Abstract
In order to characterize the roles of tyrosine kinases (TKs) and epidermal growth factor receptor (EGFR) in diabetes-induced vascular dysfunction, we investigated the ability of a chronic administration of genistein, a broad-spectrum inhibitor of TKs and AG1478, a specific inhibitor of EGFR TK activity to modulate the altered vasoreactivity of the perfused mesenteric bed to common vasoconstrictors and vasodilators in streptozotocin (STZ)-induced diabetes in rats.
The vasoconstrictor responses induced by norepinephrine (NE), endothelin-1 (ET-1) and angiotensin II (Ang II), were significantly increased, whereas vasodilator responses to carbachol and histamine were significantly reduced in the perfused mesenteric bed of STZ-induced diabetic rats in comparison with healthy rats. Treatment of diabetic animals with genistein or AG1478 produced a significant normalization of the altered agonist-induced vasoconstrictor and vasodilator responses without affecting blood glucose levels. In contrast, neither inhibitor had any effect on the vascular responsiveness of control (nondiabetic) animals. Treatment of diabetic animals with diadzein, an inactive analogue of genistein, did not affect the vasoconstrictor and vasodilator responses in control or diabetic animals. Phosphorylated EGFR levels were markedly raised in the mesenteric bed from diabetic animals and were normalized upon treatment with AG1478 or genistein.
These data suggest that activation of TK-mediated pathways, including EGFR TK signalling are involved in the development of diabetic vascular dysfunction.
Keywords: EGFR, genistein, AG1478, vascular dysfunction, diabetes
Introduction
Diabetes mellitus can induce complications such as alterations in the reactivity of blood vessels to vasoactive agents that contributes to cardiovascular dysfunction, including hypertension, atherosclerosis, microangiopathy and congestive heart failure (Garcia et al., 1974; Jarrett, 1989; Brownlee, 2001). Indeed, diabetics have an almost three times higher mortality from cardiovascular disease (CVD) than in the general population (Garcia et al., 1974; Jarrett, 1989). Enhanced vascular responsiveness to vasoconstrictors and an attenuated response to vasodilators in diabetic vascular tissues have been reported previously (Oyama et al., 1986; Kamata et al., 1989; Abebe et al., 1990; Mayhan et al., 1999; Makino et al., 2000), which may arise from increased intracellular calcium levels and/or exaggerated calcium sensitivity (Abebe et al., 1990; Mayhan et al., 1999). However, the signalling mechanisms involved in cardiovascular dysfunction in diabetes are not fully understood.
Epidermal growth factor receptor, EGFR (HER1 or erbB1), is a 170 kDa cell-surface bound glycoprotein that belongs to the erbB family of receptor tyrosine kinases, RTKs, (others are erbB2, 3 and 4) (Herbst, 2004). Upon binding of the appropriate ligand (e.g. epidermal growth factor (EGF) or heparin-binding (HB)-EGF), receptor homo- or heterodimerization results in autophosphorylation of the intracellular tyrosine kinase (TK) activity. The autophosphorylated dimer recruits adaptor proteins to lead to activation of multiple signalling pathways, including the classical cytosolic ras/raf/mitogen-activated protein kinase (MAPK) pathway (Yarden & Sliwkowski, 2001). Also, EGFR can be transactivated by G-protein coupled receptors (GPCRs) by several mechanisms including via c-src and by ADAM-12 (a disentegrin and metalloproteinase 12) (Meltrin alpha) or ADAM 10 (Kuzbanian)-mediated shedding of the HB-EGF from its cell surface bound precursor (Asakura et al., 2002; Yan et al., 2002).
Signalling via RTKs, such as the EGFR and other non-RTKs, have an influence on intracellular calcium levels and have been implicated in the development of pathologies associated with hypertension and diabetes (Patel et al., 1994; Sayed-Ahmed et al., 1996; Muthalif et al., 1998; 2000a, 2000b; 2002; Khan et al., 1999; Kassab et al., 2001; Nakajima et al., 2001). Diabetic rats fed on a diet containing genistein, a broad spectrum inhibitor of TKs including EGFR TK, had normalization of retinal vascular leakage implicating a role for TKs in diabetic retinopathy (Nakajima et al., 2001). Additionally in diabetes, enhanced levels and/or activity of EGFR have been observed in the gastric mucosa, where it is thought to lead to increased sensitivity of the gastric mucosa to injury (Khan et al., 1999), and also in the kidney of diabetic animals, where excessive local synthesis and release of EGF may contribute to the interstitial fibrosis found in kidney tubules in diabetic animals (Ziyadeh & Goldfarb, 1991). We have also previously shown in vascular smooth muscle cells (VSMCs) and experimental models of hypertension that activation of the cPLA2 (cytosolic phospholipase A2)/cytochrome P-450 (CYP-450)/Ras/Raf/MAPK pathway by angiotensin II (AngII), norepinephrine (NE), or EGF leads, through elevation of intracellular calcium levels and formation of 20-hydroxyeicosatetraenoic acid (20-HETE), to the development of hypertension and related end-organ pathologies (Muthalif et al., 1998; 2000a, 2000b; 2002). However, the contribution of EGFR TK-signalling in the development of diabetes-induced vascular dysfunction is not known. In this study, we have therefore investigated the effect of general inhibition of TKs by genistein or specific inhibition of EGFR TK activity with AG1478 on vasoreactivity to the vasoconstrictors, NE, ET-1 and AngII, and to the vasodilators, carbachol and histamine, in the perfused mesenteric vascular beds of STZ-induced diabetic rats.
