Abstract
Phorbol esters and adenosine modulate transmitter release from frog motor nerves through actions at separate sites downstream of calcium entry. However, it is not known whether these agents have calcium-independent sites of action. We therefore characterised calcium independent miniature endplate potentials (mepps) generated in response to 4-aminoquinaldine (4-AQA) and then compared the modulation of these mepps by phorbol esters and adenosine with that of normal calcium dependent mepps.
Application of 30 μM 4-AQA resulted in the appearance of a population of mepps with amplitudes greater than twice the total population mode (mepp>2M). In the presence of 4-AQA, K+ depolarisation or hypertonicity increased the numbers of normal amplitude mepps (meppN) but had no effect on the frequency of mepp>2M events, suggesting that mepp>2M are not dependent on calcium.
Treatment with the botulinum toxin (Botx) fractions C, D, or E (which selectively cleave syntaxin, synaptobrevin and SNAP-25, respectively) produced equivalent reductions in both normal and 4-AQA induced mepps, suggesting that both mepp populations have equal dependence on the intact SNARE proteins.
Phorbol dibutyrate (PDBu, 100 nM) increased the frequencies of both populations of mepps recorded in the presence of 4-AQA. Adenosine (25 μM) selectively reduced the numbers of meppN with no effect on the frequency of mepp>2M events.
These results suggest that mepp>2M events released in response to 4-AQA are dependent on intact forms of syntaxin, synaptobrevin and SNAP-25, but unlike meppN are independent of a functional calcium sensor. The selective action of adenosine, to reduce the numbers of normal amplitude mepps without effecting the frequency of mepp>2M events, suggests that adenosine normally inhibits transmitter release through a mechanism that is dependent on the presence of a functional calcium sensor.
Keywords: Ach, neurotransmitter release, neuromuscular transmission
Introduction
Studies of neurotransmitter release at the neuromuscular junction have helped shape our understanding of the means by which transmitter release can be modulated downstream of calcium entry (for a review, see Silinsky, 1985). At the frog neuromuscular junction it has now been established that the inhibitory effects of adenosine on neurotransmitter release and the increases in release produced by phorbol esters occur at distinct sites of action downstream of calcium entry (Silinsky, 1980; 1981; 1984; Redman et al., 1997; Betz et al., 1998; Robitaille et al., 1999; Searl & Silinsky, 2003). Specifically at the frog neuromuscular junction, phorbol esters are believed to act on the priming process through actions on Munc-13 (Betz et al., 1998; Brose & Rosenmund, 2002; Silinsky & Searl, 2003; for alternative mechanisms see e.g. Stevens & Sullivan, 1998), whereas adenosine receptor activation is believed to exert its actions on a late (postpriming) stage in the release process, presumably through an interaction with one or more members of the core complex proteins, that is, the SNAREs or their associated calcium sensors (Searl & Silinsky, 2003).
Given that both priming by phorbol esters, and the actions of adenosine receptor agonists, are independent of calcium entry at the amphibian neuromuscular junction, it is of interest to determine if phorbol ester and adenosine are able to modulate forms of neurotransmitter release that are themselves calcium independent.
In the 1980s, Molgo, Thesleff and co-workers found that application of 4-aminoquinoline to neuromuscular preparations resulted in the appearance of a new population of unusually large amplitude ‘giant' miniature endplate potentials (Molgo et al., 1982; Thesleff & Molgo 1983; Thesleff et al., 1983; Lupa et al., 1986). These giant miniature endplate potentials (mepps), which had longer rise times and more variable amplitude profiles than normal mepps, were found to have little or no calcium dependence. Owing to the lack of a supply for 4-aminoquinoline, we decided to use the more readily available methyl analogue 4-aminoquinaldine (4-amino-2-methylquinoline, 4-AQA) in the hope that this substitution might provide us with a similar population of giant mepps and allow us to determine if giant mepps are primed by phorbol esters and inhibited by adenosine in a manner similar to normal mepps. Such an approach would provide us with insights both into the basic mechanisms required for vesicle exocytosis at the frog neuromuscular junction, and also into the mechanisms by which adenosine and phorbol esters act at this synapse.
Methods
General
Frogs (Rana pipiens) were killed by anaesthesia with 5% ether, followed by double pithing, in accordance with guidelines laid down by our institutional animal welfare committee. Isolated cutaneous–pectoris nerve–muscle preparations were used in all experiments. Intracellular recordings were made using microelectrodes filled with 3 M KCl with resistances 3–10 MΩ. The signal from the microelectrode was fed into a conventional high-input impedance microelectrode preamplifier (Axoclamp-2A, Axon Instruments), the signal was then passed from the axoclamp through an AC coupled filter with a 10 kHz low pass filter. Responses were fed into a personal computer using either a Digidata 1200 or TL1-125 A/D converter (Axon Instruments) and digitised at a 0.1 ms sampling rate. Solutions were delivered by superfusion with a peristaltic pump and removed by vacuum suction. All experiments were carried out at room temperature (22–24°C).
