Abstract
ATP-sensitive K+ channels (KATP channels) are complexes of inwardly rectifying K+ channels (Kir6.x) and sulphonylurea receptors (SURs). Kir6.2-containing channels are closed by ATP binding to Kir6.2, and opened by MgADP binding to SUR. Channel activity is modulated by synthetic compounds such as the channel-blocking sulphonylureas and the KATP channel openers, which both act by binding to SUR. By interacting with Kir6.2, phosphatidylinositol-4,5-bisphosphate (PIP2) and oleoyl-coenzyme A (OCoA) decrease the ATP-sensitivity of the channel and abolish the effect of the synthetic channel modulators. Here, we have investigated whether lipids and related compounds interfered with the binding of the sulphonylurea, glibenclamide (GBC) and of the opener, N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine (P1075), to the SUR subtypes.
Lipids (100–300 μM) inhibited binding of [3H]GBC and [3H]P1075 to SUR subtypes in the rank order OCoA>dioleylglycerol-succinyl-nitriloacetic acid (DOGS-NTA)>oleate>malonyl-CoA>PIP2.
OCoA inhibited radioligand binding to SUR completely, with IC50 values ranging from 6 to 44 μM. Inhibition was reversed by increasing the concentration of the radioligands in agreement with an essentially competitive mechanism. MgATP and coexpression with Kir6.2 decreased the potency of OCoA.
DOGS-NTA inhibited radioligand binding to SUR by 40–88%, with IC50 values ranging from 38 to 120 μM.
Poly-lysine increased radioligand binding to SUR by up to 30% but did not affect much the inhibition of ligand binding by OCoA and DOGS-NTA.
Radioligand binding to SUR2A but not to the other SUR subtypes was slightly (10–20%) stimulated by lipids at concentrations ∼10 × lower than required for inhibition.
The data show that certain lipids, at high concentrations, interact with SUR and inhibit the binding of GBC and P1075; with SUR2A, a modest stimulation of ligand binding precedes inhibition. Regarding KATP channel activity, these effects will be overruled by the interaction of the lipids with Kir6.2 at lower (physiological) concentrations. They are, however, of pharmacological importance and must be taken into account if high concentrations of these compounds (e.g. OCoA>10 μM) are used to interfere with the action of sulphonylureas and openers on KATP channel activity.
Keywords: Lipids, sulphonylurea receptors (SURs), interaction of lipids with SUR, oleoyl-CoA, glibenclamide, P1075, inhibition of ligand binding to SUR by lipids
Introduction
ATP-sensitive K+ channels (KATP channels) are tetradimeric complexes of inwardly rectifying K+ channels (Kir6.x) and sulphonylurea receptors (SURs) (Ashcroft & Gribble, 1998; Aguilar-Bryan & Bryan, 1999). The Kir6.x subunits form the pore of the channel. The SUR subunits are members of the ATP-binding cassette protein superfamily with two intracellular nucleotide-binding domains. In addition, they carry the binding sites for the antidiabetic sulphonylureas, which close the channel, and for the KATP channel openers (Aguilar-Bryan et al., 1995; Hambrock et al., 1998; Schwanstecher et al., 1998). KATP channels have the unique property of being closed by intracellular ATP and opened by MgADP; thereby, they link the metabolic state of the cell to the cell excitability and fulfil important functions in several tissues (Ashcroft & Ashcroft, 1990; Seino & Miki, 2003). In Kir6.2-containing KATP channels, the inhibitory action of ATP is mediated by ATP binding to Kir6.2 (Tucker et al., 1997), and the activating action of MgATP and MgADP by the interaction with the nucleotide-binding domains of SUR (Gribble et al., 1997; Matsushita et al., 2002).
Phospholipids such as phosphatidylinositol-4,5-bisphosphate (PIP2) and long-chain acyl-CoA esters such as oleoyl-coenzyme A (OCoA) set the ATP-sensitivity of Kir6.2-containing KATP channels by binding to Kir6.2 in close vicinity to the ATP site (Enkvetchakul & Nichols, 2003; Schulze et al., 2003; Trapp et al., 2003). PIP2 increases the open probability of KATP channels in the absence of ATP (Hilgemann & Ball, 1996) and decreases the ATP-sensitivity of Kir6.2/SUR1 channels by shifting the ATP inhibition curve rightwards (Baukrowitz et al., 1998; Shyng & Nichols, 1998). OCoA also induces rightward shifts of the ATP-inhibition curves for cloned pancreatic (Kir6.2/SUR1) (Gribble et al., 1998) and native cardiac (Kir6.2/SUR2A) KATP channels (Liu et al., 2001), acting with similar potency at the two channels (Schulze et al., 2003). The levels of PIP2 and related phospholipids are subject to dynamic regulation in the lipid signalling cascade (Baukrowitz et al., 1998; Baukrowitz & Fakler, 2000). Similarly, in the pancreatic β-cell, the levels of OCoA and other long-chain acyl-CoA esters increase with hyperglycaemia and exposure to high levels of free fatty acids, as occurs in diabetes mellitus (Larsson et al., 1996). In the heart, acyl-CoA ester levels are about 30 nmol/g wet weight and rise sharply when the heart is subjected to ischaemia (van-der-Vusse et al., 1992). Divalent cations and polyvalent cations like spermine, neomycine and poly-lysine reverse the effect of PIP2 and OCoA on the ATP-sensitivity of Kir6.2/SURx; this shows that screening of the negative charges abolishes the effect of the lipids (Fan & Makielski, 1997; Shyng & Nichols, 1998; Schulze et al., 2003).
