Abstract
The enzyme heme oxygenase-1 (HO-1) is a cytoprotective and anti-inflammatory protein that degrades heme to produce biliverdin/bilirubin, ferrous iron and carbon monoxide (CO). The anti-inflammatory properties of HO-1 are related to inhibition of adhesion molecule expression and reduction of oxidative stress, while exogenous CO gas treatment decreases the production of inflammatory mediators such as cytokines and nitric oxide (NO). CO-releasing molecules (CO-RMs) are a novel group of substances identified by our group that are capable of modulating physiological functions via the liberation of CO. We aimed in this study to examine the potential anti-inflammatory characteristics of CORM-2 and CORM-3 in an in vitro model of lipopolysaccharide (LPS)-stimulated murine macrophages.
Stimulation of RAW264.7 macrophages with LPS resulted in increased expression of inducible NO synthase (iNOS) and production of nitrite. CORM-2 or CORM-3 (10–100 μM) reduced nitrite generation in a concentration-dependent manner but did not affect the protein levels of iNOS. CORM-3 also decreased nitrite levels when added 3 or 6 h after LPS exposure.
CORM-2 or CORM-3 did not cause any evident cytotoxicity and produced an increase in HO-1 expression and heme oxygenase activity; this effect was completely prevented by the thiol donor N-acetylcysteine.
CORM-3 also considerably reduced the levels of tumor necrosis factor-α, another mediator of the inflammatory response.
The inhibitory effects of CORM-2 and CORM-3 were not observed when the inactive compounds, which do not release CO, were coincubated with LPS.
These results indicate that CO liberated by CORM-2 and CORM-3 significantly suppresses the inflammatory response elicited by LPS in cultured macrophages and suggest that CO carriers can be used as an effective strategy to modulate inflammation.
Keywords: Carbon monoxide, carbon monoxide-releasing molecules (CO-RMs), nitric oxide, inflammation, heme oxygenase
Introduction
Heme oxygenase-1 (HO-1), the inducible isoform of heme oxygenase which catalyzes the formation of carbon monoxide (CO), biliverdin/bilirubin and ferrous iron, exhibits important anti-inflammatory properties that are beneficial for the resolution of acute inflammation (Hayashi et al., 1999; Lee & Chau, 2002; Otterbein et al., 2003). In fact, since the initial indication that HO-1 protected against a pleural model of inflammation (Willis et al., 1996), substantial experimental evidence has been accumulated to show that inhibition of heme oxygenase activity exacerbates the inflammatory response while prior induction of HO-1 significantly reduces inflammation (Wagener et al., 2003). A striking example of this phenomenon is found in a report describing the effects of adenoviral transfection of the HO-1 gene (McCarter et al., 2003), whereby the HO-1 adenovirus did not elicit the acute inflammation usually associated with this type of transfection and could also prevent the inflammation caused by a second adenovirus. The exact mechanisms underlying this effect are not clear, although hypotheses have been advanced based on the known characteristics of the products of heme oxygenase activity. Thus, the antioxidant properties of biliverdin/bilirubin combined with the sequestration of iron by ferritin and the signalling action of CO could all concertedly contribute to suppression of inflammation. In particular, heme oxygenase appears to be an important system to counteract the cytotoxicity caused by excessive production of nitric oxide (NO) (Foresti & Motterlini, 1999; Taille et al., 2001; Motterlini et al., 2002b; Lin et al., 2003). The upregulation of inducible nitric oxide synthase (iNOS) in macrophages, smooth muscle cells and hepatocytes upon exposure to bacterial lipopolysaccharide (LPS) results in a burst of NO generation that is essential for bactericidal actions. However, increased amounts of NO are also associated with deleterious effects such as hypotension and tissue damage (Macmicking et al., 1995) and induction of HO-1 in this context significantly reduces NO production and potentially restores cellular homeostasis (Wiesel et al., 2000; Wang et al., 2004). In this study, we examined the ability of two different CO-releasing molecules (CO-RMs) to modulate the inflammatory response elicited by LPS in cultured macrophages. CO-RMs have been shown to act pharmacologically in rat aortic and cardiac tissue where liberation of CO produced vasorelaxant effects (Motterlini et al., 2002a; 2003; Johnson et al., 2003; Foresti et al., 2004) and decreased myocardial ischemia–reperfusion damage (Clark et al., 2003; Guo et al., 2004), respectively. Based on these preliminary observations, it was of interest to assess whether CO-RMs could mimic some of the anti-inflammatory actions attributed to HO-1 and CO gas. For this purpose, we have analyzed tricarbonyldichlororuthenium(II) dimer (CORM-2), a commercially available DMSO-soluble CO-RM and tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), a proprietary water-soluble CO-RM.
Methods
Chemicals and reagents
Hemin (ferriprotoporphyrin IX chloride), tin protoporphyrin IX (SnPPIX) and biliverdin (biliverdin IX hydrochloride) were purchased from Porphyrin Products Inc. (Logan, UT, U.S.A.). Stock solutions of SnPPIX (10 mM) and biliverdin (10 mM) were prepared by dissolving the compounds in 0.1 M NaOH. The solutions were kept on ice and protected from light until use. Hemin (2 mM) was dissolved in 1 ml 0.1 M NaOH and the pH adjusted to 7.4 using phosphate-buffered saline (PBS). Tricarbonylchloro(glycinato)ruthenium (II) ([Ru(CO)3Cl(glycinate)] or CORM-3) was synthesized as previously described (Clark et al., 2003; Foresti et al., 2004). CORM-3 was freshly prepared as a 10 mM stock solution in pure distilled water. Tricarbonyldichlororuthenium(II) dimer ([Ru(CO)3Cl2]2 or CORM-2) was obtained from Sigma Aldrich (Poole, Dorset, U.K.) and solubilized in dimethyl sulfoxide (DMSO) to obtain a 10 mM stock. The chemical structures of CORM-2 and CORM-3 are represented in Figure 1. Inactive forms of each compound (negative controls) were also used in some experiments and they were prepared as follows: CORM-3 was ‘inactivated' (iCORM-3) by adding the compound to cell culture medium and leaving it for 18 h at 37°C in a 5% CO2 humidified atmosphere to liberate CO. The iCORM-3 solution was finally bubbled with nitrogen to remove the residual CO present in the solution. In the case of CORM-2, the inactive form was Ru(DMSO)4Cl2 (iCORM-2), a molecule where the carbonyl groups have been replaced with DMSO (Clark et al., 2003). Lipopolysaccharide (LPS – E. coli serotype 026:B6) was obtained from Sigma (Poole, Dorset, U.K.). Polyclonal antibodies against HO-1 were from Bioquote Ltd (York, U.K.) and polyclonal anti-iNOS antibodies were purchased from Santa Cruz Biotechnology Inc. (Wembley, Middlesex, U.K.). Antibodies against β-actin were purchased from Abcam (Cambridge, U.K.). All other chemicals were reagent grade and obtained from Sigma unless otherwise stated.
