Abstract
Mast cells participate in allergies, and also in immunity and inflammation by secreting proinflammatory cytokines.
Flavonoids are naturally occurring polyphenolic plant compounds, one group of which – the flavonols, inhibits histamine and some cytokine release from rodent basophils and mast cells. However, the effect of flavonols on proinflammatory mediator release and their possible mechanism of action in human mast cells is not well defined.
Human umbilical cord blood-derived cultured mast cells (hCBMCs) grown in the presence of stem cell factor (SCF) and interleukin (IL)-6 were preincubated for 15 min with the flavonols quercetin, kaempferol, myricetin and morin (0.01, 0.1, 1, 10 or 100 μM), followed by activation with anti-IgE. Secretion was quantitated for IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), histamine and tryptase levels.
Release of IL-6, IL-8 and TNF-α was inhibited by 82–93% at 100 μM quercetin and kaempferol, and 31–70% by myricetin and morin. Tryptase release was inhibited by 79–96% at 100 μM quercetin, kampferol and myricetin, but only 39% by morin; histamine release was inhibited 52–77% by the first three flavonols, but only 28% by morin. These flavonols suppressed intracellular calcium ion elevations in a dose–response manner, with morin being the weakest; they also inhibited phosphorylation of the calcium-insensitive protein kinase C theta (PKC θ).
Flavonol inhibition of IgE-mediated proinflammatory mediator release from hCBMCs may be due to inhibition of intracellular calcium influx and PKC θ signaling. Flavonols may therefore be suitable for the treatment of allergic and inflammatory diseases.
Keywords: Cytokines, flavonols, mast cells, PKC θ, tryptase, quercetin
Introduction
Mast cells are important effector cells in IgE-mediated reactions by secreting histamine, chymase, tryptase, leukotrienes (LTs), prostaglandin D2 (PGD2) and several multifunctional cytokines; they include interleukin-6 (IL-6), IL-8, IL-13, tumor necrosis factor-alpha (TNF-α), stem cell factor (SCF) and many chemotactic factors (Galli, 2000; Mekori & Metcalfe, 2000). These cytokines contribute to the late-phase allergic reactions and to allergic inflammation through the recruitment of immune cells into the site of inflammation (Wedemeyer et al., 2000; Theoharides & Cochrane, 2004). Tryptase, expressed by all subsets of human mast cells and comprising up to 25% of the total intracellular proteins, has emerged as an important mediator in allergic diseases (Shaoheng et al., 1998). Tryptase and other serine proteases are signaling molecules that cleave protease-activated surface receptor-2 (PAR-2) leading to widespread inflammation (Steinhoff et al., 2000; Cottrell et al., 2003). Calcium influx is necessary for mast cell mediator secretion (Kimata et al., 2000b) and PAR-2 activation has also been associated with a brisk increase in intracellular calcium concentration (Dery et al., 1998).
The limited number of mast cells obtained from normal human tissues has led to increasing use of human umbilical cord blood-derived cultured mast cells (hCBMCs), obtained by culturing CD34+ cells in the presence of SCF and IL-6 (Saito et al., 1996; Kempuraj et al., 1999). hCBMCs are predominantly MCT (tryptase-positive and chymase-negative) rather than MCTC (tryptase- and chymase-double-positive) type (Toru et al., 1998; Ahn et al., 2000). hCBMCs produce several mediators that include histamine, tryptase, LTs, PGD2, IL-1, IL-5, IL-6, IL-8, IL-13, SCF, TNF-α, granulocyte–macrophage colony-stimulating factor (GM-CSF) (Yanagida et al., 1995; Ahn et al., 2000; Kimata et al., 2000b; Tachimoto et al., 2000), as well as corticotropin-releasing hormone and its structurally related urocortin (Kempuraj et al., 2004). Flavonoids is a group of naturally occurring polyphenolic compounds found in fruits, vegetables, nuts, seeds, herbs, spices and red wine with antioxidant properties (Schroeter et al., 2003; Huxley et al., 2004; Lako et al., 2004). Flavonoids possess antiallergic, anti-inflammatory, cytoprotective and anticarcinogenic activity (Herrmann, 1976; Duthie et al., 1997; Middleton Jr et al., 2000; Theoharides et al., 2001). Several flavonoids with a hydroxylation pattern on the B phenolic ring, called flavonols, have been reported to inhibit histamine release from murine mast cells (Foreman, 1984; Middleton Jr, 1998) and basophils (Middleton Jr & Drzewiecki, 1985), as well as IL-6 and TNF-α release from bone marrow-derived cultured murine mast cells and rat peritoneal mast cells (Kimata et al., 2000b). Flavonoids are also known to affect many enzyme systems such as tyrosine and serine–threonine protein kinases, phospholipase A2, phospholipase C and lipoxygenase, all involved in allergic and inflammatory responses (Middleton Jr & Kandaswami, 1992). Certain flavonoids, such as quercetin, flavone and luteolin, can inhibit histamine, LTs, PGD2 and GM-CSF release from cultured human mast cells (Kimata et al., 2000b; Theoharides et al., 2001), as well as IL-4 and IL-13 from activated basophils (Hirano et al., 2004). Crosslinking of the high-affinity receptors for IgE (FcɛRI) induces the activation of protein kinase C (PKC) and the elevation of intracellular Ca2+ concentration, believed to be the primary synergistic signals required for secretion. Mast cells also express the calcium-insensitive PKC isozyme theta (PKC θ), which is functional in FcɛRI-mediated activation (Liu et al., 2001).
Here, we compared for the first time the effect of certain flavonols on the secretion of granule-stored and newly synthesized mediators, along with their effect on two different targets, intracellular calcium ions and the calcium-insensitive PKC θ in hCBMCs. We showed that quercetin and kaempferol, more than myricetin and morin, inhibited secretion of proinflammatory cytokines; morin was the only flavonol that was a weak inhibitor of tryptase and histamine release as well as of intracellular calcium levels. All flavonols inhibited activation of PKC θ.
Methods
Flavonols
Flavonols quercetin, kaempferol, myricetin and morin were purchased from Sigma (St Louis, MO, U.S.A.); they were dissolved in dimethyl sulfoxide (DMSO) as 10−1 M stock concentrations and stored at −20°C. These flavonols were selected because of previous evidence that they can inhibit rodent mast cell secretion and the fact that they only varied in the hydroxylation pattern of the B phenolic ring. They were tested at 0.01, 0.1, 1, 10 and 100 μM. The working dilutions were obtained in mast cell culture medium for the cytokine (IL-6, IL-8, TNF-α) release and phosphorylated PKC θ (phospho-PKC θ) experiments, while human Tyrode's buffer was used for the histamine, tryptase and intracellular calcium measurement experiments. The maximum DMSO concentration present in the final working dilutions was 0.1% and this concentration had no effect on the release of any of the mediators studied (results not presented). These flavonols did not affect viability of hCBMCs as determined by Trypan blue exclusion up to 6 h of culture as required for cytokine release (Table 1).
Table 1.
Effect of flavonols on hCBMCs viability
| Flavonols (μM) | % Viability | |||||
|---|---|---|---|---|---|---|
| Quercetin | Kaempferol | Myricetin | Morin | DMSO | Control | |
| 0.01 | 96.3±1.2 | 96.0±1.0 | 96.0±2.0 | 95.7±1.2 | 96.0±2.0 | 96.7±1.5 |
| 0.1 | 96.0±1.0 | 97.0±1.0 | 97.0±1.0 | 97.0±1.0 | ||
| 1 | 95.7±1.5 | 96.0±3.0 | 96.3±2.5 | 95.7±2.1 | ||
| 10 | 97.0±1.0 | 96.3±2.1 | 95.3±1.2 | 96.0±2.0 | ||
| 100 | 96.3±1.5 | 96.0±2.0 | 96.3±1.5 | 96.0±1.0 | ||
[DMSO]=0.1%, the highest concentration present in 100 μM flavonol dilutions.