Normal recording solutions contained (mM): NaCl, 115; KCl, 2; HEPES, 2; and CaCl2, 1.8 mM. Records of mepps were made (418.5 s recording duration) and analysed using CDR and SCAN programs (DOS versions, Strathclyde University Value-Packed Software; John Dempster). Amplitude histograms with a 0.1 mV bin width were generated from the mepp amplitudes. In order to determine the effects of the agents used on the two classes of mepps, giant mepps and ‘normal' mepps, it was necessary to introduce an arbitrary classification of the mepp population. From the histograms generated we determined the mode of the mepp distribution. We classified all mepps with an amplitude less than or equal to twice to mode as representing ‘normal mepps'. We then counted the number of events with amplitudes greater than twice the mode, as a measure of the giant mepps frequency.
Botulinum toxin treatments
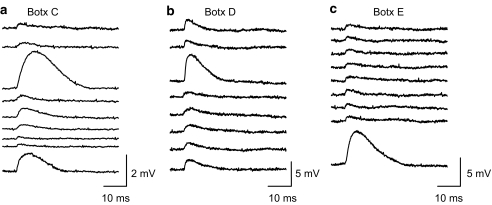
For experiments with botulinum toxins (Botxs), preparations were incubated with the appropriate fraction of Botx at a concentration of 13 μg ml−1 for a period of 2 h. The preparations were washed in fresh Ringer's solution and kept refrigerated overnight and then pinned out in the recording chamber. The nerve trunk was stimulated, until all evoked epp activity was abolished. The drug 4-AQA was then added to the preparation and mepp activity was recorded from 10 endplate regions of the muscle. For each impalement, endplate activity was recorded for 418.5 s. Owing to the very low numbers of events recorded after treatment with the Botxs, it was not practical to categorise mepps using histograms. We therefore categorised these events ‘by eye' (see Figure 4). With respect to the choice of Botx fractions used, it should be noted that Botx A cleaves only a small nine amino-acid segment of SNAP-25, and might allow some form of compromised release process to take place. In this regard it has been shown that in mammalian preparations, treatment with Botx A causes the production of calcium insensitive giant mepps, although this effect is not seen in frog neuromuscular preparations (Lupa & Yu, 1986). For these reasons, we used Botx fraction E instead of A to produce a more effective disruption of SNAP-25.
Figure 4.
Botulinum toxins C, D and E reduce the frequencies of both normal and giant mepps in the presence of 4-AQA (30 μM). All three fractions of botulinum toxins reduced mepp release to very low frequencies. Examples of individual mepps recorded in the presence of Botulinum toxin C, D, and E are shown in (a), (b), and (c) respectively. In (a) (Botx C), the examples of mepps were drawn from three separate endplates, due to the very low frequency of occurrence. The examples shown in (b) (Botx D) and (c) (Botx E) were each drawn from single endplates, which had higher than normal numbers of events. Abnormally large events can be discerned in each case. In Botx C, giant mepps made up approximately 20% of all events; in Botx D, giant mepps made up approximately 5% of all events; and in Botx E, giant mepps made up approximately 14% of all events. Overall, the number of giant mepps relative to the number of normal mepps is similar to that seen in the absence of Botx treatment (see text for more details).
Statistical methods
Data are presented as the mean±s.e.m. In most cases, the data groups were first tested for normality and then tested for significance using one-way analysis of variance (Sigma Stat, Jandel Scientific Inc.). A Student's t-test was then used to compare individual groups. Differences between groups were considered significant when P<0.05, but in some cases further statistical details are provided. Unless otherwise stated, n represents the number of single experiments carried out at single endplates on individual preparations.
Drugs
Botx fractions were obtained from Wako Chemicals U.S.A., Inc. All other drugs used in this study were obtained from Sigma, St Louis, U.S.A. A concentration of 30 μM 4-AQA was used in all the experiments presented here.
