreatment with the dopamine receptor antagonist haloperidol on those effects of glutamate receptor antagonists was also investigated.
CPA was attenuated in a dose-dependent manner by all glutamate receptor antagonists examined including the NMDA receptor antagonists (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclo-hepten-5,10-imine maleate (MK-801) and phencyclidine hydrochloride (PCP), AMPA receptor antagonist 1-(4-aminophenyl)4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI 52466), and metabotropic receptor antagonists (±)-2-amino-3-phosphonopropionic acid (AP-3) and (±)-α-methyl-4-carboxyphenylglycine (MCPG). The effects of MK-801, GYKI 52466 and MCPG were blocked by haloperidol.
These results suggest that the glutamatergic system involving multiple classes of receptors plays a role in the motivational component of withdrawal from acute morphine dependence, and the function of the glutamatergic system would be closely associated with dopaminergic neurotransmission.
Keywords: Morphine, acute dependence, naloxone, withdrawal, conditioned place aversion, glutamate receptor antagonist, dopamine
Introduction
Acute opiate dependence is defined as the precipitation of signs characteristic of withdrawal symptoms by an opiate antagonist after short-term infusion or even a single dose of an opiate agonist both in humans and in animals (Martin & Eades, 1964; Bickel et al., 1988). Recently, the maintenance of compulsive use of drugs has been suggested to be substantially associated with negative reinforcement, which is related to the aversive properties of withdrawal in dependent subjects (Rodriguez de Fonseca & Navarro, 1998; Koob, 2000). Various behavioral alterations postulated to reflect the aversive aspect of withdrawal in humans can be observed in acutely dependent animals, including suppression of operant responding, conditioned place aversion (CPA), conditioned taste aversion and increased thresholds for intracranial self-stimulation (Adams & Holtzman, 1990; Easterling & Holtzman, 1997; McDonald et al., 1997; Schulteis et al., 1997; Parker et al., 2002; Azar et al., 2003).
It is known that acute and chronic opiate dependence are not unitary phenomena. Discrepancies also appear to exist in the mechanisms underlying the negative motivational components of withdrawal that can be seen in both acutely and chronically dependent animals. Three important portions of the extended amygdala including the amygdala, the bed nucleus of the stria terminalis and the shell of the nucleus accumbens have been found to be activated in response to the low-level withdrawal from chronic dependence characterized by motivational signs (Gracy et al., 2001; Frenois et al., 2002). However, we recently demonstrated that only the amygdala among these three regions was sensitive to a negative motivational withdrawal stimulus in acute-dependent rats and appeared to serve as a common anatomic structure involved in the development of dependence from the early to fully developed stages of dependence (Jin et al., 2004). It will benefit the development of appropriate therapies for morphine dependence to further clarify the dissociation and similarity in the neuroanatomy, neurochemistry, etc., contributing to the motivational aspect of withdrawal between different stages of dependence.
It has been realized that the glutamatergic system plays a role in the development and maintenance of drug addiction (Tzschentke & Schmidt, 2003). There is evidence suggesting the involvement of the glutamatergic system in the aversive consequences of withdrawal from chronic opiate dependence. Inhibition of glutamatergic neurotransmission was found to attenuate the motivational signs of withdrawal in animals rendered chronically dependent on morphine (Higgins et al., 1992; Popik & Danysz, 1997; Watanabe et al., 2002; Kratzer & Schmidt, 2003; Maldonado et al., 2003). As to acutely dependent subjects, a similar phenomenon began to be reported recently. Blokhina et al. (2000) demonstrated that an NMDA receptor antagonist, D-CPPene, attenuated CPA induced by naloxone 4 h after acute morphine treatment in mice. In our most recent study, riluzole (a glutamate release inhibitor) blocked CPA induced by naloxone-precipitated withdrawal from a single morphine exposure 24 h before in rats (Jin et al., submitted). All these findings suggest that the glutamatergic system may be another common component in the adaptational changes of acute and chronic morphine dependence. The present study was undertaken to confirm the role of this neurotransmitter system in withdrawal aversion in animals subjected to acute morphine exposure by examining the effects of various kinds of antagonists for glutamate receptors on CPA.
A body of evidence suggests an involvement of the central dopamine system in the expression of opiate dependence. Reduction of the extracellular dopamine level in mesolimbic areas is associated with both spontaneous (Acquas & Di Chiara, 1992) and opiate receptor antagonist-appreciated withdrawal (Pothos et al., 1991; Rossetti et al., 1992). Furthermore, the role of the dopamine system in the conditioned aversive effects of opiate withdrawal has also been reported. The D2 receptor antagonist raclopride was found to produce CPA in morphine-pelleted rats (Funada & Shippenberg, 1996). In addition, our previous observation showed that the dopamine agonist apomorphine could reverse CPA induced by withdrawal of rats from a single morphine exposure (Araki et al., 2004).