Cytokines and antibodies
Recombinant human stem cell factor (rhSCF) was kindly provided by Amgen (Thousand Oaks, CA, U.S.A.). Anti-human-IgE was purchased from Dako Corporation (Carpinteria, CA, U.S.A.). Human myeloma IgE, mouse anti-human mast cell tryptase monoclonal antibody and human IL-6 were purchased from Chemicon International Inc. (Temecula, CA, U.S.A.). Phospho-PKC θ (Thr538) antibody and Phototope-HRP Western Blot Detection System (anti-rabbit IgG, HRP-linked antibody, anti-biotin, HRP-linked antibody, biotinylated Protein Ladder Detection Pack and 20X LumiGLO Reagent, 20X Peroxide) were from Cell Signaling Technology (Beverly, MA, U.S.A.).
Isolation of CD34+ cells and mast cell culture
Human umbilical cord blood was collected as approved by the Hospital's Human Investigation Review Board in tubes containing 10 U ml−1 heparin and diluted 1 : 2 with Dulbecco's phosphate-buffered saline (DPBS) from GIBCO BRL (Life Technologies, Grand Island, NY, U.S.A.) containing 2 mM ethylenediamine-tetra acetic acid (Sigma). Nonphagocytic mononuclear cells were separated by density-gradient centrifugation using Lymphocyte Separation Medium from Organon Teknika Corp. (Durham, NC, U.S.A.). The isolation of hematopoietic stem cells (CD34+) was performed by positive selection of CD34+/AC133+ cells by magnetic-associated cell sorting using an AC133+ cell isolation kit (Milltenyi Biotec, Auburn, CA, U.S.A.). CD133 expression is restricted to a subset of CD34bright-positive stem cells in human cord blood. Previously, mast cells had been obtained by culturing cord blood mononuclear cells in the presence of SCF, IL-6 and PGE2 (Shichijo et al., 1998); however, PGE2 enhances SCF and IL-6-dependent development of mast cells only from mononuclear cells, but not from CD34+ cells (Saito et al., 1996). Consequently, no PGE2 was used in the present study. Instead, hCBMCs were cultured as reported previously (Kempuraj et al., 1999, 2003). Briefly, CD34+ cells were suspended in Iscove's modified Dulbecco's medium (GIBCO BRL), supplemented with 100 ng ml−1 rhSCF, 50 ng ml−1 IL-6, 10% fetal bovine serum (FBS; Bio Whittaker, Walkesville, MD, U.S.A.), 5 × 10−5 M 2-mercaptoethanol and 1% penicillin–streptomycin (GIBCO BRL) for 12–16 weeks. During this culture period, the cells were washed with DPBS every week and resuspended using fresh complete culture medium. The purity of hCBMCs was evaluated by immunocytochemical staining for tryptase as described previously (Kempuraj et al., 1999), and mast cell viability was determined by Trypan blue (0.3%) exclusion. hCBMCs are considered MCT, but by 12 weeks of culture when we use them, they are about 80% positive for chymase and 100% positive for tryptase (Kanbe et al., 1998; Kempuraj et al., 1999). hCBMCs from 71 different cultures (ranging from 12 to 16 weeks) were used in this study.
Sensitization of mast cells
hCBMCs were washed with DPBS and plain culture medium (without any growth factors) once in each and resuspended in serum-free complete culture medium, but without IL-6 supplementation. Cells (1 × 106 cells ml−1) were then incubated with human myeloma-IgE (2 μg ml−1) at 37°C for 48 h in 24-well Falcon cell culture plates (Becton Dickinson, Franklin Lakes, NJ, U.S.A.). These sensitized hCBMCs were used in all the experiments.
Immunohistochemical staining for tryptase
The purity of hCBMCs was determined by immunostaining for mast cell-specific tryptase. Cytospin smears of hCBMCs were prepared using Cytospin 3 (Shandon, Pittsburgh, PA, U.S.A.) and fixed with Carnoy's solution (60% ethanol, 30% chloroform and 10% glacial acetic acid) for 3 min and stained for mast cell tryptase by the alkaline phosphatase antialkaline phosphatase (APAAP) procedure using Dako APAAP Kit system (Dako) as reported previously (Kempuraj et al., 1999, 2003). Briefly, the slides were incubated overnight at 4°C with mouse anti-human tryptase monoclonal antibody (Chemicon) used at 1 μg ml−1 in Tris-HCl-PBS (pH 7.6), plus 10% FBS. The slides were then brought to room temperature (RT) and were first incubated with rabbit antiserum (Ig fraction) to mouse Ig for 30 min followed by incubation with the APAAP immune complex for another 30 min. Between each incubation, the slides were rinsed in Tris-buffered saline (pH 7.6) for 10 min. The reaction was finally developed with substrate solution (napthol AS-MX phosphate, Fast Red and levamisole) for 20 min and then rinsed briefly in a water bath. Negative controls were performed either by the omission of the primary antibody or by using an isotype-matched mouse IgG1 antibody instead of the primary antibody.
IL-6, IL-8 and TNF-α assays
hCBMCs were washed with DPBS, sterile human Tyrode's buffer (133 mM NaCl, 4 mM KCl, 0.64 mM KH2PO4, 10 mM HEPES, 1 g l−1 glucose, 1 mM CaCl2, 0.6 mM MgCl2 and 0.03% human serum albumin, pH 7.4) and plain culture medium, once in each, and resuspended in complete culture medium but without IL-6. The hCBMCs (2 × 105 cells well−1) were plated in 96-well round-bottomed Falcon cell culture plates (Becton Dickinson) and were incubated for 15 min at 37°C in 5% CO2 incubator. The cells were then preincubated with the flavonols for 15 min, at the concentrations indicated in the Results and figure legends sections; following this preincubation, anti-IgE (10 μg ml−1) was added for activation and the cells were incubated for 6 h as reported previously for cytokine release assays (Tachimoto et al., 2000). After the reaction time was over, the supernatant was gently collected from the wells and stored at −80°C until IL-6, IL-8 and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (Quantikine, R&D Systems, Minneapolis, MN, U.S.A.). The minimum detectable level of IL-6, IL-8 and TNF-α was typically less than 0.7, 10 and 4.4 pg ml−1, respectively. Control cells were treated with equal volume of culture medium instead of flavonols; cells were also stimulated with anti-IgE alone for comparison. The results are expressed as the percent inhibition.
Tryptase and histamine assays
hCBMCs were washed with DPBS and human Tyrode's buffer once in each and resuspended in sterile human Tyrode's buffer. The cell suspension (5 × 104 cells tube−1, 500 μl sample−1) was preincubated for 15 min at 37°C in a shaking water bath and then treated with flavonols for 15 min before incubating with anti-IgE (10 μg ml−1) for another 30 min, as shown in the Results and figure legends sections. Control cells were treated with equal volume of only Tyrode's buffer instead of flavonols; cells were also stimulated with anti-IgE alone for comparison. After the reaction was over, the cells were centrifuged and the supernatant fluid was collected; 500 μl of 2% perchloric acid was then added to the pellet. The tryptase level in the supernatant was measured (Kempuraj et al., 2003) by a fluoroenzyme immunoassay using the Unicap Tryptase assay kit and the Unicap 100 automated instrument (Pharmacia& Upjohn, Uppsala, Sweden) as per the kit's directions. Histamine levels in the supernatant fluid and pellet were measured as described previously (Kremzner & Wilson, 1961; Kempuraj et al., 2003) using an LS-5B Luminescence Spectrometer (Perkin-Elmer, Norwalk, CT, U.S.A.). The percent histamine release was then calculated as the percent of total=(supernatant/supernatant+pellet) × 100.
