Abstract
Endogenous adenosine is a trigger for ischemic myocardial preconditioning (IPC). Although intravascular administration of adenosine has been used to further unravel the mechanism of protection by IPC, it is questionable whether adenosine and IPC employ the same signaling pathways to exert cardioprotection.
We therefore investigated whether the active metabolic barrier of the endothelium prevents an increase in myocardial interstitial adenosine concentrations by intravenous adenosine, using microdialysis, and also the role of NO and activation of a neurogenic pathway in the cardioprotection by adenosine.
In pentobarbital-anesthetized rats, area at risk and infarct size (IS) were determined 120 min after a 60-min coronary artery occlusion (CAO), using trypan blue and nitro-blue-tetrazolium staining, respectively.
IPC with a single 15-min CAO and a 15-min adenosine infusion (ADO, 200 μg min−1 i.v.) limited IS to the same extent (IS=41±6% and IS=40±4%, respectively) compared to control rats (IS=63±3%, both P<0.05). However, IPC increased myocardial interstitial adenosine levels seven-fold from 4.3±0.7 to 27.1±10.0 μM (P<0.05), while ADO had no effect on interstitial adenosine (4.1±1.2 μM), or any of the other purines.
The NO synthase inhibitor Nω-nitro-L-arginine (LNNA), which did not affect IS (IS=62±3%), attenuated the protection by ADO (IS=56±3%; P<0.05 vs ADO, P=NS vs LNNA). The ganglion blocker hexamethonium, which had also no effect on IS (IS=66±3%), blunted the protection by ADO (IS=55±4%; P<0.05 vs ADO and vs hexamethonium).
These observations demonstrate that cardioprotection by ADO is dependent on NO, and is primarily mediated by activation of a neurogenic pathway.
Keywords: Adenosine, hexamethonium, Nω-nitro-L-arginine, endothelium, nitric oxide, neurogenic pathway, ganglion blockade, remote preconditioning, rat, myocardial infarction
Introduction
Adenosine has been identified as one of the triggers of ischemic myocardial preconditioning (IPC), based on the capability of adenosine receptor antagonists to abolish and adenosine receptor agonists to mimic the cardioprotection by IPC (Mubagwa & Flameng, 2001; Liem et al., 2001; Headrick et al., 2003). However, the mechanism of protection by intravascularly administered adenosine is still incompletely understood, and doubt has been expressed as to whether exogenous adenosine and endogenous adenosine released during IPC employ the same signaling pathways (Van Winkle et al., 1994; Yao & Gross, 1994; Lasley et al., 1995; Manthei & Van Wylen, 1997). For instance, several groups of investigators have shown that myocardial interstitial adenosine levels increase during the brief ischemic episodes that are employed to precondition the myocardium (Lasley et al., 1995; Martin et al., 1997; Manthei & Van Wylen, 1997), and these increased adenosine levels has been proposed as a primary determinant of the degree of cardioprotection by IPC (Miura et al., 1992; Suzuki et al., 1998). In contrast to adenosine that is endogenously released by IPC, access of intravascular adenosine into the interstitial compartment is impeded by the active metabolic barrier function of the endothelium (Lasley et al., 1995; 1998; Headrick et al., 2003), which may explain why intravenous (i.v.) adenosine failed to decrease infarct size in some (Liu et al., 1991; Hale et al., 1993; Li & Kloner 1993) though not all studies (Toombs et al., 1992; Lasley et al., 1995), whereas high intracoronary doses (Van Winkle et al., 1994; Yao and Gross 1994; Lasley & Mentzer, 1998; Lasley et al., 1998) or coinfusion with dipyridamole (Auchampach & Gross, 1993) afforded cardioprotection. Furthermore, although several studies indicate that adenosine can reach the interstitium (Lasley et al., 1995; Manthei & Van Wylen, 1997), other investigators observed that intra-arterial infusion of adenosine into the forearm of healthy human volunteers only showed an increase in the interstitial adenosine levels of the forearm in the presence of the nucleoside transporter blocker dipyridamole (Gamboa et al., 2003), lending further support to the concept of the barrier function of the endothelium for adenosine (Lasley et al., 1998; Headrick et al., 2003). In line with this notion, several groups of investigators have shown that the cardiovascular effects of adenosine involve, at least in part, the release of endothelium-derived substances, including nitric oxide (NO) and prostanoids (Smits et al., 1995; Rubio & Ceballos, 2003), although this is not a ubiquitous finding (Costa & Biaggioni, 1998). Equally important, there is substantial, although somewhat conflicting evidence suggesting a role for NO in the second window of protection by adenosine, while very little is known about the role of NO in the first window of cardioprotection by adenosine (see Ferdinandy & Schulz, 2003).
Adenosine administered via the intravenous route does not only reach the myocardium but other organs as well. This is noteworthy, because we have previously shown that an intramesenteric artery infusion of adenosine (in a dose that did not afford cardioprotection when infused into the portal vein or i.v.), mimics remote ischemic myocardial preconditioning (Gho et al., 1996; Przyklenk et al., 2003) by activating a neurogenic pathway (Liem et al., 2002). These observations suggest that actions at extracardiac sites could contribute to the limitation of myocardial infarct size by adenosine. Furthermore, in view of earlier findings in our laboratory that blockade of the neurogenic pathway by the ganglion blocker hexamethonium does not modify the cardioprotection by IPC (Gho et al., 1996), the latter would imply that IPC and adenosine use distinctly different mechanisms to exert cardioprotection.
In light of these considerations, we used microdialysis to determine whether myocardial interstitial adenosine levels were similarly affected during IPC and adenosine in a dose that was equally effective in limiting myocardial infarct size as IPC (Liem et al., 2005). Since we observed that myocardial interstitial adenosine levels remained unchanged during adenosine infusion, but increased during IPC, we subsequently investigated the role of NO in the first window of cardioprotection by adenosine. Finally, we addressed the putative contribution of extracardiac sites to the cardioprotection by adenosine.
Methods
Experiments were performed in ad libitum fed male Wistar rats (300–380 g) in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 86–23, revised 1996) and with the approval of the Erasmus MC Animal Care Committee.