In addition to its effect on ATP-sensitivity, PIP2 abolishes the response of Kir6.2/SUR1 and Kir6.2/SUR2A channels to sulphonylurea blockers and KATP channel openers (Koster et al., 1999; Krauter et al., 2001). Similarly, OCoA (1 or 10 μM) renders the Kir6.2/SUR2A channel totally insensitive to inhibition by glibenclamide (1 and 10 μM) (Liu et al., 2001; Schulze et al., 2003). PIP2 antagonises glibenclamide (GBC) by reducing the fraction of sulphonylurea-sensitive channels without affecting the IC50 value of the drug (Koster et al., 1999; Krauter et al., 2001) and this effect is insensitive to polyvalent cations (Krauter et al., 2001). In contrast, the action of PIP2 on ATP block is apparently competitive and sensitive to polyvalent cations (see above). These differences suggest that PIP2 affects the sensitivities of the channel towards ATP and towards the synthetic modulators, sulphonylureas and openers, by different mechanisms. The lipids and ATP bind to Kir6.2, thereby stabilising different states of the channel, the open state (lipids) and a long-lived closed state (ATP); their antagonism is believed to be essentially allosteric in nature (Koster et al., 1999; Enkvetchakul & Nichols, 2003; Schulze et al., 2003). On the other hand, the synthetic KATP channel modulators bind to SUR to affect channel gating, and the insensitivity of the lipid-modified channel to the modulators is thought to reflect a functional uncoupling of Kir6.2 from SUR such that lipid-modified Kir6.2 is in an open state regardless of the regulatory input of SUR (Koster et al., 1999).
It has, however, not yet been examined whether PIP2 and OCoA, at the concentrations often used (1–100 μM), interfere with the binding of the modulators to SUR. To clarify this question, we have investigated the effects of PIP2, OCoA and related compounds, which have been shown to affect the sensitivity of KATP channels towards ATP, on the binding of [3H]GBC and [3H]P1075 to the SUR subtypes.
Methods
Cell culture, transfection and membrane preparation
Human embryonic kidney (HEK) 293 cells were cultured in minimum essential medium containing glutamine and supplemented with 10% fetal bovine serum and 20 μg ml−1 gentamycin (Hambrock et al., 1998). HEK cells lines stably expressing rat SUR1 (GenBank X97279), murine SUR2A (GenBank D86037), SUR2B (GenBank D86038), SUR2A(Y1206S) or SUR2B(Y1206S) were generated as described (Hambrock et al., 1998). The mutant SUR2 subtypes were constructed from the corresponding murine SUR2 clones as described by Hambrock et al. (2001). The expression levels of SURx were for SUR1 ∼3.6 pmol GBC bound per mg protein (in the absence of MgATP) and for SUR2x 0.5–1 pmol P1075 bound per mg protein (in the presence of 1 mM MgATP). At 3 days prior to membrane preparation, the antibiotic was withdrawn from the culture medium. Cells transiently coexpressing SUR2A and murine Kir6.2 (D50581) were transfected with the vectors pcDNA 3.1 (Invitrogen, Karlsruhe, Germany) containing the respective coding sequences at a molar ratio of 1 : 1 using lipofectAMINE and OPTIMEM (Invitrogen). Cells were used for membrane preparation 2–4 days after transfection. Membranes were prepared as described (Hambrock et al., 1998) and frozen at −80°C in Mg2+-free incubation buffer containing (in mM) HEPES (5), NaCl (139), KCl (5), and adjusted to pH 7.4 at 4°C. Protein concentration was determined according to Lowry et al. (1951) using bovine serum albumin as the standard.
Preparation of lipids and membrane treatment with lipids
1,2-dioleyl-sn-glycero-3-{[N(5-amino-1-carboxypentyl)iminodiacetic acid]succinyl} (DOGS-NTA), malonyl-CoA, OCoA, oleate and PIP2 (summarised here as ‘lipids') were added to a buffer containing (in mM) NaCl (139), KCl (5), EDTA (0.1) and HEPES (5) at pH 7.4 to give stock solutions of 1 mM, sonicated in ice-cold water for 30 min and stored in aliquots at −20°C until use. For the experiments, aliquots were diluted to the appropriate concentration and sonicated for 30 min at 0°C. Membranes in Mg2+-free solution were homogenised with a Polytron homogeniser (Kinematica, Lucerne, Switzerland) and a 200 μl aliquot was added to the lipid solution (725 μl) and sonicated for 2 min at 0°C to facilitate lipid incorporation in the membranes. Control measurements showed that sonication for 2 min did not affect [3H]P1075 or [3H]GBC binding to SUR. For experiments in the absence of lipids, membranes were treated similarly.
Radioligand-binding experiments
For radioligand-binding competition experiments in the absence/presence of MgATP, membranes (925 μl; protein concentration 100–300 μg ml−1) were supplemented with 75 μl incubation buffer containing the radioligand and MgATP so that the final concentrations were (in mM): HEPES (5), NaCl (139), KCl (5), MgCl2 (0/1), and Na2ATP (0/0.3), EDTA (0.03) and [3H]GBC ∼2 nM or [3H]P1075 ∼3 nM as the radioligand; pH was 7.4 at 37°C. In all experiments in the presence of MgATP, the nucleotide concentration was 0.3 mM. In experiments with SUR1, nonspecific binding (BNS) of [3H]GBC was determined in the presence of 100 nM GBC; in experiments with SUR2, BNS of [3H]GBC/[3H]P1075 was measured in the presence of 100/10 μM N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine (P1075) (Hambrock et al., 2001).
After equilibrium was reached (15–30 min at 37°C), incubation was stopped by diluting 0.3 ml aliquots (in triplicate) into 8 ml of ice-cold quench solution (50 mM Tris-(hydroxymethyl)-aminomethane, 154 mM NaCl, pH 7.4). Bound and free ligand were separated by rapid filtration over Whatman GF/B filters. Filters were washed twice with quench solution and counted for [3H] in the presence of 6 ml of scintillant (Ultima Gold: Packard, Meriden, CT, U.S.A.).
Data analysis
Binding equilibrium inhibition curves were analysed using the logarithmic form of the Hill equation
Here A denotes the extent (amplitude) of inhibition, n (=nH) the Hill coefficient and IC50 the midpoint of the curve with pIC50=−log IC50; x is the concentration of the compound under study with px=−log x. IC50 values were converted into inhibition constants, Ki, by correcting for the presence of the competing radioligand, L, according to the Cheng & Prusoff (1973) equation
where KD is the equilibrium dissociation constant of the radioligand. In case of homologous competition experiments, the inhibition constant Ki is identical to the KD value. Saturation experiments were analysed according to the Law of Mass Action
with BMAX (fmol mg−1 protein) denoting the concentration of specific binding sites in the preparation. Nonspecific binding (BNS) was proportional to L.