Results
General characteristics of giant mepps induced by 4-AQA (30 μM)
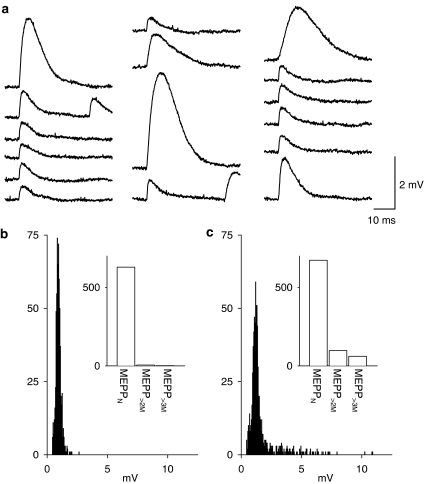
We first wished to determine if 4-AQA does induce giant mepps. As shown here, application of 4-AQA (30 μM) resulted in the appearance of large amplitude spontaneous events with unusual shapes, when compared with the control condition (Figure 1). These mepps are similar to those previously described by Molgo, Thesleff and co-workers as generated by 4-aminoquinoline (Tabti et al., 1986). Examples of the mepps recorded in the presence of 4-AQA (30 μM) are shown in Figure 1a, together with normal mepps. Amplitude histograms of mepps recorded in 4-AQA exhibited a skewed distribution with a ‘tail' of larger than normal amplitude events (see Figure 1b and c).
Figure 1.
Induction of giant mepps by 4-AQA (30 μM). (a) Consecutive examples of the mepps recorded following 15 min in the presence of 4-AQA. (b, c) Amplitude histograms of the mepps recorded from this endplate (b) before and (c) following 15 min in the presence of 30 μM 4-AQA. Insets show the same data expressed as column graphs with the number of normal mepps with amplitudes less than twice the mode (meppN) and the number of mepps with amplitudes greater than twice the mode mepp>2M and the number of mepps with amplitudes greater than three times the mode (mepp>3M) plotted, providing a further measure of the distribution of the giant mepps. The data shown were obtained from a single endplate; each recording period was 418.5 s in duration; Vm=−96 mV.
In control, the frequency of normal amplitude ‘meppN' defined as those having an amplitude equal or less than twice the mode (as measured from the amplitude histograms) was 1.21±0.195 Hz (n=6) and the numbers of events with an amplitude greater than two times the mode, mepp>2M was 0.021±0.008 Hz (n=6). Following 15 min in the presence of 30 μM 4-AQA, the frequency of meppN was 1.573±0.279 Hz and the frequency of mepp>2M was 0.324±0.077 Hz (n=6).
These events were unaffected by the application of either 3 μM TTX or 100 nM ω-conotoxin GVIA (data not shown here), but were reduced by low concentrations of (+)-tubocurarine in a graded fashion, suggesting that these mepps>2M result from the activation of nicotinic receptors. Specifically, application of 0.36 μM tubocurarine resulted in a reduction in the overall mean mepp amplitude from 2.71±0.8 to 1.01±0.15 mV (n=4). The size of the largest detected mepp (recorded over a 418.5 s period) was reduced from 14.55±2.80 to 5.25±0.21 mV (n=4). Following application of 2 μM tubocurarine, no spontaneous mepps were detectable (n=3).
The calcium independence of giant mepps induced by 4-AQA (30 μM)
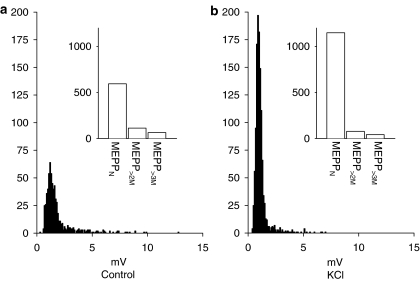
In order to determine whether these giant mepps were calcium independent, we investigated the effect of depolarisation by K+ on mepp frequencies recorded in the presence of 4AQA (see Figure 2). Increasing the KCl concentration from 3 to 8 mM resulted in a depolarisation of the muscle (18.6±2.0 mV; n=5) and an increase in mepp frequencies. The number of meppN was increased to 296.4±57% of control (n=5). In contrast, the numbers of events with an amplitude greater than two times the mode, mepp>2M were unaffected by KCl being 92.6±6.4% of control (n=5; P=0.92).
Figure 2.
Effect of K+ depolarisation on normal and giant mepps in the presence of 4-AQA (30 μM). Control (a) shows the amplitude distributions of mepps recorded in the presence of 30 μM 4-AQA in normal Ringer solution (3 mM KCl; Vm=−96 mV). (b) Effects of depolarisation by 8 mM K+ on the mepp amplitude distribution (in the continued presence of 30 μM 4-AQA; Vm=−73 mV). Insets show the same data expressed as column graphs. K+ depolarisation selectively increased the number of normal amplitude mepps. The data shown were obtained from a single endplate; each recording period was 418.5 s in duration.