Figure 1.
Chemical structures of CORM-2 and CORM-3. See Introduction and Methods for detailed information on these two compounds.
Cell culture
Murine RAW264.7 monocyte macrophages were purchased from the European Collection of Cell Cultures (Salisbury, Wiltshire, U.K.) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units ml−1 penicillin and 0.1 mg ml−1 streptomycin. Cultures were maintained at 37°C in a 5% CO2 humidified atmosphere and experiments were conducted on cells at approximately 80–90% confluence.
Experimental protocol
Macrophages were exposed for 24 h to LPS (1 μg ml−1) in the presence or absence of CORM-3 or CORM-2 (10, 50 and 100 μM) and nitrite levels and iNOS protein expression were determined at the end of the incubation. In a similar set of experiments, CORM-3 (50 or 100 μM) was added to cells 6 h after incubation with LPS and nitrite measured at 24 h. The production of nitrite was also assessed at 24 h in cells exposed simultaneously to LPS and CORM-3 (10, 50 and 100 μM) at time 0 h followed by subsequent additions of CORM-3 at 3 and/or 6 h. Experiments were repeated with the negative controls iCORM-3 and iCORM-2 to assess whether the effects observed were due to the CO liberated by the CO-RMs or caused by other components of the molecules. The effect of CO-RMs and their inactive counterparts on the heme oxygenase pathway was also investigated. Specifically, cells were treated for 6 h in the presence of 10, 50 and 100 μM CO-RMs and heme oxygenase activity as well as HO-1 protein expression determined. Furthermore, heme oxygenase activity was measured in cells treated with LPS in the presence or absence of CORM-3. Heme oxygenase activity and HO-1 protein expression were also evaluated in cells preincubated with 1 mM N-acetylcysteine (NAC) prior to exposure to 100 μM CORM-3. To exclude the possibility that endogenously generated heme degradation products would exert potential anti-inflammatory effects, the experiments were also conducted in the presence of SnPPIX (10 μM), an inhibitor of heme oxygenase activity. Furthermore, we investigated the effect of the heme oxygenase pathway on nitrite production by pretreating cells for 6 h with either hemin (10, 25 and 50 μM) or biliverdin (1, 5, 10 and 20 μM) before exposure to LPS for 24 h. Experiments were also conducted using SnPPIX, which was present both during the preincubation with hemin and the exposure to LPS. The effect of 10, 50 and 100 μM CORM-3 on the cellular redox status was assessed by measuring glutathione levels at 30 min, 2 h and 4 h after the addition of CORM-3.
Assay for nitrite levels
Nitrite levels were determined using the Griess method as previously described by our group (Foresti et al., 1997). The measurement of this parameter is widely accepted as indicative of NO production. Briefly, the medium from treated cells cultured in 24-well plates was removed and placed into a 96-well plate (50 μl per well). The Griess reagent was added to each well to begin the reaction, the plate was shaken for 10 min and the absorbance read at 550 nm on a Molecular Devices VERSAmax plate reader. The nitrite level in each sample was calculated from a standard curve generated with sodium nitrite (0–300 μM in cell culture medium).
Western blot analysis
Samples of RAW264.7 cells were analyzed by Western immunoblot technique as already reported (Foresti et al., 1997; Motterlini et al., 2000). Briefly, an equal amount of protein (30 μg) for each sample was separated by SDS–polyacrylamide gel electrophoresis, transferred overnight to nitrocellulose membranes and the nonspecific binding of antibodies was blocked with 3% nonfat dried milk in PBS. Membranes were then probed with a polyclonal rabbit anti-HO-1 antibody (Bioquote Ltd, York, U.K.) or with a polyclonal rabbit anti-iNOS antibody (Santa Cruz Biotechnology Inc., Insight Biotechnology, Wembley, Middlesex, U.K.) (1 : 1000 dilution in Tris-buffered saline, pH 7.4). After three washes with PBS containing 0.05% (v v−1) Tween 20, blots were visualized using an amplified alkaline phosphatase kit from Sigma (Extra-3A) and the densitometric ratio analyses are reported in Figures 5 and 7. To verify equal loading, samples were also probed with β-actin polyclonal antibodies (Abcam, Cambridge, U.K.).
Figure 5.
Effect of CORM-3 and CORM-2 on heme oxygenase activity. (a) RAW264.7 macrophages were incubated with increasing concentrations of CORM-3 (10–100 μM) or iCORM-3 (100 μM). Heme oxygenase activity was determined 6 h after exposure to the different compounds as described in Methods. (b) Heme oxygenase activity was measured in cells exposed to 100 μM CORM-2 or iCORM-2 for 6 h. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control.
Figure 7.