In the presence of OCoA (O), which competes with the radioligand (L), specific binding is given by
where KO designates the equilibrium dissociation constant of O binding to SUR. Comparing BS′ to BS in the absence of O, at the same radioligand concentration L, we have
Data are shown as means±s.e.m. Fits of the equations to the data were performed according to the method of least squares using the programme SigmaPlot 6.1 (SPSS Science, Chicago, IL, U.S.A.). Individual binding experiments were analysed and the parameters averaged assuming that amplitudes and pIC50 values are normally distributed (Christopoulos, 1998). In the text, KD/IC50 values are given, followed by the 95% confidence interval in parentheses. In calculations involving mean values with standard errors, propagation of errors was taken into account according to Bevington (1969). The significance of differences between two normally distributed parameters with equal variance was assessed using the two-tailed unpaired Student's t-test.
Materials and solutions
The reagents and media used for cell culture and transfection were from Invitrogen. [3H]P1075 (specific activity 4.5 TBq (117 Ci) mmol−1) was purchased from Amersham Buchler (Braunschweig, Germany) and [3H]GBC (specific activity 1.85 TBq (50 Ci) mmol−1) from Perkin Elmer Life Sciences (Bad Homburg, Germany). OCoA was from ICN Biochemicals (Eschwege, Germany), DOGS-NTA from Avanti Polar Lipids (Alabaster, AL, U.S.A.). Oleate, malonyl-CoA, PIP2, Poly-D-lysine (PDK) and GBC were purchased from Sigma (Deisenhofen, Germany); P1075 was from Leo Pharmaceuticals (Ballerup, Denmark) and Na2ATP from Roche Diagnostics (Mannheim, Germany).
Results
Effects of lipids on [3H]P1075 and [3H]GBC binding to SURs
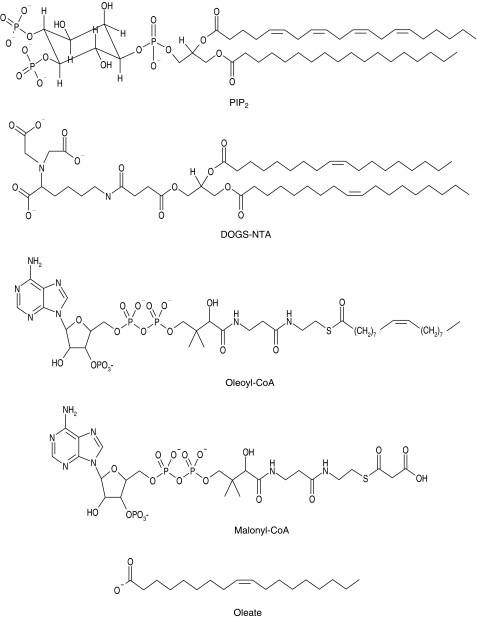
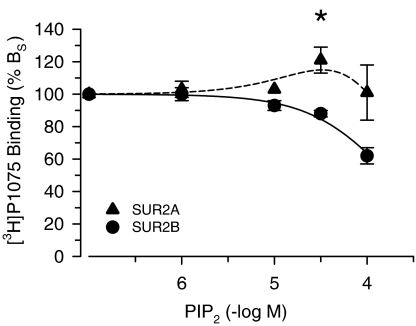
The structures of the lipids used are presented in Figure 1. In a first screen for effects on radioligand binding to SUR, high concentrations were applied. The results for SUR2A are summarised in Table 1 and show that, in general, inhibition was observed. At 100 μM, PIP2 did not affect [3H]P1075 binding. At lower concentrations, however, a small stimulation occurred (Figure 2) and at 30 μM, specific binding was increased to 117±8% of control (n=7, P=0.03). With SUR2B in contrast, only inhibition was observed (Figure 2). PIP2 concentrations >100 μM were not used since they are unrealistically high and such experiments are extremely costly.
Figure 1.
Structures of the lipids and related compounds used in this study.
Table 1.
Inhibition by lipids of [3H]P1075 and [3H]GBC binding to SUR2A and SUR2A(YS) in the presence of MgATP (0.3 mM)
| SUR | Radioligand | Lipid (μM) | Binding (% BS) (n) |
|---|---|---|---|
| SUR2A | [3H]P1075 | PIP2, 100 | 98±15a (7) |
| DOGS-NTA, 100 | 49±7 (6) | ||
| Oleoyl-CoA, 100 | 4±1 (4) | ||
| Malonyl-CoA, 300 | 64±7 (4) | ||
| Oleate, 300 | 14±3 (3) | ||
| SUR2A(YS) | [3H]GBC | DOGS-NTA, 100 | 50±4 (6) |
| Oleoyl-CoA, 100 | 38±13 (4) |
(n) denotes the number of experiments.
At 30 μM PIP2, stimulation of binding to 117±8% was observed.
Figure 2.
Modulation of [3H]P1075 binding to SUR2A and SUR2B by PIP2. Data show specific binding (BS) and are means±s.e.m. from seven experiments each. Note the small but significant stimulation of [3H]P1075 binding to SUR2A at 30 μM PIP2. Experiments were performed in the presence of MgATP (0.3 mM) at [3H]P1075 concentrations of 3 (SUR2A) and 2 nM (SUR2B). 100% BS was 260–300 fmol mg−1 and nonspecific binding (BNS) was ∼10% of total binding (BTOT).
DOGS-NTA, a lipid with structural similarity to PIP2 (Krauter et al., 2001; see also Figure 1) inhibited [3H]P1075 binding to ∼50% at 100 μM (Table 1). Of the acyl-CoA esters tested, the long-chain ester OCoA was more potent than the short-chain ester malonyl-CoA, in agreement with the observations of Larsson et al. (1996) on the pancreatic KATP channel. Interestingly, oleate also inhibited [3H]P1075 binding to SUR2A to 14% of control at 300 μM (Table 1).