There is a report demonstrating that the administration of the NMDA receptor antagonist, MK-801, to rats withdrawn from chronic morphine dependence can readily reverse the fall in the extracellular dopamine concentration in the ventral striatum (Rossetti et al., 1992). This implies an interaction between the dopaminergic and glutamatergic systems in opiate withdrawal. It remains unknown whether such an interaction exists in acutely dependent subjects and contributes to the negative motivational component of withdrawal. The present study was conducted to address this issue employing a CPA paradigm.
Methods
Subjects
Male Sprague–Dawley rats (Charles River, Japan; initial weight 205–235 g) were housed two or three per cage. The room temperature was kept at 23±1°C, and a 12-h light–dark cycle (lights on at 07:00) was maintained throughout the experiment. Food and water were available ad libitum. The experimental protocol was conducted according to the Guidelines of the Ethics Review Committee for Animal Experimentation of Okayama University Medical School. The rats were acclimatized to handling every day for about 1 week prior to the experiment.
Drugs
Morphine hydrochloride and naloxone hydrochloride were purchased from Takeda Pharmaceutical Co. Ltd (Osaka, Japan) and Sigma, respectively. Drugs examined were several glutamate receptor antagonists including (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclo-hepten-5,10-imine maleate (MK-801) (NMDA receptor antagonist, Sigma), phencyclidine hydrochloride (PCP) (NMDA receptor antagonist, Fukuyama University, Hiroshima, Japan), 1-(4-aminophenyl)4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI 52466) (AMPA receptor antagonist, Sigma), (±)-α-methyl-4-carboxyphenylglycine (MCPG) (metabotropic receptor antagonist, Sigma) and (±)-2-amino-3-phosphonopropionic acid (AP-3) (metabotropic receptor antagonist, Sigma), as well as the dopamine receptor antagonist haloperidol (injection, Dainippon Co. Ltd, Osaka, Japan). All drugs except MCPG were dissolved in or diluted with saline. MCPG was dissolved in 0.1 N NaOH. All drugs were administered subcutaneouly at a volume of 1 ml kg−1.
CPA
The method to establish CPA has been described elsewhere (Jin et al., 2004). Briefly, the apparatus consisted of two chambers with floors covered with wire mesh and check-pattern sandpaper squares, respectively. The animals experienced a preconditioning habituation to the apparatus and those showing initial bias for either compartment were eliminated from the study. On the first day of the conditioning procedure, all rats were injected with saline, and 5 min later, were confined to one side of the apparatus, either the mesh-floor chamber or the sandpaper-floor chamber in a counterbalanced manner, for 30 min. This chamber will be referred to as the ‘nontreatment-paired chamber'. On Day 2, rats were injected with 10 mg kg−1 of morphine and then returned to their home cages. On Day 3, rats were given naloxone (0.5 mg kg−1), and 5 min later, placed in the chamber opposite to that on Day 1 for 30 min. This chamber will be referred to as the ‘treatment-paired chamber'. At 48 h after the conditioning trial, all rats were allowed to freely explore the entire apparatus for 15 min and the amount of time spent in each chamber was measured. CPA scores representing the time spent in the treatment-paired chamber minus the time spent in the nontreatment-paired chamber during the place preference test were calculated.
Effects of glutamate receptor antagonists on naloxone-induced CPA in rats acutely treated with morphine
On the third day of the conditioning procedure, rats were given saline or one of the glutamate receptor antagonists prior to receiving naloxone. The intervals between these injections and the naloxone challenge were 20 min, 1 h, 30 min, 20 min, 30 min and 30 min for saline, MK-801, PCP, GYKI 52466, MCPG and AP-3, respectively. The doses used were 0.00625, 0.0125, 0.025 and 0.05 mg kg−1 for MK-801; 0.375, 0.75 and 1.5 mg kg−1 for PCP; 1.0, 2.0 and 5.0 mg kg−1 for GYKI 52466; 1.0, 2.0, 4.0 and 8.0 mg kg−1 for MCPG; and 0.75, 1.5 and 3.0 mg kg−1 for AP-3. The number of rats in each group was 5.
To determine the effects of these glutamate receptor antagonists alone on place conditioning in either morphine-naïve or morphine-exposed rats, separate groups (n=5–8) received morphine or saline on Day 2. On the next day, they were injected with saline, MK-801 (0.05 mg kg−1), PCP (1.5 mg kg−1), GYKI 52466 (5.0 mg kg−1), MCPG (4.0 mg kg−1) or AP-3 (3.0 mg kg−1) prior to receiving saline instead of naloxone.