N-acetylcysteine (NAC) abolishes the increase in heme oxygenase activity and HO-1 expression elicited by CORM-3. RAW264.7 macrophages were incubated with increasing concentrations of 100 μM CORM-3 in the presence or absence of 1 mM NAC. Heme oxygenase activity (a) and HO-1 expression (b) were assessed after 6 h incubation. Data represent the mean±s.e.m. of six independent experiments. Western blot image is representative of three different blots. β-Actin was used as an internal control for equal loading. *Indicates P<0.05 vs control.
Assay for heme oxygenase activity
Heme oxygenase activity was determined in RAW264.7 cells after various treatments as previously described by our group (Motterlini et al., 1996; Foresti et al., 1997; 2003). Briefly, harvested cells were subjected to three cycles of freeze–thawing before addition to a reaction mixture consisting of phosphate buffer (1 ml final volume, pH 7.4) containing magnesium chloride (2 mM), NADPH (0.8 mM), glucose-6-phosphate (2 mM), glucose-6-phosphate dehydrogenase (0.2 Units), rat liver cytosol as a source of biliverdin reductase, and the substrate hemin (20 μM). The reaction was conducted at 37°C in the dark for 1 h and terminated by the addition of 1 ml chloroform; the extracted bilirubin was calculated by the difference in absorbance between 464 and 530 nm (ɛ=40 mM−1 cm−1).
Determination of cellular glutathione content
The 5,5′ dithiobis-(2-nitrobenzoic acid) colorimetric assay was used for the measurement of glutathione as previously described by our group (Foresti et al., 1997). Briefly, cells at the end of the incubation period were washed with PBS and 600 μl of a 2% (w v−1) solution of 5-sulfosalicylic acid was added for cell lysis and deproteinization. The samples were centrifuged for 5 min at 10,000 × g and 500 μl aliquots were reacted with 500 μl of 5,5′ dithiobis-(2-nitrobenzoic acid) solution (0.3 M sodium phosphate buffer, 10 mM EDTA and 0.2 mM 5,5′ dithiobis-(2-nitrobenzoic acid), freshly prepared) and after 5 min the absorbance was read at 412 nm (extinction coefficient was 14.3 mM−1 cm−1). Positive and negative controls were obtained by incubating macrophages with 1 mM NAC, a precursor of glutathione, or 1 mM DL-buthionine-[S,R]sulfoximine (BSO), an inhibitor of glutathione biosynthesis.
Determination of tumor necrosis factor-α levels
The level of tumor necrosis factor-α (TNF-α) present in each sample was determined using a commercially available kit from R&D Systems (Abingdon, U.K.). The assay was performed according to the manufacturers' instructions. Briefly, cell culture supernatants were collected immediately after the treatment and spun at 13,000 × g for 2 min to remove any particulates. The medium was added to a 96-well plate precoated with affinity-purified polyclonal antibodies specific for the mouse TNF-α. An enzyme-linked polyclonal antibody specific for the mouse TNF-α was added to the wells and left to react for 2 h followed by a final wash to remove any unbound antibody-enzyme reagent. The intensity of the color detected at 450 nm (correction wavelength 570 nm) was measured after addition of a substrate solution and was proportional to the amount of TNF-α produced.
Cell viability
Cell viability was determined using an Alamar Blue assay kit and carried out according to the manufacturer's instructions (Serotec, U.K.) as previously reported by us (Clark et al., 2000). The assay is based on the detection of metabolic activity of living cells using a redox indicator, which changes from an oxidized (blue) form to a reduced (red) form. The intensity of the red color is proportional to the metabolism of the cells, which is calculated as the difference in absorbance between 570 and 600 nm and expressed as a percentage of control.
Statistical analysis
Statistical analysis was performed using one-way ANOVA combined with the Bonferroni test. Differences were considered to be significant at P<0.05.
Results
CO-RMs attenuate LPS-mediated nitrite production
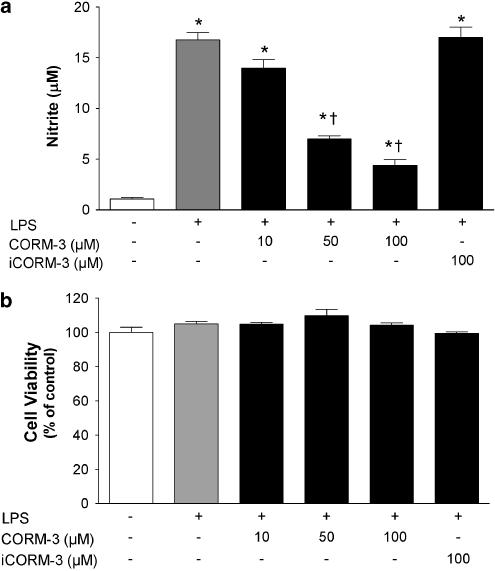
We have previously reported on some of the biological properties of CORM-2 and CORM-3 (Motterlini et al., 2002a; 2003; Clark et al., 2003), two transition carbonyl complexes that contain ruthenium as their metal center. Despite both containing ruthenium, two major differences exist between CORM-2 and CORM-3. Specifically, CORM-2 contains two ruthenium atoms whereas only one ruthenium lies at the centre of CORM-3 (see Figure 1). In addition, CORM-2 is soluble in DMSO while CORM-3 has been designed to render it water-soluble (Clark et al., 2003). Recent publications have highlighted important anti-inflammatory actions exerted by CO gas (Otterbein et al., 2000) and these findings prompted us to assess whether CO-RMs could affect the inflammatory response in LPS-activated macrophages. As shown in Figures 2a and 3a, both CORM-2 and CORM-3 significantly attenuated LPS-induced nitrite production in a concentration-dependent manner. This effect appeared to be strictly dependent on the CO released by the compounds since neither iCORM-2 nor iCORM-3, which do not liberate CO, had any effect on nitrite levels. Interestingly, CORM-3 decreased nitrite levels even when added 6 h after LPS treatment (Figure 4a), suggesting that CO directly influences the production of NO by iNOS. This is also supported by the data showing that nitrite levels were lower in cells exposed to LPS and CORM-3 at time 0 h followed by subsequent additions of CORM-3 at 3 and/or 6 h compared to treatment with LPS and CORM-3 at time 0 h only (Figure 4b). Indeed, 100 μM CORM-3 delivered in multiple additions reduced nitrite to control levels. It is important to note that cell viability was not affected by the concentrations of CO-RMs or the inactive forms used (Figures 2b and 3b), indicating that the results observed are not related to potential cytotoxicity of the compounds. These data suggest that CORM-2 and CORM-3 can modulate the production of nitrite by liberation of CO.
