Abstract
Vascular smooth muscle cell (VSMC) chemotaxis is fundamental to atherosclerosis and intimal hyperplasia. An increase in intracellular Ca2+ [Ca2+]i is an important signal in chemotaxis, but the role of L-type calcium channels (CaV1.2) in this response in human vascular smooth muscle cells (hVSMC) has not been examined.
hVSMC were grown from explant cultures of saphenous vein. Confluent hVSMC at passage 3 were studied after culture in medium containing 15% foetal calf serum (FCS) (randomly cycling) or following serum deprivation for up to 7 days. Smooth muscle α-actin was measured by immunoblotting and immunofluorescence microscopy. [Ca2+]i was measured using fura 2 fluorimetry. Chemotaxis was measured using a modified Boyden chamber technique and cell attachment to gelatin-coated plates was also quantified. The number and affinity of dihydropyridine-binding sites was assessed using [5-methyl-3H]PN 200-110 binding.
In randomly cycling cells, the calcium channel agonist, Bay K 8644a and 100 mM KCl did not affect [Ca2+]i. In addition, the rise in [Ca2+]i induced by platelet-derived growth factor-BB (PDGF) was unaffected by the CaV1.2 antagonists, amlodipine and verapamil. In randomly cycling cells amlodipine did not affect PDGF-induced migration.
In serum-deprived cells, smooth muscle α-actin was increased and Bay K 8644a and 100 mM KCl increased [Ca2+]i. PDGF-induced rises in [Ca2+]i were also inhibited by amlodipine and verapamil. The ability of Bay K 8644a to increase [Ca2+]i and verapamil to inhibit PDGF-induced rises in [Ca2+]i was evident within 3 days after serum withdrawal. In serum-deprived hVSMC Bay K 8644a induced chemotaxis and amlodipine inhibited PDGF-induced migration. Cell attachment in the presence of PDGF was unaffected by amlodipine in either randomly cycling or serum-deprived hVSMC.
Serum withdrawal was associated with a decrease in the maximum number of dihydropyridine-binding sites (Bmax) and a decrease in affinity (KD).
Serum deprivation of hVSMC results in increased expression of smooth muscle α-actin, a marker of more differentiated status, and increased [Ca2+]i responses and chemotaxis mediated by CaV1.2. These observations may have important implications for understanding the therapeutic benefits of calcium channel antagonists in cardiovascular disease.
Keywords: Vascular smooth muscle cells, L-type calcium channels, chemotaxis, intracellular calcium
Introduction
Vascular smooth muscle cells (VSMC) are responsible for the regulation of vascular tone and also participate in vascular responses to injury. Studies in animals indicate that following damage VSMC undergo dedifferentiation from a quiescent, ‘contractile' state to a ‘synthetic' phenotype (Campbell & Chamley-Campbell, 1981) and migrate into the region of injury, where they contribute to intimal hyperplasia (Ross, 1993). Smooth muscle cells in atherosclerotic plaques or intimal hyperplastic lesions more resemble dedifferentiated myofibroblasts, or cultured VSMC (Chamley-Campbell et al., 1979; Thyberg, 1996) than native contractile smooth muscle cells. In cellular terms, the transition from contractile to synthetic phenotype is characterised by reduced expression of a variety of proteins, including contractile proteins, such as smooth muscle α-actin (Owens, 1995). Synthetic cells have limited ability to contract and become more responsive to chemotactic and mitogenic factors (Ross, 1993). In culture, dedifferentiation of smooth muscle cells has been reported to be partially, though not fully, reversible by prolonged withdrawal of growth factors or serum (Chamley-Campbell et al., 1979; Chamley-Campbell & Campbell, 1981). Changes in intracellular Ca2+ ([Ca2+]i) are important in the process of cell migration (Pettit & Fay, 1998). In contractile VSMC L-type calcium channels (CaV1.2) are a major route of entry for Ca2+ ions (Hughes, 1995). Calcium antagonists, such as dihydropyridines and phenylalkylamines, block CaV1.2 and consequently their potential role in inhibiting atherosclerosis has attracted much interest. Calcium channel antagonists have been shown to inhibit smooth muscle cell migration (Nakao et al., 1983) and also inhibit formation of atherosclerosis in some animal models (Nomoto et al., 1987; Jackson et al., 1989). However, evidence in favour of a specific antiatherosclerotic effect in man is less convincing (Borhani et al., 1996; Pitt et al., 2000; Zanchetti, 2001; Hernandez et al., 2003). One possible explanation for this could be that CaV1.2 do not play such an important role in chemotaxis in human vascular smooth muscle cells (hVSMC) as in some other species. This study, therefore, examined the role of CaV1.2 in [Ca2+]i responses and chemotaxis in hVSMC.
Methods
Materials
Cell culture plastics, media, foetal calf serum (FCS), supplements and recombinant human PDGF were obtained from LifeTechnologies (Paisley, U.K.). PN200-110, (+)-[methyl-3H] (2948.9GBq mmol−1) and polycarbonate filters were purchased from NEN Life Science Products (Boston, MA, U.S.A.), and Poretics (Livermore, CA, U.S.A.) respectively. Bovine serum albumin (BSA) fraction V was obtained from Roche Diagnostics (Lewes, U.K.). Glass coverslips of 13 mm diameter and methanol were purchased from Merck (Poole, U.K.). Monoclonal anti-human smooth muscle α-actin (clone 1A4) (Skalli et al., 1986), secondary antibodies and normal rabbit serum were purchased from Dako Ltd (Ely, U.K.). Normal mouse immunoglobulins were obtained from R & D Systems Europe (Abingdon, U.K.). Nitrocellulose and the enhanced chemiluminescence kit were purchased from Amersham Pharmacia Biotech (High Wycombe, U.K.) and acrylamide and scintillant obtained from National Diagnostics (Hull, U.K). Amlodipine was a gift from Pfizer U.S. All other reagents were purchased from Sigma (Poole, U.K.).