Methods
Induction of diabetes
Female Wistar rats weighing 200–250 g were used in this study. Diabetes was induced by a single intraperitoneal injection of 55 mg kg−1 body weight streptozotocin (STZ) dissolved in citrate buffer (pH 4.5). Age-matched control rats were injected with the citrate buffer vehicle used to dissolve STZ. Basal glucose levels were determined prior to STZ injection, using an automated blood glucose analyzer (glucometer Elite XL). Blood glucose concentrations were determined 48 h after STZ injection. Rats with a blood glucose concentration above 300 mg dl−1 were declared diabetic.
Eight groups of rats were used in this study. Groups 1–4 were control (nondiabetic)-vehicle treated, control-genistein treated, control-AG1478 treated and control-diadzein treated rats, respectively. Groups 5, 6, 7 and 8 were diabetic-vehicle treated, diabetic-genistein treated, diabetic-AG1478 treated and diabetic-diadzein treated animals, respectively. Treatment with the inhibitors of TKs (1.5 mg kg−1 intraperitoneal alt diem, n=8) was started on the same day as the induction of diabetes and continued every other day for 4 weeks.
Isolation of the mesenteric vascular bed
The mesenteric beds were isolated carefully and transferred into a Petri dish containing oxygenated Krebs' solution. The mesenteric artery was cannulated, using a polyethylene cannula and the mesenteric bed was placed in a warm water-jacketed chamber at 37°C. The preparation was perfused with Krebs' solution (at 37°C), oxygenated with 95% oxygen and 5% carbon dioxide, delivered at a constant flow rate of 6 ml min−1 using a multichannel masterflex peristaltic pump. The composition of KH-solution was as follows (mM): NaCl (118.3), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), NaHCO3 (25), KH2PO4 (1.2) and glucose (11.2). Changes in perfusion pressure, which reflect peripheral resistance, were measured. Perfusion pressure was recorded via a pressure transducer connected to a Lectromed chart recorder. The preparation was always allowed to equilibrate for at least 30 min. A bolus injection of NE (100 nmol) was usually given at the beginning of the experiment as a test for tissue responsiveness.
This investigation conforms to the guidelines for the care and use of laboratory animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1985).
Vasoconstriction studies
The vasoconstrictor responses of NE (10, 100 and 1000 nmol), ET-1 (0.01, 0.1 and 1.0 nmol) and AngII (0.1 and 1.0 nmol) were investigated in the perfused mesenteric vascular bed. Following the period of equilibration, successive doses of the agonists NE, ET-1 or AngII were given at regular intervals to establish the vasoconstrictor responses (mmHg).
Vasodilation studies
The vasodilator responses of carbachol, histamine and sodium nitroprusside (SNP) were investigated in the perfused mesenteric vascular bed. Following the period of equilibration, the perfused mesenteric bed was constricted by perfusion with Krebs' solution containing NE (10−5 M). After establishing a steady level of precontraction, successive doses of carbachol (1 and 10 nmol), histamine (10 and 100 nmol) or SNP (0.1, 1.0 and 10 nmol) were given at regular intervals. The vasodilator response is expressed as % of the precontraction induced by NE (10−5 M). It should be noted that the same vascular bed was used to test each vasoconstrictor and vasodilator. The effect of the vasoconstrictors was examined first, except ET-1, followed by the vasodilators. ET-1 was the last agonist to be tested in each experiment.
Effect of acute treatment with AG1478 (10−5 M) on NE-induced vasoconstrictor response
The effect of acute treatment of the mesenteric vascular bed with AG1478 (10−5 M) was tested against the vasoconstrictor responses induced by NE. The mesenteric vascular bed from diabetic-control animals was perfused with Krebs' solution containing AG1478 (10−5 M) for 30 min. Following the period of incubation, the vasoconstrictor responses of NE (10, 100 and 1000 nmol) were investigated.
Western blotting analysis for phosphorylated EGFR
Rat mesenteric vascular beds were removed and stored at −80°C. The mesenteric beds were allowed to defrost on ice then transferred to lysis buffer (pH 7.6) containing 50 mM Tris-base, 5 mM EGTA, 150 mM NaCl, 1% Triton 100, 2 mM Na3VO4, 50 mM NaF, 1 mM PMSF, 20 μM phenylarsine, 10 mM sodium molybdate, 10 μg ml−1 leupeptin and 8 μg ml−1 aprotinin), mixed three times with sonicator probe (OMNI, 2000) at speed 2 for 20 s each time. The samples were left to lyse completely by incubation on ice for 30 min. Lysates were centrifuged at 14,000 r.p.m. for 20 min at 4°C and supernatants were collected and protein concentration was estimated by BioRad BCA protein assay. Aliquots containing equal amounts of protein were subjected to 8% SDS–PAGE gel electrophoresis (SDS–PAGE) and transferred onto Protran nitrocellulose (Schleicher & Schuell, Dassel, Germany). Membranes were incubated with rabbit Y1068 antiphosphorylated EGFR antibody (Cell Signalling Technology, U.S.A.) and then with donkey anti-rabbit IgG conjugated to horseradish peroxidase (Amersham, U.K.). Immunreactive bands were detected with SuperSignal chemiluminescent substrate (Pierce, U.K.). Densitometry was performed using GS-700 densitometer with molecular analyst software (BioRad, U.K.).
Cell culture of ECV-304 cells
Human ECV-304 cells were seeded at a density of 1 million cells in a T75-flask and incubated for 24 h in serum-free medium, after which the media was aspirated and cells were washed three times with phosphate-buffered saline (PBS). For control normal glucose conditions, cells were then grown in serum-free media containing 5.5 mM D-glucose or for high glucose media D-glucose in serum-free medium containing 25.5 mM D-glucose for varying time points. Cells were then lysed after 1, 6 and 24 h of incubation and pEGFR levels determined as described above for tissues.