Figure 2.
Effect of CORM-3 on LPS-stimulated nitrite production and cell viability. (a) RAW264.7 macrophages were exposed to 1 μg ml−1 LPS in the presence or absence of CORM-3 (10–100 μM) and nitrite production was assessed at 24 h. The inactive compound iCORM-3 (100 μM) was also used to determine the contribution of CO released by CORM-3 to the observed effect. Control cells were incubated with medium alone. (b) Cell viability was assessed 24 h after exposure of macrophages to 1 μg ml−1 LPS in the presence or absence of CORM-3 (10–100 μM) or iCORM-3 (100 μM). Viability was expressed as percentage of control. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control; †indicates P<0.05 vs LPS alone.
Figure 3.
Effect of CORM-2 on LPS-stimulated nitrite production and cell viability. (a) RAW264.7 macrophages were exposed to 1 μg ml−1 LPS in the presence or absence of CORM-2 (10–100 μM) and nitrite production was assessed at 24 h. iCORM-2 (10–100 μM), an inactive compound that does not release CO, was also used as a negative control for CORM-2. Control cells were incubated with medium alone. (b) Cell viability was assessed 24 h after exposure of macrophages to 1 μg ml−1 LPS in the presence or absence of CORM-2 (10–100 μM) or iCORM-2 (10–100 μM). Viability was expressed as percentage of control. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control; †indicates P<0.05 vs LPS alone.
Figure 4.
Multiple additions of CORM-3 or its delivery after the LPS challenge decrease nitrite production. (a) Nitrite production was measured at 24 h in macrophages incubated with LPS alone or LPS followed 6 h later by a single addition of CORM-3 at 50 or 100 μM. Data represent the mean±s.e.m. of six independent experiments. (b) RAW264.7 macrophages were exposed to 1 μg ml−1 LPS and nitrite production was assessed at 24 h. CORM-3 (10, 50 or 100 μM) was added simultaneously with LPS in all groups. In additional experiments, CORM-3 was added also at 3 h after LPS (0+3 h) or at 3 and 6 h after LPS (0+3+6 h). Data represent the mean±s.e.m. of five independent experiments. *Indicates P<0.05 vs LPS alone; †indicates P<0.05 vs CORM-3 added simultaneously with LPS (0 h).
CORM-3 and CORM-2 increase heme oxygenase activity and HO-1 protein
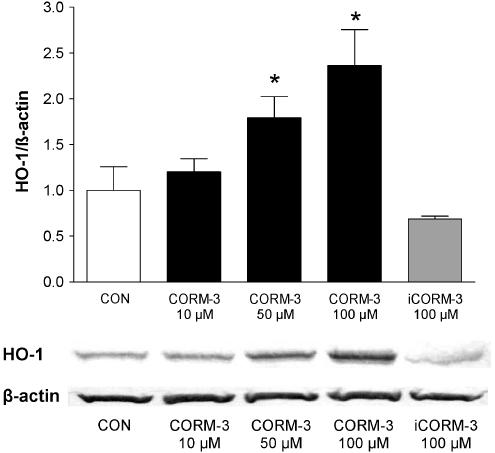
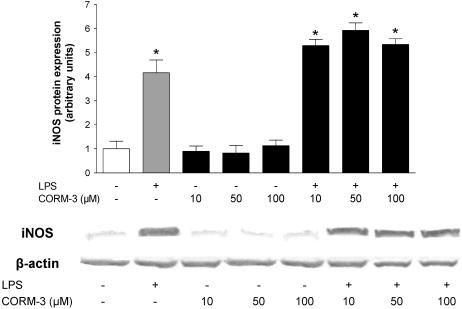
The presence of a metal and the CO released by CO-RMs could be factors stimulating the induction of HO-1, which per se may exert anti-inflammatory effects (Lee & Chau, 2002; Wagener et al., 2003). Therefore, we incubated macrophages for 6 h with different concentrations of CO-RMs or their inactive counterparts and measured heme oxygenase activity and HO-1 expression. CORM-3 caused a concomitant concentration-dependent increase in heme oxygenase activity and HO-1 expression, an effect that started to be evident at 50 μM (Figures 5a and 6). CORM-2 produced a similar effect at the concentration of 100 μM (Figure 5b). In marked contrast, both iCORM-2 and iCORM-3 (100 μM) did not change the levels of heme oxygenase activity or HO-1 protein expression (Figures 5a, b and 6) suggesting that CO liberated from the compounds is responsible for the observed effect while the rest of CORM-2 or CORM-3 molecules is inactive once CO is liberated. Since endotoxin is known to upregulate HO-1 expression and activity in a variety of tissues (Motterlini et al., 1996; Srisook & Cha, 2004) we assessed the effect of LPS and CORM-3 on macrophage heme oxygenase activity. Table 1 shows that treatment of macrophages with LPS for 6 h resulted in a slight increase of heme oxygenase activity while the combination of LPS and CORM-3 did not differ from CORM-3 alone. In contrast, heme oxygenase was significantly increased after 24 h exposure to LPS and coincubation of LPS with CORM-3 produced results similar to LPS alone. By using NAC, a thiol donor and glutathione precursor, we observed a complete suppression of HO-1 induction by CORM-3 (Figure 7), pointing to the potential of CO to modulate the redox status of the cell. Previous reports have shown that induction of HO-1 suppresses the production of nitrite in macrophages stimulated with LPS (Turcanu et al., 1998). Therefore, the above data could suggest that CORM-2 and CORM-3 decrease nitrite production not because of their ability to release CO but rather by inducing HO-1 expression. To further investigate this possibility, we performed experiments in which macrophages were exposed to LPS and CORM-3 in the presence of SnPPIX (10 μM), a known inhibitor of heme oxygenase activity. We found that the nitrite production was not significantly different between macrophages stimulated by LPS and CORM-3 versus those treated with LPS and CORM-3 in the presence of SnPPIX (Figure 8). This suggests that HO-1 induction is not responsible for the decrease in nitrite levels observed in the presence of CORM-3 and substantiates the idea that CO released by CORM-3 mediates this anti-inflammatory effect. We also examined the possibility that CORM-3 could affect the protein levels of iNOS, the inducible isoform of NO synthase which is stimulated by LPS and is accountable for the increased generation of NO during a variety of stress-related conditions. Figure 9 indicates that CORM-3 at different concentrations did not change the basal or LPS-stimulated expression of iNOS, emphasizing the possibility that CORM-3 interferes with the enzymatic activity of the protein.