Influence of haloperidol on the effects of glutamate receptor antagonists on naloxone-induced CPA in rats acutely treated with morphine
To determine whether there is a glutamatergic–dopaminergic interaction involved in the morphine withdrawal aversion, the influence of haloperidol on the effects of glutamate receptor antagonists on CPA was examined. Rats experienced the same treatments as described above, except that they received a pretreatment with haloperidol (0, 0.1 or 1.0 mg kg−1) 2 h prior to the administration of glutamate receptor antagonists on the third day of the conditioning procedure. The glutamate receptor antagonists employed here were MK-801 (0.05 mg kg−1), GYKI 52466 (5.0 mg kg−1) and MCPG (4.0 mg kg−1). The place conditioning action of haloperidol (1.0 mg kg−1) itself was also examined in both morphine-naïve and morphine-exposed rats. The number of rats in each group was 5–8.
Statistical analysis
Most of the data were analyzed using a one-way analysis of variance followed by Dunnett's multiple comparison test. In the case of comparisons between two groups, Student's t-test was carried out. The level of significance was set at P<0.05.
Results
Effects of glutamate receptor antagonists on naloxone-induced CPA in rats acutely treated with morphine
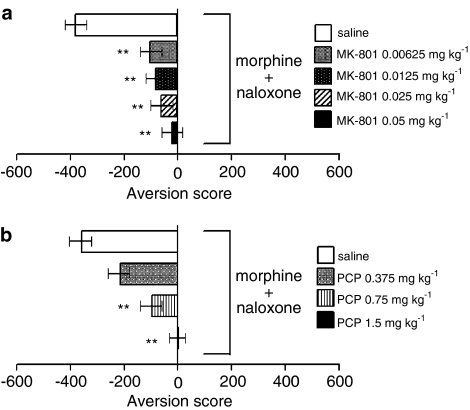
As presented in Figure 1, CPA was dose dependently attenuated by both NMDA receptor antagonists, MK-801 (Figure 1a) (F(4, 20)=7.062, P<0.01) and PCP (Figure 1b) (F(3, 16)=15.059, P<0.01). The post hoc comparisons demonstrated a significant difference for each dose (0.00625–0.05 mg kg−1) of MK-801 (P<0.01, each) and two higher doses (0.75 and 1.5 mg kg−1) of PCP (P<0.01, each) compared with the control group.
Figure 1.
Effects of NMDA receptor antagonists on CPA induced by naloxone in rats exposed to a single dose of morphine. (a) MK-801; (b) PCP. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test. **P<0.01 vs saline.
The AMPA receptor antagonist GYKI 52466 showed a similar dose-dependent inhibitory effect on CPA (Figure 2) (F(3, 16)=4.474, P<0.05). Significant differences compared with the control group were seen in the 2.0 and 5.0 mg kg−1 groups (P<0.05, each).
Figure 2.
Effect of AMPA receptor antagonist GYKI 52466 on CPA induced by naloxone in rats exposed to a single dose of morphine. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test. *P<0.05 vs saline.
The metabotropic receptor antagonists MCPG (Figure 3a) and AP-3 (Figure 3b) were also effective against CPA. Significant intergroup differences were present for both MCPG (F(4, 20)=4.010, P<0.05) and AP-3 (F(3, 16)=4.700, P<0.05). The post hoc comparisons revealed that CPA was significantly attenuated by MCPG at doses of 4.0 and 8.0 mg kg−1 (P<0.05 and 0.01, respectively) and by AP-3 at a dose of 3.0 mg kg−1 (P<0.01).
Figure 3.
Effects of metabotropic receptor antagonists on CPA induced by naloxone in rats exposed to a single dose of morphine. (a) MCPG; (b) AP-3. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test. *P<0.05, **P<0.01 vs saline.
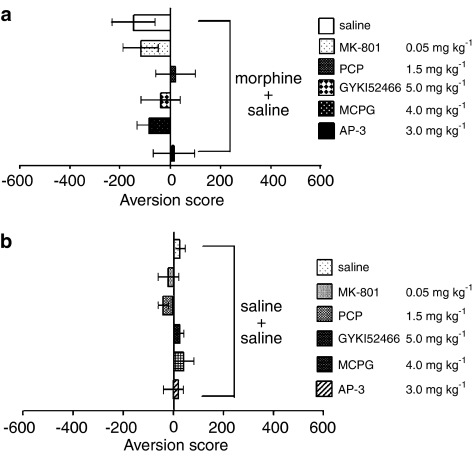
None of the agents examined including MK-801 (0.05 mg kg−1), PCP (1.5 mg kg−1), GYKI 52466 (5.0 mg kg−1), MCPG (4.0 mg kg−1) and AP-3 (3.0 mg kg−1) showed place conditioning ability (aversion or preference) on their own in either morphine-exposed (Figure 4a) or morphine-naïve (Figure 4b) rats.
