Abstract
Enaminones are a novel group of compounds that have been shown to possess anticonvulsant activity in in vivo animal models of seizures. The cellular mechanism by which these compounds produce their anticonvulsant effects is not yet known. This study examined the effects of enaminones on excitatory synaptic transmission.
We studied the effects of 3-(4′-chlorophenyl)aminocyclohex-2-enone (E118), methyl 4-(4′-bromophenyl)aminocyclohex-3-en-6-methyl-2-oxo-1-oate (E139) and ethyl 4-(4′-hydroxyphenyl)aminocyclohex-3-en-6-methyl-2-oxo-1-oate (E169) on isolated evoked, glutamate-mediated excitatory synaptic responses by recording whole-cell currents and potentials in cells of the nucleus accumbens (NAc) contained in forebrain slices.
The anticonvulsant enaminones (E118 and E139), but not E169, depressed NMDA and non-NMDA receptor-mediated synaptic responses. The inhibition of the non-NMDA response was concentration-dependent (1.0–100 μM) with a maximal depression of ∼−30%. E118 and E139 had similar potencies (EC50=3.0 and 3.5 μM, respectively) in depressing this response but E139 was more efficacious (Emax=−31.3±3.8%) than E118 (Emax=−22.6±1.6%).
The excitatory postsynaptic current (EPSC) depression caused by 10 μM E139 (−27.7±3.8%) was blocked by 1 μM CGP55845 (6.3±8.1%), a potent GABAB receptor antagonist.
Pretreatment of slices with γ-vinylGABA and 1-(2-(((diphenylmethylene)imino)oxy)ethyl)-1,2,5,6-tetrahydro-3-pyridine-carboxylic acid (NO-711), an irreversible GABA transaminase (GABA-T) inhibitor and a GABA reuptake blocker, respectively, like the anticonvulsant enaminones, also caused a depression of the evoked EPSC (−38.1±14.1 and −24.1±8.9%, respectively). In the presence of these compounds, E139 did not cause a further depression of the EPSC. Our data suggest that anticonvulsant enaminones cause EPSC depression by enhancing extracellular GABA levels possibly through the inhibition of either GABA reuptake or GABA-T enzyme, or both.
Keywords: Anticonvulsant, epilepsy, enaminones, glutamate, GABA, synaptic transmission, N-methyl-D-aspartate, non-NMDA
Introduction
Epilepsy is a common neurological disorder characterized by recurrent seizures. This condition has been described since ancient times yet it still continues to afflict a substantial number of people (1–2%) in the world today (McNamara, 1999; Browne & Holmes, 2001) with no cure in sight. This may be due in part to a complex pathophysiology and/or etiology of this disorder (see reviews by McNamara, 1999; Löscher, 2002). Epilepsy is managed medically by either pharmacotherapy or surgery where this is possible. Currently available drugs have serious shortcomings as they only provide symptomatic relief and their use is often accompanied by significant adverse or side effects. Furthermore, there is a significant proportion of patients (up to 40%) who do not respond to these agents (Regesta & Tanganelli, 1999; Kwan & Brodie, 2000; Löscher, 2002). There is therefore a need for continued research into this disorder and the development of newer agents for its management (McNamara, 1999; Brodie, 2001; Löscher, 2002).
A new class of compounds, called enaminones, with a unique structure different from currently available antiepileptic drugs has recently been synthesized (Edafiogho et al., 1992). Based on their anticonvulsant activity, they have been classified into three classes (1, 2 and 3) with class 1 being the most potent in these tests (Edafiogho et al., 1994). E139, E121 and E118, all class I anticonvulsants, have shown promise in preventing seizures in mouse and rat models of epilepsy while others (e.g. E169) are inactive as anticonvulsants. Unlike conventional antiepileptic drugs, the anticonvulsant enaminones displayed no neurotoxicity such as ataxia, and had wider safety margins as determined by their higher protective indices when compared to conventional antiepileptic drugs such as valproate, carbamazepine and phenytoin (Mulzac & Scott, 1993). They may therefore present a unique opportunity to develop anticonvulsant drugs that have a superior side effect profile as well as a wider margin of safety than currently available antiepileptic drugs.
In order to understand the mechanism(s) underlying the actions of these compounds to produce anticonvulsant activity, their effects on neuronal physiology need to be clarified. Hyperexcitability of neurons at the epileptogenic focus underlies the spontaneous synchronous discharge of neurons that precipitates an epileptic seizure (McNamara, 1999). The excitability of neurons is an integral of intrinsic membrane conductances and synaptic inputs. Both excitatory and inhibitory inputs contribute to the resting excitability (Traynelis & Dingledine, 1988; McNamara, 1994). Since the role of glutamate in the pathogenesis of seizure disorders is quite well known (McNamara et al., 1988; McBain et al., 1989; Dingledine et al., 1990; Kraus et al., 1994; McNamara, 1994), we were interested to know if enaminones affected this transmitter system to produce their anticonvulsant effects.
We tested the hypothesis that enaminones may interact with glutamate and/or its receptors to produce the reported anticonvulsant effects in rats and mice (Edafiogho et al., 1992; Abou-Zeid & Edafiogho, 2002). The rationale for this was based on the possible role of glutamate in the genesis of seizures (Dingledine et al., 1990) as well as computer modeling of these compounds and crystallographic studies, which suggest that they would fit a putative glutamate receptor site (Abou-Zeid & Edafiogho, 2002; Edafiogho et al., 2003). In this study, we hereby report our findings on the effects of three enaminones on pure NMDA and non-NMDA receptor-mediated responses recorded in neurons of the nucleus accumbens (NAc).
Methods
Synthesis of enaminones
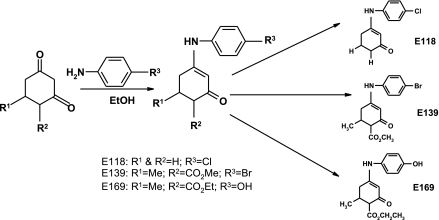
The enaminones E118, E139 and E169 were synthesized for this study according to methods reported previously (Edafiogho et al., 1992, 1994, 2003). Briefly, the condensation reaction between β-diketo intermediates and appropriate amino compounds, under carefully controlled reaction conditions, yielded the enaminones E118, E139 and E169 (Figure 1).
Figure 1.
The synthetic pathway of obtaining the enaminones used in this study. The differences in the three compounds studied are in the substituents represented by R1 to R3 on the basic enaminone structure.