To investigate whether these compounds also interfered with [3H]GBC binding to the SUR2 isoforms, we used mutant forms of SUR2 in which Tyr 1206 is replaced by Ser, the corresponding amino acid in SUR1. The amino acid in this position is of great importance for the binding of sulphonylureas to SUR (Ashfield et al., 1999), and we have shown previously that the mutation Y1206S increases the affinity of SUR2A and 2B for GBC ∼10-fold (Hambrock et al., 2001; Stephan et al., 2005). OCoA and DOGS-NTA also inhibited [3H]GBC binding to SUR2A(Y1206S) (Table 1), and more detailed investigations with these compounds are presented below.
OCoA and SUR1
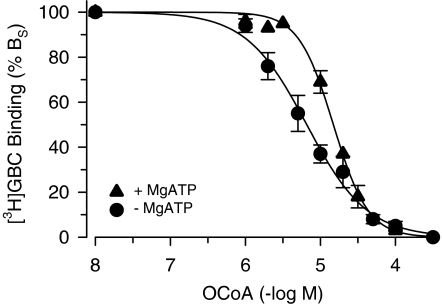
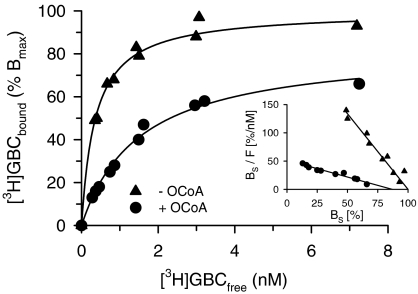
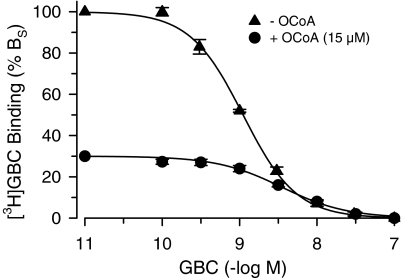
In the absence of MgATP, OCoA inhibited [3H]GBC binding to SUR1 completely with IC50=6.1 μM and Hill coefficient ∼1 (Figure 3, Table 2). In order to clarify the mechanism of inhibition, [3H]GBC-binding saturation experiments were performed in the absence and presence of OCoA (10 μM). Figure 4 shows that OCoA shifted the KD of [3H]GBC binding 4.2x from 0.36 to 1.5 nM and reduced BMAX only slightly to 83±3% (which is significantly different from 100; P<0.05). Figure 4, inset, shows the data in the Scatchard transformation; in this representation, the KD values are given by the reciprocal of the slopes and the BMAX values by the abscissa intercepts (Scatchard, 1949). It is seen that both lines essentially converge to 100% control binding. These experiments show that the inhibition of [3H]GBC binding to SUR1 by OCoA is essentially reversible and of a competitive nature. From the 4.2-fold shift in the KD of GBC, one can estimate the equilibrium dissociation constant of OCoA binding to SUR1 (=KO) by using Equation (4a) in Methods giving KO=3 μM. Owing to the essentially competitive nature of the interaction between GBC and OCoA, one can get a further estimate of KO by applying the Cheng Prusoff equation to the IC50 value of 6.1 μM for OCoA determined in Figure 3. Correcting for the presence of the competing radioligand ([3H]GBC=1.1 nM), one calculates KO=1.5 μM in reasonable agreement with the estimation obtained from the saturation experiments.
Figure 3.
Inhibition of [3H]GBC binding to SUR1 by OCoA in the absence and presence of MgATP (0.3 mM). Data are means from three to four experiments. Individual inhibition curves were analysed according to the Hill equation as described in Methods; the parameters are listed in Table 2. [3H]GBC concentration was ∼1 nM, BS was ∼600 fmol mg−1 and BNS <10% BTOT.
Table 2.
Inhibition of radioligand binding to SUR1 and SUR2 by OCoA
| SUR | Radioligand | MgATP (mM) | IC50 (μM) | nH |
|---|---|---|---|---|
| SUR1 | [3H]GBC | 0 | 6.1 (3.6,10) | 1.1±0.2 |
| SUR1 | [3H]GBC | 0.3 | 15 (12,18) | 2.1±0.2 |
| SUR2A(YS)a | [3H]GBC | 0.3 | 44 (25,60) | 1.2±0.2 |
| SUR2A | [3H]P1075 | 0.3 | 17 (15,21) | 1.5±0.1 |
| SUR2A/Kir6.2 | [3H]P1075 | 0.3 | 30 (27,32) | 1.4±0.1 |
| SUR2B | [3H]P1075 | 0.3 | 12 (7,20) | 1.8±0.2 |
Parameters are derived from experiments as shown in Figure 3. IC50 values are followed by the 95% confidence interval; nH denotes the Hill coefficient. In all cases, the inhibition curve reached completion.
At [OCoA]⩽1 μM, binding was stimulated to ∼110%.
Figure 4.
Effect of OCoA (10 μM) on [3H]GBC binding to SUR1 in the absence of MgATP. Specific binding (BS), from two individual experiments, is shown as % BMAX. The fit of BS to the Law of Mass action (equation (3) in Methods) gave KD values of 0.36 (0.32, 0.41)/1.5 (1.2,1.8) nM and BMax values of 100 and 83±3% in the absence and presence of OCoA, respectively. 100% BMAX was 3.6 pmol (mg protein)−1. Nonspecific binding increased linearly with [3H]GBC concentration and was 20% of total binding at 7.5 nM [3H]GBC. Inset: Scatchard representation of the data (F denotes the free radioligand concentration, [3H]GBCfree). The control curve was fitted to a straight line with abscissa intercept 100 and gave a slope of −2.7±0.1 nM−1; the curve in the presence of OCoA gave a slope of −0.59±0.05 nM−1 and an abscissa intercept of 88±8%, which is slightly but significantly different from 100%.
The competitive nature of the interaction between OCoA and GBC was confirmed by performing [3H]GBC-GBC competition experiments in the absence and presence of OCoA (15 μM). Figure 5 shows that OCoA reduced [3H]GBC to 30% and shifted the KD of GBC from 0.37 to 2.4 μM, that is, by a factor of 6.5. Application of Equation (4a) gave a value of 2.7 μM for KO, in excellent agreement with the estimate obtained from the saturation experiments (Figure 4). In addition, using Equation (4b), one calculates that at the [3H]GBC concentration used (∼1 nM), the increase in KD for GBC induced by OCoA accounts for a reduction in binding to 40%, in good agreement with the experimental result (30%, Figure 5).