Surgical procedures
Pentobarbital-anesthetized (60 mg kg−1 i.p.) rats were intubated for positive pressure ventilation with oxygen-enriched room air. Through the carotid artery a PE-50 catheter was positioned in the thoracic aorta for measurement of arterial blood pressure and heart rate. In the inferior caval vein a PE-50 catheter was placed for infusion of Haemaccel (Hoechst) to compensate for blood loss during surgery, and for drug infusion during the experiments. After thoracotomy, via the left third intercostal space, the pericardium was opened and a silk 6–0 suture was looped under the left anterior descending coronary artery for later CAO. A catheter was positioned in the abdominal cavity to allow intraperitoneal (i.p.) administration of pentobarbital for maintenance of anesthesia. Rectal temperature was continuously measured and maintained at 36.5–37.5°C (Gho et al., 1996; Van den Doel et al., 1998).
Microdialysis
In 17 rats a CMA/20 microdialysis probe (Carnegie Medicine AB, Stockholm, Sweden; membrane 4 × 0.5 mm, cutoff: 20 kD) was implanted in the area perfused by the left anterior descending coronary artery, to determine myocardial interstitial adenosine levels (Lameris et al., 2000; Liem et al., 2005). The probe was inserted tangentially to the epicardial surface and positioned in the left ventricular midwall; the proper probe position was confirmed at the end of each experiment. Dialysate samples were collected (with an 8-min delay to correct for 16.1 μl dead space of the probe and the distal tubing) at 15-min intervals at a rate of 2 μl min−1 (total volume of each sample was 30 μl). At the conclusion of each experiment, adenosine recovery of the probe was determined ex vivo using a solution containing 100 μM adenosine, and found to be 15±1%. All samples were stored at −50°C for later analysis.
Experimental protocols
Cardioprotection by adenosine and IPC
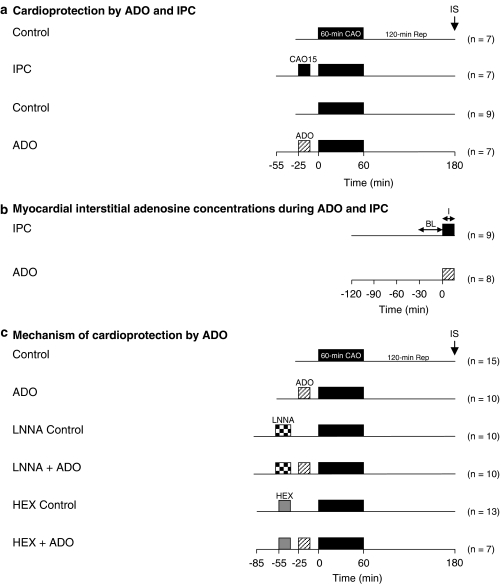
In the animals that were included in the infarct studies, a 30-min stabilization period was allowed before experimental protocols were carried out. Infarct size was determined after a 60-min CAO followed up by 120 min of reperfusion. Nine rats underwent the 60-min CAO (Control), while seven animals were preconditioned by a 15-min CAO followed by 10 min of reperfusion prior to the 60-min CAO (Figure 1a). This IPC stimulus has been shown to precondition the myocardium via an adenosine-dependent signaling pathway (Liem et al., 2001). Seven rats received a 15-min infusion of intravenous adenosine (ADO) in a dose (200 μg min−1 i.v.) that produced a similar degree of cardioprotection as IPC with a 15-min CAO (Liem et al., 2005).
Figure 1.
Experimental protocol in which the effects of IPC by 15-min CAO and of 15-min ADO (200 μg min−1 i.v.) on myocardial infarct size (panel a) and myocardial interstitial adenosine concentrations (panel b) were studied. In panel c we studied the effects of NO synthase inhibition with Nω-nitro-L-arginine (LNNA, 25 mg kg−1 i.v.) and ganglion blockade with hexamethonium (20 mg kg−1 i.v.) on cardioprotection by ADO. IS=infarct size; BL=baseline; I=intervention (ADO or IPC).
Myocardial interstitial adenosine levels during ADO and IPC
In the 17 rats in which myocardial dialysis was performed, baseline measurements were obtained 90 min after insertion of the microdialysis probe (Figure 1b). Subsequently, rats were subjected to either a 15-min CAO (n=9), or a 15-min intravenous infusion of 200 μg min−1 of adenosine (n=8).
Mechanism of cardioprotection by ADO
To investigate the involvement of endothelial NO synthase in the protection by ADO, rats were pretreated with the NO synthase inhibitor Nω-nitro-L-arginine (LNNA, 25 mg kg−1 intravenously infused over a 20-min period; Figure 1c). To investigate whether activation of a neurogenic pathway was involved, rats were pretreated with the ganglion-blocker hexamethonium (20 mg kg−1 i.v. infused over a 15-min period). Appropriate controls were added where necessary. All drugs were purchased from Sigma Chemical Co. (Germany).
Rats that encountered ventricular fibrillation during CAO or reperfusion were allowed to complete the experimental protocol, provided that conversion to normal sinus rhythm occurred spontaneously within 1 min or that defibrillation via gently thumping on the thorax was successful within 2 min of the onset of fibrillation. Occlusion and reperfusion were visually verified.
Infarct size analysis
Infarct size was determined as previously described (Gho et al., 1996; Van den Doel et al., 1998). Briefly, after 120 min of reperfusion the LAD was reoccluded, immediately followed by intravenous infusion of 10 ml trypan blue (0.4%, Sigma Chemical Co.) into the femoral vein to stain the normally perfused myocardium dark blue and delineate the nonstained area at risk (AR). Subsequently, hearts were excised, rinsed in cold NaCl 0.9%, and cut into slices of 2 mm thickness from apex to base. From each slice the right ventricle was removed and the left ventricular area at risk (nonstained) was dissected from the remaining left ventricular tissue. The area at risk was then incubated for 10 min in 37°C nitro-blue tetrazolium (Sigma Chemical Co., Germany; 1 mg ml−1 Sorensen buffer, pH 7.4), which stains viable tissue purple but leaves infarcted tissue unstained. After the infarcted area was isolated from the noninfarcted area, the different areas of the left ventricle were dried and weighed separately. Myocardial infarct size (IS) was computed as infarcted area expressed as a percentage of the AR.