Animal experiments
All the rats used in this study were obtained from the Kuwait University Animal Resource Centre. International guidelines on humane handling of animals were followed throughout this study and the minimum number of animals necessary to produce the required results was used.
Slice preparation
Parasagittal forebrain slices containing the NAc and the cortex were generated using previously published techniques (Kombian et al., 2003). Briefly, male Sprague–Dawley rats (75–150 g) were anesthetized with halothane before decapitation. The brain was quickly removed from the rat and placed in ice-cold (4°C) artificial cerebrospinal fluid (ACSF) that was bubbled with 95% O2 and 5% CO2. The composition of the ACSF was (in mM): 126 NaCl; 2.5 KCl; 1.2 NaH2PO4; 1.2 MgCl2; 2.4 CaCl2; 18 NaHCO3; 11 glucose, producing a solution with osmolarity of between 310 and 320 mosm. Thin slices (350-μm thick) were cut in the ice-cold ACSF using a Leica (VT 1000S) tissue slicer. Slices were incubated in ACSF (bubbled with 95% O2 and 5% CO2) at room temperature and allowed to recover for at least 1 h before use.
Electrophysiological recording and data acquisition
One slice was trimmed and transferred into a 500 μl capacity recording chamber and perfused submerged at a flow rate of 2–3 ml min−1 (29–31°C) with ACSF that was bubbled with 95% O2 and 5% CO2. ‘Blind patch' recordings were performed in the conventional whole-cell mode using glass electrodes with tip resistance of 4.0–8.0 MΩ. The internal recording solution had the following composition (in mM): K-gluconate (135), NaCl (8), EGTA (0.2), HEPES (10), Mg-ATP (2) and GTP (0.2). pH and osmolarity were adjusted to 7.3 (with KOH) and 270–280 mosm, respectively. Bipolar tungsten stimulating electrodes were positioned at the prefrontal cortex–accumbens border to evoke synaptic responses. Recordings were made using the Axopatch 1D amplifiers in either voltage or current clamp modes. Cells were voltage clamped at −80 mV (holding potential, Vh), and input (Rinput) and access (Ra) resistances of all cells were determined and monitored regularly throughout each experiment by applying a 75 ms, 20 mV hyperpolarizing pulse or 100 pA negative current in current clamp. All cells reported in this study had Ra of 10–30 MΩ. Data from cells that showed >15% changes in Ra during the experiment were excluded from further analysis.
All synaptic responses were recorded either as inward currents at Vh of −80 mV or depolarizing potentials at resting potential unless otherwise stated. All cells had a graded evoked response (EPSC and EPSP) to increasing stimulation intensity (ranging from 0.25 to 4.0 mA) and an intensity giving 50–60% of the maximum evoked synaptic response was used to evoke test responses. All data were acquired using pClamp Software (Clampex 7 or 8; Axon Instruments) at a sampling rate of 6.7 kHz, filtered at 1 kHz, digitized and stored for off-line analysis. Each stored trace was an average of two successive synaptic responses elicited at 10 s intervals.
Analysis and statistics
EPSC and EPSP amplitudes were measured from baseline to peak and taken as the synaptic strength at the chosen stimulus intensity. Responses were normalized by taking the mean of the last 3–4 responses prior to drug application and dividing the rest of the responses by this mean. These normalized values were then used for average plots. For these plots, all cells receiving the same treatment were aligned at the first minute of first drug application and averaged over the entire period. All values are stated as mean±standard error. One-way ANOVA and post hoc tests, as indicated in the Results section, were used to compare different values or treatments using the SigmaStat® software. Differences between groups were taken as significant at a probability level of P⩽0.05. Graphing was performed using the SigmaPlot®, GraphPad Prism® and CorelDraw® softwares.
Chemicals and solutions
All drugs were bath perfused at final concentrations indicated by dissolving aliquots of stock in the ACSF. All enaminones were synthesized in-house (see Figure 1) and were dissolved in dimethyl sulfoxide (DMSO; final concentration in bath <0.1%), aliquoted and frozen at −20°C and used within 3 weeks. Most routine laboratory chemicals as well as, DL-2-amino-5-phosphonovaleric acid (DL-APV), γ-vinyl GABA (γvG) and picrotoxin were purchased from Sigma Company (Germany). 6,7-Dinitroquinoxaline-2,3-dione (DNQX), NO-711 were obtained from RBI (U.S.A.), CGP55845 was from Tocris (U.K.).
Results
The results reported here were obtained from whole-cell recordings in 92 neurons of the rostral NAc. Out of this number, 83 neurons (∼90%) responded to enaminones with a measurable effect on synaptic transmission. The majority of cells in this region (∼95%) are medium spiny GABAergic neurons (Pennartz et al., 1994) with relatively very negative resting potentials (−72 to −90 mV). The other passive and active membrane properties of these cells were similar to those previously reported (Kombian et al., 2003).
Effects of anticonvulsant enaminones on synaptic transmission in the NAc
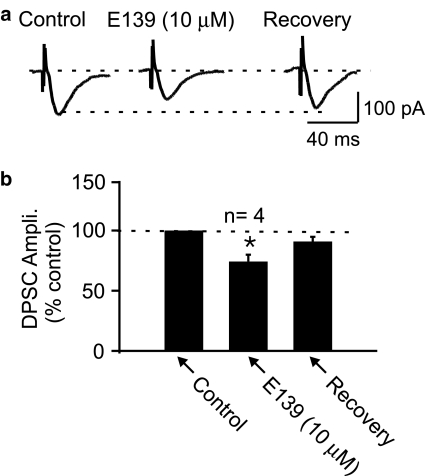
Under control conditions, stimulation of the cortico-accumbens afferents results in a mixed response mediated by glutamate and GABA (Pennartz & Kitai, 1991). The effects of enaminones on mixed synaptic responses were initially tested with E139. Figure 2 shows that bath applied E139 (10 μM) reversibly depresses this mixed response referred to as a depolarizing postsynaptic current (DPSC) by −25±5.7% (n=4). This effect was without any significant change in the holding current (−13.3±12.0 pA, P>0.05, n=4), although at higher concentrations (⩾100 μM) E139 induced an inward current.
Figure 2.
Enaminone, E139 depresses evoked depolarizing postsynaptic currents. (a) Sample depolarizing postsynaptic currents recorded in a NAc cell at a holding potential of −80 mV in control, after 5 min bath application of 10 μM E139 and following 10 min washout of E139. (b) Bar graph summarizing the above effect of 10 μM E139 (n=4). In this figure and in all other figures, * indicates statistical significance at P⩽0.05 compared to control.