Intracellular calcium measurement
hCBMCs (2 × 106 cells ml−1) were washed in DPBS and resuspended in Tyrode's buffer containing 2.5 mM probenecid and 2 μM cell-permeant fluorescent calcium indicator Calcium Green-1 AM (Molecular Probes, Eugene, OR, U.S.A.) for 30 min at RT. The cells were again washed and resuspended in fresh Tyrode's buffer (106 cells ml−1) and were pretreated with various flavonols for 15 min as shown in the Results and figure legends sections. The control hCBMCs were treated with equal volume of Tyrode's buffer alone. hCBMCs were also incubated with anti-IgE alone for comparison. After washing with Tyrode's buffer, hCBMCs at 2 × 106 cells 2 ml−1 were placed in a quartz cuvette with constant stirring with a magnetic minibar at 37°C, maintained by a closed-circuit, temperature-regulated, water pump. Samples were excited at 506 nm and the fluorescence was monitored at 530 nm using a Perkin-Elmer Fluorescence Spectrometer LS-50B (Norwalk, CT, U.S.A.). After about 5 min of baseline recordings, the cells were stimulated with anti-IgE (10 μg ml−1) with continuous recording for about 30 min. Results are presented in arbitrary fluorescence units. This technique has been used successfully to measure intracellular calcium levels (Takahashi et al., 1999) in hCBMCs and also in human leukemic mast cells (HMC-1) (Kandere-Grzybowska et al., 2003; Kempuraj et al., 2003).
Immunoblotting for PKC θ
For the detection of phospho-PKC θ, hCBMCs were washed with plain culture medium and DPBS, once in each, were resuspended in serum free complete culture medium and plated in 12-well cell culture plate (2 × 106 cells 2 ml−1 well−1). These cells were preincubated with different flavonols (10−4 M) for 15 min before incubation with anti-IgE (10 μg ml−1) for 30 min at 37°C. The reaction was stopped by the addition of cold DPBS to the wells. The cell suspension was removed from the well, centrifuged and the supernatant was discarded. The cell pellets were then lysed in 200 μl SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 2% SDS, 19% glycerol, 50 mM DTT, 0.01% bromophenol blue), sonicated for 15 s at full power (Sonicator Cell Disruptor, Model W185D, Heat System – Ultrasonics Inc., Plainview, NY, U.S.A.), heated for 5 min at 100°C and immediately cooled on ice. Cell lysates (50 μg of protein lane−1) were loaded on 10% Tris-HCl precast minigels (Bio-Rad Laboratories, Hercules, CA, U.S.A.). The separated proteins were then transferred to polyvinylidene membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Western blot analysis was performed with Rabbit anti-phospho-PKC θ (Thr538) (Cell Signaling Technology) at 1 : 1000 dilutions incubated overnight at 4°C. The secondary antibody was goat anti-rabbit HRP-linked IgG (Cell Signaling Technology) at 1 : 2000 dilution for 1 h at RT followed by HRP anti-biotin, HRP-linked antibody (Cell Signaling Technology) at 1 : 1000 dilution for 1 h at RT to detect biotinylated protein markers using the Phototope-HRP Western Blot Detection System (Cell Signaling Technology) as per the directions of the manufacturer. Blots were developed with the chemiluminescence detection system and imaged with Kodak Digital Science 1D Image Station (Eastman Kodak Company, Rochester, NY, U.S.A.). Equal loading was verified by immunoblotting the same membranes with rabbit anti-human β-actin (Cell Signaling Technology). The PKC θ Western blot was scanned and analyzed by densitometry; all samples were normalized with their respective actin bands. Each normalized flavonol-treated PKC θ value was then compared with that obtained by anti-IgE and the results are presented as % inhibition.
Data analysis
Data are represented as mean±s.e.m. The results were analyzed by one-way ANOVA followed by Tukey's multiple comparison test, and Dunn's method/Mann–Whitney Test. The level of significance was set at P<0.05 for all comparisons.
Results
Characterization of mast cells
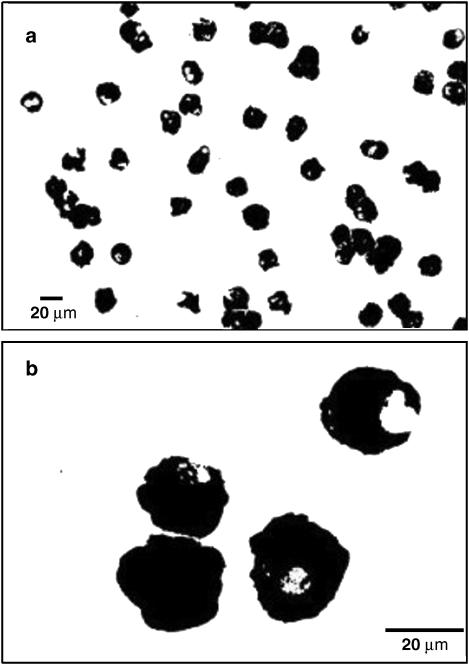
hCBMCs cultured in the presence of SCF and IL-6 for >10 weeks were 100% positive for mast cell-specific tryptase by immunohistochemical staining (Figure 1a and b). Preliminary results showed that 15 min preincubation with 100 μM flavonols resulted in maximal inhibition of cytokines, as well as tryptase and histamine release (results not shown); this preincubation time was used thereafter. Sensitized hCBMCs were preincubated with quercetin, kaempferol, myricetin or morin at 0.01, 0.1, 1, 10 and 100 μM for 15 min and then stimulated with anti-IgE for 6 h. No flavonol affected the viability of either control or stimulated hCBMCs (n=3) at any concentration used (Table 1).
Figure 1.
Photomicrographs of 14 week old hCBMCs immunostained for mast cell-specific tryptase using mouse anti-human tryptase monoclonal antibody. Note that 100% of hCBMCs are tryptase positive; panels a and b show low and high magnifications of tryptase-positive hCBMCs, respectively.
Cytokine release
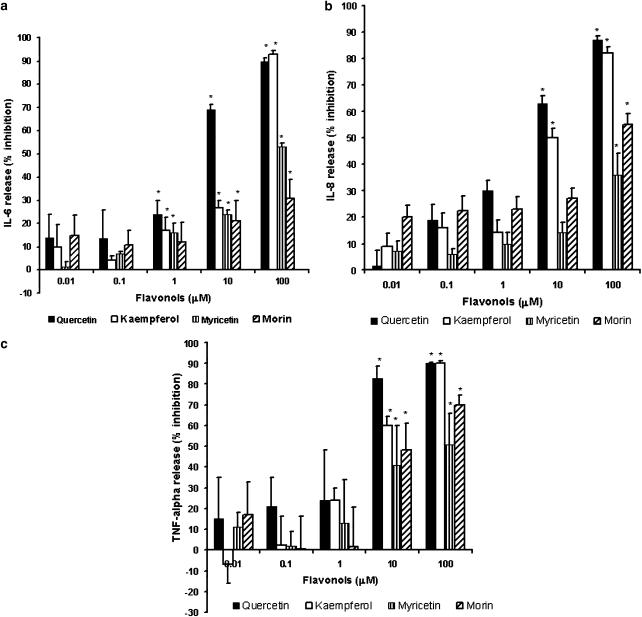
hCBMCs (n=4–7) stimulated with anti-IgE for 6 h at 37°C released significantly (P<0.05) more IL-6, IL-8 and TNF-α (223±23 pg 10−6 cells, 14,000±1700 pg 10−6 cells, 1187±100 pg 10−6 cells, respectively) when compared to control hCBMCs treated with equal amount of culture medium only (99±7 pg 10−6 cells, 3600±900 pg 10−6 cells, 107±9.0 pg 10−6 cells, respectively). Preincubation of hCBMCs for 15 min with quercetin, kaempferol, myricetin and morin significantly inhibited anti-IgE-stimulated IL-6 release, and the inhibition was 90±1.3, 93±1.5, 53±1.6 and 31±8.2%, respectively, at 100 μM and the inhibition was 69±2.4, 27±3.2, 24±1.9 and 20±9.3%, respectively, at 10 μM (Figure 2a). Only quercetin, kaempferol and myricetin inhibited IL-6 release at 1 μM and the inhibition was 24±5.7, 17±5.8 and 16±4%, respectively.