Figure 4.
Effect of glutamate receptor antagonists alone on place conditioning in either morphine-exposed (a) or morphine-naïve (b) rats. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test.
Influence of haloperidol on the effects of glutamate receptor antagonists on naloxone-induced CPA in rats acutely treated with morphine
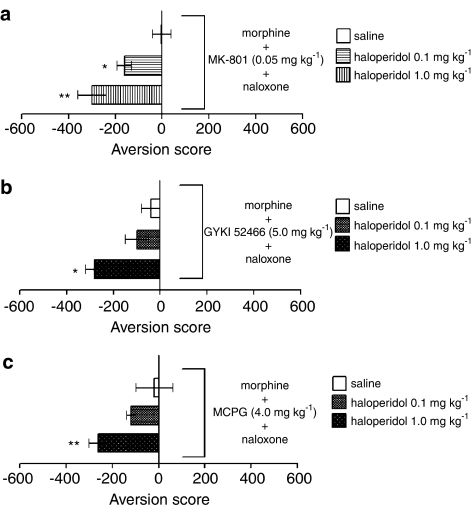
The inhibition of CPA by three kinds of glutamate receptor antagonists was blocked by the dopamine receptor antagonist haloperidol in a dose-dependent manner. Significant intergroup differences were present for 0.05 mg kg−1 of MK-801 (Figure 5a) (F(2, 12)=12.492, P<0.01), 5.0 mg kg−1 of GYKI 52466 (Figure 5b) (F(2, 12)=4.923, P<0.05) and 4.0 mg kg−1 of MCPG (Figure 5c) (F(2, 12)=8.935, P<0.01). The post hoc comparisons showed that haloperidol exerted its effect significantly at the dose of 0.1 and/or 1.0 mg kg−1 for each glutamate receptor antagonist (P<0.05 or 0.01, respectively).
Figure 5.
Influence of haloperidol on the effects of glutamate receptor antagonists on CPA induced by naloxone in rats exposed to a single dose of morphine. (a) MK-801; (b) GYKI 52466; c, MCPG. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test. *P<0.05, **P<0.01 vs saline.
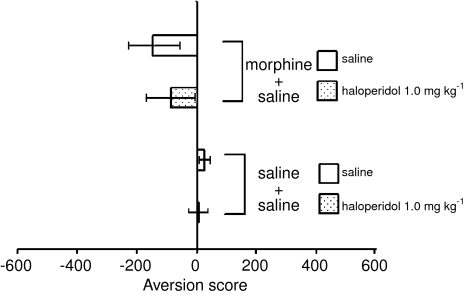
Haloperidol (1.0 mg kg−1) itself produced no place bias in either morphine-exposed or morphine-naïve rats (Figure 6).
Figure 6.
Effect of haloperidol alone on place conditioning in either morphine-exposed or morphine-naïve rats. Aversion scores are expressed as the mean time (±s.e.m.) in seconds spent in the treatment-paired chamber of the apparatus minus the mean time in seconds spent in the nontreatment-paired chamber during the place preference test.
Discussion
In the present study, all the glutamatergic antagonists examined significantly attenuated naloxone-induced CPA, a conditioned aversive behavior well established in rats experiencing a single morphine exposure. Furthermore, the effects of MK-801, GYKI 52466 and MCPG were blocked by haloperidol.
None of the glutamatergic antagonists produced any place conditioning by themselves in either morphine-naïve or morphine-treated subjects, suggesting that their effects on CPA induced by withdrawal from acute morphine exposure may not be due to a counteraction against withdrawal aversion of morphine via intrinsic reinforcing effects. Nevertheless, a considerable body of literature has demonstrated that some glutamatergic antagonists have some motivational properties, particularly two NMDA receptor antagonists PCP and MK-801. PCP is a widely abused drug in humans. Its rewarding effect has been shown in animals with certain indexes of reward including the conditioned place preference (CPP) paradigm (Marglin et al., 1989; Kitaichi et al., 1996; Nabeshima et al., 1996). However, PCP produces such a rewarding behavior only under appropriate experimental conditions. No place bias or CPA would occur with different doses and pretreatment experience (Barr et al., 1985; Acquas et al., 1989; Kitaichi et al., 1996; Nabeshima et al., 1996; Noda & Nabeshima, 1998). Here in this study, PCP failed to produce any place conditioning on its own under the present conditions: no pretreatment experience, 1.5 mg kg−1 and one-cycle conditioning training. MK-801 is another agent having some rewarding properties and eliciting CPP (Layer et al., 1993; Hoffman, 1994; Steinpreis et al., 1995; Del Pozo et al., 1996; Papp et al., 1996; Sukhotina et al., 1998; Panos et al., 1999). Similar to PCP, however, MK-801-induced CPP also appears to be dependent on the experimental conditions. Consistent with our observation, there are several reports demonstrating that this agent alone produces neither place preference nor aversion in rodents (Tzschentke & Schmidt, 1995; 1998; Kim et al., 1996; Ribeiro Do Couto et al., 2004). More importantly, MK-801 was reported to produce no motivational effects in morphine-dependent animals (Maldonado et al., 2003). All these findings suggest that a more specific mechanism rather than a simple additive one would mediate the effects of the glutamatergic antagonists examined to attenuate CPA in animals acutely dependent on morphine.