Drugs
(±) Norepinephrine-bitartrate, STZ, genistein, histamine, endothelin-1, AngII, SNP and carbachol were obtained from Sigma Biochemicals. AG1478 and diadzein were purchased from Tocris Cookson Ltd, U.K.
Statistical analysis
Results were analyzed using Graph pad Prism software. Data are presented as mean±s.e. of ‘n' number of experiments. Mean values were compared using analysis of variance followed by post hoc test (Bonferroni) or Student's T-test for Western blot data. The difference was considered to be significant when the P-value was less than 0.05. Unless otherwise stated a single asterisk (*) indicates values significantly different from nondiabetic controls and double asterisk (**) indicates values significantly different from diabetic controls.
Results
Hyperglycemia
Induction of diabetes by STZ resulted in a significant increase in blood glucose concentration. Hyperglycemia persisted in the diabetic animals and was 610±24 mg dl−1 at 4 weeks as compared with 86±5 mg dl−1 in the nondiabetic control animals. Treatment of the diabetic rats with the TK inhibitors did not affect the level of hyperglycemia. The plasma glucose levels were 538±26, 550±28 and 532±23 mg dl−1 in the diabetic animals treated with genistein, AG1478 or diadzein, respectively.
Vasoconstriction studies
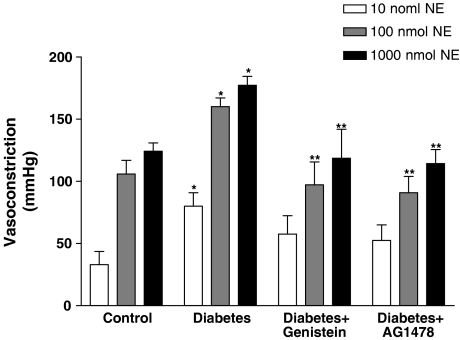
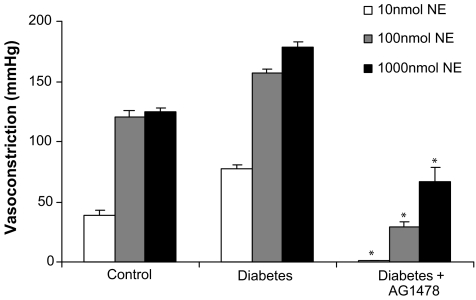
The vasoconstrictor response to NE was significantly augmented in the perfused mesenteric vascular bed from STZ-diabetic rats to 85±9, 160±7 and 177±7 mmHg compared to 39±7, 106±11 and 124±6 mmHg in the nondiabetic control rats, at 10, 100 and 1000 nmol, respectively (Figure 1). The potentiated vasopressor activity of NE was significantly (P<0.05) attenuated by 4-week treatment of diabetic rats with the TK inhibitors (genistein and AG1478) (Figure 1). The vasoconstrictor response to NE in the perfused mesenteric bed of genistein-treated animals was 97±18 and 119±23 mmHg to NE at 100 and 1000 nmol, respectively. Similarly, the vasoconstrictor response to NE in AG1478-treated perfused mesenteric beds from diabetic animals was 91±13 and 114±11 mmHg at 100 and 1000 nmol, respectively (Figure 1).
Figure 1.
Norepinephrine-induced vasoconstriction (10, 100 and 1000 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
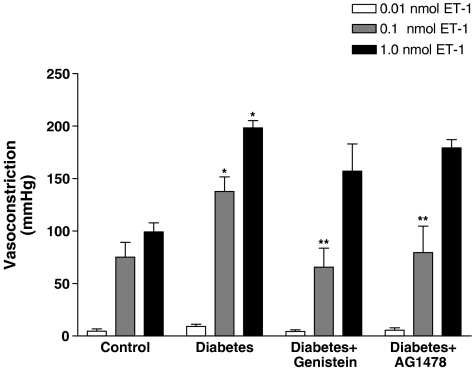
ET-1 induced vasoconstriction was also significantly (P<0.05) augmented in the perfused mesenteric beds from diabetic rats (Figure 2). ET-1-induced vasoconstriction was 138±14 and 198±7 mmHg in the mesenteric beds from diabetic rats compared to 75±14 and 99+8 mmHg at 0.1 and 1.0 nmol, respectively, to group I nondiabetic controls (Figure 2). Treatment of the diabetic rats with genistein normalized the vasoconstrictor response to ET-1 (0.1 and 1.0 nmol) to 66±18 and 157±26 mmHg. Similarly, AG1478-treatment also produced a significant attenuation of ET-1 induced vasoconstriction (0.1 nmol) to 80±25 mmHg (Figure 2).
Figure 2.
Endothelin-1-induced vasoconstriction (0.01, 0.1 and 1.0 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
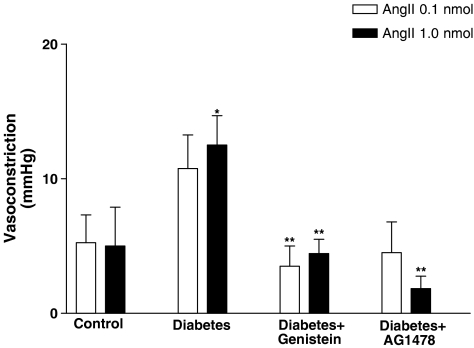
A relatively modest vasoconstrictor response to Ang II (0.1 and 1.0 nmol) was observed in the perfused mesenteric bed obtained from healthy controls, which was significantly increased in the diabetic state (Figure 3). Treatment of diabetic rats with TK inhibitors (genistein or AG1478) significantly reduced Ang II-induced vasoconstriction to levels similar to those observed in nondiabetic controls (Figure 3).