Figure 2.
Dose–response effect of flavonols on anti-IgE-mediated cytokine release from hCBMCs (n=4–7). hCBMCs were pretreated with flavonols quercetin, kaempferol, myricetin and morin each at 0.01, 0.1, 1, 10 and 100 μM for 15 min and then incubated with anti-IgE (10 μg ml−1) for 6 h at 37°C. Control cells were treated with equal volume of culture medium only. IL-6, IL-8 and TNF-α levels were measured in the supernatant fluid by ELISA. These flavonols significantly inhibited IL-6 (a), IL-8 (b) and TNF-α (c) release at certain concentrations. Values are expressed as the percent inhibition of the anti-IgE-mediated cytokine release. Each value represents the mean±s.e.m. *Significant difference (P<0.05) compared to samples treated with anti-human-IgE alone.
Only quercetin and kaempferol at 10 μM significantly inhibited IL-8 release (63±2.9 and 49±3.6% inhibition, respectively); at 100 μM, the inhibition was 87±1.7 and 82±2.4%, respectively (Figure 2b). Myricetin and morin significantly inhibited IL-8 release only at 100 μM (36±8.0 and 55±4.3% inhibition, respectively) from hCBMCs (Figure 2b). Quercetin, kaempferol, myricetin and morin significantly inhibited TNF-α release at 100 μM (90±0.7, 90±1.2, 51±15 and 70±5.0%, inhibition, respectively) as shown in Figure 2c, and at 10 μM, the inhibition was 83±5.7, 60±4.4, 41±19 and 48±12.5% inhibition, respectively.
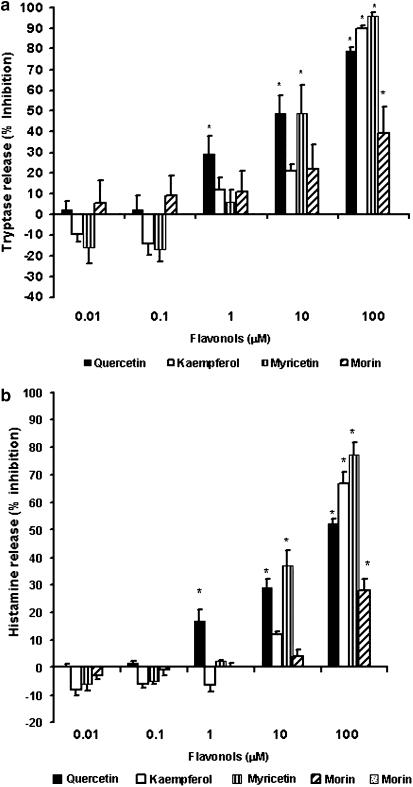
Tryptase release
hCBMCs (n=6–16) stimulated with anti-IgE for 30 min at 37°C released significantly (P<0.05) more tryptase (2070±404 ng 10−6 cells) when compared to control hCBMCs (47±4.0 ng 10−6 cells) treated with equal volume of Tyrode's buffer only. Preincubation of hCBMCs for 15 min with quercetin significantly inhibited anti-IgE-mediated tryptase release (Figure 3a) at 1, 10 and 100 μM (29±9.0, 50±8 and 79±1.9% inhibition, respectively); myricetin significantly inhibited at 10 and 100 μM (50.0±19 and 95±1.9% inhibition, respectively); kaempferol and morin significantly inhibited tryptase release only at 100 μM (90±1.1 and 39±13% inhibition, respectively).
Figure 3.
Dose–response effect of flavonols on anti-IgE-mediated tryptase (n=6–16) and histamine release (n=6–31) from hCBMCs. hCBMCs were pretreated with quercetin, kaempferol, myricetin and morin each at 0.01, 0.1, 1, 10 and 100 μM for 15 min and then incubated with anti-IgE (10 μg ml−1) for 30 min in Tyrode's buffer at 37°C in a shaking water bath. Control cells were treated with equal volume of Tyrode's buffer only. Tryptase and histamine levels were measured in the supernatant fluid by fluoroenzyme immunoassay using the Unicap Tryptase assay kit and Luminescence Spectrometer, respectively. The mean net anti-IgE-mediated tryptase and histamine release were significantly higher than the control release. These flavonols significantly inhibited tryptase release (a), as well as histamine release (b) at higher concentrations. Values are expressed as the percent inhibition of the anti-human-IgE-mediated tryptase or histamine release. Each value represents the mean±s.e.m. *Significant difference (P<0.05) compared to samples treated with anti-IgE alone.
Histamine release
hCBMCs (n=6–31) incubated with anti-IgE for 30 min at 37°C released significantly (P<0.05) more histamine (44±3.0%) when compared to control hCBMCs (14±1.1%) treated with equal amount of Tyrode's buffer only. Preincubation of hCBMCs for 15 min with quercetin significantly inhibited anti-IgE-stimulated histamine release (Figure 3b) at 1, 10 and 100 μM (17±4, 29±3.0 and 52±2.0% inhibition, respectively); myricetin significantly inhibited at 10 and 100 μM (37±5.5 and 77±4.7% inhibition, respectively). Kaempferol and morin significantly inhibited histamine release only at 100 μM (67±4.0 and 28±4% inhibition, respectively).
Intracellular calcium ion levels
In order to study the mechanism of flavonol inhibition of mediator release from hCBMCs, we investigated their effects on intracellular calcium ion levels (n=3). hCBMCs were first loaded with the cell-permeant, calcium-sensitive fluorescent indicator Calcium Green-1 AM and the fluorescence intensity was measured in cell suspensions. Control hCBMCs treated only with Tyrode's buffer did not show any increase in intracellular calcium level over 30 min recording (Figure 4a); however, hCBMCs stimulated by anti-IgE increased intracellular calcium ion levels within a few sections (Figure 4b) indicating cell activation. hCBMCs were next pretreated for 15 min with quercetin, kaempferol, myricetin or morin at 0.1, 10 and 100 μM before activation with anti-IgE for 30 min. Figure 4c–f are representative tracings of the inhibition of intracellular calcium levels by flavonols at 100 μM. Quercetin showed maximal inhibition and morin showed the least inhibition of intracellular calcium elevations when compared with hCBMCs treated with anti-IgE alone (Figure 4g). Quercetin, kaempferol and myricetin significantly decreased intracellular calcium levels at 10 and 100 μM, while morin showed inhibition only at 100 μM (Figure 4g).
Figure 4.