Cell culture
Explant cultures of human VSMC were grown from saphenous vein as described previously (Chan et al., 1993). Redundant veins were obtained from patients undergoing cardiovascular surgery in accordance with the guidelines of the local Ethics Committee. VSMC cultures were established in Dulbecco's modification of Eagle's medium (DMEM) buffered with 25 mM HEPES and supplemented with 15% (v v−1) FCS 4 mM L-alanyl-L-glutamine (Glutamax-I), penicillin (100 U ml−1), streptomycin (100 μg ml−1) and gentamicin (25 μg ml−1). HVSMC cultures were maintained in a humidified atmosphere of 5% CO2 (v v−1) in air at 37°C and characterised by positive immunostaining for smooth muscle α-actin and lack of staining for von Willebrand factor. In this study, confluent VSMC were employed at the third passage for all the experiments, either in a randomly cycling or quiescent state (following up to 7 days of serum deprivation and maintenance in NCTC 109 medium supplemented with 25 mM HEPES, 4 mM, Glutamax-I, 0.25% (w v−1) BSA fraction V (2.5 mg ml−1) and antibiotics). These conditions were used to induce maximal quiescence as we have previously reported that HVSMC remain viable and do not undergo significant apoptosis following serum withdrawal for up to 7 days (Unlu et al., 2001). Cell cycle analysis of hVSMC by flow cytometry using propidium iodide revealed that the percentages of cells in the S and G0/G1 phases, respectively, were 12.3±0.5 and 74.0±0.9% in the presence of serum, and 2.6±0.5 and 90.9±0.6% after 24 h serum deprivation in NCTC 109 medium.
Measurement of [Ca2+]i
[Ca2+]i was measured following loading of hVSMC with 5 μM fura-2AM (90 min, 37°C) as described previously (Clunn et al., 1997a). After loading, cells were washed, briefly trypsinised, centrifuged and resuspended in physiological saline comprising (mM): NaCl 127, KCl 5.9, MgCl2 1.2, CaCl2 1, glucose 14 and HEPES 10.6, adjusted to pH 7.2 with NaOH. Fluorescence was measured using excitation wavelengths of 340 and 380 nm with emission at 510 nm using a Deltascan spectrofluorimeter (PTI). Experiments were calibrated and [Ca2+]i concentration calculated using 50 μM digitonin to permeabilise the cells. All experiments were performed at 37°C.
Cell migration
Chemotaxis assays were conducted using blind well chemotaxis chambers (Neuroprobe, Cabin John, MD, U.S.A.) as described previously (Clunn et al., 1997b). Briefly, VSMC were trypsinised and suspended in standard growth medium supplemented with 15% FCS. The resultant cell suspension was centrifuged (200 × g, 10 min) and resuspended in serum-free DMEM supplemented with 0.1% (w v−1) BSA fraction V. Cell viability was assessed using Trypan blue dye exclusion and a cell suspension prepared at a density of 2.25 × 105 viable cells ml−1.
The upper and lower compartments of the blind well chambers were separated by 13 mm gelatin-coated polycarbonate filters with 8 μm pores. The chemoattractant (0.3 ml, PDGF 2 ng ml−1) was added to the lower compartment and cell suspension (0.4 ml, 2.25 × 105 cells ml−1) added to the upper chamber. Cells in suspension were either pretreated with amlodipine or serum-free DMEM (30 min, 37°C) prior to transfer into the upper chamber. The migration assay was allowed to proceed for 5 h in the presence or absence of inhibitor at 37°C in a humidified atmosphere of 5% CO2 in air. Assays were terminated by washing the cells twice in Dulbecco's phosphate-buffered saline without calcium and magnesium (PBS-A) and fixing the cells in absolute ethanol at room temperature (10 min). The polycarbonate filters were stained in a 65 mM toluidine blue solution (15 min) and the cells on the upper side of the filter gently scraped off, leaving the migrated cells on the underside of the filters for counting. The cells present in four fields of view (× 200 magnification) were counted on duplicate filters.
Cell attachment
Cell adhesion was assessed using a modification of the method described by Bilato et al. (1997). Briefly, gelatin-coated 96-well plates were prepared by coating overnight with 50 μg ml−1 gelatin at 4°C and blocked with 5 mg ml−1 denatured BSA (1 h, 37°C). A cell suspension was prepared at a density of 2 × 105 viable cells ml−1 and 100 μl aliquots of cell suspension added to the wells in triplicate and maintained in a humidified atmosphere of 5% CO2 in air (5 h, 37°C). The optimal cell density and time course for cell adhesion were determined in preliminary experiments (data not shown). All subsequent steps were conducted at ambient temperature. Nonadherent VSMC were removed by washing twice with PBS-A and adherent cells fixed with 4% formaldehyde in PBS-A. The fixative was removed and the cells stained with 1% (w v−1) toluidine blue in 4% (v v−1) formaldehyde for 15 min. The wells were extensively rinsed with water and the stain eluted from the attached cells using 1% (w v−1) sodium dodecyl sulphate solution. Cell attachment was quantified by the dye adsorption which was determined spectrophotometrically at 630 nm using a microplate reader.