Figure 6.
Effect of CORM-3 on HO-1 protein expression. RAW264.7 macrophages were incubated with increasing concentrations of CORM-3 (10–100 μM) or 100 μM iCORM-3 and HO-1 protein levels were determined by Western blot analysis. The graph shows the average expression of HO-1 following densitometric analysis of three different blots from three independent experiments and one representative image is reported. β-Actin was used as an internal control for equal loading. *Indicates P<0.05 vs control.
Table 1.
Heme oxygenase activity in macrophages exposed to LPS and/or CORM-3
| Groups | Heme oxygenase activity (pmol bilirubin mg protein−1 h−1) | |
|---|---|---|
| 6 h | 24 h | |
| Control | 310.7±9.2 | 408.9±27.2 |
| LPS (1 μg ml−1) | 342.6±9.3 | 672.4±27.9* |
| CORM-3 (50 μM) | 418.2±16.3* | 434.4±8.1 |
| CORM-3+LPS | 452.8±30.2* | 623.3±22.5* |
| iCORM-3 (50 μM) | 387.4±13.8 | 339.7±23.9 |
| iCORM-3+LPS | 350.8±6.5 | 541.2±85.6 |
Macrophages were incubated for 6 or 24 h in the presence of medium alone (control), LPS, CORM-3 alone or a combination of CORM-3 and LPS. Experiments were also conducted with the negative control iCORM-3. At the end of the incubation, cells were collected for the heme oxygenase activity assay as described in the Methods. Data represent the mean±s.e.m. of four independent experiments.
Indicates P<0.05 vs control.
Figure 8.
Effect of CORM-3 on LPS-stimulated nitrite production in the presence of SnPPIX, an inhibitor of heme oxygenase activity. RAW264.7 macrophages were exposed to 1 μg ml−1 LPS in the presence or absence of CORM-3 (10–100 μM) and SnPPIX (10 μM) and nitrite production was assessed after 24 h. Control cells were incubated with medium alone. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control; †indicates P<0.05 vs LPS alone.
Figure 9.
Effect of CORM-3 on LPS-stimulated expression of inducible NO synthase (iNOS). Macrophages were exposed to 1 μg ml−1 LPS in the presence or absence of CORM-3 (10–100 μM) and iNOS protein expression was examined by Western blot analysis. The graph shows the average expression of iNOS protein following densitometric analysis of three different blots from three independent experiments and one representative image is reported. β-Actin was used as an internal control for equal loading. *Indicates P<0.05 vs control.
Effect of CORM-3 on cellular glutathione content
The results showing that NAC abolished the induction of HO-1 by CORM-3 suggested that CO exerted a degree of cellular oxidative stress. To examine this phenomenon more closely, we assessed the changes in glutathione levels during exposure to CORM-3 (10, 50 and 100 μM). As reported in Table 2, the glutathione content did not vary between control and CORM-3-treated cells following a 30 min incubation. However, after 2 h there was a significant decrease in glutathione in macrophages exposed to 100 μM CORM-3, while 10 and 50 μM CORM-3 caused only a slight change. Interestingly, at 4 h there was a complete recovery of the glutathione content with all three concentrations of CORM-3, suggesting that cells were rapidly reacting to the CO-mediated stress by stimulating de novo glutathione synthesis and thus re-establishing the normal endogenous redox balance. Since iCORM-3 did not affect glutathione (data not shown), we conclude that the effect caused by CORM-3 is due to CO liberated by the molecule.
Table 2.
Effect of CORM-3 on intracellular glutathione content in murine macrophages
| Groups | Glutathione (% of control) | ||
|---|---|---|---|
| 30 min | 2 h | 4 h | |
| Control | 100.0±9.5 | 100.0±5.3 | 100.0±4.8 |
| CORM-3 (10 μM) | 110.0±1.5 | 98.0±2.6 | 102.5±2.3 |
| CORM-3 (50 μM) | 107.3±1.8 | 92.4±0.7 | 99.7±3.5 |
| CORM-3 (100 μM) | 110.0±1.0 | 81.1±5.6* | 106.2±3.1 |
Macrophages were exposed to medium alone (control) or CORM-3 (10, 50 or 100 μM) and the intracellular glutathione content was assessed after 30 min, 2 h or 4 h using a spectrophotometric assay. Experiments conducted using the negative control iCORM-3 showed no significant changes (data not shown). Data represent the mean±s.e.m. of 3–5 independent experiments.
Indicates P<0.05 vs control.