Isolation of synaptic responses
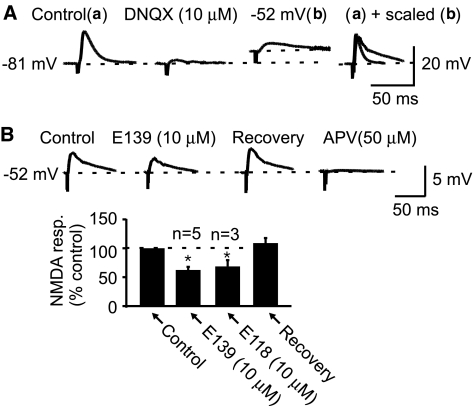
Glutamate-induced, pure EPSCs were isolated by applying 50 μM of picrotoxin, a GABAA receptor-chloride channel blocker. At a holding potential of −80 mV, the response was entirely non-NMDA receptor-mediated as it could be completely blocked by DNQX (10 μM, n=8; see Figure 3a). NMDA receptor-mediated pure EPSPs were isolated by combining pharmacological and biophysical approaches. In the presence of picrotoxin (50 μM), addition of 10 μM DNQX abolished the non-NMDA and GABA receptor-mediated responses recorded at rest. Cells were then depolarized to between −45 and −55 mV. Stimulation at slightly higher intensity at these potentials produced a slow rising and decaying response characteristic of the NMDA receptor kinetics (n=8). This response in the presence of picrotoxin and DNQX and at depolarized potentials was purely NMDA receptor-mediated as it was blocked by the selective NMDA receptor antagonist DL-2-amino-5-phosphonovaleric acid as previously reported (n=3; Kombian et al., 2003).
Figure 3.
E139 depresses isolated, pure NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs). (A) Sample control EPSP (a) recorded in a cell at its resting potential in the presence of only picrotoxin (50 μM). Application of DNQX (10 μM) completely abolishes this response (middle trace). The cell was then depolarized to −52 mV (by positive current injection) and stimulated at a slightly higher stimulus intensity to generate an NMDA receptor-mediated EPSP with the characteristic slow rise and decay rates (b). Far right panel: superimposed control EPSP (a) and the NMDA receptor-mediated response (b) scaled to the amplitude of (a) and baseline adjusted to show the differences in the kinetics of these two responses. (B) A summary bar graph showing that E139 and E118 reversibly depress the pure NMDA receptor-mediated EPSP (n=5 and 3, respectively). Above this are sample traces taken from the cell in (A) illustrating this effect (note the differences in scale here). Far right trace shows that application of DL-APV (50 μM) blocks this synaptic response. In these experiments and the rest of the study, picrotoxin (50 μM) was present throughout each experiment.
Anticonvulsant enaminones inhibit glutamate-mediated synaptic responses
To characterize the effects of anticonvulsant enaminones on excitatory synaptic transmission, we pharmacologically and/or biophysically isolated and studied glutamate-mediated fast synaptic transmission. In this region, both NMDA and non-NMDA receptor-mediated responses can be recorded (Pennartz et al., 1990; Kombian & Malenka, 1994). Figure 3a shows the protocol used to isolate the NMDA receptor-mediated EPSP for the purpose of this study. We chose to record the NMDA response in current clamp mode because it was much easier to observe the characteristic slow rise and decay kinetics of the potential. Figure 3b reveals that 10 μM E139 and E118 both reversibly inhibit this pure, NMDA receptor-mediated EPSP by −37.5±5.3% (n=5) and −28.2±8.1% (n=3) respectively.
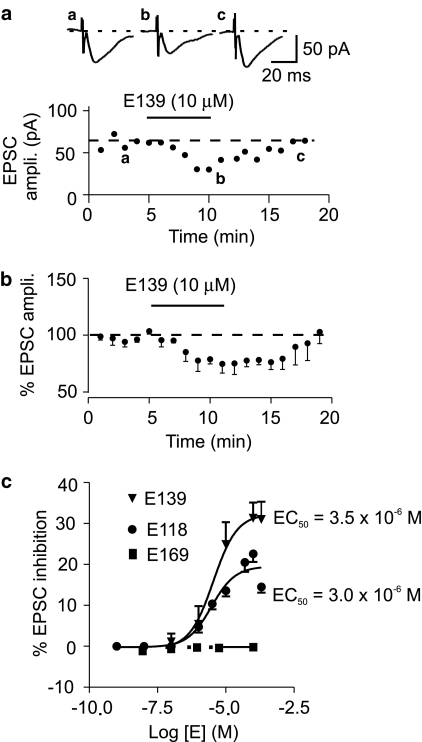
The other glutamate-mediated response is through the activation of AMPA/kainate or non-NMDA receptors, which are responsible for basal, fast excitatory synaptic transmission because unlike the NMDA receptors, they do not display voltage-dependent activation (Mayer et al., 1984). These pure non-NMDA receptor-mediated currents were used to investigate the effects of three enaminones on fast excitatory synaptic transmission. Bath application of E118 or E139 caused a reversible depression of the evoked EPSC. The onset of action was about 2 min with a maximum effect observed at 5 min (Figure 4a and b). Recovery from the EPSC depression was complete and relatively quick, with responses returning to baseline levels in 5–10 min after the commencement of washout (Figure 4a and b). The synaptic depressant effects of both of these enaminones were concentration-dependent. The estimated EC50 of E139 was 3.5 μM with a maximum depression of −31.3±3.8% (Figure 4c; n=4) obtained at 100 μM. For E118, the estimated EC50 was 3.0 μM but with a maximal response of −22.6±1.6% (Figure 4c; n=5) at 100 μM. The E118- and E139-induced EPSC depression at concentrations ⩽10 μM was without significant changes in holding current. E169, on the other hand, was inactive as it neither depressed the evoked EPSCs nor induced any currents at all concentrations tested. These findings indicate that while E118 and E139 are equipotent, E139 is more efficacious than E118 in depressing evoked EPSC, with E169 having no efficacy. E139 was used for the rest of the study because it was more efficacious and produced the most consistent responses. A concentration of 10 μM was selected as this concentration produced a robust effect on the evoked EPSC that could be subjected to pharmacological characterization. This concentration of E139 was shown to cause a consistent EPSC depression following repeated applications. In six cells, a second application of E139 (10 μM) shortly after recovery from the first application produced an almost identical level of EPSC depression (−23.7±1.4% in the first compared to −23.3±2.5% in the second; P>0.05, n=6; paired t-test).