Dihydropyridine binding
The binding of [5-methyl-3H]-PN 200-110 to human VSMC was conducted according to the method of Navarro (1987). Briefly, VSMC were plated into 24-well plates at a density of 105 cells ml−1 (1 ml well−1) in standard growth medium supplemented with 15% FCS and allowed to attach overnight. One set of the 24-well plates was used to conduct a binding assay on randomly cycling cells, while cells in the remaining 24-well plates were rendered quiescent (as described earlier) for up to 7 days.
Prior to conducting the binding assays, the hVSMC were washed twice with pre-warmed serum-free DMEM. Cell counts were obtained in triplicate by treatment of the hVSMC with trypsin (0.25% in 1 mM EDTA in PBS-A) in order to normalise the ligand binding with respect to cell number. [5-methyl-3H]-PN 200-110 was prepared in serum-free DMEM to provide a range of final concentrations (10 pM–10 nM). Assays were performed under dim light in triplicate wells (30 min, 37°C) and terminated by removing the ligand and washing the cells three times (5 min each wash) with ice-cold wash buffer comprising 0.9% (w v−1) sodium chloride, 5% (v v−1) dimethyl sulphoxide and 1% (w v−1) BSA (pH 7.0). Nonspecific binding was determined by incubation under the same conditions with excess unlabelled nitrendipine (10 μM). Cells were solubilised with 1 M sodium hydroxide solution and aliquots of the cell lysates transferred to scintillation vials to which Ecoscint was added. Radioactivity was measured in a 1900CA TriCarb liquid scintillation counter (Canberra Packard, Pangbourne, U.K.) to determine the amount of [3H]-PN 200-110 bound to the cells.
HVSMC were plated at a density of 2 × 104 cells well−1 onto sterile 13 mm diameter glass coverslips that had been placed in 24-well plates. After overnight attachment, randomly cycling cells were washed twice with PBS-A and fixed with ice-cold methanol (10 min). Parallel cultures were rendered quiescent (as described above) by maintenance in supplemented NCTC 109 growth medium and fixed as described above.
Immunocytochemical studies were conducted at ambient temperatures. Fixed cells were hydrated by incubation in PBS-A (10 min) and blocked with 10% (v v−1) normal rabbit serum in PBS-A for 20 min to reduce nonspecific staining. Cells were incubated with either α-actin antibody (Skalli et al., 1986) (1/25) or normal mouse immunoglobulins at the same IgG1 concentration for 1 h and extensively washed in PBS-A containing 0.1% v v−1 Tween 20. Secondary antibody (1/15) was applied for 1 h in PBS-A containing 10% normal rabbit serum and the cells thoroughly washed in PBS-A containing 0.1% (v v−1) Tween 20. The coverslips were mounted in Citifluor mountant and viewed on a Carl Zeiss Axiophot microscope under epifluorescence. Photographs were taken using Kodak EliteChrome 400ASA film.
Immunoblotting studies
Proteins were separated on 10% SDS-polyacrylamide gels prior to transfer to nitrocellulose using a Bio-Rad wet gel transfer system as described previously (Patel et al., 2002). Following successful transfer, nitrocellulose blots were blocked using 5% BSA (w v−1) in tris-buffered saline containing 0.05% Tween 20 (TTBS) for 1 h and washed thoroughly in TTBS prior to probing with primary antibody for 1 h. Blots were probed with the appropriate secondary antibody prepared in TTBS containing 3% BSA, washed three times in TTBS and developed using the enhanced chemiluminescence kit.
Statistics and data analysis
All data are presented as means±s.e.m. of n observations. Radioligand-binding data were fitted to a logistic function by nonlinear regression using Prism 3.03 (GraphPad Software, San Diego, CA, U.S.A.) to calculate the maximum binding site number (Bmax) and binding affinity (KD). Statistical comparisons were made with Instat 3.05 (GraphPad Software, San Diego, CA, U.S.A.) using a repeated-measures ANOVA followed by Dunnett's multiple comparison test, or by a Student's paired t-test as appropriate. P<0.05 was considered significant.