Figure 3.
Angiotensin-II-induced vasoconstriction (0.1, 1.0 and 10 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
Diadzein, the inactive analogue of genistein, did not affect agonist-induced vasoconstrictor responses in the diabetic animals (data not shown). Furthermore, inhibition of TKs or EGFR did not affect agonist-induced vasoconstrictor responses in the control animals.
Vasodilation studies
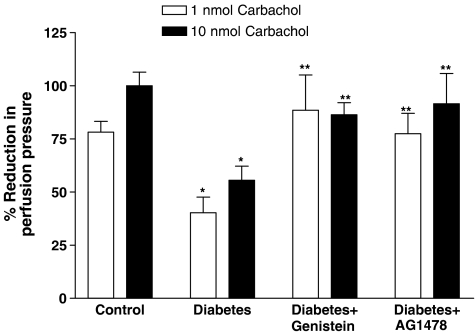
The vasodilator response to carbachol was significantly reduced in the perfused mesenteric vascular bed from STZ-diabetic rats to 44±7 and 59±6% compared to 78±5 and 100±6% in the nondiabetic control rats, at 1 and 10 nmol, respectively (Figure 4). Treatment of the diabetic rats with genistein or AG1478 prevented the diabetes-induced attenuation of carbachol-mediated vasodilation (Figure 4). The vasodilator responses to carbachol (1 and 10 nmol) were 89±17 and 86±6% in genistein-treated diabetic rats. Similarly, carbachol-mediated vasodilation in AG1478-treated diabetic rats was 78±9% and 92±14% at 1 and 10 nmol (Figure 4).
Figure 4.
Carbachol-induced vasodilation (1 and 10 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
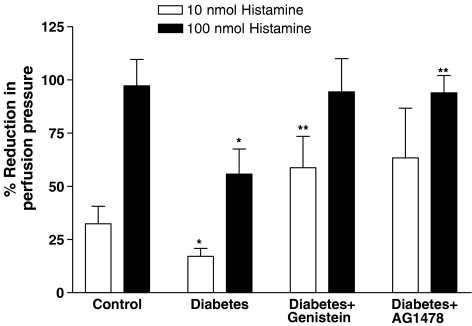
The vasodilator response to histamine (100 nmol) was significantly reduced in the perfused mesenteric vascular bed from STZ-diabetic rats to 56±12% compared to 97±12% in the nondiabetic control rats (Figure 5). Treatment of the diabetic rats with genistein or AG1478 prevented the diabetes-induced reduction in histamine-induced vasodilation at some of the tested doses (Figure 5). The vasodilator response to histamine (10 nmol) was 59±14% in genistein-treated diabetic rats compared to 17±4% in diabetes. Histamine-induced vasodilation (100 nmol) was normalized to a value of 94±8% in AG1478-treated diabetic rats (Figure 5).
Figure 5.
Histamine-induced vasodilation (10 and 100 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
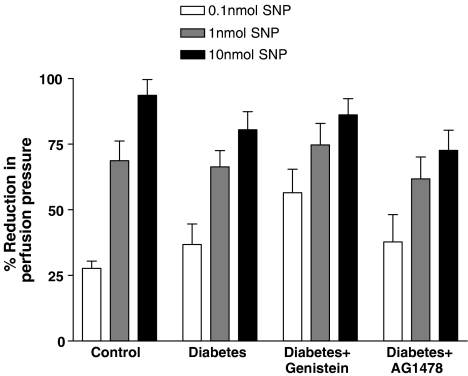
The vasodilator response to SNP was not significantly affected in the perfused mesenteric vascular bed of the STZ-diabetic rats or by any treatments (Figure 6).
Figure 6.
SNP-induced vasodilation (0.1, 1 and 10 nmol) in the perfused mesenteric vascular bed of control, diabetic, genistein- and AG1478-treated diabetic rats (mean±s.e., n=8) (*significantly different from control, **significantly different from diabetic).
Diadzein, the inactive analogue of genistein, did not affect agonist-induced vasodilator responses in the diabetic animals (data not shown). Furthermore, inhibition of TKs or EGFR did not affect agonist-induced vasodilator responses in the control animals.
Effect of acute treatment with AG1478 (10−5 M) on NE-induced vasoconstrictor response in isolated mesenteric bed
In the perfused mesenteric vascular bed, the vasoconstrictor response to NE was markedly exaggerated in diabetic animals compared to control animals (similar to that seen in Figure 1). Upon acute treatment of the perfused mesenteric vascular bed from STZ-diabetic rats with AG1478 (10−5 M), a significant reduction in the vasoconstrictor response to NE was observed (P<0.05) (see Figure 7).
Figure 7.
Effect of (10−5 M) AG1478 on norepinephrine-induced vasoconstriction (10, 100 and 1000 nmol) in the perfused mesenteric vascular bed of control and diabetic rats (mean±s.e., n=4). (*significantly different from diabetic mesenteric bed P<0.05).