HPLC analysis of purine concentrations
The adenosine, inosine, hypoxanthine, xanthine and uric acid concentrations in the dialysate samples were determined by reversed-phase high-performance liquid chromatography as described by Smolenski et al. (1990). In brief, adenosine and its metabolites were determined by reversed-phase high-performance liquid chromatography using a C18 column (Hypersil ODS 3 μm, 150 × 4.6 mm, Alltech, Deerfield, IL, U.S.A.) combined with a C18 guard column (Hypersil ODS 5 μm, 7.5 × 4.6 mm). We used an AS 3000 cooled autosampler, a SCM 1000 vacuum membrane degasser, a P2000 gradient pump, a 50 μl sample loop and PC 1000 software from Thermo Separation Products, Riviera Beach, FL, U.S.A.) in combination with a Spectra Focus forward optical scanning detector (Spectra-Physics, San Jose, CA, U.S.A.). Peaks were detected (and concentrations determined) at 254 nm (hypoxanthine, xanthine, inosine and adenosine) and at 280 nm (uric acid). Purines were identified based on external standards, retention times and the ratios of the areas under the curve at 254 and 280 nm (Smolenski et al., 1990).
Data analysis and presentation
Infarct data were analyzed using one-way analysis of variance followed by posthoc testing using Student–Newman–Keuls method. Hemodynamic variables were compared using two-way analysis of variance for repeated measures followed by post hoc testing using Student–Newman–Keuls method. Purine data were analysed using the paired t-test. Statistical significance was accepted when P<0.05. Data are presented as mean±s.e.m.
Results
Mortality and exclusions
Of the 78 rats that entered the infarction protocol, four rats were excluded because of pump failure during the 60-min index ischemia. Several rats fibrillated during the 60-min index ischemia CAO period (no more than three rats per group), but were successfully reverted to sinus rhythm and completed the experimental protocol. Infarct size was not different in rats that fibrillated and were thus included in the final analysis. Finally, one rat was excluded due to technical failure and one rat due to an AR<10% of the left ventricle.
Heart rate and arterial blood pressure
Baseline heart rate and mean arterial blood pressure for all animals were 351±3 b.p.m. and 99±1 mmHg, with no differences in heart rate (P=0.55) and mean arterial blood pressure (P=0.11) between the experimental groups. ADO produced a small decrease in heart rate (5.4±2.5%), while decreasing mean arterial blood pressure by up to 41±3% (both P<0.05) at the end of the infusion (Table 1). After discontinuation of ADO, both heart rate and arterial pressure recovered to baseline values well before the onset of the 60-min CAO. Infusion of LNNA caused a marked pressor response, as arterial pressure increased by up to 37±3%, which was accompanied by an 11±2% decrease in heart rate (both P<0.05). These changes were sustained until the onset of the 60-min CAO. Administration of hexamethonium produced decreases in both heart rate (11±3%) and arterial pressure (33±4%), which had recovered partly at the onset of the 60-min CAO. LNNA and hexamethonium did not blunt the hemodynamic responses to ADO.
Table 1.
Heart rate and arterial blood pressure
| n | Baseline | Control/ADO | Coronary artery occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|---|
|
Pre (−25 min) |
End (−10 min) |
Pre (−1 min) |
End (60 min) |
End (120 min) |
||||
| 1 | Control | 15 | ||||||
| HR | 345±8 | 346±10 | 348±11 | 348±12 | 365±12* | 394±12* | ||
| MAP | 91±3 | 93±2 | 98±3 | 96±3 | 92±3 | 79±4* | ||
| 2 | ADO | 10 | ||||||
| HR | 361±9 | 358±7 | 337±6*† | 368±9 | 368±12 | 385±13 | ||
| MAP | 104±5 | 106±5 | 63±3*† | 118±4* | 106±4 | 93±6* | ||
| 3 | LNNA Control | 10 | ||||||
| HR | 338±6 | 301±6* | 304±7 | 299±6* | 319±8 | 311±17 | ||
| MAP | 101±2 | 138±4* | 145±3* | 140±3* | 107±7 | 72±6* | ||
| 4 | LNNA+ADO | 10 | ||||||
| HR | 348±6 | 316±6* | 335±8† | 325±6* | 323±8* | 324±14* | ||
| MAP | 103±3 | 150±4* | 81±5*† | 151±3* | 117±7 | 74±9* | ||
| 5 | HEX Control | 13 | ||||||
| HR | 353±8 | 312±6* | 318±8*† | 327±8 | 343±11 | 377±13* | ||
| MAP | 100±5 | 67±2* | 83±2*† | 89±4 | 95±4 | 96±5 | ||
| 6 | HEX+ADO | 7 | ||||||
| HR | 346±11 | 311±8* | 307±5* | 340±12 | 349±9 | 399±11* | ||
| MAP | 93±4 | 70±3* | 49±1*† | 89±4 | 96±4 | 89±5 | ||
HR=heart rate (b.p.m.);
MAP=mean aortic pressure (mmHg);
Data are mean±s.e.m.;
P<0.05 vs Baseline
P<0.05 vs Baseline
Cardioprotection and myocardial interstitial adenosine concentrations
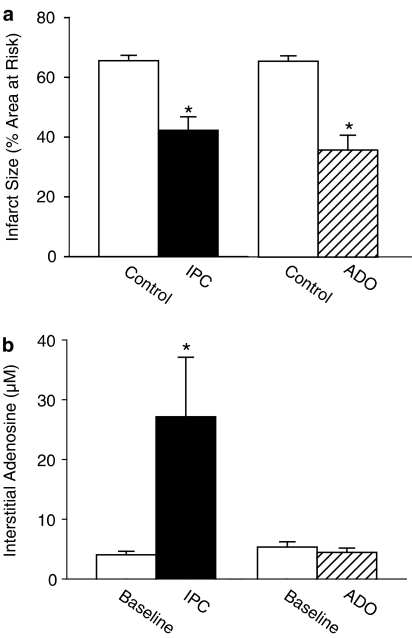
IPC with a 15-min CAO and a 15-min ADO produced similar marked reductions in IS (Figure 2a). However, while IPC produced marked increments in myocardial interstitial adenosine levels from 4.3±0.7 μM at baseline to 27.1±10.0 μM during the 15-min CAO (P<0.05), as well as increases in dialysate concentrations of the other purines, ADO had no effect on myocardial interstitial adenosine levels (4.1±1.2 μM; Figure 2b), or on dialysate concentrations of any of the other purines (Table 2).