Results
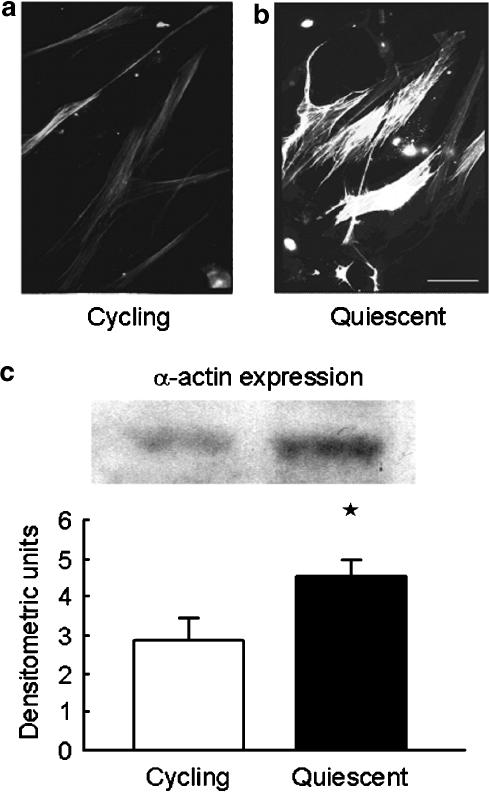
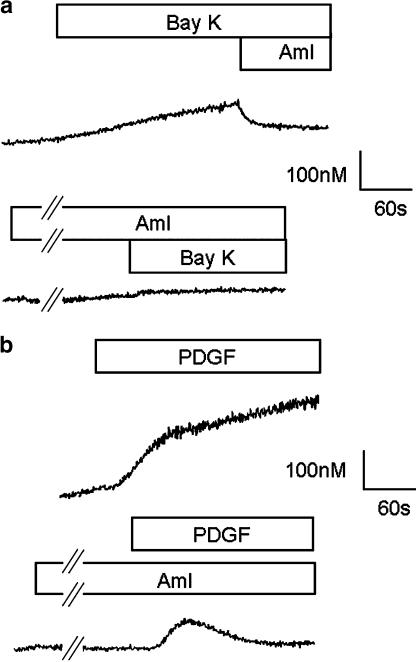
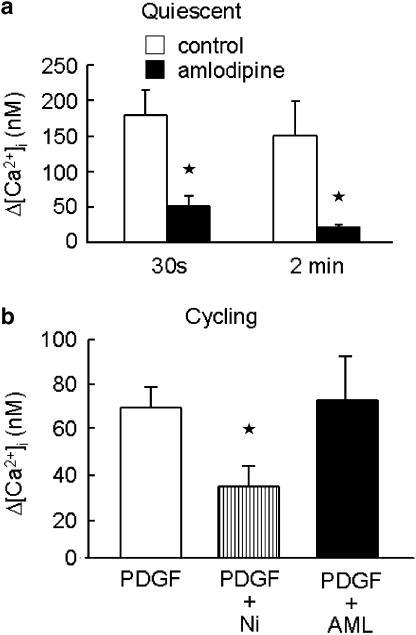
Serum withdrawal for 7 days resulted in increased levels of smooth muscle α-actin measured by immunoblotting and immunofluorescence microscopy (Figure 1). The effect of 7 days serum deprivation was also examined on changes in [Ca2+]i due to CaV1.2 in hVSMC. The calcium channel agonist Bay K 8644a (100 nM) or 100 mM KCl had no effect on [Ca2+]i in randomly cycling human VSMC (n=3; data not shown). However, following serum deprivation for 7 days 100 mM KCl (data not shown) or Bay K 8644a increased [Ca2+]i (Figure 2a) and this effect was inhibited by amlodipine (3 μM), an inhibitor of CaV1.2 (Figure 2a). In serum-deprived VSMC, pre-incubation with amlodipine (3 μM) also inhibited the rise in [Ca2+]i following application of PDGF (10ng ml−1) (Figure 2b). The effect of amlodipine was more marked on the later sustained rise in [Ca2+]i measured 2 min after the initial response to PDGF than the early rise in [Ca2+]i measured at 30 s (Figure 3a). In contrast, in randomly cycling cells amlodipine (3 μM) had no effect on the rise in [Ca2+]i, although NiCl2 (2 mM), a nonselective blocker of Ca2+ entry was able to partially inhibit the response to PDGF (Figure 3b).
Figure 1.
Effect of serum deprivation on smooth muscle α-actin in human saphenous vein-derived VSMC. (a) randomly cycling VSMC and (b) quiescent cells (following serum deprivation for 3 days) were stained with antibody for smooth muscle α-actin and examined using immunofluorescence microscopy (scale bar 50 μm, original magnification × 750). (c) Representative Western blot and average data derived from densitometry of three separate Western blots show α-actin content of cell lysates from randomly cycling and quiescent cells following 7 days serum deprivation. *P<0.05.
Figure 2.
Effect of (a) Bay K 8644a (Bay K; 100 nM) and amlodipine (AML; 3 μM) on [Ca2+]i in human VSMC rendered quiescent by 7 days of serum deprivation. (b) Effect of amlodipine (AML; 3 μM) on the rise in [Ca2+]i induced by PDGF (10 ng ml−1) in human VSMC rendered quiescent by 7 days serum deprivation. Traces are representative of 3–4 similar experiments.
Figure 3.
(a) Effect of amlodipine (3 μM) or vehicle (control) on the increase in intracellular calcium (Δ[Ca2+]i) induced by PDGF (10ng ml−1) in hVSMC deprived of serum for 7 days (quiescent) 30 s and 2 min after the initial response to PDGF. (b) Effect of NiCl2 (Ni; 2 mM) or amlodipine (AML; 3 μM) on the change in intracellular calcium (Δ[Ca2+]i) induced by PDGF (10ng ml−1) in randomly cycling human VSMC. Data are mean±s.e.m. of 3–5 observations. *P<0.05.