Figure 5.
Inhibition of [3H]GBC binding to SUR1 by GBC in the absence (n=3) and presence (n=4) of OCoA (15 μM). Experiments were performed in the absence of MgATP; [3H]GBC was ∼1 nM, BS ∼300 fmol mg−1 and BNS<10% BTOT. The fit of Equation (1) to the data gave KD values of 0.37 (0.30, 0.47) and 2.4 (2.1, 2.8) nM, respectively, and Hill coefficients ∼1.
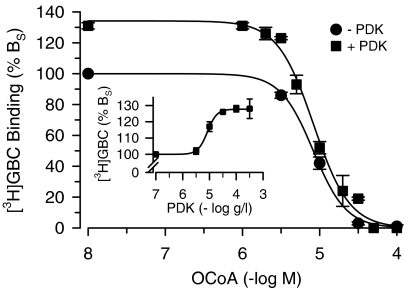
Polyvalent cations reverse the effect of phospholipids on the sensitivity of KATP channels towards ATP but not towards sulphonylureas and openers (see Introduction). We have examined here the effect of PDK as the polyvalent cation on the OCoA-induced inhibition of [3H]GBC binding. Surprisingly, PDK increased specific [3H]GBC binding to SUR1 in the absence of MgATP by up to 28% (Figure 6, inset). To study the effect on OCoA, two PDK concentrations were chosen, 3 and 100 μg ml−1. At 3 μg ml−1, which does not increase [3H]GBC binding, PDK did not affect the inhibition curve of OCoA and the same IC50 value for OCoA was obtained (8.9 μM; not shown). The experiments performed at 100 μg ml−1 PDK, that is, at the plateau of the effect on [3H]GBC binding, are illustrated in Figure 6. PDK alone increased binding to 131±2% of control. OCoA inhibited [3H]GBC binding completely with a IC50 value of ∼8 μM regardless of the absence or presence of PDK. This showed that OCoA was able to inhibit also the increase in [3H]GBC binding induced by PDK and that PDK did not affect the inhibition of [3H]GBC binding by OCoA.
Figure 6.
Effect of poly-D-lysine (PDK) on the inhibition of [3H]GBC binding to SUR1 by OCoA. Experiments (n=5) were performed in the absence of MgATP with membranes sonicated with the OCoA concentration indicated or with buffer before the addition of PDK (0 or 100 μg ml−1); [3H]GBC was ∼1 nM, BS ∼2 pmol mg−1 and BNS<10% BTOT. Individual experiments were analysed according to Equation (1); after averaging, IC50 values of 8.9 (8.1,9.8)/7.8 (6.5, 9.3) nM and Hill coefficients of 2.1±0.2/2.1±0.2 were obtained in the absence/presence of PDK. PDK increased [3H]GBC binding to 131±2% of control. Inset: Increase in [3H]GBC binding by PDK. Evaluation of the concentration dependence (n=4–9 per point) gave the following parameters: EC50=8.3 (7.9,8.7) μg ml−1, maximum increase=28±1%; Hill coefficient=2.5±0.2.
Experiments were also performed in the presence of MgATP (0.3 mM). Under these conditions, the KD value of GBC binding was 2.3 (1.7, 2.9). Comparison with the KD in the absence of MgATP (0.37 nM; Figure 5) showed that MgATP reduced the affinity of GBC binding to SUR1 by a factor of 6 in agreement with earlier experiments (Hambrock et al., 2002; see also Schwanstecher et al. (1991) for experiments in islet membranes). Inhibition of [3H]GBC binding by OCoA is shown in Figure 3 and gave an IC50 value of 15 μM and a Hill coefficient of 2.1 (Table 2). Hence, MgATP rendered the inhibition curve steeper and induced a rightward shift. The mechanism of inhibition of OCoA on [3H]GBC binding in the presence of MgATP was investigated in experiments analogous to those presented in Figure 5. In the absence of OCoA, GBC inhibited [3H]GBC (2 nM) binding with KD=2.3 (1.7, 2.9) nM; the presence of OCoA (25 μM) reduced binding to 30% of control and shifted the KD value to 5.6 (5.4,5.9) nM (n=4, data not illustrated). Using Equation (4b), one calculates from these values a reduction in binding to 60%, which is less than the experimental value of 30%. This shows that in the presence of MgATP, the OCoA-induced increase in KD is not sufficient to explain the observed inhibition. Hence, the inhibitory mechanism of OCoA is noncompetitive and both the affinity of GBC binding and the number of GBC sites are decreased. The high value of the Hill coefficient also indicates that, in the presence of MgATP, the inhibitory mechanism of OCoA is complex.
OCoA and radioligand binding to SUR2
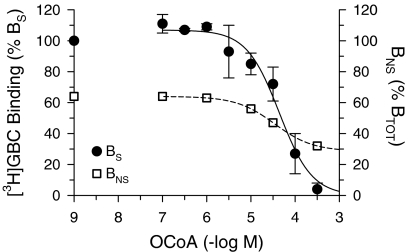
In order to study the interference of OCoA with [3H]GBC binding to SUR2A, the mutant SUR2A(Y1206S) was used; all experiments were performed in the presence of MgATP (0.3 mM). At low concentrations (0.1 μM), OCoA stimulated [3H]GBC binding by ∼10%; at higher concentrations, inhibition was observed with an IC50 value of 44 μM (Figure 7). The small size of the stimulatory effect together with a marked variability in size and the high level of nonspecific binding in these experiments (∼65%) prevented a detailed investigation of this phenomenon. The inhibitory effect of OCoA on [3H]GBC binding was slightly alleviated by PDK (100 μg ml−1). OCoA (30 μM) alone decreased specific [3H]GBC (2.1 nM) binding to SUR2A(Y1206S) to 46±8% of control; addition of PDK increased binding to 67±11% (P<0.05; corrected for a PDK-induced increase in binding to 124±6% of control and taking propagation of errors into account).
Figure 7.
