Abstract
There is some dispute concerning the extent to which the uptake inhibitor VDM11 (N-(4-hydroxy-2-methylphenyl) arachidonoyl amide) is capable of inhibiting the metabolism of the endocannabinoid anandamide (AEA) by fatty acid amide hydrolase (FAAH). In view of a recent study demonstrating that the closely related compound AM404 (N-(4-hydroxyphenyl)arachidonylamide) is a substrate for FAAH, we re-examined the interaction of VDM11 with FAAH.
In the presence of fatty acid-free bovine serum albumin (BSA, 0.125% w v−1), both AM404 and VDM11 inhibited the metabolism of AEA by rat brain FAAH with similar potencies (IC50 values of 2.1 and 2.6 μM, respectively). The compounds were about 10-fold less potent as inhibitors of the metabolism of 2-oleoylglycerol (2-OG) by cytosolic monoacylglycerol lipase (MAGL).
The potency of VDM11 towards FAAH was dependent upon the assay concentration of fatty acid-free bovine serum albumin (BSA). Thus, in the absence of fatty acid-free BSA, the IC50 value for inhibition of FAAH was reduced by a factor of about two (from 2.9 to 1.6 μM). A similar reduction in the IC50 value for the inhibition of membrane bound MAGL by both this compound (from 14 to 6 μM) and by arachidonoyl serinol (from 24 to 13 μM) was seen.
An HPLC assay was set up to measure 4-amino-m-cresol, the hypothesised product of FAAH-catalysed VDM11 hydrolysis. 4-Amino-m-cresol was eluted with a retention time of ∼2.4 min, but showed a time-dependent degradation to compounds eluting at peaks of ∼5.6 and ∼8 min. Peaks with the same retention times were also found following incubation of the membranes with VDM11, but were not seen when the membranes were preincubated with the FAAH inhibitors URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate) and CAY10401 (1-oxazolo[4,5-b]pyridin-2-yl-9-octadecyn-1-one) prior to addition of VDM11. The rate of metabolism of VDM11 was estimated to be roughly 15–20% of that for anandamide.
It is concluded that VDM11 is an inhibitor of FAAH under the assay conditions used here, and that the inhibition may at least in part be a consequence of the compound acting as an alternative substrate.
Keywords: Anandamide, 2-arachidonoyl glycerol, fatty acid amide hydrolase, monoacylglycerol lipase, AM404, VDM11
Introduction
It is now well established that the endocannabinoid system is involved in a number of physiological processes, including modulation of neurotransmitter release, pain perception, learning and memory, to mention but a few (for a recent review, see Rodríguez de Fonseca et al., 2005). The two most well-established endocannabinoid molecules are anandamide (AEA, arachidonoylethanolamide) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Sugiura et al., 1995; Mechoulam et al., 1995), although other molecules, mainly arachidonoyl derivatives, have been suggested to act as endocannabinoids (see De Petrocellis et al., 2004; Bradshaw & Walker, 2005).
A prerequisite for a signalling molecule is that mechanisms should be present that allow for its removal from the site of action. In the case of AEA and 2-AG, this is achieved by intracellular uptake followed by enzymic metabolism, primarily to arachidonic acid (see Piomelli, 2004). While the enzymes involved (fatty acid amide hydrolase, FAAH, for AEA and, in the brain, monoacylglycerol lipase, MAGL, for 2-AG, Deutsch & Chin, 1993; Dinh et al., 2002) have been well characterised, the transport mechanisms involved are a matter of controversy. In the case of AEA, it was initially suggested that the compound was transported into the cell by a mechanism of facilitated transport (Di Marzo et al., 1994; Hillard et al., 1997), although now a variety of different models have been suggested including sequestration and/or FAAH-driven uptake, intracellular shuttling proteins and endocytosis (Day et al., 2001; Deutsch et al., 2001; Hillard & Jarrahian, 2003; Fowler et al., 2004; McFarland et al., 2004; Ortega-Gutiérrez et al., 2004).