Figure 4.
Anticonvulsant enaminones depress fast, non-NMDA receptor-mediated synaptic responses in a concentration-dependent manner. (a) A time–effect graph of EPSCs recorded in a representative cell that was treated with E139 (10 μM). In this graph and in all other graphs, above each are sample traces taken at the times indicated by letters. (b) A normalized and averaged time–effect plot of the effect of E139 on evoked EPSCs obtained from five cells that received the same concentration of E139 (10 μM). (c) Concentration–response curves generated by applying different concentrations of 3 different enaminones (E118, E139 and E169). Each point on the graph has an n value of 3–5 cells.
To determine if the anticonvulsant enaminones produced the synaptic depression by interacting with the postsynaptic non-NMDA receptor, we analyzed the activation and inactivation kinetics of the non-NMDA receptor-mediated EPSC in the absence and presence of E139 (10 μM). The rise time constant tau-1 (τ1) and the decay time constant tau 2 (τ2) were fitted to a single exponential. E139 (10 μM) did not significantly alter τ1 (3.9±0.5 ms in control and 3.7±0.6 ms in the presence of E139; P>0.05, paired t-test, n=8) or τ2 (23.3±5.3 ms in control compared to 23.0±8.1 ms in the presence of E139, P>0.05, paired t-test, n=8). These values indicate that E139 does not affect the rise time or the decay rate of the non-NMDA receptor-mediated EPSC and hence may not cause the decrease in EPSC amplitude by altering the kinetics of this receptor channel.
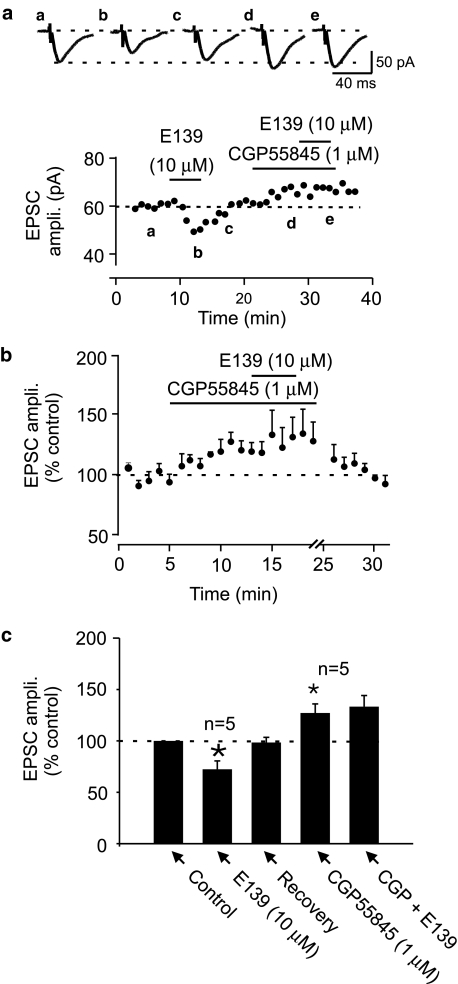
Enaminone-induced depression of evoked EPSC is blocked by a GABAB receptor antagonist
To further characterize the mechanism of action of anticonvulsant enaminones in depressing EPSC amplitude, we hypothesized that it may act indirectly through an intermediate neuromodulator. Since several currently available anticonvulsants work by enhancing GABA mechanisms, we tested if GABA was a possible intermediate that these compounds employed to depress the evoked EPSC amplitude. We have previously reported that, GABA which is abundant in this nucleus depresses EPSCs through GABAB receptors (Kombian et al., 2004). Bath application of 1 μM CGP55845, a potent GABAB receptor antagonist caused an increase in evoked EPSC amplitude (27.1±8.2%; n=5; P<0.05; Figure 5) confirming the presence of a tonic GABA effect on excitation in this nucleus (Kombian et al., 2004). When E139 (10 μM) was applied at the peak of the CGP55845 effect, the change in EPSC amplitude was 6.3±8.1% compared to a depression of −27.7±3.8% observed in these same cells prior to CGP55845 pretreatment (P<0.05; n=5; paired t-test; Figure 5). The lack of effect of E139 in the presence of CGP55845 was not due to desensitization of the E139 effect from the previous exposure as we had earlier shown that the effect of E139 on evoked EPSC in this region does not desensitize. Therefore, the complete block of the E139-induced synaptic depression by CGP55845 suggests that enaminones cause EPSC depression indirectly through GABA and GABAB receptors.
Figure 5.
GABAB receptor antagonist blocks E139-induced EPSC depression. (a) A time–effect plot of evoked EPSCs recorded in a representative cell showing the effect of E139 (10 μM) before and after pretreatment with 1 μM CGP55845, a GABAB receptor antagonist. Note the unmasking by CGP55845 of an endogenous GABA effect on the EPSC. Subsequent application of E139 in the presence of CGP55845 produced no EPSC inhibition. (b) A normalized and averaged time–effect plot in five cells that were pretreated with CGP55845 prior to E139 (10 μM). (c) A bar graph summarizing the effect of E139 in control and in the presence of CGP55845 (n=5).
Enhancement of extracellular GABA levels occludes enaminone effects on EPSCs
The fact that the EPSC depression produced by E139 was blocked by a GABAB receptor antagonist suggests that anticonvulsant enaminones either bind to the GABA binding site on GABAB receptors to produce the observed effect or act to increase extracellular GABA levels, which then mediates the synaptic depression. The former possibility is unlikely to be the case as an anticonvulsant enaminone, ADD 196022, with close structural similarity with E139, is reported not to affect [3H]-GABA binding to neuronal membranes (Mulzac & Scott, 1993). The other alternative, which is to alter extracellular GABA levels, was therefore examined in this study.
There is a tonic presence of GABA in the NAc (Pennartz et al., 1994; Kombian et al., 2004) and this appears to be controlled mainly by plasma membrane transporters called GATs that remove extracellular GABA back into terminals and glial cells, which may then subsequently be degraded by the enzyme GABA transaminase (GABA-T) (Borden et al., 1992; Isaacson et al., 1993; Minelli et al., 1995; Brickley et al., 1996; Rossi & Hamann, 1998). Blockade or inhibition of GATs and GABA-T usually results in the extracellular accumulation of GABA that may lead to prolonged GABA effects (Otis et al., 1991; Isaacson et al., 1993). In the CNS, change in extracellular GABA levels can be monitored by measuring its effect on other responses such as GABAA receptor-mediated currents or GABAB receptor-mediated synaptic depression (see Overstreet & Westbrook, 2001). To verify these facts electrophysiologically, we manipulated the extracellular levels of GABA with known pharmacological agents and asked if the effects of E139 remained unaltered under these conditions.