Effect of OCoA on specific (BS) and nonspecific (BNS) binding of [3H]GBC to SUR2A(Y1206S). Data are means±s.e.m. from six to eight experiments performed in the presence of MgATP (0.3 mM) and at a [3H]GBC concentration of 2.3 nM. BS (solid curve) is normalised to 100% specific binding in the absence of OCoA. Owing to the stimulation of BS at [OCoA]⩽1 μM, the curve starts above 100%. The fit of the Hill equation to the descending part of the individual inhibition curves gave the parameters listed in Table 2. BNS (broken curve): OCoA decreased BNS from 65 to 30% of BTOT (109±6 fmol mg−1) with IC50=35 μM.
OCoA also inhibited [3H]P1075 binding to SUR2A; in this case, an IC50 value of 17 μM was obtained (Table 2). In order to characterise the mechanism of inhibition, [3H]P1075–P1075 inhibition curves were determined in the absence and presence of OCoA (30 μM). OCoA decreased [3H]P1075 (2.2 nM) binding to 30% and shifted the KD value of P1075 from 15 to 48 nM (not illustrated). Inserting these values in Equation (4b) one calculates a reduction in binding to 30%, in agreement with the experimental result and with a competitive inhibition of [3H]P1075 binding by OCoA. From the 3.2-fold shift in the KD of P1075 binding by 30 μM OCoA, one further calculates the KO of OCoA binding to SUR2A in the presence of MgATP to 14 μM. PDK (30 and 100 μM) did not reduce the inhibition (not shown).
Next, the effect of coexpression with Kir6.2 on the inhibition of [3H]P1075 binding to SUR2A by OCoA was examined. In three experiments performed side by side at a radioligand concentration of 2.3 nM, OCoA inhibited [3H]P1075 binding to SUR2A expressed alone with IC50=17 and that to Kir6.2/SUR2A with IC50=30 μM (Table 2). This shows that coexpression weakly protected [3H]P1075 binding to SUR2A against the inhibitory effect of OCoA by a factor of 1.7 (1.4,2.0) (comparison of the normally distributed pIC50 values by t-test, P=0.007).
OCoA also inhibited [3H]P1075 binding to SUR2B with an IC50 value of 12 μM and nH=1.8 (Table 2).
DOGS-NTA and radioligand binding to SUR
DOGS-NTA inhibited binding of both radioligands to SUR subtypes; however, the inhibition was incomplete (Table 3). DOGS-NTA was weakest in inhibiting [3H]GBC binding to SUR1 both in potency (IC50=120 μM) and in efficacy (48%). For inhibition of [3H]GBC binding to SUR2A(Y1206S) and of [3H]P1075 to SUR2A and SUR2B, potencies were between 40 and 60 μM and efficacies between 85 and 88% (Table 3). DOGS-NTA increased specific binding of [3H]P1075 to SUR2A slightly (at 3 μM to 107±2%), an effect not seen with SUR2B (data not illustrated). PDK (100 μM) increased binding of [3H]P1075 to SUR2A to 139±12% but did not weaken the inhibition induced by 100 μM DOGS-NTA (49±8% in the absence and 55±15% in the presence of PDK, respectively; the latter value is corrected for the PDK-produced increase in BS).
Table 3.
Concentration-dependent inhibition of radioligand binding to SURs by DOGS-NTA in the presence of MgATP (0.3 mM)
| SUR | Radioligand | IC50 (μM) | A (% BS) | nH |
|---|---|---|---|---|
| SUR1 | [3H]GBC | 120 (48,280) | 48±11 | 1.5±0.1 |
| SUR2A(YS) | [3H]GBC | 38 (29,50) | 82±11 | 1.0 |
| SUR2Aa | [3H]P1075 | 72 (52,100) | 88±8 | 1.0 |
| SUR2B | [3H]P1075 | 43 (28,65) | 87±4 | 1.0 |
A denotes the extent of inhibition (amplitude), nH the Hill coefficient. Parameters are derived from three to six inhibition curves.
At [DOGS-NTA]=3 μM, binding was stimulated to 107%.
Discussion
We have shown here that OCoA, DOGS-NTA and related compounds modulate the binding of the standard blocker, GBC, and of the standard opener, P1075, to all SUR subtypes. Considering first the inhibition, several observations suggest that this is not an artefact such as denaturation of the protein by high concentrations of amphiphilic compounds; rather, it reflects a specific interaction of the compounds with SUR. In case of OCoA and in the absence of MgATP, the inhibition of [3H]GBC binding to SUR1 was almost completely reversed by increasing the concentration of the radioligand. This is in accordance with a (essentially) competitive interaction between OCoA and [3H]GBC, and it was calculated that OCoA binds to SUR1 with an equilibrium dissociation constant KO∼3 μM. Similarly, the inhibition of [3H]P1075 binding to SUR2A in the presence of MgATP was compatible with an apparent competition between OCoA and [3H]P1075, and KO ∼14 μM was estimated for the binding of OCoA to SUR2A. Two possibilities are envisaged: either OCoA and the radioligands compete for the same site of SUR or they bind to different sites linked by strong negative allosteric coupling. Whatever the case, the important point here is that the inhibition was reversible in the continued presence of OCoA, suggesting a specific interaction of OCoA with SUR. Circumstantial evidence for this comes from the observation that MgATP and coexpression with Kir6.2 shifted the inhibition curve towards higher concentrations.
The binding sites of the lipids on SUR remain to be identified. ABC proteins play an important role in the transport of lipids including phospholipids and long-chain fatty acids; however, the lipid-binding site has remained elusive (Borst & Elferink, 2002; Higgins & Linton, 2004). Looking at multidrug resistance protein 2 (MRP2, ABCC2), which is structurally related to SUR and is involved in the transport of estradiole glucuronide, it has been found that many negatively charged compounds, including GBC, at micromolar concentrations affect transport (Zelcer et al., 2003).