CORM-3 attenuates LPS-mediated TNF-α production
Having established that CORM-3 could attenuate LPS-induced nitrite production, we investigated its effect on the generation of TNF-α, an additional marker of inflammation (Otterbein et al., 2000). As shown in Figure 10a, LPS caused a significant production of TNF-α in macrophages and the addition of 10 μM CORM-3 completely abolished this inflammatory response. Conversely, iCORM-3 did not change TNF-α levels, suggesting once again that CO is the active component of the CORM-3 molecule that elicits this effect. Considering that CORM-3 can induce HO-1 (see earlier results of Figures 5a and 6), we assessed whether heme oxygenase activity was involved in the mechanisms modulating TNF-α production. Interestingly, treatment of macrophages with LPS in the presence of SnPPIX caused an even greater increase in TNF-α levels (Figure 10b), implying that endogenous heme oxygenase activity inhibits the production of this proinflammatory molecule. However, the amount of TNF-α was reduced to that of LPS alone when CORM-3 was added in the presence of LPS and SnPPIX (Figure 10b). These data suggest that CO plays an important role in the attenuation of LPS-induced TNF-α production, highlighting that inhibition of heme oxygenase activity also profoundly influences the levels of TNF-α.
Figure 10.
CORM-3 mitigates the production of TNF-α stimulated by LPS. (a) Macrophages were exposed to 1 ng ml−1 LPS in the presence or absence of 10 μM CORM-3 or iCORM-3 and the level of TNF-α in the culture medium was measured at 24 h by a commercially available immunoassay (see Methods for more details). (b) TNF-α production was measured in macrophages stimulated by LPS in the presence or absence of CORM-3 and SnPPIX (10 μM). Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 compared to control; †indicates P<0.05 vs LPS alone.
Effect of hemin and biliverdin or bilirubin on nitrite production stimulated by LPS
To assess the contribution of heme oxygenase and endogenously produced metabolites of its enzymatic activity (i.e. biliverdin/bilirubin, CO and ferrous iron) in regulating nitrite levels, we incubated cells with hemin, an inducer and substrate of heme oxygenase. As expected, macrophages exposed to hemin (10–50 μM) exhibited a concentration-dependent increase in heme oxygenase activity (Figure 11a). This effect was accompanied by a significant reduction in LPS-stimulated nitrite production (Figure 11b) and was partially reversed by inhibition of heme oxygenase activity with SnPPIX (Figure 12a). We also noted that stimulation of macrophages with LPS in the presence of SnPPIX resulted in an enhancement of nitrite levels compared to LPS alone (Figure 12a), suggesting that heme oxygenase exerts a negative feedback mechanism on NO generation during endotoxin treatment. As shown in Figure 12b, biliverdin (or bilirubin, data not shown) did not affect this response, suggesting that the nitrite reduction caused by hemin treatment is mediated by CO or possibly by degradation of the iNOS cofactor heme by heme oxygenase (Albakri & Stuehr, 1996).
Figure 11.
Preincubation with hemin increases heme oxygenase activity and reduces LPS-stimulated nitrite production. (a) Macrophages were treated with hemin (10–100 μM) for 6 h and heme oxygenase activity was measured at the end of the incubation as described. (b) Pretreatment of macrophages with hemin (6 h) was followed by exposure to 1 μg ml−1 LPS and nitrite production was assessed after 24 h. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control; †indicates P<0.05 vs LPS alone.
Figure 12.
Heme oxygenase activity, but not biliverdin, contributes to the reduction in nitrite production elicited by hemin. (a) Macrophages were stimulated with 1 μg ml−1 LPS in the presence or absence of SnPPIX (10 μM) and nitrite production was measured at 24 h. In some experiments, nitrite levels in the medium of macrophages preincubated with 10 μM hemin and SnPPIX prior to exposure to LPS were determined. (b) Nitrite levels were measured in medium of macrophages preincubated with biliverdin (1–20 μM) prior to exposure to LPS. Data represent the mean±s.e.m. of six independent experiments. *Indicates P<0.05 vs control; †indicates P<0.05 vs LPS alone.
Discussion
The importance of the HO-1 pathway in physiology and pathophysiology has now been confirmed by many experimental studies. In particular, the anti-inflammatory and antioxidant activities of this system are perhaps the most crucial in view of the fact that oxidative stress and inflammatory reactions are major underlying causes of many common diseases. This concept is best represented by the human HO-1-deficient case described in the literature (Yachie et al., 1999) and by HO-1-deficient mice (Poss & Tonegawa, 1997), which exhibit increased susceptibility to stress and manifestations of an inflammatory state. The degradation of the pro-oxidant heme combined with the production of antioxidant and signalling molecules by HO-1 likely explain HO-1-mediated protection; however, recent studies have highlighted the specific and independent role of CO gas in the modulation of inflammation (Otterbein et al., 2000; Nakao et al., 2003). In the last few years, our group has identified and synthesized new metal carbonyl-based compounds (CO-RMs) that have the ability to release CO in biological systems (Johnson et al., 2003; Motterlini et al., 2003). We have demonstrated that the vasoactive (Motterlini et al., 2002a; Foresti et al., 2004), antihypertensive (Motterlini et al., 2002a) and antirejection effects (Clark et al., 2003) of CO-RMs are due to the CO liberated by the compounds and are continuing our studies to uncover other potential benefits of these and other CO-releasing agents (Motterlini et al., 2005). We report here that CORM-2 and CORM-3, respectively a DMSO- and a water-soluble CO-RM, exhibited antiinflammatory actions in an in vitro model of LPS-stimulated murine macrophages. This effect was linked mainly to a marked reduction of nitrite levels and TNF-α production, although the increased expression of iNOS by LPS did not seem to be affected by CO-RMs. In addition, incubation of cells with hemin, an inducer of HO-1, prior to the LPS challenge, decreased nitrite production in a manner similar to CO-RMs while biliverdin or bilirubin were ineffective. These results strongly suggest that CORM-2 and CORM-3 exert an anti-inflammatory action and that their use can be manipulated to control the cellular response to inflammatory stimuli.