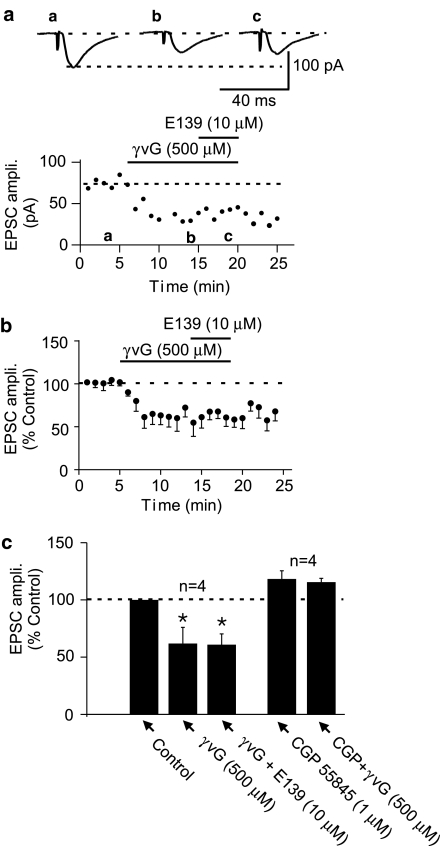
By monitoring EPSC amplitude, we first tested if γvG, an irreversible GABA-T inhibitor (Löscher & Horstermann, 1994; Qume et al., 1995; Qume & Fowler, 1997) and an activator of reverse GABA transport (Wu et al., 2001), depressed evoked EPSC. When 500 μM γvG was bath applied to these cells, there was depression of the evoked EPSC amplitude (−38.1±14.1%, n=4; Figure 6). This effect of γvG did not recover following 15–20 min washout consistent with the fact that it is an irreversible inhibitor of GABA-T. At the peak of the γvG-induced EPSC depression, application of E139 (10 μM) did not cause a further decrease in EPSC amplitude (−39.1±9.4%; P>0.05 compared to depression with γvG alone, n=4, paired t-test; Figure 6). The effect of γvG was confirmed to be through GABA, acting on GABAB receptors, as it was blocked when slices were pretreated with CGP55845 (1 μM; −6.4±5.7%; P<0.05 compared to γvG effect in control; n=4; unpaired t-test; Figure 6c). The occlusion of the EPSC depressant effect of E139 by γvG suggests that E139 may work to enhance extracellular GABA levels by either inhibiting GABA-T and/or inducing nonvesicular GABA release through reversal of GABA transporters (Levi & Raiteri, 1993; Wu et al., 2001, 2003).
Figure 6.
The EPSC depressing effect of E139 is occluded by pretreatment with γ-vinyl GABA (γvG), an irreversible GABA transaminase inhibitor. (a) A representative time–effect plot of the effect of 500 μM γvG on evoked EPSCs and the subsequent application of E139 showing no additional synaptic depression. (b) A normalized averaged time–effect plot obtained from four cells that received the above treatment. (c) A summary bar graph of the effect of 500 μM γvG and the lack of effect of E139 in the presence of γvG. This graph also summarizes the blockade of γvG effect in four additional cells that were pretreated with 1 μM CGP55845 prior to γvG.
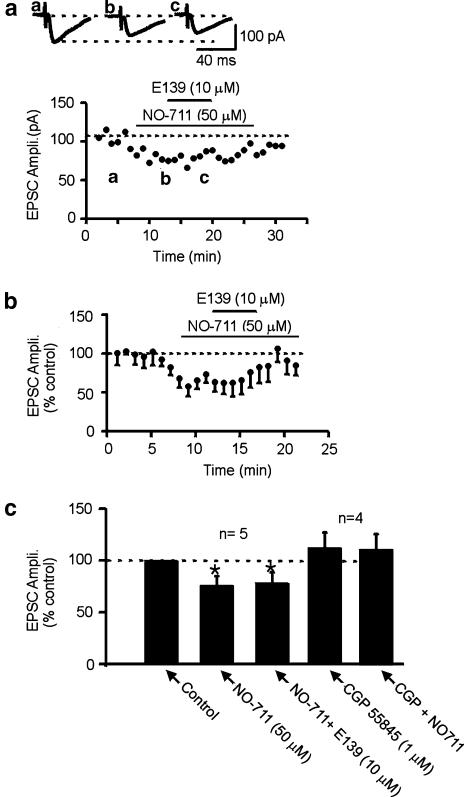
To determine if anticonvulsant enaminones also interacted with GABA reuptake to enhance extracellular GABA levels, we used a selective GABA reuptake inhibitor, NO-711 to test if we could also occlude the E139 effect. When NO-711 (50 μM) was applied, it depressed the evoked EPSC amplitude (−24.1±8.9%, n=5; Figure 7). In contrast to γvG however, this effect was reversible upon washout and was also shown to be via GABA as it was blocked by pretreatment with 1 μM CGP55845 (−1.9±2.2%; P<0.05 compared to the effect of NO-711 in control; n=4; unpaired t-test, Figure 7c). At the peak of the NO-711 effect, application of 10 μM E139 could no longer produce a further decrease in the evoked EPSC amplitude (−23.6±10.8%, P>0.05 compared to the effect of NO-711 alone; n=5, paired t-test, Figure 7). These results suggest that E139 may also inhibit GABA reuptake leading to the observed GABA-mediated effects.
Figure 7.
The EPSC depressing effect of E139 is occluded by pretreatment with NO-711, a GABA reuptake blocker. (a) A representative time–effect plot showing that 50 μM NO-711 reversibly depresses evoked EPSC amplitude. At the peak of the NO-711 effect, coapplication of E139 produces no additional EPSC inhibition. (b) A normalized and averaged time–effect plot generated from five cells that received the treatment above. (c) A summary bar graph of the effect of NO-711 (50 μM) and the lack of effect of E139 in the presence of NO-711. This graph also summarizes the blockade of the NO-711 effect in four additional cells that were pretreated with CGP55845 (1 μM) prior to NO-711.