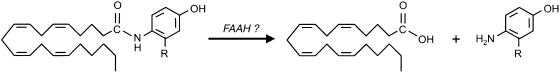
While the molecular mechanism for AEA uptake remains a matter of debate, it is clear that a number of compounds can inhibit its intracellular accumulation, and that these compounds are active in vivo. The first of these compounds, AM404 (N-(4-hydroxyphenyl)arachidonoylamide, structure, see Figure 1) (Beltramo et al., 1997) has been shown to produce a variety of effects in vivo, including potentiation of the effects of a low dose of AEA upon hypothermia (that was blocked by a CB1 cannabinoid receptor antagonist) in FAAH knockout mice (Fegley et al., 2004; for a review of the in vivo properties of AEA uptake inhibitors, see Fowler et al., 2005). AM404, however, has additional effects, including activation of TRPV1 receptors (Zygmunt et al., 2000) and nonspecific effects upon calcium signalling and cell proliferation (Chen et al., 2001; Jonsson et al., 2003; Kelley & Thayer, 2004). It also inhibits the activity of FAAH (Jarrahian et al., 2000), presumably as a result of its ability to act as an alternative FAAH substrate (Fegley et al., 2004; for expected reaction pathway, see Figure 1).
Figure 1.
Schematic drawing of the expected FAAH-catalysed hydrolysis pathway of AM404 and, by extension, VDM11. The R substituent is a hydrogen atom for AM404, and a methyl group for VDM11.
VDM11 (N-(4-hydroxy-2-methylphenyl) arachidonoyl amide) is a closely related analogue of AM404 (see Figure 1) that is equipotent as an uptake inhibitor as AM404 (De Petrocellis et al., 2000) and shares its nonspecific effects upon cell proliferation (Jonsson et al., 2003; see also Kelley & Thayer, 2004). In addition, VDM11 reduces the activity of the AEA synthetising enzyme N-acyl-phosphatidylethanolamine-specific phospholipase D (Fezza et al., 2005), but does not activate TRPV1 receptors (De Petrocellis et al., 2000). In the original study, it was reported that VDM11 was a weak inhibitor of AEA hydrolysis by N18TG2 cell membranes (IC50>50 μM, De Petrocellis et al., 2000). However, in a subsequent study, it was reported that the observed sensitivity of rat brain FAAH to the inhibition by VDM11 was highly dependent upon the assay used: in one method, IC50 values in the range 1.2–3.7 μM were found depending upon the source of the compound and the assay pH used, whereas in the other method 39% inhibition was found at a VDM11 concentration of 50 μM (Fowler et al., 2004).
One possible reason for this assay dependency could be an influence of fatty acid-free bovine serum albumin (BSA) upon the observed inhibitory potency, since this was one of the differences between the two assays. Consistent with this hypothesis is published data for AM404: thus, for this compound, IC50 values (with AEA concentrations given in parentheses) for inhibition of rat brain FAAH of 0.5 μM (0.2 nM), 3.6 μM (2 μM) and 6 μM (30 μM) have been reported from three different laboratories using assays containing fatty acid-free BSA (Jarrahian et al., 2000; Jonsson et al., 2001; Glaser et al., 2003). A subsequent study found that the inhibition was not dependent upon the assay pH (Fowler et al., 2004). In contrast, when N18TG2 cells were used as a source of FAAH and fatty acid-free BSA was not included in the medium, the inhibition of the hydrolysis of 6 μM AEA by AM404 was less potent (IC50 value 22 μM, De Petrocellis et al., 2000). Calculations of substrate concentration-independent Ki values require information both as to the mode of inhibition plus the ratio of substrate concentration to the Km value for AEA assayed under the same conditions. However, Km values reported from other work by the laboratories concerned (see e.g. Maurelli et al., 1995; Jonsson et al., 2001) would suggest that the variation in potency is not related to any great degree to the substrate concentration used.
Although the above discussion would suggest that the assay concentration of fatty acid-free BSA may be an important determinant of the FAAH inhibitory potency of VDM11, it is important to investigate this systematically in a single assay. In addition, the structural similarity of AM404 and VDM11 raise the distinct possibility that the latter compound is also a substrate for FAAH. Finally, in view of the recent finding that VDM11 can increase 2-AG levels in rat thyroid carcinomas (Bifulco et al., 2004), the ability of this compound to inhibit MAGL requires investigation. These aims have been studied here.