Figure 2.
Panel a displays the protective effect of IPC (n=7) and ADO (n=7) compared to control rats that underwent only the 60-min CAO (n=7 and n=9, respectively). Panel b displays the increase in myocardial interstitial adenosine concentrations from baseline produced by IPC (n=9) and the lack of increase by ADO (n=8). *P<0.05 vs corresponding Control or Baseline.
Table 2.
Dialysate concentrations of purines
| Concentration (μM) | |||
|---|---|---|---|
| Baseline | Intervention | ||
| IPC (n=9) | Adenosine | 0.6±0.1 | 4.7±1.9* |
| Inosine | 3.0±0.4 | 9.4±2.7* | |
| Hypoxanthine | 0.3±0.1 | 3.9±1.3* | |
| Xanthine | 0.7±0.2 | 3.6±0.8* | |
| Uric acid | 5.0±0.5 | 5.9±0.5 | |
| Total purines | 9.6±0.9 | 27.5±6.8* | |
| ADO (n=8) | Adenosine | 0.9±0.2 | 0.7±0.1 |
| Inosine | 2.8±0.3 | 2.7±0.2 | |
| Hypoxanthine | 0.2±0.1 | 0.3±0.1 | |
| Xanthine | 0.6±0.1 | 0.6±0.1 | |
| Uric acid | 5.9±0.4 | 6.9±0.4 | |
| Total purines | 10.5±0.8 | 11.4±0.8 | |
Data are mean±s.e.m.
P<0.05 vs corresponding baseline.
Mechanism of protection by ADO
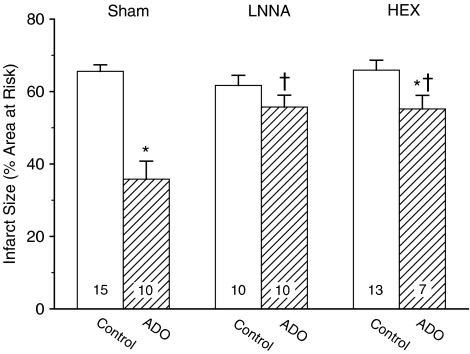
There were no differences (P=0.32) in the area at risk of the various experimental groups (Table 3). In agreement with earlier reports on rats (Van den Doel et al., 1998; Liem et al., 2005), rabbits (Miura et al., 1992) and pigs (Koning et al., 1994), we observed no significant linear correlation between the rate-pressure product at the onset of the 60-min CAO and the corresponding infarct size (linear regression: r2=0.02; P=0.31). LNNA, which had no significant effect on IS by itself (IS=62±3% vs IS=66±2% in sham-treated control rats), virtually abolished the protection by ADO (Figure 3). Pretreatment with hexamethonium, which also did not affect IS (IS=66±3%) by itself, attenuated the amount of protection by ADO by 65% (Figure 3).
Table 3.
Area at risk and infarct area
| n | AR (% LV) | IA (%LV) | |
|---|---|---|---|
| Control | 15 | 38±2 | 24±2 |
| IPC | 12 | 32±3 | 13±2* |
| ADO | 10 | 32±3 | 12±2* |
| LNNA Control | 10 | 38±3 | 23±1 |
| LNNA+ADO | 10 | 39±3 | 22±2† |
| HEX Control | 13 | 38±2 | 26±2 |
| HEX+ADO | 7 | 36±4 | 20±3† |
AR=area at risk;
LV=left ventricle;
IA=infarct area.
Data are mean±s.e.m.
P<0.05 vs corresponding control.
P<0.05 vs ADO.
Figure 3.
Infarct size in control rats and in rats receiving ADO, without (Sham) or after NO-synthase blockade (LNNA) or ganglion blockade (hexamethonium, HEX). Infarct size is expressed as percentage of the area at risk. The number of animals in each group is shown within the bars. *P<0.05 vs corresponding Control; †P<0.05 vs corresponding Sham.
Discussion
The mechanism by which IPC protects the myocardium has been the topic of numerous studies since the first description of the phenomenon by Murry et al. (1986) in the expectation that knowledge of the mechanism would permit the development of pharmacological exploitation in the clinical setting (Kloner & Jennings, 2001; Vinten-Johansen et al., 2003). The discovery that activation of adenosine receptors is one of the triggers of IPC has led to the investigation of the usefulness of adenosine in the treatment of a coronary artery stenosis by elective percutaneous coronary intervention (Strauer et al., 1996; Leesar et al., 1997), as adjuvant to thrombolysis (Mahaffey et al., 1999) or percutaneous coronary intervention (Garratt et al., 1998) in myocardial infarction and as adjuvant to the cardioplegic solution during cardiac surgery (Lee et al., 1995).
Adenosine has been shown to be a trigger of IPC in all animal species studied. However, based on several studies including those in which the selective adenosine A1-receptor antagonist PD 115,199 and the nonselective antagonist SPT failed to block IPC (Liu & Downey, 1992; Li & Kloner, 1993), Ganote & Armstrong (2000) concluded that adenosine does not play a role in the myocardial infarct size limitation by IPC in rats. Importantly, in these studies (Liu & Downey, 1992; Li & Kloner, 1993) the duration of the multiple IPC stimuli was 3–5 min. We subsequently confirmed the observations by Li & Kloner (1993) that the cardioprotection by a triple 3-min CAO did not depend on intact adenosine receptors, but in contrast, that cardioprotection by a single 15-min CAO was completely abolished by the adenosine receptor antagonist 8-SPT (Liem et al., 2001). Thus, similar to the porcine heart (Schulz et al., 1998), in the rat heart the role of adenosine in IPC depends critically on the type of IPC stimulus.