Effect of flavonols on anti-IgE-mediated elevation of intracellular calcium levels in hCBMCs (n=3). Intracellular calcium levels were measured using an automated spectrometer. hCBMCs were loaded with the cell permeant fluorescent calcium indicator Calcium Green-1 AM for 30 min at room temperature. The samples were excited at 506 nm and the fluorescence level was recorded. Control hCBMCs treated with equal volume of Tyrode's buffer alone did not show any increase in intracellular calcium ion levels in 30 min (a); however, hCBMCs treated with anti-IgE (10 μg ml−1) for 30 min showed substantial elevation of intracellular calcium ion levels (b) indicating mast cell activation. hCBMCs were pretreated for 15 min with quercetin, kaempferol, myricetin or morin at 0.1, 10, and 100 μM before activation with anti-IgE for 30 min; these flavonols at 10 and 100 μM concentrations showed substantial inhibition of intracellular calcium ion elevation (c, d, e and f, respectively) compared to hCBMCs treated only with anti-IgE (b). c, d, e and f show representative of the inhibition of intracellular calcium levels by flavonols at 100 μM. The % inhibition of intracellular calcium elevations by flavonols has been shown in (g) Quercetin showed more inhibition of anti-IgE-induced intracellular calcium ion elevation. Results are presented as arbitrary fluorescence units and are representative of three or more similar experiments. *Significant difference (P<0.05) compared to samples treated with only anti-IgE.
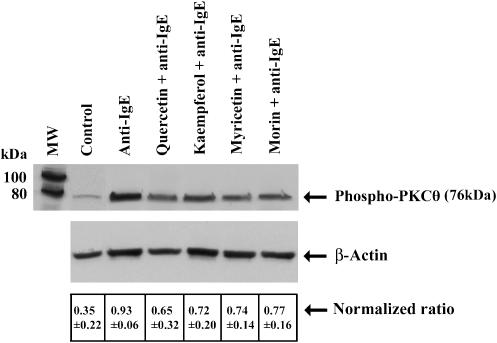
Phospho-PKC θ levels
Anti-IgE stimulated phosphorylation of PKC θ in hCBMCs (n=3) as determined with anti-phospho-PKC θ (Thr538) (Figure 5). Preliminary studies showed that 30 min incubation (selected from 1 to 120 min time course) with anti-IgE yielded maximal PKC θ phosphorylation (results not shown). The preincubation of hCBMCs with flavonols (100 μM) for 15 min prior to incubation with anti-IgE for 30 min inhibited PKC θ phosphorylation (Figure 5). Quercetin showed more inhibition than all other flavonols used (Figure 5). Equal loading was verified by immunoblotting the same membranes with antibody to β-actin (Figure 5). The band intensity was then normalized by obtaining the ratio of the densitometric value of β-actin over that of phospho-PKC θ. These ratios were 0.35±0.22 for the control; 0.93±0.06 for anti-IgE; 0.65±0.32 for quercetin and anti-IgE; 0.72±0.20 for kaempferol and anti-IgE; 0.74±0.14 for myricetin and anti-IgE and 0.77±0.16 for morin and anti-IgE (Table 2). The corresponding inhibition for quercetin, kaempferol, myricetin and morin was 48, 36, 32 and 28%, respectively.
Figure 5.
Effect of flavonols on anti-human-IgE-mediated PKC θ phosphorylation in hCBMCs (n=3). Incubation of hCBMCs with anti-human-IgE (10 μg ml−1)-stimulated phosphorylation of PKC θ as determined with phospho-PKC θ (Thr538) antibody. However, preincubation of hCBMCs with various flavonols (100 μM)for 15 min prior to incubation with anti-IgE for 30 min inhibited PKC θ phosphorylation. Figure is a representative of three similar experiments. Equal loading was verified by immunoblotting the same membranes with antibody to actin.
Table 2.
Densitometry of PKC θ and β-actin bands (arbitrary units)
| No. | Control | Anti-IgE | Quercetin+anti-IgE | Kaempferol+anti-IgE | Myricetin+anti-IgE | Morin+anti-IgE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PKC θ | β-actin | PKC θ | β-actin | PKC θ | β-actin | PKC θ | β-actin | PKC θ | β-actin | PKC θ | β-actin | |
| 1 | 69 | 115 | 148 | 148 | 125 | 123 | 131 | 138 | 117 | 136 | 120 | 130 |
| 2 | 35 | 144 | 130 | 145 | 74 | 142 | 84 | 146 | 83 | 142 | 86 | 143 |
| 3 | 27 | 129 | 105 | 119 | 52 | 122 | 79 | 125 | 92 | 117 | 96 | 124 |
| Normalized ratio (PKC θ/β-actin) | 0.60 0.24 0.21 |
1.00 0.90 0.88 |
1.02 0.52 0.43 |
0.95 0.58 0.63 |
0.86 0.58 0.79 |
0.92 0.60 0.77 |
||||||
| Mean±s.d. | 0.35±0.22 | 0.93±0.06 | 0.65±0.32 | 0.72±0.20 | 0.74±0.14 | 0.77±0.16 | ||||||
| % Inhibition | 48 | 36 | 32 | 28 | ||||||||
Discussion
In the present study, we report on the comparative inhibitory effect of certain flavonols on hCBMCs secretion of inflammatory cytokines (IL-6, IL-8 and TNF-α) and preformed granule-stored tryptase and histamine, as well as on intracellular calcium ion levels and phosphorylation of PKC θ. These flavonols included quercetin, kaempferol, myricetin and morin, which differ only on the hydroxylation pattern of the B phenolic ring. The extent of the inhibition, for at least TNF-α, was much more potent than that reported for the ‘mast cell stabilizer drug disodium cromoglycate' and nedocromil, which inhibited TNF-α release from rat peritoneal mast cells by 20% at 10−5 M (Bissonnette et al., 1995). All flavonols but not morin significantly inhibited anti-IgE-mediated release of IL-6 from 1 μM. Cytokine levels were measured 6 h after stimulation because cytokine production by hCBMCs after stimulation with anti-IgE reached a plateau by 6 h (Tachimoto et al., 2000); histamine release was measured after 30 min incubation as described previously (Igarashi et al., 1996). The results on mediator release are presented as the percent inhibition in order to normalize baseline differences between cultures; that might be due to variation among the individual batches of hCBMCs obtained from different donors, as reported previously (Yamaguchi et al., 1999; Tachimoto et al., 2000). The different flavonols were not equally effective in inhibiting histamine and tryptase release. Morin showed less inhibition than other flavonols. In general, all the flavonoids inhibited cytokine release more efficiently than tryptase or histamine release. Kaempferol inhibited cytokine release more than histamine or tryptase release; it may be that kaempferol's inhibition of PKC θ is more directly involved with the release of cytokines than preformed mediators such as tryptase and histamine.
We and others had previously reported that anti-IgE induces the release of cytokines, histamine and tryptase from hCBMCs (Igarashi et al., 1996; Kempuraj et al., 2002). Quercetin had been reported to inhibit histamine release from rat connective tissue mast cells and mucosal mast cells (Pearce et al., 1984), as well as from human lung and intestinal mast cells (Fox et al., 1988). Quercetin and luteolin were also shown to inhibit histamine, IL-6 and TNF-α production from bone marrow-derived cultured murine mast cells (Kimata et al., 2000a), while myricetin inhibited histamine and LTB4 release from rat peritoneal exudate cells (Yamada et al., 1999) and downregulated transcription factor NF-κB/IκB involved in immune and inflammatory responses (Tsai et al., 1999). These flavonols also inhibited IL-4, IL-5 and IL-13 secretion from human basophils (Higa et al., 2003). A similar rank of potency (quercetin>kaempferol>morin) was also reported in the inhibition of proliferation and mediator accumulation in human leukemic mast cells (HMC-1) (Alexandrakis et al., 2003).