Methods
Compounds
Radiolabelled arachidonoylethanolamide ([3H]AEA: [ethanolamine 1-3H], 60 Ci mmol−1, for the FAAH studies and 2-monooleoylglycerol [glycerol-1,2,3-3H] ([3H]2-OG, 20 Ci mmol−1) were obtained from American Radiolabeled Chemicals, Inc. (St Louis, MO, U.S.A.). Nonradioactive AEA, URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate), oleoyltrifluoromethyl ketone (OTMK), arachidonoyl serotonin, CAY10401 (1-oxazolo[4,5-b]pyridin-2-yl-9-octadecyn-1-one, compound 59 of Boger et al., 2000) and arachidonoyl serinol were obtained from the Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). VDM11 and AM404 were purchased from Tocris Cookson (Bristol, U.K.). Non radioactive 2-OG was obtained from the Sigma Chemical Co. (St Louis, MO, U.S.A.). Fatty acid-free BSA was obtained either from Calbiochem-Novabiochem (La Jolla, CA, U.S.A.), or from the Sigma Chemical Co. 4-Amino-m-cresol was obtained from Acros Organics (Geel, Belgium). Cell culture media, sera and supplements were purchased from Invitrogen (Sweden).
Assay of FAAH and MAGL
Cerebella previously obtained from adult Sprague–Dawley rats were used. These were thawed and homogenised at 4°C in sodium phosphate buffer (50 mM, pH 8) containing 0.32 M sucrose. Homogenates were centrifuged at 100,000 × g for 60 min (Figure 2) or 120 min (Figures 3 and 4) at 4°C. The supernatants (‘cytosol fractions') were collected. The pellets were suspended in sodium phosphate buffer (50 mM, pH 8) to give the membrane fractions used in this study. The fractions were stored frozen in aliquots at −70°C until used for assay. Protein concentrations of the fractions were determined by using the method described by Harrington (1990), with BSA as standard.
Figure 2.
Inhibition of membrane-bound [3H]AEA and cytosolic [3H]2-OG hydrolysis by AM404 and VDM11. Data are means±s.e.m. (when not enclosed by the symbols), n=3, of the activities expressed as % of controls treated with the same concentration of vehicle. The assays contained 0.125% fatty acid-free BSA (from Calbiochem). The substrate concentrations were 2 μM.
Figure 3.
HPLC chromatograms showing the metabolism of 4-amino-m-cresol (100 μM, Panels a–c) and VDM11 (400 μM, panels d–f). Rat cerebellar membranes (100 μg protein) were incubated with the compounds at 37°C and pH 7.2 for either 0 min (a), 120 min (d) or 24 h (b, c, e, f) prior to chloroform:methanol extraction and HPLC analysis. In panels (c) and (f), the membranes were preincubated for 15 min with 3 μM URB597 prior to addition of 4-amino-m-cresol or VDM11. The traces were scanned into a computer after which the contrast was increased and the retention times that were originally printed on the traces were removed digitally.
Figure 4.
HPLC chromatograms showing the metabolism of VDM11 (400 μM) following preincubation with CAY10401 at concentrations of 10 nM (a), 100 nM (b) or 1 μM (c). For experimental and digital treatment details, see legend to Figure 3. The retention times were slightly longer in these experiments, but this was also seen for a sample run concurrently but with 4-amino-m-cresol (100 μM) rather than VDM11 (data not shown).
Rates of hydrolysis of [3H]AEA (labelled in the ethanolamine part of the molecule) and [3H]2-OG (labelled in the glycerol part of the molecule) as substrates for FAAH and MAGL, respectively, were determined essentially as described by Omeir et al. (1995) and Dinh et al. (2002). Briefly, aliquots (165 μl) of membrane (1.5–3 μg assay−1) or cytosol (1–2 μg assay−1) fractions, diluted to the appropriate assay protein concentrations in Tris-HCl buffer (10 mM, pH 7.2) containing 1 mM EDTA, were added to glass tubes containing 10 μl of test compound. Blanks contained assay buffer instead of membrane or cytosol samples. Substrate (25 μl, final concentration 2 μM) was then added and the samples were incubated for 10 min at 37°C. The substrate solution contained fatty acid-free BSA, when appropriate. The fatty acid-free BSA concentrations given in the text are the final assay concentrations. For the experiments using membrane fractions and [3H]2-OG, the samples were coincubated with 3 μM URB597 in order to inhibit the FAAH. After the incubation phase, reactions were stopped by adding 400 μl chloroform: methanol (1/1 v v−1), vortex mixing the tubes two times and placing them on ice. Phases were separated by centrifugation (10 min, 2500 r.p.m.), and 200 μl aliquots of the methanol/buffer phase were taken and measured for tritium content by liquid scintillation spectroscopy with quench correction. This assay is the same as that used in the previous study from this laboratory on the pharmacology of MAGL (Ghafouri et al., 2004), although unfortunately the description of the method in that paper inadvertantly omitted to indicate the fatty acid-free BSA concentration used (0.125% w v−1).