The mechanism of protection by intravascular adenosine is still incompletely understood, but a large number of studies indicate that it may differ from that of endogenous adenosine in IPC (Van Winkle et al., 1994; Yao & Gross, 1994; Lasley et al., 1995; Manthei & Van Wylen, 1997; Headrick et al., 2003). For example, while myocardial interstitial adenosine levels increase during IPC (Lasley et al., 1995; 1998; Martin et al., 1997; Harrison et al., 1998; Mei et al., 1998; Liem et al., 2005), access of intravascular adenosine into the interstitial compartment is impeded by the active metabolic barrier function of the endothelium (Nees et al., 1985; Lasley et al., 1995; Manthei & Van Wylen, 1997; Headrick et al., 2003). This barrier function may explain why in some studies, though not all (Toombs et al., 1992; Lasley et al., 1995), intravascular adenosine failed to decrease infarct size (Auchampach & Gross, 1993; Hale et al., 1993; Li & Kloner 1993), or increase interstitial adenosine concentrations (Gamboa et al., 2003), unless the adenosine transport inhibitor dipyridamole was coadministered (Auchampach & Gross, 1993; Gamboa et al., 2003). In contrast, studies employing high doses of intra-arterial adenosine observed cardioprotection (Liu et al., 1991; Van Winkle et al., 1994; Yao and Gross 1994; Lasley et al., 1998), and increases in interstitial adenosine concentrations (Lasley et al., 1995; 1998; Manthei & Van Wylen, 1997). In the present study, we observed that ADO produced a marked reduction in IS, which contrasts with Li & Kloner (1993) who reported a lack of cardioprotection by adenosine in the rat heart in situ. These divergent findings are difficult to explain but could be related to differences in the employed anesthesia. Thus, the signaling pathway involved in IPC has been shown to differ in ketamine-xylazine vs pentobarbital anesthesia (Miura et al., 1995). In addition, differences in rat strain (Sprague–Dawley vs Wistar) and gender (female vs male), the dose and duration of intravenous adenosine infusion (1.5 mg administered over 5 min vs 3 mg administered over 15 min) and CAO duration (90 vs 60 min) may also have contributed to the different outcomes.
Interestingly, we observed that while ADO produced marked cardioprotection, it failed to increase myocardial interstitial adenosine concentration. These findings are at variance with the increases in myocardial interstitial adenosine concentrations produced by intravenous adenosine, in a dose that produced a degree of cardioprotection in the rabbit (Lasley et al., 1995), that was comparable to the cardioprotection observed in the present study. Failure to detect an increase in interstitial adenosine does not appear to be due to increased adenosine catabolism in the rat heart, because concentrations of the adenosine metabolites remained similarly unchanged (Table 2). It could also be argued that the probe recovery was too low to detect changes in adenosine concentrations. The recovery rate of our microdialysis fibers was 15±1%, which is considerably lower than that reported in other studies (64–66%; Lasley et al., 1995; 1998). However, the lower recovery in the present study is at least in part due to the higher dialysate flow rate (2 μl min−1 compared to 0.75 μl min−1 in the studies by Lasley et al., 1995; 1998), which is inversely related to recovery percentage of the probe (Lameris et al., 1999). Furthermore, we readily detected marked increases in adenosine and other purine concentrations during total coronary artery occlusion, that are comparable to the increases observed in the rabbit heart (Lasley et al., 1995). An alternative explanation could be that adenosine produced an increase in coronary blood flow that caused enhanced adenosine washout, thereby masking a small increase in interstitial adenosine concentrations (Lasley & Mentzer, 1998). Although this would not explain the increase in interstitial adenosine that was observed in the rabbit heart (Lasley et al., 1995), we cannot entirely exclude that this effect may have increased importance in the in situ rat heart, in which we observed relatively high intersititial adenosine concentrations under baseline conditions.
The observation in the present study that ADO did not result in elevated myocardial interstitial adenosine levels, suggests that adenosine remained principally confined to the intravascular compartment. In support of that concept, there is evidence to suggest that the cardiovascular effects of adenosine involve, at least in part, the release of endothelium-derived substances, including NO and prostanoids (Smits et al., 1995; Rubio & Ceballos, 2003). Furthermore, there is, albeit somewhat controversial, evidence that NO plays a role during the second window of protection (Ferdinandy & Schulz, 2003). Since the involvement of NO in the early phase of protection by adenosine has not been previously studied, we investigated the role of NO in the early phase of protection by ADO. In the presence of LNNA, ADO no longer afforded cardioprotection, which in conjunction with the lack of increase in myocardial interstitial adenosine levels, could be interpreted to suggest that ADO affords cardioprotection via (coronary) endothelium-derived NO. However, from our in vivo experiments we cannot determine the site of NO production by ADO. For example, recent evidence suggests that adenosine may not only stimulate eNOS in the endothelium, but also in cardiomyocytes (Xu et al., 2005). Moreover, we cannot exclude the fact that interstitial adenosine concentrations may have increased in tissues other than the heart, which would implicate the involvement of NO production at sites other than the endothelium, for example, downstream of the neurogenic pathway. Another limitation is that LNNA is a nonspecific NO synthase inhibitor, and hence we cannot exclude the fact that isoforms other than eNOS are involved in the cardioprotection by ADO. Future studies, using microdialysis in other organs and using selective inhibitors of the various NOS isoforms are required to address these important issues.