Elevation of phosphorylated EGFR in the mesenteric tissue from STZ-diabetic rats
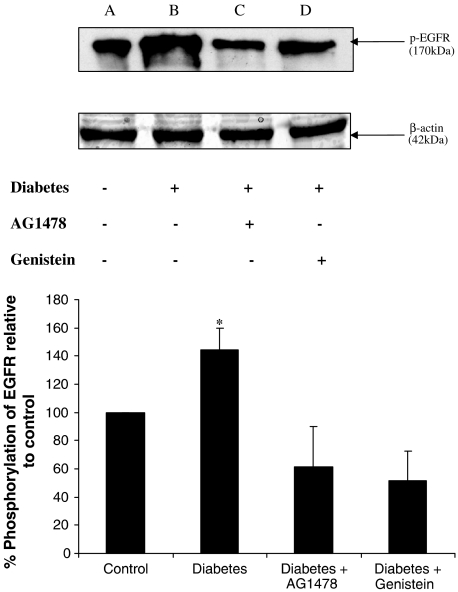
Mesenteric tissue from nondiabetic controls, diabetic animals and those treated with AG1478 or genistein were lysed as in Methods and equal amounts of protein was run on 8% SDS–PAGE gels. Proteins were transferred onto nitrocellulose membranes by electrobloting and probed for activated EGFR using a specific phospho (p)-EGFR rabbit polyclonal (Y1068) antibody. Mesenteric tissue from diabetic animals showed a higher level of p-EGFR 144±15% compared to controls that was then reduced by treatment with either AG1478 or genistein to 61±29 and 52±21%, respectively, compared to control (see Figure 8).
Figure 8.
Western blotting analysis of phosphorylated EGFR using p-EGFR (Y1068) primary antibody. Top panel: a marked increase of p-EGFR in mesenteric tissue from STZ-diabetic rats (B) compared to nondiabetic control (A). Treatment with either AG1478 (C) or genistein (D) altered such increase. The protein β-actin was used as control to verify an equal protein loading in each lane. Lower panel: Histogram showing % phosphorylation of EGFR relative to control (mean±s.e., n=3) (*significantly different from control, P<0.05).
The time course of EGFR phosphorylation in conditions of hyperglycemia
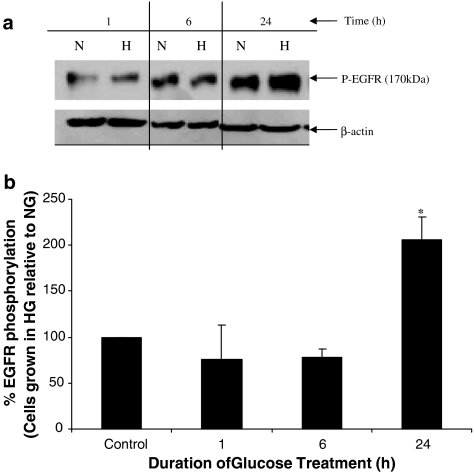
To discount any possible contribution of STZ (or the acute inflammatory response associated with STZ-induced diabetes) in elevating mesenteric levels of pEGFR in our animal model, we examined phosphorylation of EGFR in cultured cells grown in high glucose medium (25 mM D-glucose). Human ECV-304 cells grown in high glucose medium for 1or 6 h did not significantly alter EGFR phosphorylation from control levels observed in ECV-304 cells grown in 5.5 mM D-glucose. In contrast, cells incubated with high glucose for 24 h lead to a robust and significant increase in EGFR phosphorylation (P<0.05) (see Figure 9).
Figure 9.
Time course of EGFR phosphorylation in cultured ECV-304 cells grown in high glucose conditions. Cells were grown in either media containing 5.5 mM (Normal, N) or 25.5 mM high gluose media (H) for the stated times and EGFR phosphorylation relative to actin levels was monitored. (a) Western blot of pEGFR and β-actin for cells grown in N or H glucose is shown, whereas (b) a histogram of the densitometric analyses of the blots expressed as % of the normal (N) controls is shown. *Indicates a significant elevation in pEGFR relative to control.
Discussion
Diabetes mellitus affects the structural and functional integrity of many organ systems including the cardiovasculature. Diabetes-induced abnormalities in vascular function are now well established despite conflicting reports on whether the vascular responsiveness is enhanced or attenuated for a given vasoactive agonist (Head et al., 1987; Bhardwaj & Moore, 1988; Kamata & Hosokawa, 1997; Makino & Kamata, 1998). However, the underlying mechanisms for the altered vasoreactivity are not well characterized. In the perfused mesenteric vascular bed from STZ-induced diabetic rats used in the present study, we observed an exaggerated response to some vasoconstrictors, Ang II, NE and ET-1, and an attenuated response to some vasodilators, carbachol and histamine. Furthermore, we have demonstrated for the first time that phosphorylated EGFR levels in the mesenteric bed were elevated in diabetes compared to nondiabetic controls. Cell culture studies with human ECV-304 cells grown in high glucose (Figure 9) showed that chronic hyperglycemia (∼24 h) was required to show a marked (∼2-fold) increase in EGFR phosphorylation relative to normo-glycemic controls. This is in contrast to EGFR phosphorylation induced upon exogenous addition of EGF ligand to cells that typically occurs within 5 min (e.g. Sturla et al., 2005). Chronic treatment with AG1478 or genistein led to normalization of phosphorylated EGFR levels (Figure 8) as well as the altered vasoconstrictor (Figures 1, 2 and 3) and vasodilator responses (Figures 4, 5 and 6) in the perfused mesenteric vascular bed of STZ-diabetic rats implying that EGFR signalling is an important contributor to the development of vascular dysfunction during diabetes. In addition, acute treatment of the mesenteric vascular bed with AG1478 were also able to significantly reduce the exaggerated vasconstrictor response to NE (Figure 7), suggesting that short-term treatment with appropriate doses of EGFR inhibitors may be sufficient to correct diabetes-induced vascular dysfunction.