Discussion
The results of this study show that some, but not all enaminones cause a concentration-dependent depression of glutamate-mediated excitatory synaptic transmission in the CNS. The concentrations of 0.1–100 μM used in this study (which are equivalent to 0.034–33.8 μg g−1) are therapeutically relevant since quantities of approximately 1–10 μg g−1 of enaminone have been reported in the brains of rats, 15–200 min after intraperitoneal injection of an anticonvulsant dose (10 mg kg−1) of E121 (Khurana et al., 2003). In this study, we mainly perfused 10 μM, a molar concentration that works out to 3.38 μg ml−1, a quantity close to that reported above. Concentrations ⩽10 μM did not have a significant effect on resting membrane currents in the cells tested. As well, at or below this concentration, the anticonvulsant enaminones did not significantly alter the kinetics of the non-NMDA receptor-mediated currents. These findings indicate that a direct action of enaminones on the non-NMDA receptor did not contribute significantly to the observed synaptic depression and are not consistent with the modeling predictions (Abou-Zeid & Edafiogho, 2002). Our data, however, support a GABA-dependent action of enaminones to depress excitatory synaptic transmission as their synaptic depressing effect was blocked by an antagonist of GABAB receptors and occluded by pharmacological manipulations that are known to enhance extracellular GABA concentration. The blockade of the E139 effect by a GABAB receptor antagonist suggests that E139 interacts with this receptor directly to produce the synaptic depression. This is, however, unlikely as an enaminone, ADD 196022 which differs from E139 only in a chloro-substitution on the phenyl group, has been reported not to affect [3H]GABA binding (Mulzac & Scott, 1993). Our data are therefore consistent with an indirect action of the anticonvulsant enaminones to produce the GABA-dependent synaptic depression. Thus, the anticonvulsant enaminones may act to increase extracellular GABA concentration and GABA then acts on GABAB receptors, located on presynaptic glutamate terminals, to decrease EPSC amplitude (Uchimura & North, 1991).
Mechanism(s) of action of anticonvulsant enaminones to depress excitatory synaptic transmission
Our data indicate that anticonvulsant enaminones caused a decrease in non-NMDA receptor-mediated currents at concentrations ranging from 0.1 to 100 μM. At concentrations of 10 μM and below, no significant changes in holding current or membrane potential was observed indicating that the active enaminones did not affect the excitability of the recorded postsynaptic cells at rest. However, at concentrations above 10 μM, we consistently observed an inward current or depolarization. The nature of this current and its effect on membrane excitability is currently being investigated.
A tonic presence of GABA in this region has been reported (Pennartz et al., 1994; Kombian et al., 2004), and altering the level of ambient GABA may be a mechanism by which enaminones work to cause the GABA effects observed in this study. After release, GABA is rapidly cleared from the extracellular space by two main mechanisms: reuptake into the presynaptic terminal and surrounding glial cells followed by enzymatic degradation by GABA-T (Löscher et al., 1989; Qume & Fowler, 1997; Conti et al., 1998) and diffusion (Isaacson et al., 1993; Rossi & Hamann, 1998; Sipila et al., 2004). Reuptake of GABA from the extracellular space back into the terminal and glial cells in the CNS by plasma membrane GABA transporters (Borden, 1996; Jensen et al., 2003) is very important in terminating and/or limiting GABA actions (Overstreet & Westbrook, 2003) as well as playing a role in maintaining extracellular GABA level at equilibrium. Four types of GATs have so far been described in the mammalian CNS, GAT1–3 and betaine/GABA transporter, with GAT1 being present in large amounts in regions that are rich in GABAergic neurons (Guastella et al., 1990; Borden, 1996; Jensen et al., 2003). Indeed, GAT1 has been found on GABAergic terminals as well as on glia using immunocytochemical and electron microscopic techniques (Minelli et al., 1995; Conti et al., 1998). As a large fraction (>90%) of neurons in the NAc are GABAergic (Pennartz et al., 1994), GAT1 may play a very important function here to regulate the extracellular levels of GABA. Our finding that NO-711, a selective GABA reuptake inhibitor and a potent GAT1 inhibitor (Borden et al., 1994; Soudijn & Wijngaarden, 2000), caused a GABAB receptor-mediated depression of the evoked EPSC that occluded the actions of E139 suggests that this enaminone may utilize the same transporter and/or mechanism as NO-711 to enhance extracellular GABA levels. The exact mechanism by which the enaminones may alter the function of GAT1 to produce the observed effects is not yet known. Several possibilities however exist. For example, if an anticonvulsant enaminone works like NO-711, it may inhibit the transporter causing a pooling of GABA in the extracellular space. Alternatively, it may also engage this transporter at the expense of GABA with the same end result. Finally, it may cause the transporter to reverse and engage in nonvesicular GABA release (Wu et al., 2001, 2003; Richerson & Wu, 2003).
In addition to a possible interaction with GAT1 to alter GABA levels, our results also indicate that anticonvulsant enaminones may inhibit GABA-T as the suicide GABA-T inhibitor, γvG also occluded the ability of E139 to depress EPSCs. If E139 works by inhibiting GABA-T, its mechanism of action may be different from that of γvG as its effects reversed quite rapidly whereas γvG effect was irreversible. From our results, E139 and other anticonvulsant enaminones may enhance extracellular GABA levels by one or the other of the above mechanisms, or even both. It is possible that anticonvulsant enaminones may enter neurons to inhibit GABA-T resulting in an increase in cytosolic GABA concentration. This can then trigger a reversal of the GABA transporter leading to nonvesicular release (Wu et al., 2001, 2003) that is thought to contribute to the anticonvulsant effects of established drugs such as γvG.
Structural requirements for anticonvulsant activity of enaminones
Our data on synaptic transmission is consistent with data obtained in in vivo studies that show that the enaminones E118 and E139 are potent (class 1) anticonvulsant agents, while E169 is inactive (Edafiogho et al., 1994). The main structural difference in these enaminones is the hydroxyl (OH) group in the inactive E169 compared to the halide moieties (Cl and Br) in the anticonvulsant analogues. This is a classical example of pharmacologically active compounds whereby activity is abolished by hydroxylation at the para position of the phenyl ring. For example, carbamazepine, a well-known anticonvulsant agent is inactivated by hydroxylation at the para position of a phenyl ring in vivo (Edafiogho & Scott, 1996). In vivo metabolic studies of E121 in rats detected de-esterification and decarboxylation products as the major metabolites (Khurana et al., 2003). Since no metabolites were found that had a hydroxyl group in place of the halide on the phenyl ring, these metabolites may also possess anticonvulsant activity. Further studies need to be conducted on these metabolites to verify if they retain anticonvulsant activity as the basic pharmacophoric structure is still maintained.