Recently, the concept of IPC has been expanded to include remote preconditioning, the phenomenon that a brief period of ischemia in an organ or tissue not only elicits a local preconditioning effect, but also provides protection against prolonged ischemia in virgin tissue and organs at a distance (Gho et al., 1996; Przyklenk et al., 2003) For instance, Gho et al. (1996) have shown that a brief episode of intestinal ischemia produced by a 15-min mesenteric artery occlusion, limited myocardial IS produced by a subsequent 60-min CAO. Remote preconditioning was mimicked by a low dose of intramesenteric adenosine infusion, but not by infusion of the same dose into the portal vein (Liem et al., 2002). Both cardioprotection by remote preconditioning and intramesenteric adenosine infusion were abolished by ganglion blockade, implying the involvement of a neurogenic pathway. In light of these considerations, we investigated whether an action at extracardiac sites contributed to the protection by ADO. The observation that hexamethonium, which does not modify the protection by IPC with a 15-min CAO (Gho et al., 1996) attenuated the protection by 65%, indicates that the ADO-induced cardioprotection originates, at least in part, at extracardiac sites where it initiates cardioprotection via activation of a neurogenic pathway. The design of the study does not permit one to draw any conclusions about the location of these extracardiac sites. The small intestine is a prime candidate, considering our earlier observations with the intramesenteric artery infusion of adenosine (Liem et al., 2002), but other organs such as the kidney may also be involved (see Przyklenk et al., 2003). There is evidence that remote preconditioning by skeletal muscle ischemia may not depend on a neurogenic pathway (Addison et al., 2003; Wang et al., 2004). Hence, we cannot simply ascribe the residual protection by ADO that was not amenable to ganglion blockade to direct intracardiac action of ADO, as we cannot exclude that a humoral factor released from skeletal muscle may also have contributed.
The dose of adenosine that produced the cardioprotection caused a 44±4 mmHg decrease in mean arterial blood pressure and it might be argued that it is therefore not clinically relevant. It must be kept in mind, however, that sodium pentobarbital was used to anesthetize the animals and that this anesthetic regimen suppresses baroreceptor-mediated reflexes (Zimpfer et al., 1982) and thereby exaggerates the hypotension (Verdouw et al., 1987). Indeed, we observed a small decrease in heart rate during ADO, consistent with observations by Li & Kloner (1993), suggesting the absence of significant baroreflex activity. In the present study, it should also be considered that ADO was administered to animals with a normal endothelial function and it cannot be excluded that ADO would increase the interstitial adenosine levels when administered to animals with endothelial dysfunction. Since this may be of clinical relevance, we also administered the same dose of ADO to rats that had been exposed to four sequences of 15-min CAO. In this model, that causes 10% of the area at risk to become infarcted (Liem et al., 2005) and which is likely associated with endothelial dysfunction (Pearson et al., 1990), we also did not find a rise in interstitial adenosine levels (2.1±0.5 μM before vs 2.0±0.4 μM during ADO).
In conclusion, the findings in the present study demonstrate that the early phase of cardioprotection by ADO: (i) is not associated with a detectable increase in myocardial interstitial purine concentrations, (ii) depends critically on NO production, and (iii) involves the activation of a neurogenic pathway. These findings indicate that ADO administered as adjunct therapy to reperfusion treatment in patients with a pending myocardial infarction may not require access to the jeopardized myocardium, but rather may initiate cardioprotection at remote extracardiac sites.
Acknowledgments
The present study was supported by Grants NHS99.143 and 2000T038 from the Netherlands Heart Foundation.
Abbreviations
- ADO
15-min intravenous adenosine infusion
- CAO
coronary artery occlusion
- IPC
ischemic preconditioning
- LNNA
Nω-nitro-L-arginine
- NO
nitric oxide
References
- ADDISON P.D., NELIGAN P.C., ASHRAFPOUR H., KHAN A., ZHONG A., MOSES M., FORREST C.R., PANG C.Y. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am. J. Physiol. Heart. Circ. Physiol. 2003;285:H1435–H1443. doi: 10.1152/ajpheart.00106.2003. [DOI] [PubMed] [Google Scholar]
- AUCHAMPACH J.A., GROSS G.J. Adenosine A1 receptors, K+ATP channels, and ischemic preconditioning in dogs. Am. J. Physiol. 1993;264:H1327–H1336. doi: 10.1152/ajpheart.1993.264.5.H1327. [DOI] [PubMed] [Google Scholar]
- COSTA F., BIAGGIONI I. Role of nitric oxide in adenosine-induced vasodilation in humans. Hypertension. 1998;31:1061–1064. doi: 10.1161/01.hyp.31.5.1061. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBOA A., ERTL A.C., COSTA F., FARLEY G., MANIER M.L., HACHEY D.L., DIEDRICH A., BIAGGIONI I. Blockade of nucleoside transport is required for delivery of intraarterial adenosine into the interstitium: relevance to therapeutic preconditioning in humans. Circulation. 2003;108:2631–2635. doi: 10.1161/01.CIR.0000101927.70100.41. [DOI] [PubMed] [Google Scholar]
- GANOTE C.E., ARMSTRONG S.C. Adenosine and preconditioning in the rat heart. Cardiovasc. Res. 2000;45:134–140. doi: 10.1016/s0008-6363(99)00312-0. [DOI] [PubMed] [Google Scholar]