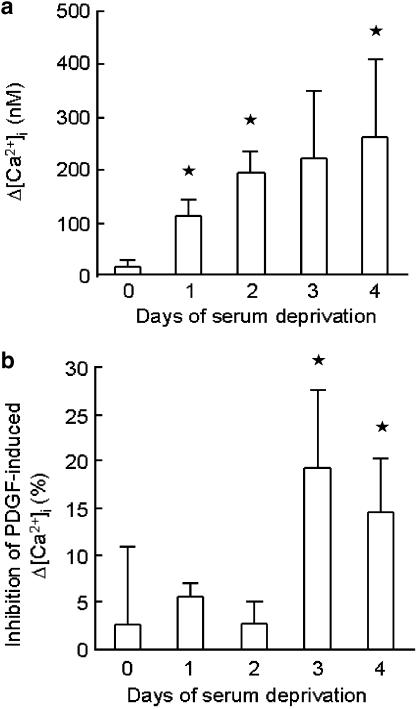
The time course of appearance of CaV1.2 was examined in more detail in subsequent studies using Bay K 8644a (100 nM) and another CaV1.2 inhibitor, verapamil (10 μM). Responses to Bay K 8644a (100 nM) increased progressively over a 4-day period (Figure 4) and by day 3 following serum withdrawal verapamil significantly inhibited the rise in [Ca2+]i induced by PDGF (10 ng ml−1) (Figure 4).
Figure 4.
(a) Effect of Bay K 8644a (100 nM) on the peak increase in intracellular calcium (Δ[Ca2+]i) after serum deprivation up to four days. (b) Effect of verapamil (10 μM) on the peak increase in intracellular calcium (Δ[Ca2+]i) induced by PDGF (10ng ml−1) after serum deprivation up to 4 days. Data represent mean±s.e.m. of 3–5 observations. *P<0.05.
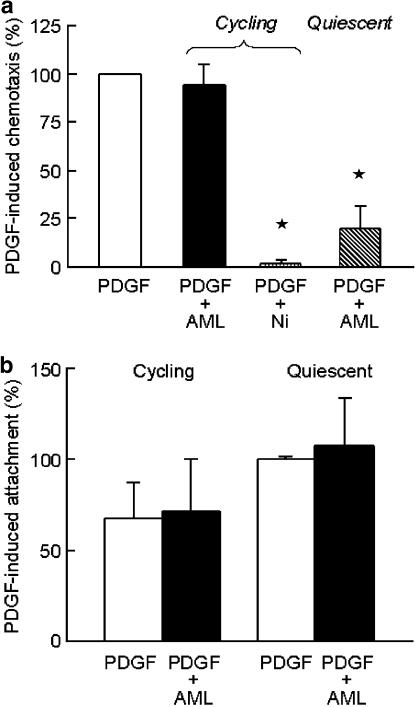
In order to assess the functional significance of these observations, we examined the effect of Bay K 8644a alone, and amlodipine on PDGF-induced chemotaxis of both randomly cycling hVSMC and hVSMC rendered quiescent by serum withdrawal for 7 days. In randomly cycling hVSMC, Bay K 8644a (100 nM) had no effect on chemotaxis and amlodipine (10 μM) did not affect migration in response to PDGF (2 ng ml−1), but the nonselective voltage-operated and receptor-operated channel blocker NiCl2 (2 mM) reduced PDGF-induced chemotaxis to nonstimulated levels (Figure 5a).
Figure 5.
(a) Effect of calcium channel blockers on chemotaxis of hVSMC in response to PDGF (2ng ml−1). Studies were performed in cycling and quiescent cells (after 7 days of serum deprivation). PDGF was placed in the lower chambers of the modified Boyden chambers and amlodipine (AML; 10 μM) or NiCl2 (Ni; 2 mM) was present in both chambers. (b) Effect of amlodipine (AML; 10 μM) on attachment of human VSMC in the presence of PDGF (2ng ml−1). Both randomly cycling and quiescent cells (after 7 days of serum deprivation) were preincubated with either serum-free medium or amlodipine (10 μM) for 30 min prior to conducting the attachment assays in gelatin-coated 96-well plates. Data are mean±s.e.m. of 4–7 separate experiments. *P<0.05.
In contrast, following serum deprivation Bay K 8644a (100 nM) increased chemotaxis to 156±30% of control (n=3; P=0.05), and amlodipine (10 μM) also significantly inhibited PDGF-induced chemotaxis (Figure 5a). Since the migration of cells through the pores in the filters in the modified Boyden chamber involves the process of attachment as well as directed movement of cells, we examined the effect of amlodipine on attachment to the gelatin-coated filters to establish which process involved CaV1.2. PDGF-induced attachment was unaffected by amlodipine (10 μM) in either randomly cycling or quiescent human VSMC (Figure 5b), suggesting that inhibition of CaV1.2 reduced cell migration rather than attachment.
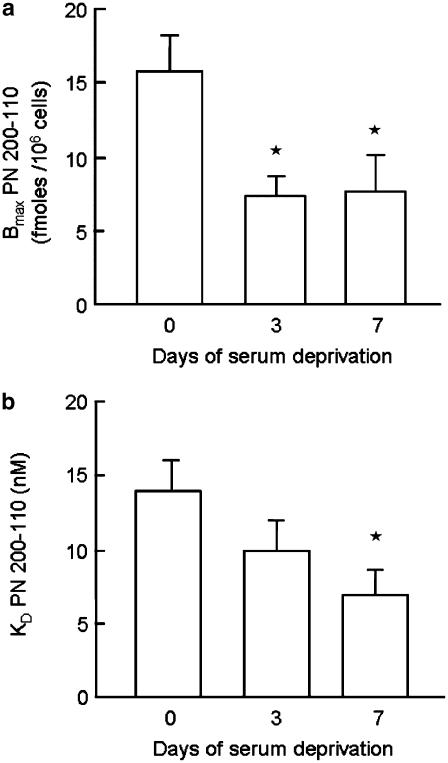
In view of the evidence suggesting an increased functional role of CaV1.2 in [Ca2+]i responses and chemotaxis in serum-deprived cells, we examined whether this was due to changes in the number and/or affinity of dihydropyridine-binding sites. Radioligand-binding studies using [3H]-PN 200-110 showed a significant reduction in the Bmax and a progressive decrease in KD in hVSMC after serum deprivation for 3 or 7 days (Figure 6a) compared with randomly cycling cells.