Despite the differential acceleration of the frequencies of normal mepps by the Ca2+ influx produced by K+ depolarisation, the effects of the KCl depolarisation on mepp amplitudes were equivalent for both classes of mepps (P=0.85) with each class being reduced to approximately 73–74% of their amplitudes in normal KCl (meppN, 74.1+2.5%; mepp>2M, 73.2+4.0% of control amplitudes, n=5). Thus, while the numbers of giant mepps is unaffected by K+ depolarisation their amplitudes are reduced to a similar extent as the ‘normal mepps', suggesting that the postsynaptic nicotinic receptors they activate have similar biophysical properties. Giant mepps represented 18.4±0.04% of the total mepp population in control KCl solution; in 8 mM KCl solution they represented 8.6±2.8% of the total population (n=5).
The effect of hypertonicity on giant mepps induced by 4-AQA (30 μM)
Previously, Molgo, Thesleff and co-workers (Thesleff et al., 1983; Thesleff & Molgo, 1983) found that hypertonic solutions reduced giant mepp frequency, while normal mepps were increased in frequency, at the rat neuromuscular junction. In order to determine if a similar effect of tonicity occurred with giant mepps induced by 4-AQA in the frog, we tested the effects of 40 mM sucrose on mepps following treatment with 4-AQA (Figure 3).
Figure 3.
Effect of sucrose (40 mM) on normal and giant mepps in the presence of 4-AQA (30 μM). mepp amplitude histograms recorded in the presence of 30 μM 4-AQA before (a, control) and after application of 40 mM sucrose (b) are shown. Insets show the same data expressed as column graphs. As shown, sucrose selectively increased the number of normal amplitude mepps. The data shown were obtained from a single endplate; each recording period was 418.5 s in duration; Vm=−86 mV.
In 4AQA solutions, overall mepp frequencies were 1.42±0.3 Hz (n=4). The frequency of meppN was 1.02±0.2 Hz and the frequency of mepp>2M was 0.40±0.12 Hz. Application of 40 mM sucrose in the presence of 4AQA increased the overall mepp frequencies by 217±30%. However, this overall increase in mepp frequencies was solely as a result of an increase in meppN (see Figure 3b, inset). Specifically, the meppN were increased in numbers by 262±33% of control; whereas mepp>2M were not significantly changed in frequency, being 89±11% of control (P=0.3). As a result, the ratio of giant mepps as a proportion of the total mepp population fell from 26.6±4.7% in control to 12.3±1.2% in sucrose solution (n=4). Thus, the giant mepps induced by 4-AQA are not increased in frequency by moderate hypertonic solutions.
The effect of Botx fractions C, D and E on giant mepps induced by 4-AQA (30 μM)
We then employed Botxs in order to address two issues related to the generation and release of the calcium insensitive mepps. Firstly, given the unusual characteristics of the giant mepps stimulated by 4-AQA, it seemed possible that they might utilise a different release mechanism from that of normal mepps. Thus, the use of Botx fractions that selectively cleave the core components of the secretory apparatus, namely the SNARES (syntaxin, synaptobrevin and SNAP-25) (see Sutton et al., 1998; Gerst, 1999), should demonstrate if those core complex elements are necessary for release of the calcium insensitive mepps. If this is the case, the release of mepps>2M would be reduced to a similar extent as the meppN population. Secondly, these experiments should determine whether the generation of giant mepps results from the impaired recycling of synaptic vesicles as has been previously suggested (Rizzoli & Betz, 2002). Thus, following paralysis of the muscle by Botx where the release of neurotransmitter and the concomitant recycling of synaptic vesicles were greatly reduced as to be negligible, it might be predicted that no giant mepps would be seen in response to 4-AQA.
Botx C
Botx C cleaves syntaxin between K253 and A254 near the transmembrane region of this intrinsic presynaptic membrane SNARE (Jahn et al., 1995; Raciborska et al., 1998). As expected, treatment with Botx C resulted in a block in nerve-evoked transmitter release. Application of 4-AQA resulted in the release of a greatly reduced number of giant mepps (see Figure 4a). In total, 20 spontaneous events were observed out of 40 individual endplates recorded from four separate preparations. Out of these events, four might be tentatively be classified as giant events and 16 as normal events (see Methods). The overall frequency of mepps was 0.0011±0.0006 Hz (n=4). These results suggest that syntaxin is necessary for the release of giant mepps.
Botx D
Botx D cleaves the vesicle SNARE, synaptobrevin (also known as VAMP) between K59 and L60 (Jahn et al., 1995; Raciborska et al., 1998). Treatment with Botx D also resulted in a block in nerve-evoked transmitter release. As with Botx C treatment, application of 4-AQA resulted in a very low frequency of giant mepps. In total, 142 spontaneous events were observed out of 40 individual endplates recorded from four separate preparations. Out of these events, seven might be tentatively be classified as giant events and the remaining 135 as normal events (see Figure 4b). The overall frequency of mepps was 0.0085±0.0054 Hz (n=4). These results suggest that synaptobrevin is also necessary for the release of giant mepps.