In conclusion, the effects of the anticonvulsant enaminones on fast excitatory synaptic transmission observed in this study may contribute to their anticonvulsant effects reported in vivo. Although our current data are consistent with an action of these enaminones to alter GABAergic transmission, these compounds may have additional actions on neurons that may also contribute to their anticonvulsant effects (Scott et al., 1995). Further studies, including specific binding and biochemical experiments with these anticonvulsant enaminones, are required to determine the nature of their interaction with GABAB receptors, GABA transporters and/or GABA-T enzyme.
Acknowledgments
This work was supported by Kuwait University Grant # PT02/02 to SBK. We thank Dr W. Matowe for comments on the manuscript and Dr S. Parvathy for technical assistance.
Abbreviations
- AMPA
(s)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl) propanoate
- DL-APV
DL-2-amino-5-phosphonovalerate
- DMSO
dimethyl sulfoxide
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- E118
3-(4′-chlorophenyl)aminocyclohex-2-enone
- E139
methyl 4-(4′-bromophenyl)aminocyclohex-3-en-6-methyl-2-oxo-1-oate
- E169
ethyl 4-(4′-hydroxyphenyl)aminocyclohex-3-en-6-methyl-2-oxo-1-oate
- Emax
maximum effect
- γvG
gamma vinyl GABA
- Nac
nucleus accumbens
- NMDA
N-methyl-D-aspartate
- NO-711
1-(2-(((diphenylmethylene)imino)oxy)ethyl)-1,2,5,6-tetrahydro-3-pyridine-carboxylic acid
References
- ABOU-ZEID L.A., EDAFIOGHO I.O.Glutamate receptor is a likely target for anticonvulsant enaminones: a molecular dynamics-based evidence Abstract of 7th Health Science Poster day and Ibn Sina Forum 2002178p
- BORDEN L.A. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- BORDEN L.A., DHAR T.G., SMITH K.E., BRANCHEK T.A., GLUCHOWSKI C., WEINSHANK R.L. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Receptors. Channels. 1994;2:207–213. [PubMed] [Google Scholar]
- BORDEN L.A., SMITH K.E., HARTIG P.R., BRANCHEK T.A., WEINSHANK R.L. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J. Biol. Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- BRICKLEY S.G., CULL-CANDY S.G., FARRANT M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996;497 Part 3:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE M.J. Do we need any more new antiepileptic drugs. Epilepsy Res. 2001;45:3–6. doi: 10.1016/s0920-1211(01)00203-0. [DOI] [PubMed] [Google Scholar]
- BROWNE T.R., HOLMES G.L. Epilepsy. N. Engl. J. Med. 2001;344:1145–1151. doi: 10.1056/NEJM200104123441507. [DOI] [PubMed] [Google Scholar]
- CONTI F., MELONE M., DE BIASI S., MINELLI A., BRECHA N.C., DUCATI A. Neuronal and glial localization of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in human cerebral cortex: with a note on its distribution in monkey cortex. J. Comp. Neurol. 1998;396:51–63. doi: 10.1002/(sici)1096-9861(19980622)396:1<51::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- DINGLEDINE R., MCBAIN C.J., MCNAMARA J.O. Excitatory amino acid receptors in epilepsy. Trends Pharmacol. Sci. 1990;11:334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- EDAFIOGHO I.O., SCOTT K.R.Anticonvulsants Burger's Medicinal Chemistry and Drug Discovery 19963175–259.5th edn., ed. Wolff, M.E, Vol [Google Scholar]
- EDAFIOGHO I.O., ALEXANDER M.S., MOORE J.A., FAWAR V.A., SCOTT K.R. Anticonvulsant enaminoes: with emphasis on methyl 4-(p-chlorophenyl)amino-6-methyl-2-oxocyclohex-3-en-1-oate (ADD196022) Curr. Med. Chem. 1994;1:159–175. [Google Scholar]
- EDAFIOGHO I.O., DENNY B.J., SCHWALBE C.H., LOWE P.R. X-ray crystallographic and theoretical studies of an anticonvulsant enaminone: methyl 4-(4′-bromophenyl)amino-6-methyl-2-oxocyclohex-3-en-1-oate. Med. Principles Practice. 2003;12:237–242. doi: 10.1159/000072290. [DOI] [PubMed] [Google Scholar]
- EDAFIOGHO I.O., HINKO C.N., MOORE J.A., MULZAC D., NICHOLSON J.M., SCOTT K.R. Synthesis and anticonvulsant activity of enaminones. J. Med. Chem. 1992;35:2798–2805. doi: 10.1021/jm00093a012. [DOI] [PubMed] [Google Scholar]
- GUASTELLA J., NELSON N., NELSON H., CZYZYK L., KEYNAN S., MIEDEL M.C., DAVIDSON N., LESTER H.A., KANNER B.I. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- ISAACSON J.S., SOLIS J.M., NICOLL R.A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- JENSEN K., CHIU C.S., SOKOLOVA I., LESTER H.A., MODY I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J. Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- KHURANA M., SALAMA N.N., SCOTT K.R., NEMIEBOKA N.N., BAUER JR K.S., EDDINGTON N.D. Preclinical evaluation of the pharmacokinetics, brain uptake and metabolism of E121, an antiepileptic enaminone ester in rats. Biopharm. Drug Dispos. 2003;24:397–407. doi: 10.1002/bdd.376. [DOI] [PubMed] [Google Scholar]
- KOMBIAN S.B., MALENKA R.C. Simultaneous LTP of non-NMDA- and LTD of NMDA- receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- KOMBIAN S.B., ANANTHALAKSHMI K.V.V., PARVATHY S.S., MATOWE W.C. Substance P depresses excitatory synaptic transmission in the nucleus accumbens through dopaminergic and purinergic mechanisms. J. Neurophysiol. 2003;89:728–737. doi: 10.1152/jn.00854.2002. [DOI] [PubMed] [Google Scholar]
- KOMBIAN S.B., ANANTHALAKSHMI K.V.V., PARVATHY S.S., MATOWE W.C. Cholecystokinin activates CCKB receptors to excite cells and depress EPSCs in the rat rostral nucleus accumbens in vitro. J. Physiol. 2004;555:71–84. doi: 10.1113/jphysiol.2003.056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUS J.E., YEH G.C., BONHAUS D.W., NADLER J.V., MCNAMARA J.O. Kindling induces the long-lasting expression of a novel population of NMDA receptors in hippocampal region CA3. J. Neurosci. 1994;14:4196–4205. doi: 10.1523/JNEUROSCI.14-07-04196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAN P., BRODIE M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- LEVI G., RAITERI M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993;16:415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- LÖSCHER W. Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol. Sci. 2002;23:113–118. doi: 10.1016/S0165-6147(00)01974-X. [DOI] [PubMed] [Google Scholar]