Figure 6.
Changes in (a) Bmax and (b) KD for the binding of [5-methyl-3H]-PN 200-110 to human VSMC. Radioligand-binding assays were conducted as described in Methods. Both Bmax and KD were determined from experiments performed on randomly cycling VSMC (day 0) and following serum deprivation for 3 and 7 days. Data represent mean±s.e.m. from five separate experiments. *P<0.05.
Discussion
In this study, we have examined the role of CaV1. 2 channels in [Ca2+]i responses and chemotaxis in randomly cycling and serum-deprived hVSMC derived from saphenous vein. In randomly cycling cells cultured in medium containing 15% FCS depolarisation by KCl or the selective CaV1.2 opener Bay K 8644a had no effect on [Ca2+]i and inhibition of CaV1.2 with amlodipine did not affect migration in response to PDGF. Serum withdrawal caused growth arrest as evidenced by the reduced number of cells in S phase. In addition, there was an increase in smooth muscle α-actin expression, suggesting that serum withdrawal results in some degree of re-differentiation towards a contractile state as reported previously (Fager et al., 1989). Serum deprivation was also accompanied by evidence of a contribution of CaV1.2 to [Ca2+]i responses and chemotaxis. In the case of [Ca2+]i responses, this was evident after 3–4 days of serum withdrawal. An action of amlodipine on cell attachment was excluded as the basis for its inhibitory action on cell migration and this effect is therefore likely to involve interference with Ca2+-dependent processes involved in migration (Nomoto et al., 1988). The concentrations of amlodipine used in these studies were quite high, but we have previously reported that the IC50 for amlodipine under nondepolarised conditions is ∼0.5 μM (Garcha et al., 1993) and consequently high concentrations are required for effective inhibition of CaV1.2. The absence of an effect on amlodipine on randomly cycling cells argues against nonspecific inhibitory effects of these concentrations of amlodipine. These observations contrast with earlier studies in animal-derived VSMC in which dihydropyridines and other calcium channel antagonists have been reported to inhibit chemotaxis in randomly cycling cells (Nomoto et al., 1988; Corsini et al., 1996).
Previous studies have reported that cell culture conditions affect expression of calcium channels. In rat aortic myocytes, Richard et al. (1992) reported that low-voltage-activated calcium channel currents were expressed transiently, while cells were proliferating, whereas high-voltage-activated currents were seen in the first 5 days in primary culture and after the cells had reached confluence. Ihara et al. (2002) reported L-type calcium channels were lost during proliferation and reappeared following serum deprivation, inhibition of MEK, or once cells had achieved confluence. Similarly, Kuga et al. (1996) reported that expression of both L- and T-type currents in rat aortic myocytes varied between different phases of the cell cycle with greatest expression of L-type currents in G1 phase and T-type currents in S phase. In human coronary myocytes, only dihydropyridine-sensitive L-type currents were seen in freshly isolated myocytes, whereas both L- and T-type currents were recorded from cells in primary culture (Quignard et al., 1997); the effect of more prolonged culture was not examined.
Surprisingly, in our studies of hVSMC, radioligand-binding assays showed a decrease in the number and affinity of dihydropyridine-binding sites following serum withdrawal for up to 7 days. These data suggest that reduced expression of α1-subunits of CaV1.2 (which correspond to dihydropyridine binding sites) does not account for the reduced functional role of CaV1.2 in randomly cycling hVSMC. This finding contrasts with a previous study, examining the A7r5 cell line, derived from rat embryonic thoracic aorta, which reported that retinoic acid-induced re-differentiation resulted in increased calcium channel currents, higher levels of dihydropyridine binding and increased expression of mRNA for α1 calcium channel subunits (Gollasch et al., 1998). While the decrease in dihydropyridine-binding sites with serum deprivation in hVSMC was unexpected and remains to be explained, it should be noted that the number of dihydropyridine-binding sites does not correspond to functional CaV1.2 in the membrane. Indeed, the number of dihydropyridine-binding sites generally exceeds the estimated number of functional CaV1.2 channels by 50–100-fold (Schwartz et al., 1985; Aiba & Creazzo, 1993). It has been reported that the level of expression of β subunits is important in regulating membrane localisation of α1 subunits (Perez-Garcia et al., 1995; Gao et al., 1999) and further studies exploring the effect of serum deprivation on calcium channel β (and other accessory) subunit expression would be of interest in understanding this issue.
The majority of evidence supporting the use of calcium antagonists as antiatherosclerotic agents has been based on animal studies in which these agents reduce the severity of atherosclerosis, independent of actions on plasma lipids and blood pressure (Paoletti et al., 1995). In humans, however, studies which have examined the action of calcium channel antagonists on atherosclerosis and intimal hyperplasia have been less clear cut (Borhani et al., 1996; Pitt et al., 2000; Zanchetti, 2001; Hernandez et al., 2003). It is possible that this is related to the influence of phenotypic state on CaV1.2 and its function in the migration of hVSMC.