Botx E
Botx E acts on SNAP-25 (a SNARE that is loosely associated with the plasma membrane by palmitoylation), cleaving a 26 amino-acid segment between R180 and I181 (Jahn et al., 1995; Raciborska et al., 1998). Treatment with Botx E also resulted in a block in nerve-evoked transmitter release. As with the other Botx treatments, application of 4-AQA resulted in the appearance of a small number of giant mepps. In total, 167 spontaneous events were observed out of 40 individual endplates recorded from four separate preparations. Out of these events, 23 might be tentatively be classified as giant events and the remaining 144 as normal events (see Figure 4c). It should be noted that the majority of the spontaneous events (163) were observed in just one of the Botx E treated preparations. The overall frequency of mepps was 0.0099±0.0096 Hz (n=4). These results suggest that SNAP-25 is also necessary for the release of giant mepps.
In the absence of Botx treatment, the proportion of giant mepps as a percentage of the overall mepps recorded in the presence of 4-AQA was 14.5±3.9% (n=9 preparations), with values ranging from 3.1 to 44.5%. When the mepps are combined from all Botx treated preparations, the proportion of giant mepps is 12.9±4.3%. Thus, the proportion of giant to normal mepps recorded in 4AQA following Botx treatments is similar to that seen in the absence of Botx treatment (n=12 preparations, P=0.79).
These experiments with the Botx fractions C, D and E thus demonstrate that the release of both normal mepps and the giant mepps stimulated by 4-AQA have a similar dependence on the presence of intact forms of each of the three SNARE proteins: syntaxin, synaptobrevin and SNAP-25.
The effect of phorbol esters on the giant mepps induced by 4-AQA (30 μM)
The failure of either K+ depolarisation or hypertonicity to increase the frequency of giant mepps suggests that their release is independent of nerve terminal calcium entry. We therefore decided to determine if phorbol esters, which are thought to act by increasing the numbers of primed vesicles (see Brose & Rosenmund, 2002; Silinsky & Searl, 2003), were able to increase the frequency of giant mepps as well as the frequency of normal mepps.
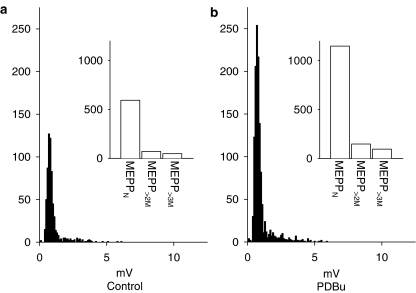
The application of phorbol dibutyrate (PDBu) (100 nM) to preparations treated with 4-AQA resulted in an increase in the numbers of all mepps (Figure 5). Normal mepps were increased in numbers by 208.6±14.4% of control while mepp>2M were increased in frequency by 236.8±35.1% of control (n=6). PDBu had no measurable effect on mepp amplitudes (meppN, 101.7±5.6; mepp>2M, 109.3±6.1% of control for mepps; P>0.05, n=6). As a result, the proportion of giant mepps as a percentage of the total mepp population was unchanged (8.3±2.6% in control; 7.7±1.3% in PDBu, n=6). Hence, priming via phorbol esters, presumably mediated through Munc-13 at the amphibian neuromuscular junction (Redman et al., 1997; Betz et al., 1998; Searl & Silinsky, 1998), occurs for both normal and calcium independent giant mepps.
Figure 5.
Effect of PDBu (100 nM) on normal and giant mepps in the presence of 4-AQA (30 μM). mepp amplitude histograms recorded in the presence of 30 μM 4-AQA before (a, control) and after application of 100 nM PDBu (b) are shown. Insets show the same data expressed as column graphs. As shown, PDBu increased the number of all mepps. The data shown were obtained from a single endplate; each recording period was 418.5 s in duration; Vm=−86 mV.