- LÖSCHER W., HONACK D., GRAMER M. Use of inhibitors of gamma-aminobutyric acid (GABA) transaminase for the estimation of GABA turnover in various brain regions of rats: a reevaluation of aminooxyacetic acid. J. Neurochem. 1989;53:1737–1750. doi: 10.1111/j.1471-4159.1989.tb09239.x. [DOI] [PubMed] [Google Scholar]
- LÖSCHER W., HORSTERMANN D. Differential effects of vigabatrin, gamma-acetylenic GABA, aminooxyacetic acid, and valproate on levels of various amino acids in rat brain regions and plasma. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:270–278. doi: 10.1007/BF00169293. [DOI] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L., GUTHRIE P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- MULZAC D., SCOTT K.R. Profile of anticonvulsant activity and minimal toxicity of methyl 4-[(p-chlorophenyl)amino]-6-mrhtyl-2-oxo-cyclohex-3-en-1-oate amd some prototype antiepileptic drugd in mice and rats. Epilepsia. 1993;34:1141–1146. doi: 10.1111/j.1528-1157.1993.tb02147.x. [DOI] [PubMed] [Google Scholar]
- MCBAIN C.J., BODEN P., HILL R.G. Rat hippocampal slices ‘in vitro' display spontaneous epileptiform activity following long-term organotypic culture. J. Neurosci. Methods. 1989;27:35–49. doi: 10.1016/0165-0270(89)90051-4. [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O. Identification of genetic defect of an epilepsy: strategies for therapeutic advances. Epilepsia. 1994;35 Suppl 1:S51–S57. doi: 10.1111/j.1528-1157.1994.tb05929.x. [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O., RUSSELL R.D., RIGSBEE L., BONHAUS D.W. Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology. 1988;27:563–568. doi: 10.1016/0028-3908(88)90176-1. [DOI] [PubMed] [Google Scholar]
- MINELLI A., BRECHA N.C., KARSCHIN C., DEBIASI S., CONTI F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J. Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTIS T.S., STALEY K.J., MODY I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- OVERSTREET L.S., WESTBROOK G.L. Paradoxical reduction of synaptic inhibition by vigabatrin. J. Neurophysiol. 2001;86:596–603. doi: 10.1152/jn.2001.86.2.596. [DOI] [PubMed] [Google Scholar]
- OVERSTREET L.S., WESTBROOK G.L. Synapse density regulates independence at unitary inhibitory synapses. J. Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNARTZ C.M., BOEIJINGA P.H., LOPES DA SILVA F.H. Locally evoked potentials in slices of the rat nucleus accumbens: NMDA and non-NMDA receptor mediated components and modulation by GABA. Brain Res. 1990;529:30–41. doi: 10.1016/0006-8993(90)90808-o. [DOI] [PubMed] [Google Scholar]
- PENNARTZ C.M., GROENEWEGEN H.J., LOPES DA SILVA F.H. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- PENNARTZ C.M., KITAI S.T. Hippocampal inputs to identified neurons in an in vitro slice preparation of the rat nucleus accumbens: evidence for feed-forward inhibition. J. Neurosci. 1991;11:2838–2847. doi: 10.1523/JNEUROSCI.11-09-02838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUME M., FOWLER L.J. Effect of chronic treatment with the GABA transaminase inhibitors gamma-vinyl GABA and ethanolamine O-sulphate on the in vitro GABA release from rat hippocampus. Br. J. Pharmacol. 1997;122:539–545. doi: 10.1038/sj.bjp.0701383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUME M., WHITTON P.S., FOWLER L.J. The effect of chronic treatment with the GABA transaminase inhibitors gamma-vinyl-GABA and ethanolamine-O-sulphate on the in vivo release of GABA from rat hippocampus. J. Neurochem. 1995;64:2256–2261. doi: 10.1046/j.1471-4159.1995.64052256.x. [DOI] [PubMed] [Google Scholar]
- REGESTA G., TANGANELLI P. Clinical aspects and biological bases of drug-resistant epilepsies. Epilepsy Res. 1999;34:109–122. doi: 10.1016/s0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- RICHERSON G.B., WU Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- ROSSI D.J., HAMANN M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- SCOTT K.R., RANKIN G.O., STABLES J.P., ALEXANDER M.S., EDAFIOGHO I.O., FARRAR V.A., KOLEN K.R., MOORE J.A., SIMS L.D., TONNU A.D. Synthesis and anticonvulsant activity of enaminones. 3. Investigations on 4′-, 3′-, and 2′-substituted and polysubstituted anilino compounds, sodium channel binding studies, and toxicity evaluations. J. Med. Chem. 1995;38:4033–4043. doi: 10.1021/jm00020a019. [DOI] [PubMed] [Google Scholar]
- SIPILA S., HUTTU K., VOIPIO J., KAILA K. GABA uptake via GABA transporter-1 modulates GABAergic transmission in the immature hippocampus. J. Neurosci. 2004;24:5877–5880. doi: 10.1523/JNEUROSCI.1287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUDIJN W., WIJNGAARDEN I. The GABA transporter and its inhibitors. Curr. Med. Chem. 2000;7:1063–1079. doi: 10.2174/0929867003374363. [DOI] [PubMed] [Google Scholar]
- TRAYNELIS S.F., DINGLEDINE R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J. Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- UCHIMURA N., NORTH R.A. Baclofen and adenosine inhibit synaptic potentials mediated by gamma- aminobutyric acid and glutamate release in rat nucleus accumbens. J. Pharmacol. Exp. Ther. 1991;258:663–668. [PubMed] [Google Scholar]
- WU Y., WANG W., RICHERSON G.B. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J. Neurosci. 2001;21:2630–2639. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU Y., WANG W., RICHERSON G.B. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J. Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]