Reports in the literature suggest that EGFR is most likely differentially regulated in tissues obtained from diabetic animals. For example, EGFR activity has been reported to be decreased in the liver, placenta and pancreas of diabetic animals (Korc et al., 1984; Sissom et al., 1987; Okamoto et al., 1988), whereas it is elevated in the gastric mucosa and the kidney tubules (Ziyadeh & Goldfarb, 1991; Khan et al., 1999). In addition, as many feedback systems may impact on EGFR activity and regulation, there may also be differences in measured EGFR activity and levels as a function of time and severity of disease. Indeed, in the present study, we show that after 4-weeks of STZ-induced diabetes, EGFR phosphorylation was also elevated in the mesenteric vasculature and contributes to the associated vascular dysfunction (Figure 8).
EGFR-signalling pathways appear to be important in mediating diabetic nephropathy (Yang et al., 1997) and sensitizing the diabetic gastric mucosa to damaging agents (Khan et al., 1999). Here we show that EGFR signalling is also important in mediating diabetic vascular dysfunction. However, the precise mechanisms responsible for EGFR pathway-induced vascular damage in diabetes are not known. It is possible that EGFR-led changes in calcium homeostasis may be important. It is known that EGFR-signalling pathways impact on calcium homeostasis and that abnormalities in calcium homeostasis are responsible for exaggerated vascular reactivity in CVDs such as hypertension and diabetes (Beenen et al., 1995; Makino & Kamata, 1998; Mayhan et al., 1999). For example, it has been shown that enhanced contractile responses of the diabetic aortas and mesenteric arteries to α1-agonists are due to altered levels of intra- and extracellular calcium (Abebe et al., 1990). Our hypothesis that TKs, including EGFR TK, are involved in mediating vascular dysfunction via modulation of calcium homeostasis that leads to altered responsiveness to vasoconstrictors/vasodilators is consistent with several recent reports. It has been shown in aortic strips that TKs contribute to agonist-induced vascular smooth muscle contraction mediated through increased calcium sensitivity and increased calcium entry (Carter & Kanagy, 2001). Kawanabe et al. (2003) have also reported that both EGFR phosphorylation and calcium influx appear to have important roles in mediating ET-I-induced arachidonic acid release in VSMC.
GPCRs, including angiotensin type 1 and ET-1 receptors, transactivate growth factor receptors including EGFR. It is thought that EGFR transactivation plays an important role in ET-1-induced vascular contraction as blockade of EGFR phosphorylation by AG1478 in the rabbit basilar artery (BA) reduced contraction in BA ring preparations (Kawanabe et al., 2004). Similarly, transactivation is important for many of the VSMC effects reported for Ang II including proliferation, hypertophy, migration and vasoconstriction (Bokemeyer et al., 2000; Eguchi & Inagami, 2000; Eguchi et al., 2003; Hao et al., 2004). Clinically, it has been established that angiotensin converting enzyme inhibitors or Ang II receptor blockers can reduce cardiovascular events and renal failure in patients with diabetes, suggesting an important interaction between Ang II and hyperglycemia. Based on the present study, we may speculate that inhibitors of EGFR may have important benefits in diabetes-induced vascular disease by limiting events produced by GPCR-mediated EGFR transactivation.
Functional and anatomical abnormalities of the vascular endothelium are also commonly associated with diabetes (Beckman et al., 2002). Hyperglycemia results in the impairment of endothelial cell nitric oxide (NO) production possibly by protein kinase C (PKC) activation (Beckman et al., 2002), and this has recently been shown in human subjects also that leads to leukocyte adhesion to the endothelium through upregulation of cell-surface-specific adhesion molecules that possibly require NF-κB activation (Morigi et al., 1998). Since PKC is a potentially downstream signalling molecule of EGFR, impaired NO synthesis may be partially responsible for the exaggerated effects of vasoconstrictors and the reduced effects of vasodilators observed in diabetic animals. Furthermore, the fact that SNP-mediated vasodilation was unchanged in diabetes from normal animals (Figure 6) implies that the responsiveness of the smooth muscle to NO is unaffected by the disease, at least at the 4-weeks following STZ-induction that was examined in this study.
It has also been shown that diabetes and chronic hyperglycemia induce oxidative stress and increased production of reactive oxygen species (ROS), a possible common element in hyperglycemia induced pathologies that occur via several different mechanisms including via the polyol pathway (for a review see Brownlee, 2001). There is already some evidence to suggest that EGFR signalling can be activated by ROS. For example, Nishinaka & Yabe-Nishimura (2001) have reported that ROS-mediated elevation of aldose reductase activity, the first enzyme step in the polyol pathway that meatbolizes excess glucose to sorbitol, occurs via an EGFR-MAP kinase pathway. Since our cell culture data (see Figure 9) also suggests that chronic hyperglycemia is necessary for increasing EGFR phosphorylation (∼2-fold increase after 24 h), it is possible that ROS-mediated mechanism may also be at play in our model systems and requires further study.
In summary, we have shown that treatment with the RTK inhibitors, genistein or AG1478 produced a significant normalization of the altered agonist-induced vasoconstrictor and vasodilator responses in diabetes. These data suggest that EGFR signalling is an essential component in the development of diabetic vascular dysfunction. Hence, potential strategies aimed at inhibiting EGFR may represent promising novel approaches for the treatment of vascular complications in diabetes.
Acknowledgments
This work was supported by grants from Kuwait University Research Administration (Project Number MR02/02) and the British Heart Foundation. We are deeply appreciative of the technical support of Dr S. Abraham and A. Cherian in this project.