It may be argued that the effects of glutamatergic antagonists observed in the present study are a result of impairment in the rats' ability to associate the environmental cues to naloxone-precipitated opiate withdrawal. Indeed, it is well known that NMDA receptors are involved in some types of learning and memory, and NMDA receptor antagonists can interfere with these processes (Riedel et al., 2003). However, this explanation appears unlikely. If this is the case, it is difficult to interpret the phenomenon that these antagonists can produce CPP under suitable experimental conditions as mentioned above. In addition, the effective doses of MK-801 (0.00625–0.05 mg kg−1) in the present study are lower than those (0.06 and 0.12 mg kg−1) required to impair passive avoidance learning (Venable & Kelly, 1990), implying that the rats employed here were capable of developing certain conditioned associations under the MK-801 exposure. Furthermore, although MK-801 exerts an inhibitory influence on both CPA induced by opiate withdrawal (Higgins et al., 1992; Watanabe et al., 2002; Maldonado et al., 2003) and CPP induced by morphine (Tzschentke & Schmidt, 1995; Del Pozo et al., 1996; Kim et al., 1996) and cocaine (Cervo & Samanin, 1995), it had no effect on CPP induced by amphetamine (Hoffman, 1994). This phenomenon would be difficult to understand if the effect of MK-801 is due to a general nonselective disruption of place conditioning as the establishment of both CPA and CPP involves associative learning. In contrast to the well-established contribution of NMDA receptors to learning and memory, the contribution of AMPA receptors is less clearcut (Riedel et al., 2003). With respect to metabotropic receptors (Riedel et al., 2003), numerous studies have examined the functions of this kind of glutamatergic receptor with various agonists and antagonists (including MCPG) employing many different forms of learning. The functions of metabotropic receptors appear variable and dependent on the learning task (Riedel et al., 2003). So far, the data suggest little or no involvement of metabotropic receptors in the actual acquisition of new information (Riedel et al., 2003).
Taken together, although the possibility of impairing learning and memory processes could not be explicitly ruled out for all agents examined in the present study, such an impairment may not be of major importance for the antagonism of withdrawal-induced CPA in acutely dependent animals. The effects of glutamatergic antagonists might be attributable, at least in part, to a reduction in aversive or negative motivational aspects of opiate withdrawal. This is supported by the similar effect of riluzole observed in our most recent study using the same place-conditioning paradigm (Jin et al., submitted) and the inhibitory effect of D-CPPene on naloxone-induced CPA in mice acutely treated with morphine (Blokhina et al., 2000). These findings imply that the glutamatergic system may be involved in the motivational component of morphine withdrawal in acutely dependent animals and this involvement appears to include multiple classes of receptors. The existing literature has demonstrated that the neurobiological mechanisms underlying the aversive consequences of withdrawal from chronic opiate dependence also involve the glutamatergic system (Higgins et al., 1992; Popik & Danysz, 1997; Watanabe et al., 2002; Kratzer & Schmidt, 2003; Maldonado et al., 2003). Therefore, it is presumable that the glutamatergic system would serve as a common substrate for the negative motivational aspects of both chronic- and acute-dependent subjects.
Another finding in the present study is that the inhibition by MK-801, GYKI 52466 and MCPG of morphine withdrawal-induced CPA was blocked by the dopamine receptor antagonist haloperidol. The result that haloperidol itself showed no action to produce place conditioning in morphine-naïve or morphine-treated rats suggests a specific interactive effect of glutamatergic and dopaminergic neurotransmission in acute-dependent animals, and the inhibitory effects of glutamatergic antagonists on CPA may be related to the activation of the dopaminergic system. In animals rendered chronically dependent on opiate, withdrawal has been found to be associated with increased extracellular glutamate release (Aghajanian et al., 1994; Zhang et al., 1994; Sepulveda et al., 1998; Tokuyama et al., 2001) and a reduction of the dopamine level (Pothos et al., 1991; Acquas & Di Chiara, 1992; Rossetti et al., 1992) in the brain. Such a fall in the extracellular dopamine concentration in the ventral striatum could be reversed by MK-801, implying that the modification to the dopamine level caused by opiate withdrawal is related to NMDA-mediated glutamatergic activity (Rossetti et al., 1992). This glutamatergic–dopaminergic interaction is supported by an observation made in brain slices from normal rats. That is, glutamate mediated an inhibitory postsynaptic potential in dopamine neurons (Fiorillo & Williams, 1998). As we demonstrated above, the glutamatergic system may be involved in the negative motivational aspects of opiate withdrawal from both chronic and acute dependence. The dopaminergic system appears to play a similar role. The D2 receptor antagonist raclopride was found to induce CPA in morphine-pelleted rats (Funada & Shippenberg, 1996). The dopamine agonist apomorphine was shown to attenuate CPA in animals withdrawn from acute morphine exposure (Araki et al., 2004). The results of the present study suggest that the negative motivational aspects of opiate withdrawal involve an interaction between the glutamatergic and dopaminergic systems at least in acutely dependent subjects.