Comparing the potency of the compounds in inhibiting ligand binding to SUR gave the rank order OCoA>DOGS-NTA>oleate>malonyl-CoA>PIP2. From this, it seems that a long fatty acid tail and a strongly charged negative head group are required for potency. The surprisingly weak effect of PIP2 in comparison to DOGS-NTA obviously contradicts this statement and one may speculate that the rather rigid sterical arrangement of the charges in the head of PIP2 does not fit well with the binding pocket of SUR. In any event, the rank order of potency of the lipids interacting with SUR differs substantially from that established for Kir6.2. DOGS-NTA, which is of similar potency to PIP2 when acting on Kir6.2 (Krauter et al., 2001), is considerably more potent than PIP2 when acting on SUR. In addition, the inhibition of ligand binding by DOGS-NTA did not reach completion, with SUR1 being particularly resistant (Table 3), and this suggested an allosteric mechanism of inhibition. Of interest also is the activity of oleate for which an IC50 value of ∼50 μM is estimated for the inhibition of 3HP1075 binding to SUR2A. Oleate, which carries only a single charge (Figure 1), does not open but blocks the cardiac channel at 10 μM (Liu et al., 2001). Regarding the effects of PDK, we found that the agent increased 3HGBC binding to SUR1 and SUR2A(Y1206S) and 3HP1075 binding to SUR2A by ∼30%, an effect too small to allow further characterisation. However, it did not reverse (or only very weakly reduced) the inhibition of radioligand binding by OCoA and DOGS-NTA. The latter is in agreement with the total (Krauter et al., 2001) or partial (Koster et al., 1999) inability of poly-lysine to restore the sensitivity of lipid-modified KATP channels towards sulphonylureas and openers.
A major result of the study is that the concentrations of OCoA and the other compounds required to affect ligand binding to SUR were up to 100 × higher than those active at Kir6.2. Regarding OCoA interacting with Kir6.2, concentrations of 100 and 200 nM are sufficient to prevent run down of KATP channel activity in inside-out patches from mouse β-cells (Larsson et al., 1996) and guinea-pig cardiomyocytes (Liu et al., 2001), and concentrations of 1–10 μM are fully effective in opening the channel closed by ATP (Bränström et al., 1998; Liu et al., 2001; Rohács et al., 2003; Schulze et al., 2003) and in reversing channel closure by GBC (1 μM) (Liu et al., 2001; Schulze et al., 2003). In contrast, the IC50 values for OCoA inhibiting ligand binding to the SUR subtypes ranged from 6 to 44 μM (Table 2). The concentration of acyl-CoA esters in the heart is in the high μM range; however, the esters are almost exclusively localised in the mitochondria (van-der-Vusse et al., 1992). Regarding PIP2 and DOGS-NTA, 10 μM completely reverses or prevents the Kir6.2/SUR2A channel-blocking effect of GBC (10 μM) and the channel-opening effect of P1075 (10 μM) against 1 mM MgATP (Koster et al., 1999; Krauter et al., 2001). Tables 1 and 3 show that for DOGS-NTA, 10–100 × higher concentrations were required to inhibit ligand binding to SUR and that PIP2 was almost inactive in the accessible concentration range.
At concentrations ∼10 × lower than those inducing inhibition, the lipids induced a modest stimulation of [3H]P1075 binding to SUR2A (PIP2: +17%, DOGS-NTA: +7%) and of [3H]GBC binding to SUR2A(Y1206S) (OCoA: +10 %). In experiments done side by side, it was confirmed that the stimulation was confined to the SUR2A subtype and did not occur with SUR2B. This indicates that the carboxy-terminal 42 amino acids, the only difference between the two SUR2 isoforms, are important for this effect. It is recalled that the carboxy-terminal sequence of SUR2A differs substantially from that of SUR2B, which, in turn, resembles that of SUR1 (Isomoto et al., 1996). Unfortunately, the stimulatory effect was small and variable, and therefore it was not amenable to further characterisation.
In conclusion, we have shown here that acyl-CoA esters, lipids and oleate interact with SUR and inhibit the binding of the sulphonylurea, GBC and of the opener, P1075. For the SUR2A subtype, a modest stimulation occurs at concentrations ∼10 × lower than that required for inhibition. The concentrations at which both effects occur are higher than those present in the cytoplasm and which are required for the interaction with Kir6.2, which in turn decreases ATP-sensitivity and decouples Kir6.2 from the input of SUR on channel activity. Therefore, the effects described here are not of physiological relevance. The interaction of lipids with SUR should, however, be taken into account if high concentrations (e.g. OCoA>10 μM) are used. In this case, the lack of response of the channel to sulphonylureas and openers is caused both by the interaction of the lipid with SUR and with Kir6.2.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Qu 100/3-1). We thank Drs Y. Kurachi and Y. Horio (Osaka) for the generous gift of the murine clones of SUR2A, 2B and Kir6.x and to Dr C. Derst for rat SUR1. We are grateful to Dr T. Baukrowitz (Jena) for helpful discussions and for the kind gift of DOGS-NTA. We thank Dr U Russ (Tübingen) for help with the manuscript.