Metabolic stability of VDM11
The membrane fractions (100 μg of protein) from the cerebella of adult Sprague–Dawley rats were incubated at 37°C in Tris-HCl buffer (10 mM, pH 7.2) containing either ethanol, 50–100 μM 4-amino-m-cresol or 400 μM VDM11 and incubated at 37°C for the times shown in the text and figures. Reactions were stopped by adding 400 μl chloroform: methanol (1 : 1 v v−1). The phases were separated by centrifugation (10 min, 2500 r.p.m.). When FAAH inhibitors were used, they were preincubated with the membranes for 15 min at 37°C prior to addition of 4-amino-m-cresol or VDM11. Aliquots (20 μl) of the methanol/buffer phase were then injected to the high-performance liquid chromatography (HPLC) system. The system used comprised of a pump (constametric Model III, laboratory data control, FL, U.S.A.), and a UV absorbance detector (Waters 486, France), which was set at 250 nm (4-amino-m-cresol shows highest UV absorbance at 250 nm). Chromatographic separations were performed with the Chromolith Performance RP-18e 4.6 × 100 mm column from Merck (Darmstadt, Germany). The mobile phase consisted of water/acetonitrile (95 : 5 v v−1) and the flow rate was 2.0 ml min−1. Injection volume was 20 μl. In these conditions, unchanged 4-amino-m-cresol is detected with a retention time of ∼2.4 min and a detection limit of 20 ng, corresponding to a concentration of ∼8 μM.
Analysis of data
For the MAGL and FAAH assays, the pooled data for each test compound, expressed as % of control activity containing the same carrier concentration, was analysed using the built-in equation ‘sigmoidal dose–response (variable slope)' of the GraphPad Prism computer programme (GraphPad Software Inc., San Diego, CA, U.S.A.) with ‘top' and ‘bottom' values set to 100 and 0%, respectively.
Results
Comparison of the effect of AM404 and VDM11 upon FAAH and MAGL
Initial experiments, undertaken in the presence of 0.125% fatty acid-free BSA, investigated the relative potencies of AM404 and VDM11 towards FAAH and cytosolic MAGL. Both compounds showed very similar potency profiles, with a ∼10-fold selectivity for FAAH (IC50 values of 2.1 and 2.6 μM for AM404 and VDM11, respectively) over cytosolic MAGL (IC50 values of 20 and 21 μM for AM404 and VDM11, respectively) (Figure 2).
In Table 1, a comparison has been made between the sensivitity of FAAH to inhibition by high concentrations of VDM11 and a battery of FAAH inhibitors using a low (2 μM) and a high concentration of [3H]AEA (nominal 400 μM, see legend to Table 1). The assays were undertaken in the presence of 0.125% fatty acid-free BSA. At the low concentration of [3H]AEA, the FAAH inhibitors inhibited the enzyme in a manner consistent with their reported potencies (see Bisogno et al., 1998; Boger et al., 2000; Kathuria et al., 2003; Ghafouri et al., 2004). VDM11 behaved like CAY10401, arachidonoyl serotonin and OTMK in that the sensitivity to inhibition was greater at the lower [3H]AEA concentration. Indeed, for arachidonoyl serotonin (30 μM) and oleoyltrifluoromethyl ketone (3 μM), complete inhibition of hydrolysis at the low [3H]AEA concentration was seen, whereas little or no inhibiton was seen, at the high [3H]AEA concentration (Table 1). These data are consistent with the suggestion that VDM11 and these reversible inhibitors are either competitive or mixed in their mode of action, as has been shown previously for arachidonoyl serotonin (Bisogno et al., 1998). In contrast, little dependency upon the assay concentration of [3H]AEA was seen for URB597 (Table 1), which is reported to act as an irreversible or alternatively tight binding inhibitor of FAAH (Kathuria et al., 2003).
Table 1.