In conclusion, the present study suggests that the glutamatergic system involving multiple classes of receptors plays a role in the motivational component of withdrawal from acute dependence on morphine, and the function of the glutamatergic system would be closely associated with dopaminergic neurotransmission.
Acknowledgments
This work was supported in part by a Grant-in-aid for Drug Abuse Research from the Ministry of Health and Welfare, Japan, and the Uehara Memorial Foundation. We thank Ms E. Tatewaki for the donation of PCP.
Abbreviations
- AP-3
(±)-2-amino-3-phosphonopropionic acid
- CPA
conditioned place aversion
- CPP
conditioned place preference
- GYKI 52466
1-(4-aminophenyl)4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride
- MCPG
(±)-α-methyl-4-carboxyphenylglycine
- MK-801
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclo-hepten-5,10-imine maleate
- PCP
phencyclidine hydrochloride
References
- ACQUAS E., CARBONI E., LEONE P., DI CHIARA G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade. Psychopharmacology. 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- ACQUAS E., DI CHIARA G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J. Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- ADAMS J.U., HOLTZMAN S.G. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J. Pharmacol. Exp. Ther. 1990;253:483–489. [PubMed] [Google Scholar]
- AGHAJANIAN G.K., KOGAN J.H., MOGHADDAM B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- ARAKI H., KAWAKAMI K.Y., JIN C., SUEMARU K., KITAMURA M., FUTAGAMI K., SHIBATA K., KAWASAKI H., GOMITA Y. Nicotine attenuates place induces by naloxone in single-dose, morphine-treated rats. Psychopharmacology. 2004;171:398–404. doi: 10.1007/s00213-003-1595-7. [DOI] [PubMed] [Google Scholar]
- AZAR M.R., JONES B.C., SCHULTEIS G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology. 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- BARR G.A., PAREDES W., BRIDGER W.H. Place conditioning with morphine and phencyclidine: dose dependent effects. Life Sci. 1985;36:363–368. doi: 10.1016/0024-3205(85)90122-5. [DOI] [PubMed] [Google Scholar]
- BICKEL W.K., STITZER M.L., LIEBSON I.A., BIGELOW G.E. Acute physical dependence in man: effects of naloxone after brief morphine exposure. J. Pharmacol. Exp. Ther. 1988;244:126–132. [PubMed] [Google Scholar]
- BLOKHINA E.A., SUKHOTINA I.A., BESPALOV A.Y. Pretreatment with morphine potentiates naloxone-conditioned place aversion in mice: effects of NMDA receptor antagonists. Eur. J. Pharmacol. 2000;406:227–232. doi: 10.1016/s0014-2999(00)00689-0. [DOI] [PubMed] [Google Scholar]
- CERVO L., SAMANIN R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- DEl POZO E., BARRIOS M., BAEYENS J.M. The NMDA receptor antagonist dizocilpine (MK-801) stereoselectively inhibits morphine-induced place preference conditioning in mice. Psychopharmacology. 1996;125:209–213. doi: 10.1007/BF02247330. [DOI] [PubMed] [Google Scholar]
- EASTERLING K.W., HOLTZMAN S.G. Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J. Pharmacol. Exp. Ther. 1997;281:188–199. [PubMed] [Google Scholar]
- FIORILLO C.D., WILLIAMS J.T. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- FRENOIS F., CADOR M., CAILLE S., STINUS L., LE MOINE C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur. J. Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- FUNADA M., SHIPPENBERG T.S. Differential involvement of D1 and D2 dopamine receptors in the expression of morphine withdrawal signs in rats. Behav. Pharmacol. 1996;7:448–453. [PubMed] [Google Scholar]
- GRACY K.N., DANKIEWICZ L.A., KOOB G.F. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdale parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- HIGGINS G.A., NGUYEN P., SELLERS E.M. The NMDA antagonist dizocilpine (MK801) attenuates motivational as well as somatic aspects of naloxone precipitated opioid withdrawal. Life Sci. 1992;50:PL167–PL172. doi: 10.1016/0024-3205(92)90452-u. [DOI] [PubMed] [Google Scholar]