The effect of adenosine on the giant mepps induced by 4-AQA (30 μM)
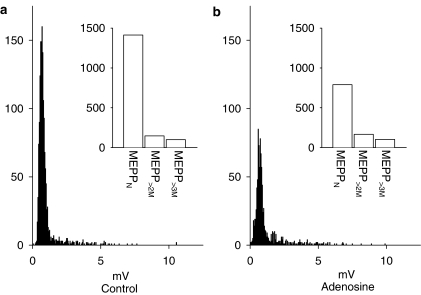
Adenosine has also been shown to work downstream of calcium entry at the frog neuromuscular junction (Silinsky, 1981; 1984; Robitaille et al., 1999) through a mechanism that is independent of the action of phorbol esters (Searl & Silinsky, 2003). It is believed that the activation of A1 adenosine receptors leads to inhibition of ACh release at a late postpriming stage in the release process (Silinsky, 1984; Searl & Silinsky, 2003). As shown in Figure 6, application of 25 μM adenosine produced the expected reduction in the numbers of meppN (52.8±5.8%; P=0.005, n=7). In contrast, adenosine had no significant effect on the frequencies of giant mepps, mepp>2M frequencies were 89.1±7.9% of control (P=0.78, n=7; see Figure 6). As a result, the proportion of giant mepps as a percentage of the total mepp population increased from 14.5±3.9% in control to 19.3+3.0% in adenosine. The effects of adenosine on the mepp frequencies were reversible on wash into adenosine free solution (meppN, 106±8.8%; mepp>2M, 103±8.7% of control, n=7). As might be expected, adenosine had no effect on the mean amplitudes of the two classes of mepps (meppN, 101.5±5.1%; mepp>2M 105.5±5.1% of control amplitudes, P⩾0.05). These results demonstrate a differential sensitivity of normal mepps and giant mepps to adenosine.
Figure 6.
Effect of adenosine (25 μM) on normal and giant mepps in the presence of 4-AQA (30 μM). mepp amplitude histograms recorded in the presence of 30 μM 4-AQA before (a, control) and after application of 25 μM adenosine (b) are shown. Insets show the same data expressed as column graphs. As shown, adenosine selectively reduces the number of normal amplitude mepps without affecting those with amplitudes greater than twice the mode. The data shown were obtained from a single endplate; each recording period was 418.5 s in duration; Vm=−103 mV.
Discussion
The results we present here mirror those of Molgo, Thesleff and co-workers (Molgo et al., 1982; Thesleff & Molgo 1983; Thesleff et al., 1983), who found that application of the structually related compound 4-aminoquinoline resulted in the appearance of a similar population of giant mepps insensitive to changes in intraterminal Ca2+ concentration. The exact nature and origin of giant mepps at the skeletal neuromuscular junction is unclear. It has recently been suggested that giant mepps might result from impaired processing of recycled synaptic vesicles (Rizzoli & Betz, 2002). However, this explanation does not hold for 4-AQA, which induced giant mepps under conditions where the levels of neurotransmitter release were very low (i.e. following treatment with Botx C, D or E) and after relatively short time periods (5–10 min).
Interestingly, the structurally similar compound tacrine, an agent used to treat Alzheimer's disease, also generates giant mepps at the skeletal neuromuscular junction (Thesleff et al., 1990). Tacrine has major effects on subcellular elements of hepatocytes including degranulation and vesiculation of the endoplasmic reticulum and increases in the cellular lysosome content (Monteith & Theiss, 1996; Plymale & De La Iglesia, 1999). While the molecular mechanism by which tacrine exerts these effects is unknown, it is tempting to speculate that the 4-AQA derivatives produce similar effects in nerve terminals and this is the source of giant mepps.
As reported here, the giant mepps induced by 4-AQA were calcium insensitive, such that increases in intraterminal [Ca2+] caused by K+ depolarisation increased the number of normal mepps but had no effect on the frequency of giant mepps (see Table 1). This suggests that the release of giant mepps is not subject to control by the same calcium sensor as are the normal mepps. The lack of calcium sensitivity of giant mepps previously led to the suggestion that giant mepps might represent a form of constitutive exocytosis (Sellin et al., 1986), lacking synaptotagmins as a component of the release machinery (Morimoto et al., 1995).
Table 1.
Summary of the effects of various agents and treatments on the frequency of normal mepps (meppN) and giant mepps (mepp>2M) recorded in the presence of 30 μM 4-AQA
| meppN | mepp>2M | |
|---|---|---|
| K+ depolarisation (8 mM) | ↑↑ | ← → |
| Sucrose (40 mM) | ↑↑ | ← → |
| Botx C | ↓↓↓ | ↓↓↓ |
| Botx D | ↓↓↓ | ↓↓↓ |
| Botx E | ↓↓↓ | ↓↓↓ |
| Phorbol dibutyrate (100 nM) | ↑ | ↑ |
| Adenosine (25 μM) | ↓ | ← → |
Symbols: ↓, decreases mepp frequency; ↑, increases mepp frequency; ← →, mepp frequency does not change. The number of arrows that represent increases or decreases in mepp frequency reflect the relative order of magnitude of the change in mepp frequency.