Inhibition of FAAH by high concentrations of VDM11 and a battery of FAAH inhibitors
| FAAH activity (% of control) | ||
|---|---|---|
| [3H]AEA assay concentration | 2 μM | 400 μMa |
| VDM11 | ||
| 400 μM | 0.3±3 | 45±3 |
| URB597 | ||
| 10 nM | 49±3 | 66±6 |
| 100 nM | 4±2 | 14±1 |
| 1 μM | −0.2±2 | 7±0.8 |
| 3 μM | 8±9 | 12±0.3 |
| Arachidonoyl serotonin | ||
| 3 μM | 35±5 | 95±0.4 |
| 30 μM | 4±1 | 91±2 |
| CAY10401 | ||
| 10 nM | 6±1 | 40±2 |
| 100 nM | −0.1±1 | 9±0.4 |
| 1 μM | 6±0.8 | 8±1 |
| 10 μM | 1±1 | 7±0.5 |
| OTMK | ||
| 3 μM | 0±3 | 78±4 |
| 10 μM | 0±4 | 57±4 |
| 50 μM | −2±4 | 24±4 |
| 100 μM | 9±3 | 21±1 |
Data are means±s.e.m. from analyses of data from three preparations. The compounds were preincubated with the membrane preparations for 15 min prior to addition of substrate and further incubation. For 2 μM [3H]AEA, the incubation time and membrane protein concentrations were 10 min and 2 μg assay−1, respectively. For 400 μM [3H]AEA, the incubation time and membrane protein concentrations were 60 min and 100 μg assay−1, respectively.
This concentration is probably an overestimate, due to the limited solubility of AEA.
Effect of BSA upon the sensitivity of FAAH and MAGL to inhibition by VDM11
Two sources of fatty acid-free BSA were used. Analyses of two experiments gave pI50 values of 5.80, 5.66, 5.49, 5.58 and 5.54 for 0, 0.0125% (Sigma), 0.0375% (Sigma), 0.125% (Sigma) and 0.125% (Calbiochem) w v−1 fatty acid-free BSA, respectively. Thus, the addition of fatty acid-free BSA from either source produced a ∼50% reduction in the potency of VDM11 towards FAAH. It should be pointed out that in the absence of BSA, the concentration of [3H]AEA is nominal rather than exact, since the ‘stickiness' of this compound will reduce the absolute concentration (see Karlsson et al., 2004). Indeed, the added radioactivity was 62±5 and 64±5% of that seen when 0.125% (Sigma) and 0.125% (Calbiochem) w v−1 fatty acid-free BSA, respectively, were present. This reduction was not seen for [3H]2-OG, where the added radioactivity was 91±4 and 89±3% of that seen when 0.125% (Sigma) and 0.125% (Calbiochem) w v−1 fatty acid-free BSA, respectively, were present. In the remaining experiments described below, 0.125% fatty acid-free BSA from Calbiochem was used.
The effect of fatty acid-free BSA upon the potency of VDM11 towards MAGL was also determined. In this case, membranes were used, in order to provide more information as to the pharmacology of this enzyme. 2-AG (and presumably therefore 2-OG) can be metabolised by FAAH (Goparaju et al., 1998), and so it is important to ensure that FAAH is completely inhibited in these experiments. To this end, the membranes were coincubated with 3 μM URB597, since this compound does not affect MAGL activity (Kathuria et al., 2003). Under these conditions, the rate of [3H]2-OG metabolism was 77±3% (no fatty acid-free BSA) and 95±3% (fatty acid-free BSA, means±s.e.m., n=9) of the activity seen in concomitant assays run without URB597. Thus, at the concentration of [3H]2-OG used, FAAH is responsible for only a small fraction of the metabolism in the membrane fractions, a result in line with the study of Saario et al. (2004), who used 2-AG as substrate. Under these conditions, VDM11 inhibited membrane MAGL with pI50 values (IC50 values given in brackets) of 5.22±0.06 (6 μM) and 4.86±0.05 (14 μM) in the absence and presence of 0.125% w v−1 fatty acid-free BSA, respectively (data not shown). The corresponding values for arachidonoylserinol were 4.93±0.03 (12 μM) and 4.61±0.03 (24 μM), respectively (data not shown). Thus, for both VDM11 and arachidonoylserinol, the inhibitory potency was about two-fold higher in the absence of fatty acid-free BSA than in its presence. This would suggest that the ‘BSA shift' is not unique to VDM11, but is found at least with other arachidonoyl-based compounds.