- HOFFMAN D.C. The noncompetitive NMDA antagonist MK-801 fails to block amphetamine-induced place conditioning in rats. Pharmacol. Biochem. Behav. 1994;47:907–912. doi: 10.1016/0091-3057(94)90295-x. [DOI] [PubMed] [Google Scholar]
- JIN C., ARAKI H., NAGATA M., SUEMARU K., SHIBATA K., KAWASAKI H., HAMAMURA T., GOMITA Y. Withdrawal-induced c-Fos expression in the rat centromedial amygdala 24 h following a single morphine exposure. Psychopharmacology. 2004;175:428–435. doi: 10.1007/s00213-004-1844-4. [DOI] [PubMed] [Google Scholar]
- JIN C., ARAKI H., KAWASAKI Y., NAGATA M., SUEMARU K., SHIBATA K., HAMAMURA T., KAWASAKI H., GOMITA Y.Effect of riluzole on conditioned place aversion and c-Fos expression within the amygdala induced by naloxone in rats undergoing a single-morphine exposure Eur. Neuropsychopharmacolsubmitted, JVR000635/JVR000716
- KIM H.S., JANG C.G., PARK W.K. Inhibition by MK-801 of morphine-induced conditioned place preference and postsynaptic dopamine receptor supersensitivity in mice. Pharmacol. Biochem. Behav. 1996;55:11–17. doi: 10.1016/0091-3057(96)00078-0. [DOI] [PubMed] [Google Scholar]
- KITAICHI K., NODA Y., HASEGAWA T., FURUKAWA H., NABESHIMA T. Acute phencyclidine induces aversion, but repeated phencyclidine induces preference in the place conditioning test in rats. Eur. J. Pharmacol. 1996;318:7–9. doi: 10.1016/s0014-2999(96)00875-8. [DOI] [PubMed] [Google Scholar]
- KOOB G.F. Neurobiology of addiction: toward the development of new therapies. Ann. NY Acad. Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- KRATZER U., SCHMIDT W.J. Caroverine inhibits the conditioned place aversion induced by naloxone-precipitated morphine withdrawal in rats. Neurosci. Lett. 2003;349:91–94. doi: 10.1016/s0304-3940(03)00783-3. [DOI] [PubMed] [Google Scholar]
- LAYER R.T., KADDIS F.G., WALLACE L.J. The NMDA receptor antagonist MK-801 elicits conditioned place preference in rats. Pharmacol. Biochem. Behav. 1993;44:245–247. doi: 10.1016/0091-3057(93)90306-e. [DOI] [PubMed] [Google Scholar]
- MALDONADO C., CAULI O., RODRIGUZ-ARIAS M., AGUILAR M.A., MINARRO J. Memantine presents different effects from MK-801 in motivational and physical signs of morphine withdrawal. Behav. Brain Res. 2003;144:25–35. doi: 10.1016/s0166-4328(03)00044-5. [DOI] [PubMed] [Google Scholar]
- MARGLIN S.H., MILANO W.C., MATTIE M.E., REID L.D. PCP and conditioned place preferences. Pharmacol. Biochem. Behav. 1989;33:281–283. doi: 10.1016/0091-3057(89)90500-5. [DOI] [PubMed] [Google Scholar]
- MARTIN W.R., EADES C.G. A comparison between acute and chronic physical dependence in the chronic spinal dog. J. Pharmacol. Exp. Ther. 1964;146:385–394. [PubMed] [Google Scholar]
- MCDONALD R.V., PARKER L.A., SIEGEL S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacol. Biochem. Behav. 1997;58:1003–1008. doi: 10.1016/s0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., KITAICHI K., NODA Y. Functional changes in neuronal systems induced by phencyclidine administration. Ann. NY Acad. Sci. 1996;801:29–38. doi: 10.1111/j.1749-6632.1996.tb17429.x. [DOI] [PubMed] [Google Scholar]
- NODA Y., NABESHIMA T. Neuronal mechanisms of phencyclidine-induced place aversion and preference in the conditioned place preference task. Methods Find Exp. Clin. Pharmacol. 1998;20:607–611. doi: 10.1358/mf.1998.20.7.485726. [DOI] [PubMed] [Google Scholar]
- PANOS J.J., RADEMACHER D.J., RENNER S.L., STEINPREIS R.E. The rewarding properties of NMDA and MK-801 (dizocilpine) as indexed by the conditioned place preference paradigm. Pharamacol. Biochem. Behav. 1999;64:591–595. doi: 10.1016/s0091-3057(99)00155-0. [DOI] [PubMed] [Google Scholar]
- PAPP M., MORYL E., MACCECCHINI M.L. Differential effects of agents acting at various sites of the NMDA receptor complex in a place preference conditioning model. Eur. J. Pharmacol. 1996;317:191–196. doi: 10.1016/s0014-2999(96)00747-9. [DOI] [PubMed] [Google Scholar]