Abbreviations
- ADAM
a disintegrin and metalloproteinase
- Ang II
angiotensin II
- cPLA2
cytosolic phospholipase A2
- CVD
cardiovascular disease
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ET-1
endothelin-1
- GPCRs
G-protein coupled receptor
- HB-EGF
heparin-binding EGF
- 20-HETE
20-hydroxyeicosatetraenoic acid
- MAPK
mitogen-activated protein kinase
- NE
norepinephrine
- NO
nitric oxide
- PKC
protein kinase C
- RTKs
receptor tyrosine kinases
- STZ
streptozotocin
- TK
tyrosine kinase
- VSMC
vascular smooth muscle cell
References
- ABEBE W., HARRIS K.H., MACLEOD K.M. Enhanced contractile responses of arteries from diabetic rats to alpha 1-adrenoceptor stimulation in the absence and presence of extracellular calcium. J. Cardiovasc. Pharmacol. 1990;16:239–248. doi: 10.1097/00005344-199008000-00010. [DOI] [PubMed] [Google Scholar]
- ASAKURA M., KITAKAZE M., TAKASHIMA S., LIAO Y., ISHIKURA F., YOSHINAKA T., OHMOTO H., NODE K., YOSHINO K., ISHIGURO H., ASANUMA H., SANADA S., MATSUMURA Y., TAKEDA H., BEPPU S., TADA M., HORI M., HIGASHIYAMA S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.A., GOLDFINE A.B., GORDON M.B., GARRETT L.A., CREAGER M.A. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ. Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- BEENEN O.H., PFAFFENDORF M., VAN ZWIETEN P.A. Contractile responses to various inotropic agents in isolated hearts obtained from hypertensive diabetic rats. Blood Pressure. 1995;4:372–378. doi: 10.3109/08037059509077624. [DOI] [PubMed] [Google Scholar]
- BHARDWAJ R., MOORE P.K. Increased vasodilator response to acetylcholine of renal blood vessels from diabetic rats. J. Pharm. Pharmacol. 1988;40:739–742. doi: 10.1111/j.2042-7158.1988.tb07009.x. [DOI] [PubMed] [Google Scholar]
- BOKEMEYER D., SCHMITZ U., KRAMER H.J. Angiotensin II-induced growth of vascular smooth muscle cells requires an Src-dependent activation of the epidermal growth factor receptor. Kidney Int. 2000;58:549–558. doi: 10.1046/j.1523-1755.2000.t01-1-00201.x. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414 6865:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- CARTER R.W., KANAGY N.L.NOS inhibition hypertension and alpha2 adrenoreceptors activate a tyrosine kinase to enhance calcium sensitivity Hypertension 200138521(abstr) [Google Scholar]
- EGUCHI S., FRANK G.D., MIFUNE M., INAGAMI T. Metalloprotease-dependent ErbB ligand shedding in mediating EGFR transactivation and vascular remodelling. Biochem. Soc. Trans. 2003;31:1198–1202. doi: 10.1042/bst0311198. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., INAGAMI T. Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul. Peptides. 2000;91:13–20. doi: 10.1016/s0167-0115(00)00126-9. [DOI] [PubMed] [Google Scholar]
- GARCIA M.J., MCNAMARA P.M., GORDON T., KANNEL W.B. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- HAO L., DU M., LOPEZ-CAMPISTROUS A., FERNANDEZ-PATRON C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ. Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- HEAD R.J., LONGHURST P.A., PANEK R.L., STITZEL R.E. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br. J. Pharmacol. 1987;91:275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBST R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- JARRETT R.J. Cardiovascular disease and hypertension in diabetes mellitus. Diabetes Metab. Rev. 1989;5:547–558. doi: 10.1002/dmr.5610050702. [DOI] [PubMed] [Google Scholar]
- KAMATA K., HOSOKAWA M. Endothelial dysfunction in the perfused kidney from the streptozotocin-induced diabetic rat. Res. Commun. Mol. Pathol. Pharmacol. 1997;96:57–70. [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASSAB E., MCFARLANE S.I., SOWER J.R. Vascular complications in diabetes and their prevention. Vasc. Med. 2001;6:249–255. doi: 10.1177/1358836x0100600409. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., MASAKI T., HASHIMOTO N. Involvement of epidermal growth factor receptor-protein tyrosine kinase transactivation in endothelin-1-induced vascular contraction. J. Neurosurg. 2004;100:1066–1071. doi: 10.3171/jns.2004.100.6.1066. [DOI] [PubMed] [Google Scholar]
- KAWANABE Y., NOZAKI K., HASHIMOTO N., MASAKI T. Involvement of extracellular Ca2+ influx and epidermal growth factor receptor tyrosine kinase transactivation in endothelin-1-induced arachidonic acid release. Br. J. Pharmacol. 2003;139:1516–1522. doi: 10.1038/sj.bjp.0705386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAN A.J., FLIGIEL S.E., LIU L., JASZEWSKI R., CHANDOK A., MAJUMDAR A.P. Induction of EGFR tyrosine kinase in the gastric mucosa of diabetic rats. Proc. Soc. Exp. Biol. Med. 1999;221:105–110. doi: 10.1046/j.1525-1373.1999.d01-62.x. [DOI] [PubMed] [Google Scholar]
- KORC M., MATRISIAN L.M., NAKAMURA R., MAGUN B.E. Epidermal growth factor binding is altered in pancreatic acini from diabetic rats. Life Sci. 1984;35:2049–2055. doi: 10.1016/0024-3205(84)90562-9. [DOI] [PubMed] [Google Scholar]