We also found that a moderate increase in tonicity, provided by 40 mM sucrose, selectively increased normal mepp frequency, without affecting the giant mepp population (see Table 1). This might seem surprising, as it has been suggested that hypertonic solutions evoke release through a calcium independent mechanism (Stevens & Sullivan, 1998). However, it has recently been shown that the calcium sensor protein synaptotagmin 1 is necessary for the hypertonic stimulation of mepp release at the Drosophila neuromuscular junction (Kidokoro, 2003), suggesting that increases in spontaneous release in response to hypertonic solutions might be dependent on the presence of a functional calcium sensor as a component of the release apparatus.
Given the unusual release characteristics of the giant mepp population induced by AQA, it seemed possible that the giant mepps utilised a release mechanism that was dependent on a different set of SNAREs than normal synaptic vesicles. We tested this using three fractions of Botx, which are known to selectively cleave individual components of the core complex, namely syntaxin (Botx C), synaptobrevin (Botx D) and SNAP-25 (Botx E). Each fraction of Botx tested, individually inhibited the secretion of both normal mepps and giant mepps induced by 4-AQA equally (see Table 1). This suggests that intact forms of syntaxin, synaptobrevin and SNAP-25 are all essential components of the giant mepp release machinery. Thus, the only apparent difference in the release machinery controlling giant mepps compared with normal mepps relates to the presence of a functional calcium sensor.
In contrast to the selective increase in normal mepp frequencies produced by K+ depolarisation and sucrose, we found that application of PDBu resulted in an increase in the frequencies of all mepps (both normal amplitude and giant mepps). This suggests that both giant mepps and normal mepps undergo a similar Munc-13 dependent priming mechanism in order for them to be available for release (Betz et al., 1998; Searl & Silinsky, 1998). This is further supported by the observation that Botx C reduced the numbers of giant mepps induced by 4-AQA in proportion to the reduction in normal mepps, which suggests that syntaxin (which works in consort with Munc-13 as a major determinant of priming) is essential for the release of giant mepps. Furthermore, the lack of effect of PDBu on the amplitudes of the giant mepps argues against them being the result of multiquantal releases, but rather suggests they are a truly a univesicular release form.
Adenosine failed to reduce either the numbers of giant mepps or their amplitudes, but did reduce the frequencies of meppN. This suggests that the release machinery of the calcium independent giant mepps either lacks the component that is responsible for the inhibition of release caused by adenosine, or alternatively that component is nonfunctional. It might also be tentatively suggested that either synaptotagmin or a related calcium sensor molecule may act as the end target mediating the action of adenosine at the frog neuromuscular junction.
Acknowledgments
We thank Ms Shirley Foster for her technical assistance with the botulinum toxin treatments, and Dr Stanley M. Parsons for the suggestion of 4-aminoquinaldine substitution. This work was supported by a grant from the NIH (NS 12782).
Abbreviations
- 4-AQA
4-aminoquinaldine
- Botx
botulinum toxin
- mepps
miniature endplate potentials
- mepp>2M
mepps with amplitudes greater than twice the mode
- meppN
normal amplitude mepps
- PDBu
phorbol dibutyrate
References
- BETZ A., ASHERY U., RICKMANN M., AUGUSTIN I., NEHER E., SUDHOF T.C., RETTIG J., BROSE N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- BROSE N., ROSENMUND C. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- GERST J.E. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol. Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHN R., HANSON P.I., OTTO H., AHNERT-HILGER G. Botulinum and tetanus neurotoxins: emerging tools for the study of membrane fusion. Cold Spring Harbor Symp. Quant. Biol. 1995;LX:329–335. doi: 10.1101/sqb.1995.060.01.037. [DOI] [PubMed] [Google Scholar]
- KIDOKORO Y. Roles of SNARE proteins and synaptotagmin I in synaptic transmission: studies at the Drosophila neuromuscular synapse. NeuroSignals. 2003;12:13–30. doi: 10.1159/000068912. [DOI] [PubMed] [Google Scholar]
- LUPA M.T., TABTI N., THESLEFF S., VYSKOCIL F., YU S.P. The nature and origin of calcium-insensitive miniature end-plate potentials at rodent neuromuscular junctions. J. Physiol. 1986;381:607–618. doi: 10.1113/jphysiol.1986.sp016346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUPA M.T., YU S.P. A comparison of miniature end-plate potentials at normal, denervated, and long-term botulinum toxin type A poisoned frog neuromuscular junctions. Pflugers Archiv. 1986;407:476–481. doi: 10.1007/BF00657503. [DOI] [PubMed] [Google Scholar]