In summary, this study shows that serum deprivation increases functional CaV1.2 in hVSMC and that this is not attributable to a reduction in the number of dihydropyridine-binding sites (i.e. the α1 subunit of CaV1.2). These findings may have implications for better understanding the effects of calcium channel antagonists in human cardiovascular disease.
Acknowledgments
We are grateful for financial support provided by the British Heart Foundation and Pfizer International. We thank Laura Sampson and Karen Gallagher for assistance in performing the immunocytochemical studies and maintaining the VSMC cultures, respectively. We also thank the surgeons and theatre staff at St Mary's Hospital for help with collection of saphenous vein.
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- hVSMC
human vascular smooth muscle cell(s)
- PBS-A
Dulbecco's phosphate-buffered saline (without calcium and magnesium)
- PDGF
platelet-derived growth factor-BB isoform
References
- AIBA S., CREAZZO T.L. Comparison of the number of dihydropyridine receptors with the number of functional L-type calcium channels in embryonic heart. Circ. Res. 1993;72:396–402. doi: 10.1161/01.res.72.2.396. [DOI] [PubMed] [Google Scholar]
- BILATO C., CURTO K.A., MONTICONE R.E., PAULY R.R., WHITE A.J., CROW M.T. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the alphavbeta3 integrin complex is reversed by activated calcium/calmodulin-dependent protein kinase II. J. Clin. Invest. 1997;100:693–704. doi: 10.1172/JCI119582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORHANI N.O., MERCURI M., BORHANI P.A., BUCKALEW V.M., CANOSSA-TERRIS M., CARR A.A., KAPPAGODA T., ROCCO M.V., SCHNAPER H.W., SOWERS J.R., BOND M.G. Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA. 1996;276:785–791. [PubMed] [Google Scholar]
- CAMPBELL G.R., CHAMLEY-CAMPBELL J.H. Smooth muscle phenotypic modulation: role in atherogenesis. Med. Hypotheses. 1981;7:729–735. doi: 10.1016/0306-9877(81)90084-0. [DOI] [PubMed] [Google Scholar]
- CHAMLEY-CAMPBELL J., CAMPBELL G.R., ROSS R. The smooth muscle cell in culture. Physiol. Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- CHAMLEY-CAMPBELL J.H., CAMPBELL G.R. What controls smooth muscle phenotype. Atherosclerosis. 1981;40:347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- CHAN P., MUNRO E., PATEL M., BETTERIDGE L., SCHACHTER M., SEVER P., WOLFE J. Cellular biology of human intimal hyperplastic stenosis. Eur. J. Vasc. Surg. 1993;7:129–135. doi: 10.1016/s0950-821x(05)80752-2. [DOI] [PubMed] [Google Scholar]
- CLUNN G.F., LYMN J.S., SCHACHTER M., HUGHES A.D. Differential effects of lovastatin on mitogen induced calcium influx in human cultured vascular smooth muscle cells. Br. J. Pharmacol. 1997a;121:1789–1795. doi: 10.1038/sj.bjp.0701299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORSINI A., BONFATTI M., QUARATO P., ACCOMAZZO M.R., RAITERI M., SARTANI A., TESTA R., NICOSIA S., PAOLETTI R., FUMAGALLI R. Effect of the new calcium antagonist lercanidipine and its enantiomers on the migration and proliferation of arterial myocytes. J. Cardiovasc. Pharmacol. 1996;28:687–694. doi: 10.1097/00005344-199611000-00012. [DOI] [PubMed] [Google Scholar]
- CLUNN G.F., REFSON J.S., LYMN J.S., HUGHES A.D. Platelet-derived growth factor beta-receptors can both promote and inhibit chemotaxis in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997b;17:2622–2629. doi: 10.1161/01.atv.17.11.2622. [DOI] [PubMed] [Google Scholar]
- FAGER G., HANSSON G.K., GOWN A.M., LARSON D.M., SKALLI O., BONDJERS G. Human arterial smooth muscle cells in culture: inverse relationship between proliferation and expression of contractile proteins. In vitro Cell. Dev. Biol. 1989;25:511–520. doi: 10.1007/BF02623563. [DOI] [PubMed] [Google Scholar]
- GAO T., CHIEN A.J., HOSEY M.M. Complexes of the alpha1C and beta subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J. Biol. Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- GARCHA R.S., SEVER P.S., HUGHES A.D. The action of amlodipine on human subcutaneous resistance arteries studied in vitro. J. Pharmacol. Exp. Ther. 1993;265:860–865. [PubMed] [Google Scholar]
- GOLLASCH M., HAASE H., RIED C., LINDSCHAU C., MORANO I., LUFT F.C., HALLER H. L-type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. 1998;12:593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ R.H., ARMAS-HERNANDEZ M.J., VELASCO M., ISRAILI Z.H., ARMAS- PADILLA M.C. Calcium antagonists and atherosclerosis protection in hypertension. Am. J. Ther. 2003;10:409–414. doi: 10.1097/00045391-200311000-00006. [DOI] [PubMed] [Google Scholar]