- MAKINO A., KAMATA K. Possible modulation by endothelin-1, nitric oxide, prostaglandin I2 and thromboxane A2 of vasoconstriction induced by an alpha-agonist in mesenteric arterial bed from diabetic rats. Diabetologia. 1998;41:1410–1418. doi: 10.1007/s001250051086. [DOI] [PubMed] [Google Scholar]
- MAKINO A., OHUCHI K., KAMATA K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br. J. Pharmacol. 2000;130:549–556. doi: 10.1038/sj.bjp.0703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYHAN W.G., IRVINE S.D., SHARPE G.M. Constrictor responses of resistance arterioles during diabetes mellitus. Diabetes Res. Clin. Pract. 1999;44:147–156. doi: 10.1016/s0168-8227(99)00031-5. [DOI] [PubMed] [Google Scholar]
- MORIGI M., ANGIOLETTI S., IMBERTI B., DONADELLI R., MICHELETTI G., FIGLIUZZI M., REMUZZI A., ZOJA C., REMUZZI G. Leukocyte–endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-κB-dependent fashion. J. Clin. Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTHALIF M.M., BENTER I.F., KARZOUN N., FATIMA S., HARPER J., UDDIN M.R., MALIK K.U. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTHALIF M.M., BENTER I.F., KHANDEKAR Z., GABER L., ESTES A., MALIK S., PARMENTIER J.H., MANNE V., MALIK K.U. Contribution of Ras GTPase/MAP kinase and cytochrome P450 metabolites to deoxycorticosterone-salt-induced hypertension. Hypertension. 2000a;35:457–463. doi: 10.1161/01.hyp.35.1.457. [DOI] [PubMed] [Google Scholar]
- MUTHALIF M.M., KARZOUN N.A., BENTER I.F., GABER L., LJUCA F., UDDIN M.R., KHANDEKAR Z., ESTES A., MALIK K.U. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II-induced vascular hyperplasia and hypertension. Hypertension. 2002;39:704–709. doi: 10.1161/hy0202.103823. [DOI] [PubMed] [Google Scholar]
- MUTHALIF M.M., KARZOUN N.A., GABER L., KHANDEKAR Z., BENTER I.F., SAEED A.E., PARMENTIER J.H., ESTES A., MALIK K.U. Angiotensin II-induced hypertension: contribution of Ras GTPase/Mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension. 2000b;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA M., COONEY M.J., TU A.H., CHANG K.Y., CAO J., ANDO A., AN G.J., MELIA M., DE JUAN E., Jr Normalization of retinal vascular permeability in experimental diabetes with genistein. Invest. Ophthalmol. Vis. Sci. 2001;42:2110–2114. [PubMed] [Google Scholar]
- NISHINAKA T., YABE-NISHIMURA C. EGF receptor-ERK pathway is the major signaling pathway that mediates upregulation of aldose reductase expression under oxidative stress. Free Radical Biol. Med. 2001;31:205–216. doi: 10.1016/s0891-5849(01)00571-8. [DOI] [PubMed] [Google Scholar]
- OKAMOTO M., KAHN C.R., MARON R., WHITE M.F. Decreased autophosphorylation of EGF receptor in insulin-deficient diabetic rats. Am. J. Physiol. 1988;254:E429–E434. doi: 10.1152/ajpendo.1988.254.4.E429. [DOI] [PubMed] [Google Scholar]
- OYAMA Y., KAWASAKI H., HATTORI Y., KANNO M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur. J. Pharmacol. 1986;132:75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- PATEL B., HISCOTT P., CHARTERIS D., MATHER J., MCLEOD D., BOULTON M. Retinal and preretinal localisation of epidermal growth factor, transforming growth factor alpha, and their receptor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 1994;78:714–718. doi: 10.1136/bjo.78.9.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAYED-AHMED N., BESBAS N., MUNDY J., MUCHANETA-KUBARA E., COPE G., PEARSON C., EL NAHAS M. Upregulation of epidermal growth factor and its receptor in the kidneys of rats with streptozotocin-induced diabetes. Exp. Nephrol. 1996;4:330–339. [PubMed] [Google Scholar]
- SISSOM J.F., STENZEL W.K., WARSHAW J.B. Decreased binding of epidermal growth factor in placentas from streptozotocin-diabetic rats. J. Clin. Invest. 1987;80:242–247. doi: 10.1172/JCI113054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURLA L.M., AMORINO G., ALEXANDER M.S., MIKKELSEN R.B., VALERIE K., SCHMIDT-ULLRICH R.K.Requirement of Tyr992 and Tyr1173 in phosphorylation of the epidermal growth receptor by ionising radiation and modulation of SHP2 J. Biol. Chem. 2005. Feb 11 (epub ahead of print) [DOI] [PubMed]
- YAN Y., SHIRAKABE K., WERB Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J. Cell Biol. 2002;158:221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M.L., GUH J.Y., LAI Y.H., YANG Y.L., CHANG C.C., TSAI J.H., CHUANG L.Y. Effects of high glucose culture on EGF effects and EGF receptors in the LLC-PK1 cells. Am. J. Nephrol. 1997;17:193–198. doi: 10.1159/000169097. [DOI] [PubMed] [Google Scholar]
- YARDEN Y., SLIWKOWSKI M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell. Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- ZIYADEH F.N., GOLDFARB S. The renal tubulointerstitium in diabetes mellitus. Kidney Int. 1991;39:464–475. doi: 10.1038/ki.1991.57. [DOI] [PubMed] [Google Scholar]