- PARKER L.A., CYR J.A., SANTI A.N., BURTON P.D. The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine evaluation by taste and place conditioning. Pharmacol. Biochem. Behav. 2002;72:87–92. doi: 10.1016/s0091-3057(01)00724-9. [DOI] [PubMed] [Google Scholar]
- POPIK P., DANYSZ W. Inhibition of reinforcing effects of morphine and motivational aspects of naloxone-precipitated opioid withdrawal by N-methyl-D-aspartate receptor antagonist, memantine. J. Pharmacol. Exp. Ther. 1997;280:854–865. [PubMed] [Google Scholar]
- POTHOS E., RADA P., MARK G.P., HOEBEL B.G. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- RIBEIRO DO COUTO B., AGUILAR M.A., MANZANEDO C., RODRIGUEZ-ARIAS M., MINARRO J. Effects of NMDA receptor antagonists (MK-801 and memantine) on the acquisition of morphine-induced conditioned place preference in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:1035–1043. doi: 10.1016/j.pnpbp.2004.05.038. [DOI] [PubMed] [Google Scholar]
- RIEDEL G., PLATT B., MICHEAU J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ DE FONSECA F., NAVARRO M. Role of the limbic system in dependence on drugs. Ann. Med. 1998;30:397–405. doi: 10.3109/07853899809029940. [DOI] [PubMed] [Google Scholar]
- ROSSETTI Z.L., HMAIDAN Y., GESSA G.L. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur. J. Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- SCHULTEIS G., HEYSER C.J., KOOB G.F. Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology. 1997;129:56–65. doi: 10.1007/s002130050162. [DOI] [PubMed] [Google Scholar]
- SEPULVEDA M.J., HERNANDEZ L., RADA P., TUCCI S., CONTRERAS E. Effect of precipitated withdrawal on extracellular glutamate and aspartate in the nucleus accumbens of chronically morphine-treated rats: an in vivo microdialysis study. Pharmacol. Biochem. Behav. 1998;60:255–262. doi: 10.1016/s0091-3057(97)00550-9. [DOI] [PubMed] [Google Scholar]
- STEINPREIS R.E., KRAMER M.A., MIX K.S., PIWOWARCZYK M.C. The effects of MK801 on place conditioning. Neurosci. Res. 1995;22:427–430. doi: 10.1016/0168-0102(95)00919-k. [DOI] [PubMed] [Google Scholar]
- SUKHOTINA I., DRAVOLINA O., BESPALOV A. Place conditioning of mice with the NMDA receptor antagonists, eliprodil and dizocilpine. Eur. J. Pharmacol. 1998;362:103–110. doi: 10.1016/s0014-2999(98)00737-7. [DOI] [PubMed] [Google Scholar]
- TOKUYAMA S., ZHU H., OH S., HO I.K., YAMAMOTO T. Further evidence for a role of NMDA receptors in the locus coeruleus in the expression of withdrawal syndrome from opioids. Neurochem. Int. 2001;39:103–109. doi: 10.1016/s0197-0186(01)00019-5. [DOI] [PubMed] [Google Scholar]
- TZSCHENTKE T.M., SCHMIDT W.J. N-methyl-D-aspartic acid-receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci. Lett. 1995;193:37–40. doi: 10.1016/0304-3940(95)11662-g. [DOI] [PubMed] [Google Scholar]
- TZSCHENTKE T.M., SCHMIDT W.J. Discrete quinolinic acid lesions of the rat prelimbic medial prefrontal cortex affect cocaine- and MK-801-, but not morphine- and amphetamine-induced reward and psychomotor activation as measured with the place preference conditioning paradigm. Behav. Brain. Res. 1998;97:115–127. doi: 10.1016/s0166-4328(98)00034-5. [DOI] [PubMed] [Google Scholar]
- TZSCHENTKE T.M., SCHMIDT W.J. Glutamatergic mechanisms in addiction. Mol. Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- VENABLE N., KELLY P.H. Effects of NMDA receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacology. 1990;100:215–221. doi: 10.1007/BF02244409. [DOI] [PubMed] [Google Scholar]
- WATANABE T., NAKAGAWA T., YAMAMOTO R., MAEDA A., MINAMI M., SATOH M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn. J. Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- ZHANG T., FENG Y., ROCKHOLD R.W., HO I.K. Naloxone-precipitated morphine withdrawal increases pontine glutamate levels in the rat. Life Sci. 1994;55:PL25–PL31. doi: 10.1016/0024-3205(94)90108-2. [DOI] [PubMed] [Google Scholar]