- GARRATT K.N., HOLMES D.R., JR, MOLINA-VIAMONTE V., REEDER G.S., HODGE D.O., BAILEY K.R., LOBL J.K., LAUDON D.A., GIBBONS R.J. Intravenous adenosine and lidocaine in patients with acute mycocardial infarction. Am. Heart J. 1998;136:196–204. doi: 10.1053/hj.1998.v136.89910. [DOI] [PubMed] [Google Scholar]
- GHO B.C., SCHOEMAKER R.G., VAN DEN DOEL M.A., DUNCKER D.J., VERDOUW P.D. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- HALE S.L., BELLOWS S.D., HAMMERMAN H., KLONER R.A. An adenosine A1 receptor agonist, R(−)-N-(2-phenylisopropyl)-adenosine (PIA), but not adenosine itself, acts as a therapeutic preconditioning-mimetic agent in rabbits. Cardiovasc. Res. 1993;27:2140–2145. doi: 10.1093/cvr/27.12.2140. [DOI] [PubMed] [Google Scholar]
- HARRISON G.J., WILLIS R.J., HEADRICK J.P. Extracellular adenosine levels and cellular energy metabolism in ischemically preconditioned rat heart. Cardiovasc. Res. 1998;40:74–87. doi: 10.1016/s0008-6363(98)00123-0. [DOI] [PubMed] [Google Scholar]
- HEADRICK J.P., HACK B., ASHTON K.J. Acute adenosinergic cardioprotection in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1797–H1818. doi: 10.1152/ajpheart.00407.2003. [DOI] [PubMed] [Google Scholar]
- KLONER R.A., JENNINGS R.B. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- KONING M.M., SIMONIS L.A., DE ZEEUW S., NIEUKOOP S., POST S., VERDOUW P.D. Ischaemic preconditioning by partial occlusion without intermittent reperfusion. Cardiovasc. Res. 1994;28:1146–1151. doi: 10.1093/cvr/28.8.1146. [DOI] [PubMed] [Google Scholar]
- LAMERIS T.W., DE ZEEUW S., ALBERTS G., BOOMSMA F., DUNCKER D.J., VERDOUW P.D., MAN IN'T VELD A.J., VAN DEN MEIRACKER A.H. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation. 2000;101:2645–2650. doi: 10.1161/01.cir.101.22.2645. [DOI] [PubMed] [Google Scholar]
- LAMERIS T.W., VAN DEN MEIRACKER A.H., BOOMSMA F., ALBERTS G., DE ZEEUW S., DUNCKER D.J., VERDOUW P.D., MAN IN'T VELD A.J. Catecholamine handling in the porcine heart: a microdialysis approach. Am. J. Physiol. 1999;277:H1562–H1569. doi: 10.1152/ajpheart.1999.277.4.H1562. [DOI] [PubMed] [Google Scholar]
- LASLEY R.D., HEGGE J.O., NOBLE M.A., MENTZER R.M., JR Comparison of interstitial fluid and coronary venous adenosine levels in in vivo porcine myocardium. J. Mol. Cell Cardiol. 1998;30:1137–1147. doi: 10.1006/jmcc.1998.0683. [DOI] [PubMed] [Google Scholar]
- LASLEY R.D., KONYN P.J., HEGGE J.O., MENTZER R.M., JR Effects of ischemic and adenosine preconditioning on interstitial fluid adenosine and myocardial infarct size. Am. J. Physiol. 1995;269:H1460–H1466. doi: 10.1152/ajpheart.1995.269.4.H1460. [DOI] [PubMed] [Google Scholar]
- LASLEY R.D., MENTZER R.M., JR Dose-dependent effects of adenosine on interstitial fluid adenosine and postischemic function in the isolated rat heart. J. Pharmacol. Exp. Ther. 1998;286:806–811. [PubMed] [Google Scholar]
- LEE H.T., LAFARO R.J., REED G.E. Pretreatment of human myocardium with adenosine during open heart surgery. J. Card Surg. 1995;10:665–676. doi: 10.1111/j.1540-8191.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- LEESAR M.A., STODDARD M., AHMED M., BROADBENT J., BOLLI R. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation. 1997;95:2500–2507. doi: 10.1161/01.cir.95.11.2500. [DOI] [PubMed] [Google Scholar]
- LI Y., KLONER R.A. The cardioprotective effects of ischemic ‘preconditioning' are not mediated by adenosine receptors in rat hearts. Circulation. 1993;87:1642–1648. doi: 10.1161/01.cir.87.5.1642. [DOI] [PubMed] [Google Scholar]
- LIEM D.A., TE LINTEL HEKKERT M., MANINTVELD O.C., BOOMSMA F., VERDOUW P.D., DUNCKER D.J. Myocardium tolerant to ischemic preconditioning can still be protected by preconditioning stimuli that employ alternative pathways. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1165–H1172. doi: 10.1152/ajpheart.00899.2004. [DOI] [PubMed] [Google Scholar]
- LIEM D.A., VAN DEN DOEL M.A., DE ZEEUW S., VERDOUW P.D., DUNCKER D.J. Role of adenosine in ischemic preconditioning in rats depends critically on the duration of the stimulus and involves both A1 and A3 receptors. Cardiovasc. Res. 2001;51:701–708. doi: 10.1016/s0008-6363(01)00321-2. [DOI] [PubMed] [Google Scholar]
- LIEM D.A., VERDOUW P.D., PLOEG H., KAZIM S., DUNCKER D.J. Sites of action of adenosine in interorgan preconditioning of the heart. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H29–H37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- LIU G.S., THORTON J., VAN WINKLE D.M., STANLEY A.W., OLSSEN R.A., DOWNEY J.M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- LIU Y., DOWNEY J.M. Ischemic preconditioning protects against infarction in rat heart. Am. J. Physiol. 1992;263:H1107–H1112. doi: 10.1152/ajpheart.1992.263.4.H1107. [DOI] [PubMed] [Google Scholar]
- MAHAFFEY K.W., PUMA J.A., BARBAGELATA N.A., DICARLI M.F., LEESAR M.A., BROWNE K.F., EISENBERG P.R., BOLLI R., CASAS A.C., MOLINA-VIAMONTE V., ORLANDI C., BLEVINS R., GIBBONS R.J., CALIFF R.M., GRANGER C.B. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J. Am. Coll. Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- MANTHEI S.A., VAN WYLEN D.G. Purine metabolite accumulation during myocardial ischemia: adenosine pretreatment versus brief ischemia. Basic Res. Cardiol. 1997;92:368–377. doi: 10.1007/BF00796210. [DOI] [PubMed] [Google Scholar]
- MARTIN B.J., MCCLANAHAN T.B., VAN WYLEN D.G., GALLAGHER K.P. Effects of ischemia, preconditioning, and adenosine deaminase inhibition on interstitial adenosine levels and infarct size. Basic Res. Cardiol. 1997;92:240–251. doi: 10.1007/BF00788519. [DOI] [PubMed] [Google Scholar]