The most reasonable explanation for the decreased nitrite formation when macrophages were exposed to LPS in the presence of CO-RMs is that the activity of NO synthase could be inhibited by CO. This is highly feasible since CO gas has already been shown to potently inhibit the conversion of L-arginine to NO and citrulline by neuronal and macrophage NO synthases because of two heme moieties contained in the active enzymes (White & Marletta, 1992). The hypothesis could also be supported by the fact that CORM-3 did not directly affect the protein levels of iNOS (which were actually slightly higher in cells treated with LPS and CORM-3 compared to cells exposed to LPS alone) and by the data showing that nitrite levels were significantly reduced by CORM-3 added in multiple additions or after LPS treatment, when iNOS protein was already present. Moreover, the negative controls (iCORM-2 and iCORM-3), which are depleted of CO, did not change nitrite production indicating that CO liberated by CO-RMs is responsible for the observed effect. At the same time, and of major importance, these data exclude the possibility that the transition metal complex per se might interfere with NO generation or act as an NO scavenger. Nevertheless, these findings are intriguing in light of the short half-life that these types of CO-RMs have in physiological buffers. For instance, the myoglobin assay (which allows us to assess the ability of CO-RMs to release CO and to calculate the amount of CO liberated over time) indicates that CORM-3 has a half-life of 10.2 min in DMEM culture medium (Motterlini et al., 2003); however, this half-life is considerably reduced (1–2 min) in the presence of myoglobin (Clark et al., 2003; Motterlini et al., 2005) and a similar profile is expected in the presence of cellular components. In addition, the release of CO from CORM-2 once added to physiological solutions is nearly instantaneous (Motterlini et al., 2002a). Therefore, how could CO derived from CORM-2 and CORM-3 elicit a significant reduction of nitrite 24 h after coincubation with LPS? We can exclude a priori a cytotoxic effect, since both CORM-2 and CORM-3 did not affect cell viability at the concentrations used (10–100 μM). We are tempted to speculate that once CO is liberated, it will tightly bind to cellular targets that preserve CO bioactivity over time, in a manner similar to S-nitrosothiols, such as S-nitrosoalbumin and S-nitrosoglutathione (Stamler et al., 2001), which are long-lived species acting apparently as a reservoir of NO bioactivity. Cellular targets we propose for such a role are heme-dependent proteins or proteins containing metal centers, due to the high affinity of heme/metal prosthetic groups for CO. An alternative explanation for our results is that CO could activate (or inactivate) pathways that influence NO production by NO synthase.
In strong support of the anti-inflammatory action exerted by CORM-3 are also the data showing that this transition metal carbonyl complex causes a considerable decrease in LPS-mediated TNF-α production. This set of results emphasizes two important issues that: (1) heme oxygenase intrinsically counteracts inflammation, since its inhibition during the LPS challenge enhances TNF-α production and (2) during blockade of the heme oxygenase pathway, external interventions with a CO carrier are an effective strategy to modulate TNF-α levels. With the use of CORM-3 as a source of CO, we confirm in the present study the initial observations by Otterbein and colleagues showing that low concentrations of CO gas inhibited the expression of LPS-induced TNF-α both in vitro and in vivo (Otterbein et al., 2000). Interestingly, we have recently observed in a collaborative study that treatment of pigs with CORM-3 prevents the release of TNF-α by isolated peripheral blood mononuclear cells stimulated with LPS (unpublished observations).
The finding of HO-1 induction by CORM-2 or CORM-3 points to the possibility that the release of CO causes some forms of cellular stress, possibly oxidative stress. This concept is sustained by the data showing that NAC completely abolished CORM-3-mediated HO-1 upregulation and by the measurements of total glutathione contents, which significantly decreased 2 h after incubation of cells with 100 μM CORM-3 and started to increase at 4 h. However, since 10 and 50 μM CORM-3 did not change glutathione levels, it can be inferred that cellular stress only occurred following exposure to high concentrations of CORM-3 (>50 μM), a finding that strictly correlates with the fact that CORM-2 and CORM-3 induced HO-1 at concentrations above 50 μM. Despite this upregulation, it did not appear that heme oxygenase activity contributed to reduce nitrite levels, possibly due to a limitation in substrate availability. In this respect, it was important to compare the effect of CO-RMs on nitrite production with that of hemin, the substrate and inducer of HO-1. As already reported by others (Turcanu et al., 1998; Dulak et al., 2002), we found that hemin pretreatment resulted in a decreased nitrite production and inhibition of the heme oxygenase pathway partially reversed the effect, implicating HO-1-derived products in the modulation of NO generation. Biliverdin and bilirubin (data not shown) were also tested without significant changes, suggesting that other heme metabolites can affect nitrite levels.
In conclusion, the present study supports an anti-inflammatory action of CO-RMs and HO-1 in a cell culture system of LPS challenge and encourages the testing of CO carriers in in vivo models of inflammation. More investigations are now required to understand the mechanisms underlying the anti-inflammatory effects mediated by CO-RMs.
Acknowledgments
We thank the British Heart Foundation (PG/2000-047 to R.F.; FS/02/027 to R.M.) and the Dunhill Medical Trust (R.M.) for their financial support. B.E.M. and R.M. have financial interest with hemo CORM Ltd.