Abbreviations
- BMAX
maximum specific binding of radioligand
- BNS
nonspecific binding
- BS
specific binding
- BTOT
total binding
- GBC
glibenclamide
- DOGS-NTA
1,2-dioleyl-sn-glycero-3-{[N(5-amino-1-carboxypentyl)iminodiacetic acid]succinyl}
- HEK cells
human embryonic kidney 293 cells
- KATP channel
ATP-sensitive K+ channel
- Kir
inwardly rectifying K+ channel
- P1075
N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine
- PDK
poly-D-lysine
- PIP2
phosphatidylinositol-4,5-bisphosphate
- OCoA
oleoyl-coenzyme A
- SUR
sulphonylurea receptor
References
- AGUILAR-BRYAN L., BRYAN J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocrine Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- AGUILAR-BRYAN L., NICHOLS C.G., WECHSLER S.W., CLEMENT IV J.P., BOYD A.E., III, GONZÁLES G., HERRERA-SOZA H., NGUY K., BRYAN J., NELSON D.A. Cloning of the b cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. Correlating structure and function in ATP–sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- ASHCROFT S.J.H., ASHCROFT F.M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- ASHFIELD R., GRIBBLE F.M., ASHCROFT S.J.H., ASHCROFT F.M. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- BAUKROWITZ T., FAKLER B. K-ATP channels gated by intracellular nucleotides and phospholipids. Eur. J. Biochem. 2000;267:5842–5848. doi: 10.1046/j.1432-1327.2000.01672.x. [DOI] [PubMed] [Google Scholar]
- BAUKROWITZ T., SCHULTE U., OLIVER D., HERLITZE S., KRAUTER T., TUCKER S.J., RUPPERSBERG J.P., FAKLER B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- BEVINGTON P.R. Data Reduction and Error Analysis for the Physical Sciences 1969New York: McGraw-Hill; 55–65.92–118 [Google Scholar]
- BORST P., ELFERINK R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- BRÄNSTRÖM R., LEIBIGER I.B., LEIBIGER B., CORKEY B.E., BERGGREN P.-O., LARSSON O. Long chain coenzyme A esters activate the pore-forming subunit (Kir6.2) of the ATP-regulated potassium channel. J. Biol. Chem. 1998;273:31395–31400. doi: 10.1074/jbc.273.47.31395. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS A. Assessing the distribution of parameters in models of ligand–receptor interaction: to log or not to log. Trends Pharmacol. Sci. 1998;19:351–357. doi: 10.1016/s0165-6147(98)01240-1. [DOI] [PubMed] [Google Scholar]
- ENKVETCHAKUL D., NICHOLS C.G. Gating mechanism of KATP channels: function fits form. J. Gen. Physiol. 2003;122:471–480. doi: 10.1085/jgp.200308878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN Z., MAKIELSKI J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- GRIBBLE F.M., PROKS P., CORKEY B.E., ASHCROFT F.M. Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA. J. Biol. Chem. 1998;273:26383–26387. doi: 10.1074/jbc.273.41.26383. [DOI] [PubMed] [Google Scholar]
- GRIBBLE F.M., TUCKER S.J., ASHCROFT F.M. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., KURACHI Y., QUAST U. Mg2+ and ATP dependence of KATP channel modulator binding to the recombinant sulphonylurea receptor, SUR2B. Br. J. Pharmacol. 1998;125:577–583. doi: 10.1038/sj.bjp.0702109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., QUAST U. Glibenclamide binding to sulphonylurea receptor subtypes: dependence on adenine nucleotides. Br. J. Pharmacol. 2002;136:995–1004. doi: 10.1038/sj.bjp.0704801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LÖFFLER-WALZ C., RUSS U., LANGE U., QUAST U. Characterization of a mutant sulfonylurea receptor SUR2B with high affinity for sulfonylureas and openers: differences in the coupling to Kir6.x subtypes. Mol. Pharmacol. 2001;60:190–199. doi: 10.1124/mol.60.1.190. [DOI] [PubMed] [Google Scholar]
- HIGGINS C.F., LINTON K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- HILGEMANN D.W., BALL R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KONDO C., YAMADA M., MATSUMOTO S., HIGASHIGUCHI O., HORIO Y., MATSUZAWA Y., KURACHI Y. A novel sulfonylurea receptor forms with BIR (KIR6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- KOSTER J.C., SHA Q., NICHOLS C.G. Sulfonylurea and K+-channel opener sensitivity of KATP channels – functional coupling of Kir6.2 and SUR1 subunits. J. Gen. Physiol. 1999;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUTER T., RUPPERSBERG J.P., BAUKROWITZ T. Phospholipids as modulators of KATP channels: distinct mechanisms for control of sensitivity to sulphonylureas, K+ channel openers, and ATP. Mol. Pharmacol. 2001;59:1086–1093. [PubMed] [Google Scholar]
- LARSSON O., DEENEY J.T., BRÄNSTRÖM R., BERGGREN P.O., CORKEY B.E. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J. Biol. Chem. 1996;271:10623–10626. doi: 10.1074/jbc.271.18.10623. [DOI] [PubMed] [Google Scholar]
- LIU G.X., HANLEY P.J., RAY J., DAUT J. Long-chain acyl-coenzyme A esters and fatty acids directly link metabolism to KATP channels in the heart. Circ. Res. 2001;88:918–924. doi: 10.1161/hh0901.089881. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MATSUSHITA K., KINOSHITA K., MATSUOKA T., FUJITA A., FUJIKADO T., TANO Y., NAKAMURA H., KURACHI Y. Intramolecular interaction of SUR2 subtypes for intracellular ADP-induced differential control of KATP channels. Circ. Res. 2002;90:554–561. doi: 10.1161/01.res.0000012666.42782.30. [DOI] [PubMed] [Google Scholar]
- ROHÁCS T., LOPES C.M.B., JIN T., RAMDYA P.P., MOLNÁR Z., LOGOTHETIS D.E. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. U.S.A. 2003;100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCATCHARD G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949;51:660–672. [Google Scholar]
- SCHULZE D., RAPEDIUS M., KRAUTER T., BAUKROWITZ T. Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism. J. Physiol. (London) 2003;552:357–367. doi: 10.1113/jphysiol.2003.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWANSTECHER M., LÖSER S., RIETZE I., PANTEN U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:83–89. doi: 10.1007/BF00180681. [DOI] [PubMed] [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DÖRSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C., BRYAN J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEINO S., MIKI T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- SHYNG S.-L., NICHOLS C.G. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- STEPHAN D., STAUSS E., LANGE U., FELSCH H., LÖFFLER-WALZ C., HAMBROCK A., RUSS U., QUAST U. The mutation Y1206S increases the affinity of the sulphonylurea receptor SUR2A for glibenclamide and enhances the effects of coexpression with Kir6.2. Br. J. Pharmacol. 2005;144:1078–1088. doi: 10.1038/sj.bjp.0706142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAPP S., HAIDER S., JONES P., SANSOM M.S., ASHCROFT F.M. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 2003;22:2903–2912. doi: 10.1093/emboj/cdg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER S.J., GRIBBLE F.M., ZHAO C., TRAPP S., ASHCROFT F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- VAN-DER-VUSSE G.J., GLATZ J.F., STAM H.C., RENEMAN R.S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol. Rev. 1992;72:881–940. doi: 10.1152/physrev.1992.72.4.881. [DOI] [PubMed] [Google Scholar]
- ZELCER N., HUISMAN M.T., REID G., WIELINGA P., BREEDVELD P., KUIL A., KNIPSCHEER P., SCHELLENS J.H.M., SCHINKEL A.H., BORST P. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2) J. Biol. Chem. 2003;278:23538–23544. doi: 10.1074/jbc.M303504200. [DOI] [PubMed] [Google Scholar]