- HUGHES A.D. Calcium channels in vascular smooth muscle. J. Vasc. Res. 1995;32:353–370. doi: 10.1159/000159111. [DOI] [PubMed] [Google Scholar]
- IHARA E., HIRANO K., HIRANO M., NISHIMURA J., NAWATA H., KANAIDE H. Mechanism of down-regulation of L-type Ca(2+) channel in the proliferating smooth muscle cells of rat aorta. J. Cell Biochem. 2002;87:242–251. doi: 10.1002/jcb.10295. [DOI] [PubMed] [Google Scholar]
- JACKSON C.L., BUSH R.C., BOWYER D.E. Mechanism of antiatherogenic action of calcium antagonists. Atherosclerosis. 1989;80:17–26. doi: 10.1016/0021-9150(89)90063-4. [DOI] [PubMed] [Google Scholar]
- KUGA T., KOBAYASHI S., HIRAKAWA Y., KANAIDE H., TAKESHITA A. Cell cycle-dependent expression of L- and T-type Ca2+ currents in rat aortic smooth muscle cells in primary culture. Circ. Res. 1996;79:14–19. doi: 10.1161/01.res.79.1.14. [DOI] [PubMed] [Google Scholar]
- NAKAO J., ITO H., OOYAMA T., CHANG W.C., MUROTA S. Calcium dependency of aortic smooth muscle cell migration induced by 12-L-hydroxy-5,8,10,14- eicosatetraenoic acid. Effects of A23187, nicardipine and trifluoperazine. Atherosclerosis. 1983;46:309–319. doi: 10.1016/0021-9150(83)90180-6. [DOI] [PubMed] [Google Scholar]
- NAVARRO J. Modulation of [3H]dihydropyridine receptors by activation of protein kinase C in chick muscle cells. J. Biol. Chem. 1987;262:4649–4652. [PubMed] [Google Scholar]
- NOMOTO A., HIROSUMI J., SEKIGUCHI C., MUTOH S., YAMAGUCHI I., AOKI H. Antiatherogenic activity of FR34235 (Nilvadipine), a new potent calcium antagonist. Effect on cuff-induced intimal thickening of rabbit carotid artery. Atherosclerosis. 1987;64:255–261. doi: 10.1016/0021-9150(87)90253-x. [DOI] [PubMed] [Google Scholar]
- NOMOTO A., MUTOH S., HAGIHARA H., YAMAGUCHI I. Smooth muscle cell migration induced by inflammatory cell products and its inhibition by a potent calcium antagonist, nilvadipine. Atherosclerosis. 1988;72:213–219. doi: 10.1016/0021-9150(88)90083-4. [DOI] [PubMed] [Google Scholar]
- OWENS G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- PAOLETTI R., BERNINI F., CORSINI A., SOMA M.R. The antiatherosclerotic effects of calcium antagonists. J. Cardiovasc. Pharmacol. 1995;25 Suppl 3:S6–S10. [PubMed] [Google Scholar]
- PATEL M.K., MULLOY B., GALLAGHER K.L., O'BRIEN L., HUGHES A.D. The antimitogenic action of the sulphated polysaccharide fucoidan differs from heparin in human vascular smooth muscle cells. Thromb. Haemost. 2002;87:149–154. [PubMed] [Google Scholar]
- PEREZ-GARCIA M.T., KAMP T.J., MARBAN E. Functional properties of cardiac L- type calcium channels transiently expressed in HEK293 cells. Roles of alpha 1 and beta subunits. J. Gen. Physiol. 1995;105:289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTIT E.J., FAY F.S. Cytosolic free calcium and the cytoskeleton in the control of leukocyte chemotaxis. Physiol. Rev. 1998;78:949–967. doi: 10.1152/physrev.1998.78.4.949. [DOI] [PubMed] [Google Scholar]
- PITT B., BYINGTON R.P., FURBERG C.D., HUNNINGHAKE D.B., MANCINI G.B., MILLER M.E., RILEY W. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–1510. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.F., FRAPIER J.M., HARRICANE M.C., ALBAT B., NARGEOT J., RICHARD S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J. Clin. Invest. 1997;99:185–193. doi: 10.1172/JCI119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARD S., NEVEU D., CARNAC G., BODIN P., TRAVO P., NARGEOT J. Differential expression of voltage-gated Ca(2+)-currents in cultivated aortic myocytes. Biochim. Biophys. Acta. 1992;1160:95–104. doi: 10.1016/0167-4838(92)90042-c. [DOI] [PubMed] [Google Scholar]
- ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ L.M., McCLESKEY E.W., ALMERS W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. Nature. 1985;314:747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- SKALLI O., ROPRAZ P., TRZECIAK A., BENZONANA G., GILLESSEN D., GABBIANI G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J. Cell. Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THYBERG J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int. Rev. Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- UNLU S., CLUNN G., SCHACHTER M., DEMOLIOU-MASON C., HUGHES A.D. Action of an HMG CoA reductase inhibitor, lovastatin, on apoptosis of untransformed and ts-SV40 transformed human smooth muscle cells derived from saphenous vein. J. Cardiovasc. Pharmacol. 2001;38:161–173. doi: 10.1097/00005344-200108000-00001. [DOI] [PubMed] [Google Scholar]
- ZANCHETTI A. The antiatherogenic effects of antihypertensive treatment: trials completed and ongoing. Curr. Hypertens. Rep. 2001;3:350–359. doi: 10.1007/s11906-001-0098-3. [DOI] [PubMed] [Google Scholar]