We chose to study two different signal transduction steps, intracellular calcium elevations and the calcium-insensitive PKC θ. Previous studies had reported that activation of hCBMCs by anti-IgE increased intracellular calcium levels (Saito et al., 1996; Kempuraj et al., 2003), a necessary step for mediator secretion (Douglas, 1974). Fewtrell & Gomperts (1977) had first shown that quercetin could inhibit calcium influx in rat peritoneal mast cells. Previous reports showed that IgE-mediated release of histamine and LTs was abolished after calcium depletion (Kimata et al., 1999). It was also shown that quercetin could inhibit ionophore-induced histamine release from rat peritoneal mast cells, suggesting that it had actions other than antigen-induced calcium influx (Ennis et al., 1981). Another report also suggested that cytokine release from HMC-1 stimulated by a combination of the cation ionophore A23187 and phorbol myristate acetate for 24 h was not dependent on extracellular calcium (Lippert et al., 2000). Morin's inhibition of calcium ion is disproportionate to the inhibition of histamine and tryptase; this discrepancy points to the fact that inhibition of calcium entry is not sufficient to inhibit degranulation that may also utilize calcium released from intracellular stores.
PKC translocation and phosphorylation has also been shown to be critical for secretion of mast cells (Kimata et al., 1999) and rat basophil leukemia (RBL) cells (Sagi-Eisenberg et al., 1985). About 12 closely related PKC isozymes have been described and classified into three subfamilies based on their ability to respond to Ca2+ and diacylglycerol (DAG) (Trushin et al., 2003). The calcium-dependent or conventional PKC isoforms (α, β and γ) are regulated by DAG, which binds to the C1 domain (Trushin et al., 2003). The calcium-independent or novel PKC isoforms (δ, ɛ, η, μ and θ) are not regulated by Ca2+, but respond to DAG (Trushin et al., 2003). The atypical PKC isoforms (ζ and μ) are regulated neither by Ca2+ nor DAG. Of the novel PKC isoforms, only θ is associated with FcɛRI-mediated activation of mast cells (Liu et al., 2001), while most of the others are either involved in the inhibition of secretion or not at all. We selected to study PKC θ, the activity of which is not regulated by Ca2+, because it is also involved in allergic activation of mast cells (Liu et al., 2001). Here, we showed that flavonols at 10 and 100 μM inhibited anti-IgE-mediated intracellular calcium ion levels, except morin at 10 μM. These flavonols also inhibited phospho-PKC θ at 100 μM; this concentration was selected for testing because it showed the highest inhibition of mediator release from hCBMCs. However, our preliminary studies indicate that lower concentrations of flavonols show corresponding inhibition of PKC θ phosphorylation. These results suggest that PKC θ activation may be important in IL-6 release, such as the selective release induced by IL-1 reported recently not to depend on extracellular calcium (Kandere-Grzybowska et al., 2003).
It was previously reported that quercetin inhibited histamine release and calcium influx, as well as extracellular signal-regulated kinase translocation and c-Jun NH2-terminal kinase, but not activation of p38-mitogen-activated protein kinase from hCBMCs (Kimata et al., 2000b). Moreover, quercetin also inhibited collagen-stimulated platelet activation, as well as tyrosine phosphorylation of the Fc receptor γ-chain, Syk, LAT and phospholipase Cγ2 (Hubbard et al., 2003). Another flavonol, fisetin, reduced cation ionophore A23187 and phorbol ester-induced IL-4, IL-13 and IL-15 synthesis, but not IL-6 and IL-8 production from the human basophilic cell line KU812 (Higa et al., 2003).
Recent reviews have discussed the possible structural requirements for the inhibitory action of flavonols on secretory processes (Middleton Jr et al., 2000; Theoharides et al., 2001), as well as on adhesion molecule expression (Chen et al., 2004). The flavonoid basic structure is comprised of two phenolic rings (A and B) linked through a heterocyclic pyran or pyrone ring (C) in the middle. The inhibitory effect of flavonols requires in the presence of the C ring of an oxy group at position 4, a double bond between carbon atoms 2 and 3 and a hydroxyl group in position 3. The catechol (o-dihydroxy) group in the B ring, as in quercetin, confers further potent inhibitory ability, as does the presence of hydroxy groups in kaempferol and myricetin. Structural requirements were shown to be similar to those for fisetin's inhibitory action on antigen-induced IL-4 and TNF-α secretion from RBL cells (Higa et al., 2003). Our present study extends these findings (Middleton Jr et al., 2000; Theoharides et al., 2001) and confirms that addition of one hydroxyl group at position 2′ of the B ring (as in morin) reduces its ability to inhibit especially histamine and tryptase secretion and intracellular calcium ion levels, even though it can still inhibit PKC θ phosphorylation and cytokine secretion. This finding is supported by previous reports that morin did not inhibit polymorphonuclear leukocyte functions (Berton et al., 1980) or mitogen-induced lymphocyte proliferation (Namgoong et al., 1994). Quercetin, myricetin, kaempferol, but not morin, inhibited RBL cell secretion (Trnovsky et al., 1993).
Cytokines produced by mast cells and basophils are associated with the development of allergic inflammation (Costa et al., 1997). Flavonoids have been reported to improve allergic symptoms or prevent the development of allergic diseases (Miller, 2001; Hirano et al., 2004). Moreover, oral administration of astragalin, which is absorbed and converted into kaempferol, suppressed the onset of dermatitis in NC/Nga mice (Kotani et al., 2000), while the naturally occurring quercetin glycoside rutin, which is converted into quercetin in the intestine, showed antiarthritic actions (Ostrakhovitch & Afanas'ev, 2001). The principal sources of dietary flavonols are tea, onions, apples and seeds. In plants, flavonoids generally occur in glycosylated form (e.g. rutin is the glycosylated form of quercetin). Biotransformation in the gut occurs by ring scission under the influence of intestinal microorganisms that release these phenolic acid derivatives. For instance, plasma quercetin concentration following ingestion of fried onions containing quercetin glycosides equivalent to 64 mg of quercetin aglycone (a typical daily diet) led to peak plasma levels of 196 ng ml−1 after 2.9 h, with a half-life of 16.8 h (Theoharides et al., 2001). This actually translates to a concentration of 0.65 μM (assuming one compartment model), while the best inhibition in our studies was obtained with 10–100 μM, which could be attained with a dietary supplement of about 1000 mg quercein per day (Theoharides, 2003). Although 1–100 μM appear to be high, this concentration may be achieved in the body with diet containing high flavonols/flavonol dietary supplements, as reported previously for the dietary sources and intake of flavonoids (Aherne & O'Brien, 2002).
In conclusion, our results indicate that quercetin>kaempferol>myricetin and >morin can inhibit FcɛRI-mediated release of proinflammatory cytokines, tryptase and histamine from hCBMCs; this inhibition appears to involve inhibition of calcium influx, as well as phospho-PKC θ. Some flavonols could, therefore, be useful in allergic and inflammatory diseases (Theoharides & Cochrane, 2004).
Acknowledgments
We thank all Obstetricians and Nurses of Labor and Delivery division of the Department of Obstetrics and Gynecology, Tufts-New England Medical Center, Boston, MA, U.S.A. for collecting the umbilical cord blood used in this study. We also thank Amgen (Thousand Oaks, CA, U.S.A.) for their supply of the recombinant human SCF used for the mast cell cultures. We also thank Ms Jessica Christian for her patience and word processing skills. This work was funded in part by Theta Biomedical Consulting and Development Co. Inc. (Brookline, MA, U.S.A.).