Abbreviations
- CO
carbon monoxide
- CO-RMs
carbon monoxide-releasing molecules
- CORM-2
tricarbonyldichlororuthenium(II) dimer
- CORM-3
tricarbonylchloro(glycinato)ruthenium(II)
- HO-1
heme oxygenase-1
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NAC
N-acetylcysteine
- NO
nitric oxide
- SnPPIX
tin protoporphyrin IX
References
- ALBAKRI Q.A., STUEHR D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996;271:5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- CLARK J.E., FORESTI R., GREEN C.J., MOTTERLINI R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem. J. 2000;348:615–619. [PMC free article] [PubMed] [Google Scholar]
- CLARK J.E., NAUGHTON P., SHUREY S., GREEN C.J., JOHNSON T.R., MANN B.E., FORESTI R., MOTTERLINI R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- DULAK J., JOZKOWICZ A., FORESTI R., KASZA A., FRICK M., HUK I., GREEN C.J., PACHINGER O., WEIDINGER F., MOTTERLINI R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox. Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- FORESTI R., CLARK J.E., GREEN C.J., MOTTERLINI R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J. Biol. Chem. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- FORESTI R., HAMMAD J., CLARK J.E., JOHNSON R.A., MANN B.E., FRIEBE A., GREEN C.J., MOTTERLINI R. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br. J. Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORESTI R., HOQUE M., BAINS S., GREEN C.J., MOTTERLINI R. Haem and nitric oxide: synergism in the modulation of the endothelial haem oxygenase-1 pathway. Biochem. J. 2003;372:381–390. doi: 10.1042/BJ20021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORESTI R., MOTTERLINI R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Rad. Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- GUO Y., STEIN A.B., WU W.J., TAN W., ZHU X., LI Q.H., DAWN B., MOTTERLINI R., BOLLI R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am. J. Physiol Heart Circ. Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., TAKAMIYA R., YAMAGUCHI T., MATSUMOTO K., TOJO S.J., TAMATANI T., KITAJIMA M., MAKINO N., ISHIMURA Y., SUEMATSU M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress. Role of bilirubin generated by the enzyme. Circ. Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- JOHNSON T.R., MANN B.E., CLARK J.E., FORESTI R., GREEN C.J., MOTTERLINI R. Metal carbonyls: a new class of pharmaceuticals. Angew. Chem. Int. Ed. Engl. 2003;42:3722–3729. doi: 10.1002/anie.200301634. [DOI] [PubMed] [Google Scholar]
- LEE T.S., CHAU L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- LIN H.Y., JUAN S.H., SHEN S.C., HSU F.L., CHEN Y.C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003;66:1821–1832. doi: 10.1016/s0006-2952(03)00422-2. [DOI] [PubMed] [Google Scholar]
- MACMICKING J.D., NATHAN C., HOM G., CHARTRAIN N., FLETCHER D.S., TRUMBAUER M., STEVENS K., XIE Q.W., SOKOL K., HUTCHINSON N., CHEN H., MUDGETT J.S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- MCCARTER S.D., SCOTT J.R., LEE P.J., ZHANG X., CHOI A.M., MCLEAN C.A., BADHWAR A., DUNGEY A.A., BIHARI A., HARRIS K.A., POTTER R.F. Cotransfection of heme oxygenase-1 prevents the acute inflammation elicited by a second adenovirus. Gene Therapy. 2003;10:1629–1635. doi: 10.1038/sj.gt.3302063. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., CLARK J.E., FORESTI R., SARATHCHANDRA P., MANN B.E., GREEN C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 2002a;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., FORESTI R., BASSI R., CALABRESE V., CLARK J.E., GREEN C.J. Endothelial heme oxygenase-1 induction by hypoxia: modulation by inducible nitric oxide synthase (iNOS) and S-nitrosothiols. J. Biol. Chem. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., FORESTI R., INTAGLIETTA M., WINSLOW R.M. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am. J. Physiol. Heart Circ. Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., GREEN C.J., FORESTI R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antiox. Redox Signal. 2002b;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., MANN B.E., JOHNSON T.R., CLARK J.E., FORESTI R., GREEN C.J. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr. Pharm. Des. 2003;9:2525–2539. doi: 10.2174/1381612033453785. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., SAWLE P., BAINS S., HAMMAD J., ALBERTO R., FORESTI R., GREEN C.J. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- NAKAO A., MOORE B.A., MURASE N., LIU F., ZUCKERBRAUN B.S., BACH F.H., CHOI A.M., NALESNIK M.A., OTTERBEIN L.E., BAUER A.J. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278–1285. doi: 10.1136/gut.52.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTERBEIN L.E., BACH F.H., ALAM J., SOARES M., TAO LU H., WYSK M., DAVIS R.J., FLAVELL R.A., CHOI A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., SOARES M.P., YAMASHITA K., BACH F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- POSS K.D., TONEGAWA S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRISOOK K., CHA Y.N. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochem. Pharmacol. 2004;68:1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., LAMAS S., FANG F.C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- TAILLE C., FORESTI R., LANONE S., ZEDDA C., GREEN C.J., AUBIER M., MOTTERLINI R., BOCZKOWSKI J. Protective role of heme oxygenases against endotoxin-induced diaphragmatic dysfunction in rats. Am. J. Respir. Crit. Care Med. 2001;163:753–761. doi: 10.1164/ajrccm.163.3.2004202. [DOI] [PubMed] [Google Scholar]
- TURCANU V., DHOUIB M., POINDRON P. Nitric oxide synthase inhibition by haem oxygenase decreases macrophage nitric-oxide-dependent cytotoxicity: a negative feedback mechanism for the regulation of nitric oxide production. Res. Immunol. 1998;149:741–744. doi: 10.1016/s0923-2494(99)80050-9. [DOI] [PubMed] [Google Scholar]
- WAGENER F.A., VOLK H.D., WILLIS D., ABRAHAM N.G., SOARES M.P., ADEMA G.J., FIGDOR C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- WANG W.W., SMITH D.L., ZUCKER S.D. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology. 2004;40:424. doi: 10.1002/hep.20334. [DOI] [PubMed] [Google Scholar]
- WHITE K.A., MARLETTA M.A. Nitric oxide synthase is a cytochrome P-450 type protein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- WIESEL P., PATEL A.P., DIFONZO N., MARRIA P.B., SIM C.U., PELLACANI A., MAEMURA K., LEBLANC B.W., MARINO K., DOERSCHUK C.M., YET S.F., LEE M.E., PERRELLA M.A. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- WILLIS D., MOORE A.R., FREDERICK R., WILLOUGHBY D.A. Heme oxygenase: a novel target for the modulation of inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- YACHIE A., NIIDA Y., WADA T., IGARASHI N., KANEDA H., TOMA T., OHTA K., KASAHARA Y., KOIZUMI S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]