- MOLGO J., GOMEZ S., POLAK R.L., THESLEFF S. Giant miniature endplate potentials induced by 4-aminoquinoline. Acta Physiol. Scand. 1982;115:201–207. doi: 10.1111/j.1748-1716.1982.tb07066.x. [DOI] [PubMed] [Google Scholar]
- MONTEITH D.K., THEISS J.C. Comparison of tacrine-induced cytotoxicity in primary cultures of rat, mouse, monkey, dog, rabbit, and human hepatocytes. Drug Chem. Toxicol. 1996;19:59–70. doi: 10.3109/01480549609002196. [DOI] [PubMed] [Google Scholar]
- MORIMOTO T., POPOV S., BUCKLEY K.M., POO M.M. Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron. 1995;15:689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- PLYMALE D.R., DE LA IGLESIA F.A. Acridine-induced subcellular and functional changes in isolated human hepatocytes in vitro. J. Appl. Toxicol. 1999;19:31–38. doi: 10.1002/(sici)1099-1263(199901/02)19:1<31::aid-jat535>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- RACIBORSKA D.A., TRIMBLE W.S., CHARLTON M.P. Presynaptic protein interactions in vivo: evidence from botulinum A, C, D and E action at neuromuscular junctions. Eur. J. Neurosci. 1998;10:2617–2628. doi: 10.1046/j.1460-9568.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- REDMAN R.S., SEARL T.J., HIRSH J.K., SILINSKY E.M. Opposing effects of phorbol esters on transmitter release and calcium currents at frog motor nerve endings. J. Physiol. 1997;501:41–48. doi: 10.1111/j.1469-7793.1997.041bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZZOLI S.O., BETZ W.J. Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction. J. Neurosci. 2002;22:10680–10689. doi: 10.1523/JNEUROSCI.22-24-10680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBITAILLE R., THOMAS S., CHARLTON M.P. Effects of adenosine on Ca2+ entry in the nerve terminal of the frog neuromuscular junction. Can. J. Physiol. Pharmacol. 1999;77:707–714. [PubMed] [Google Scholar]
- SEARL T.J., SILINSKY E.M. Increases in acetylcholine release produced by phorbol esters are not mediated by protein kinase C at motor nerve endings. J. Pharmacol. Exp. Ther. 1998;285:247–251. [PubMed] [Google Scholar]
- SEARL T.J., SILINSKY E.M. Phorbol esters and adenosine affect the readily releasable neurotransmitter pool by different mechanisms at amphibian motor nerve endings. J. Physiol. 2003;553:445–456. doi: 10.1113/jphysiol.2003.051300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLIN L.C., MOLGO J., TORNQUIST K., HANSSON B., THESLEFF S. On the possible origin of giant or slow-rising miniature end-plate potentials at the neuromuscular junction. Pflugers Archiv. 1986;431:325–334. doi: 10.1007/BF02207269. [DOI] [PubMed] [Google Scholar]
- SILINSKY E.M. Evidence for specific adenosine receptors at cholinergic nerve endings. Br. J. Pharmacol. 1980;71:191–194. doi: 10.1111/j.1476-5381.1980.tb10925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br. J. Pharmacol. 1981;73:413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J. Physiol. 1984;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol. Rev. 1985;37:81–132. [PubMed] [Google Scholar]
- SILINSKY E.M., SEARL T.J. Phorbol esters and neurotransmitter release; more than just protein kinase C. Br. J. Pharmacol. 2003;138:1191–1201. doi: 10.1038/sj.bjp.0705213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS C.F., SULLIVAN J.M. Regulation of the readily-releasable vesicle pool by protein kinase C. Neuron. 1998;21:885–893. doi: 10.1016/s0896-6273(00)80603-0. [DOI] [PubMed] [Google Scholar]
- SUTTON R.B., FASSHAUER D., JAHN R., BRUNGER A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- TABTI N., LUPA M.T., THESLEFF S. Effects of some aminoquinolines on spontaneous quantal acetylcholine release. Acta. Physiol. Scand. 1986;127:553–555. doi: 10.1111/j.1748-1716.1986.tb07940.x. [DOI] [PubMed] [Google Scholar]
- THESLEFF S., MOLGO J. A new type of transmitter release at the neuromuscular junction. Neuroscience. 1983;9:1–8. doi: 10.1016/0306-4522(83)90041-6. [DOI] [PubMed] [Google Scholar]
- THESLEFF S., MOLGO J., LUNDH H. Botulinum toxin and 4-aminoquinoline induce a similar abnormal type of spontaneous quantal transmitter release at the rat neuromuscular junction. Brain Res. 1983;264:89–97. doi: 10.1016/0006-8993(83)91123-x. [DOI] [PubMed] [Google Scholar]
- THESLEFF S., SELLIN L.C., TAGERUD S. Tetrahydroaminoacridine (tacrine) stimulates neurosecretion at mammalian motor endplates. Br. J. Pharmacol. 1990;100:487–490. doi: 10.1111/j.1476-5381.1990.tb15834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]