Abbreviations
- hCBMCs
human umbilical cord blood-derived cultured mast cells
- HMC-1
human leukemic mast cells
- IL-6
interleukin-6
- PKC θ
protein kinase C theta
- SCF
stem cell factor
References
- AHERNE S.A., O'BRIEN N.M. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- AHN K., TAKAI S., PAWANKAR R., KURAMASU A., OHTSU H., KEMPURAJ D., TOMITA H., LIDA M., MATSUMOTO K., AKASAWA A., MIYAZAKI M., SAITO H. Regulation of chymase production in human mast cell progenitors. J. Allergy Clin. Immunol. 2000;106:321–328. doi: 10.1067/mai.2000.108107. [DOI] [PubMed] [Google Scholar]
- ALEXANDRAKIS M.G., LETOURNEAU R., KEMPURAJ D., KANDERE K., HUANG M., CHRISTODOULOU S., BOUCHER W., SERETAKIS D., THEOHARIDES T.C. Flavones inhibit proliferation and increase mediator content in human leukemic mast cells (HMC-1) Eur. J. Haematol. 2003;71:448–454. doi: 10.1046/j.0902-4441.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- BERTON G., SCHNEIDER C., ROMEO D. Inhibition by quercetin of activation of polymorphonuclear leucocyte functions. Stimulus-specific effects. Biochim. Biophys. Acta. 1980;595:47–55. doi: 10.1016/0005-2736(80)90246-1. [DOI] [PubMed] [Google Scholar]
- BISSONNETTE E.Y., ENCISO J.A., BEFUS A.D. Inhibition of tumour necrosis factor-alpha (TNF-α) release from mast cells by the anti-inflammatory drugs, sodium cromoglycate and nedocromil sodium. Clin. Exp. Immunol. 1995;102:78–84. doi: 10.1111/j.1365-2249.1995.tb06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C.C., CHOW M.P., HUANG W.C., LIN Y.C., CHANG Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol. Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- COSTA J.J., WELLER P.F., GALLI S.J. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA. 1997;278:1815–1822. [PubMed] [Google Scholar]
- COTTRELL G.S., AMADESI S., SCHMIDLIN F., BUNNETT N. Protease-activated receptor 2: activation, signalling and function. Biochem. Soc. Trans. 2003;31:1191–1197. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W.W. Involvement of calcium in exocytosis and the exocytosis–vesiculation sequence. Biochem. Soc. Symp. 1974;39:1–28. [PubMed] [Google Scholar]
- DUTHIE S.J., JOHNSON W., DOBSON V.L. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimidines) and growth in human cells. Mutat. Res. 1997;390:141–151. doi: 10.1016/s0165-1218(97)00010-4. [DOI] [PubMed] [Google Scholar]
- ENNIS M., TRUNEH A., WHITE J.R., PEARCE F.L. Inhibition of histamine secretion from mast cells. Nature. 1981;289:186–187. doi: 10.1038/289186a0. [DOI] [PubMed] [Google Scholar]
- FEWTRELL C.M., GOMPERTS B.D. Quercetin: a novel inhibitor of Ca2+ influx and exocytosis in rat peritoneal mast cells. Biochim. Biophys. Acta. 1977;469:52–60. doi: 10.1016/0005-2736(77)90325-x. [DOI] [PubMed] [Google Scholar]
- FOREMAN J.C. Mast cells and the actions of flavonoids. J. Allergy Clin. Immunol. 1984;73:769–774. doi: 10.1016/0091-6749(84)90446-9. [DOI] [PubMed] [Google Scholar]
- FOX C.C., WOLF E.J., KAGEY-SOBOTKA A., LICHTENSTEIN L.M. Comparison of human lung and intestinal mast cells. J. Allergy Clin. Immunol. 1988;81:89–94. doi: 10.1016/0091-6749(88)90225-4. [DOI] [PubMed] [Google Scholar]
- GALLI S.J. Mast cells and basophils. Curr. Opin. Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- HERRMANN K. Flavonols and flavones in food plants. J. Food Technol. 1976;11:433–448. [Google Scholar]
- HIGA S., HIRANO T., KOTANI M., MATSUMOTO M., FUJITA A., SUEMURA M., KAWASE I., TANAKA T. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003;111:1299–1306. doi: 10.1067/mai.2003.1456. [DOI] [PubMed] [Google Scholar]
- HIRANO T., HIGA S., ARIMITSU J., NAKA T., SHIMA Y., OHSHIMA S., FUJIMOTO M., YAMADORI T., KAWASE I., TANAKA T. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int. Arch. Allergy Immunol. 2004;134:135–140. doi: 10.1159/000078498. [DOI] [PubMed] [Google Scholar]
- HUBBARD G.P., STEVENS J.M., CICMIL M., SAGE T., JORDAN P.A., WILLIAMS C.M., LOVEGROVE J.A., GIBBINS J.M. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J. Theomb. Haemost. 2003;1:1088. doi: 10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- HUXLEY R.R., LEAN M., CROZIER A., JOHN J.H., NEIL H.A., OXFORD FRUIT AND VEGETABLE STUDY GROUP Effect of dietary advice to increase fruit and vegetable consumption on plasma flavonol concentrations: results from a randomised controlled intervention trial. J. Epidemiol. Community Health. 2004;58:288–289. doi: 10.1136/jech.2003.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGARASHI Y., KUROSAWA M., ISHIKAWA O., MIYACHI Y., SAITO H., EBISAWA M., IIKURA Y., YANAGIDA M., UZUMAKI H., NAKAHATA T. Characteristics of histamine release from cultured human mast cells. Clin. Exp. Allergy. 1996;26:597–602. [PubMed] [Google Scholar]
- KANBE N., KUROSAWA M., MIYACHI Y., KANBE M., KEMPURAJ D., TACHIMOTO H., SAITO H. Carnoy's fixative reduces the number of chymase-positive cells in immunocytochemical staining of cord-blood-derived human cultured mast cells. Allergy. 1998;53:981–985. doi: 10.1111/j.1398-9995.1998.tb03800.x. [DOI] [PubMed] [Google Scholar]
- KANDERE-GRZYBOWSKA K., LETOURNEAU R., BOUCHER W., BERY J., KEMPURAJ D., POPLAWSKI S., ATHANASSIOU A., THEOHARIDES T.C. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J. Immunol. 2003;171:4830–4836. doi: 10.4049/jimmunol.171.9.4830. [DOI] [PubMed] [Google Scholar]
- KEMPURAJ D., HUANG M., KANDERE K., BOUCHER W., LEUTOURNEAU R., JEUDY S., FITZGERALD K., SPEAR K., ATHANASIOU A., THEOHARIDES T.C. Azelastine is more potent than olopatadine in inhibiting interleukin-6 and tryptase release from human umbilical cord blood-derived cultured mast cells. Ann. Allergy Asthma Immunol. 2002;88:501–506. doi: 10.1016/s1081-1206(10)62389-7. [DOI] [PubMed] [Google Scholar]
- KEMPURAJ D., HUANG M., KANDERE-GRZYBOWSKA K., BASU S., BOUCHER W., LETOURNEAU R., ATHANASIOU A., THEOHARIDES T.C. Azelastine inhibits secretion of IL-6, TNF-α and IL-8 as well as NF-κB activation and intracellular calcium ion levels in normal human mast cells. Int. Arch. Allergy Immunol. 2003;132:231–239. doi: 10.1159/000074304. [DOI] [PubMed] [Google Scholar]
- KEMPURAJ D., PAPADOPOULOU N.G., LYTINAS M., HUANG M., KANDERE-GRZYBOWSKA K., MADHAPPAN B., BOUCHER W., CHRISTODOULOU S., ATHANASSIOU A., THEOHARIDES T.C. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- KEMPURAJ D., SAITO H., KANEKO A., FUKAGAWA K., NAKAYAMA M., TORU H., TOMIKAWA M., TACHIMOTO H., EBISAWA M., AKASAWA A., MIYAGI T., KIMURA H., NAKAJIMA T., TSUJI K., NAKAHATA T. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- KIMATA M., INAGAKI N., NAGAI H. Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med. 2000a;66:25–29. doi: 10.1055/s-2000-11107. [DOI] [PubMed] [Google Scholar]
- KIMATA M., SHICHIJO M., MIURA T., SERIZAWA I., INAGAKI N., NAGAI H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy. 2000b;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- KIMATA M., SHICHIJO M., MIURA T., SERIZAWA I., INAGAKI N., NAGAI H. Ca2+ and protein kinase C signaling for histamine and sulfidoleukotrienes release from human cultured mast cells. Biochem. Biophys. Res. Commun. 1999;257:895–900. [Google Scholar]