- MEI D.A., NITHIPATIKOM K., LASLEY R.D., GROSS G.J. Myocardial preconditioning produced by ischemia, hypoxia, and a KATP channel opener: effects on interstitial adenosine in dogs. J. Mol. Cell Cardiol. 1998;30:1225–1236. doi: 10.1006/jmcc.1998.0687. [DOI] [PubMed] [Google Scholar]
- MIURA T., GOTO M., MIKI T., SAKAMOTO J., SHIMAMOTO K., IIMURA O. Glibenclamide, a blocker of ATP-sensitive potassium channels, abolishes infarct size limitation by preconditioning in rabbits anesthetized with xylazine/pentobarbital but not with pentobarbital alone. J. Cardiovasc. Pharmacol. 1995;25:531–538. doi: 10.1097/00005344-199504000-00004. [DOI] [PubMed] [Google Scholar]
- MIURA T., OGAWA T., IWAMOTO T., SHIMAMOTO K., IIMURA O. Dipyridamole potentiates the myocardial infarct size-limiting effect of ischemic preconditioning. Circulation. 1992;86:979–985. doi: 10.1161/01.cir.86.3.979. [DOI] [PubMed] [Google Scholar]
- MUBAGWA K., FLAMENG W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc. Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- NEES S., HERZOG V., BECKER B.F., BOCK M., DES ROSIERS C., GERLACH E. The coronary endothelium: a highly active metabolic barrier for adenosine. Basic Res. Cardiol. 1985;80:515–529. doi: 10.1007/BF01907915. [DOI] [PubMed] [Google Scholar]
- PEARSON P.J., SCHAFF H.V., VANHOUTTE P.M. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ. Res. 1990;67:385–393. doi: 10.1161/01.res.67.2.385. [DOI] [PubMed] [Google Scholar]
- PRZYKLENK K., DARLING C.E., DICKSON E.W., WHITTAKER P. Cardioprotection ‘outside the box' – the evolving paradigm of remote preconditioning. Basic Res. Cardiol. 2003;98:149–157. doi: 10.1007/s00395-003-0406-y. [DOI] [PubMed] [Google Scholar]
- RUBIO R., CEBALLOS G. Sole activation of three luminal adenosine receptor subtypes in different parts of coronary vasculature. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H204–H214. doi: 10.1152/ajpheart.00068.2002. [DOI] [PubMed] [Google Scholar]
- SCHULZ R., POST H., VAHLHAUS C., HEUSCH G. Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation. 1998;98:1022–1029. doi: 10.1161/01.cir.98.10.1022. [DOI] [PubMed] [Google Scholar]
- SMITS P., WILLIAMS S.B., LIPSON D.E., BANITT P., RONGEN G.A., CREAGER M.A. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. doi: 10.1161/01.cir.92.8.2135. [DOI] [PubMed] [Google Scholar]
- SMOLENSKI R.T., LACHNO D.R., LEDINGHAM S.J., YACOUB M.H. Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation. J. Chromatogr. 1990;527:414–420. doi: 10.1016/s0378-4347(00)82125-8. [DOI] [PubMed] [Google Scholar]
- STRAUER B.E., HEIDLAND U.E., HEINTZEN M.P., SCHWARTZKOPFF B. Pharmacologic myocardial protection during percutaneous transluminal coronary angioplasty by intracoronary application of dipyridamole: impact on hemodynamic function and left ventricular performance. J. Am. Coll. Cardiol. 1996;28:1119–1126. doi: 10.1016/S0735-1097(96)00307-5. [DOI] [PubMed] [Google Scholar]
- SUZUKI K., MIURA T., MIKI T., TSUCHIDA A., SHIMAMOTO K. Infarct-size limitation by preconditioning is enhanced by dipyridamole administered before but not after preconditioning: evidence for the role of interstitial adenosine level during preconditioning as a primary determinant of cardioprotection. J. Cardiovasc. Pharmacol. 1998;31:1–9. doi: 10.1097/00005344-199801000-00001. [DOI] [PubMed] [Google Scholar]
- TOOMBS C.F., MCGEE S., JOHNSTON W.E., VINTEN-JOHANSEN J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992;86:986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- VAN DEN DOEL M.A., GHO B.C., DUVAL S.Y., SCHOEMAKER R.G., DUNCKER D.J., VERDOUW P.D. Hypothermia extends the cardioprotection by ischaemic preconditioning to coronary artery occlusions of longer duration. Cardiovasc. Res. 1998;37:76–81. doi: 10.1016/s0008-6363(97)00222-8. [DOI] [PubMed] [Google Scholar]
- VAN WINKLE D.M., CHIEN G.L., WOLFF R.A., SOIFER B.E., KUZUME K., DAVIS R.F. Cardioprotection provided by adenosine receptor activation is abolished by blockade of the KATP channel. Am. J. Physiol. 1994;266:H829–H839. doi: 10.1152/ajpheart.1994.266.2.H829. [DOI] [PubMed] [Google Scholar]
- VERDOUW P.D., SASSEN L.M., DUNCKER D.J., SCHMEETS I.O., RENSEN R.J., SAXENA P.R. Nicorandil-induced changes in the distribution of cardiac output and coronary blood flow in pigs. Naunyn. Schmiedebergs Arch. Pharmacol. 1987;336:352–358. doi: 10.1007/BF00172690. [DOI] [PubMed] [Google Scholar]
- VINTEN-JOHANSEN J., ZHAO Z.Q., CORVERA J.S., MORRIS C.D., BUDDE J.M., THOURANI V.H., GUYTON R.A. Adenosine in myocardial protection in on-pump and off-pump cardiac surgery. Ann. Thorac. Surg. 2003;75:S691–S699. doi: 10.1016/s0003-4975(02)04694-5. [DOI] [PubMed] [Google Scholar]
- WANG W.Z., STEPHESON L.L., FANG X.H., KHIABANI K.T., ZAMBONI W.A. Ischemic preconditioning-induced microvascular protection at a distance. J. Reconstr. Microsurg. 2004;20:175–181. doi: 10.1055/s-2004-820775. [DOI] [PubMed] [Google Scholar]
- XU Z., PARK S.S., MUELLER R.A., BAGNELL R.C., PATTERSON C., BOYSEN P.G. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc. Res. 2005;65:803–812. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- YAO Z., GROSS G.J. A comparison of adenosine-induced cardioprotection and ischemic preconditioning in dogs. Efficacy, time course, and role of K+ATP channels. Circulation. 1994;89:1229–1236. doi: 10.1161/01.cir.89.3.1229. [DOI] [PubMed] [Google Scholar]
- ZIMPFER M., MANDERS W.T., BARGER A.C., VATNER S.F. Pentobarbital alters compensatory neural and humoral mechanisms in response to hemorrhage. Am. J. Physiol. 1982;243:H713–H721. doi: 10.1152/ajpheart.1982.243.5.H713. [DOI] [PubMed] [Google Scholar]