- KOTANI M., MATSUMOTO M., FUJITA A., HIGA S., WANG W., SUEMURA M., KISHIMOTO T., TANAKA T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000;106:159–166. doi: 10.1067/mai.2000.107194. [DOI] [PubMed] [Google Scholar]
- KREMZNER L.T., WILSON J.B. A procedure for the determination of histamine. Biochim. Biophys. Acta. 1961;50:364–367. doi: 10.1016/0006-3002(61)90339-0. [DOI] [PubMed] [Google Scholar]
- LAKO J., TRENERRY C., WAHLQVIST M.L., WATTANAPENPAIBOON N., SOTHEESWARAN S., PREMIER R. Total antioxidant capacity and selected flavonols and carotenoids of some Australian and Fijian fruits and vegetables. Asia Pac. J. Clin. Nutr. 2004;13:S127. [Google Scholar]
- LIPPERT U., MOLLER A., WELKER P., ARTUC M., HENZ B.M. Inhibition of cytokine secretion from human leukemic mast cells and basophils by H1- and H2-receptor antagonists. Exp. Dermatol. 2000;9:118–124. doi: 10.1034/j.1600-0625.2000.009002118.x. [DOI] [PubMed] [Google Scholar]
- LIU Y., GRAHAM C., PARRAVICINI V., BROWN M.J., RIVERA J., SHAW S. Protein kinase C theta is expressed in mast cells and is functionally involved in Fcepsilon receptor I signaling. J. Leukocyte Biol. 2001;69:831–840. [PubMed] [Google Scholar]
- MEKORI Y.A., METCALFE D.D. Mast cells in innate immunity. Immunol. Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., JR. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998;439:175–182. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., JR., DRZEWIECKI G. Naturally occurring flavonoids and human basophil histamine release. Int. Arch. Allergy Appl. Immunol. 1985;77:155–157. doi: 10.1159/000233771. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., JR., KANDASWAMI C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E., JR., KANDASWAMI C., THEOHARIDES T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- MILLER A.L. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern. Med. Rev. 2001;6:20–47. [PubMed] [Google Scholar]
- NAMGOONG S.Y., SON K.H., CHANG H.W., KANG S.S., KIM H.P. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1994;54:313–320. doi: 10.1016/0024-3205(94)00787-x. [DOI] [PubMed] [Google Scholar]
- OSTRAKHOVITCH E.A., AFANAS'EV I.B. Oxidative stress in rheumatoid arthritis leukocytes: suppression by rutin and other antioxidants and chelators. Biochem. Pharmacol. 2001;62:743–746. doi: 10.1016/s0006-2952(01)00707-9. [DOI] [PubMed] [Google Scholar]
- PEARCE F.L., BEFUS A.D., BIENENSTOCK J. Mucosal mast cells. III. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J. Allergy Clin. Immunol. 1984;73:819–823. doi: 10.1016/0091-6749(84)90453-6. [DOI] [PubMed] [Google Scholar]
- SAGI-EISENBERG R., LIEMAN H., PECHT I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukemia cells. Nature. 1985;313:59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., EBISAWA M., TACHIMOTO H., SHICHIJO M., FUKAGAWA K., MATSUMOTO K., IIKURA Y., AWAJI T., TSUJIMOTO G., YANAGIDA M., UZUMAKI H., TAKAHASHI G., TSUJI K., NAKAHATA T. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J. Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- SCHROETER H., HOLT R.R., OROZCO T.J., SCHMITZ H.H., KEEN C.L. Nutrition: milk and absorption of dietary flavanols. Nature. 2003;426:787–788. doi: 10.1038/426787b. [DOI] [PubMed] [Google Scholar]
- SHAOHENG H.E., GACA M.D.A., WALLS A.F. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J. Pharmacol. Exp. Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- SHICHIJO M., INAGAKI N., NAKAI N., KIMATA M., NAKAHATA T., SERIZAWA I., IIKURA Y., SAITO H., NAGAI H. The effects of anti-asthma drugs on mediator release from cultured human mast cells. Clin. Exp. Allergy. 1998;28:1228–1236. doi: 10.1046/j.1365-2222.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S.H., TOGNETTO M., AMADESI S., ENNES H.S., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G.H., MITCHELL S.E., WILLIAMS L.M., GEPPETTI P., MAYER E.A., BUNNETT N.W. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- TACHIMOTO H., EBISAWA M., HASEGAWA T., KASHIWABARA T., RA C., BOCHNER B., MIURA K., SAITO H. Reciprocal regulation of cultured human mast cell cytokine production by IL-4 and IFN-γ. J. Allergy Clin. Immunol. 2000;106:141–149. doi: 10.1067/mai.2000.107043. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI A., CAMACHO P., LECHLEITER J.D., HERMAN B. Measurement of intracellular calcium. Physiol. Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- THEOHARIDES T.C. Dietary supplements for arthritis and other inflammatory conditions: key role of mast cells and benefit of combining anti-inflammatory and proteoglycan products. Eur. J. Inflamm. 2003;1:1–8. [Google Scholar]
- THEOHARIDES T.C., ALEXANDRAKIS M., KEMPURAJ D., LYTINAS M. Anti-inflammatory actions of flavonoids and structural requirements for new design. Int. J. Immunopathol. Pharmacol. 2001;14:119–127. [PubMed] [Google Scholar]
- THEOHARIDES T.C., COCHRANE D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- TORU H., EGUCHI M., MATSUMOTO R., YANAGIDA M., YATA J., NAKAHATA T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–195. [PubMed] [Google Scholar]
- TRNOVSKY J., LETOURNEAU R.J., HAGGAG E., BOUCHER W., THEOHARIDES T.C. Quercetin-induced expression of rat mast cell protease II and accumulation of secretory granules in rat basophilic leukemia cells. Biochem. Pharmacol. 1993;46:2315–2326. doi: 10.1016/0006-2952(93)90623-5. [DOI] [PubMed] [Google Scholar]
- TRUSHIN S.A., PENNINGTON K.N., CARMONA E.M., ASIN S., SAVOY D.N., BILLADEAU D.D., PAYA C.V. Protein kinase Calpha (PKCalpha) acts upstream of PKCtheta to activate IkappaB kinase and NF-kappaB in T lymphocytes. Mol. Cell. Biol. 2003;23:7068–7081. doi: 10.1128/MCB.23.19.7068-7081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI S.-H., LIANG Y.-C., LIN-SHIAU S.-Y., LIN J.-K. Suppression of TNFα- mediated NFκB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J. Cell. Biochem. 1999;74:606–615. [PubMed] [Google Scholar]
- WEDEMEYER J., TSAI M., GALLI S.J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- YAMADA K., SHOJI K., MORI M., UEYAMA T., MATSUO N., OKA S., NISHIYAMA K., SUGANO M. Structure–activity relationship of polyphenols on inhibition of chemical mediator release from rat peritoneal exudate cells. In vitro Cell Dev. Biol. Anim. 1999;35:169–174. doi: 10.1007/s11626-999-0020-x. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI M., SAYAMA K., YANO K., LANTZ C.S., NOBEN-TRAUTH N., COSTA J.J., GALLI S.J. IgE enhances Fcɛreceptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fcɛ receptor I expression and mediator release. J. Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- YANAGIDA M., FUKAMACHI H., OHGAMI K., KUWAKI T., ISHII H., UZUMAKI H., AMANO K., TOKIWA T., MITSUI H., SAITO H., LIKURA Y., ISHIZAKA T., NAKAHATA T. Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]