Metabolic stability of VDM11
FAAH-catalysed hydrolysis of VDM11 would be expected to yield arachidonic acid and 4-amino-m-cresol (Figure 1). An HPLC assay was therefore set up to detect 4-amino-m-cresol. Under the assay conditions used, 4-amino-m-cresol was detected with a retention time of about 2.4 min (detection limit 20 ng). Addition of 100 μM 4-amino-m-cresol to a membrane preparation (100 μg protein) and immediate assay gave the expected peak, together with minor peaks at ∼5.6 and ∼11 min retention times (Figure 3a). The ∼11 min retention time was also seen for samples to which ethanol carrier rather than 4-amino-m-cresol had been added (data not shown). When the gain was reduced to be able to quantitate the early peak, it was found that the ratio of the two peaks changed as the samples were incubated further. Thus, the AUC values for the ∼5.6 min peak as a % of the AUC values of the ∼2.4 min peak were 8, 13, 19, 30 and 42% following incubation of the membranes for 0, 10, 30, 60 and 120 min (data not shown). The peak at ∼5.6 min was also seen if the solution of 4-amino-m-cresol used in the experiments was left overnight at room temperature and then injected into the HPLC (data not shown) and so presumably represents a nonenzymatic oxidation product of this compound. At the 120 min time point, an additional peak was noted at a retention time of around 8 min (data not shown). In two separate experiments, membrane fractions (100 μg protein) were incubated with the 4-amino-m-cresol for 24 h. In both cases, the major peak was now the ∼8 min peak (see Figure 3b). This peak was not seen when 4-amino-m-cresol was incubated for 24 h with distilled water at 37°C, but was seen when it was incubated with the tris buffer, pH 7.2, suggesting once again a nonenzymic oxidation product. No such peaks were seen with either water, buffer or membranes incubated with ethanol alone (data not shown). Preincubation of the membranes with 3 μM URB597 for 15 min prior to addition of 4-amino-m-cresol did not affect the major peaks (Figure 3c).
Addition of VDM11 (400 μM) to the membranes and incubation gave no obvious peaks other than the ∼11 min peak for incubation times of 0 or 10 min (data not shown). At 30 and 60 min of incubation, however, peaks at retention times of ∼2.4 min were seen, and by 120 min a small additional peak at ∼5.4 min was seen (Figure 3d). Three preparations were incubated for 120 and 180 min with 400 μM VDM11, and the peak at ∼2.4 min was quantitated and compared with the values seen for the combined ∼2.4 and ∼5.4 min peaks for incubation of the same preparations for 0 min with 50 μM 4-amino-m-cresol followed by extraction and assay. From these data, the rate of breakdown of VDM11 was determined to be 160±12 pmol min−1 mg protein−1 (data not shown). For comparison, the rate of hydrolysis of 2 μM [3H]AEA for the control samples for Table 1 was 560±46 pmol min−1 mg protein−1.
Although incubations of 1–3 h were sufficient to demonstrate metabolism of VDM11, the peaks were too small to be able to undertake studies of inhibitor sensitivity of VDM11. In consequence, long incubation times (24 h) were used. Under these conditions, clear peaks were seen (Figure 3e) at the same retention times as were seen when 4-amino-m-cresol was added to the samples. These peaks were not seen when either water or buffer was incubated with the VDM for 24 h at 37°C (data not shown), or, more importantly, when the membranes were preincubated for 15 min with 3 μM URB597 prior to the addition of VDM11 and incubation for a further 24 h (Figure 3f). Preliminary experiments indicated that 1 μM URB597 also produced a complete blockade of VDM11 metabolism, whereas residual metabolic activity was seen at lower concentrations (10 and 100 nM) of URB597(data not shown).
Although URB597 is highly selective for FAAH vs cannabinoid receptors and MAGL (Kathuria et al., 2003), it has been reported to inhibit other serine hydrolase enzymes (Lichtman et al., 2004), raising the possibility that a URB597-sensitive activity unrelated to FAAH may be involved in the metabolism of VDM11. In consequence, the battery of FAAH inhibitors shown in Table 1 were tested for their ability to inhibit VDM11 metabolism. Given that the assay concentration of VDM11 is high relative to its potency (and hence predicted Km value) towards FAAH, the sensitivity to inhibition by these compounds should be compared with the data for the high concentration of [3H]AEA shown in Table 1. The results were found to be consistent with these data. Thus, CAY10401 produced a concentration-dependent inhibition of VDM11 metabolism with a complete blockade being seen at a concentration of 1 μM (Figure 4), 10, 50 and 100 μM (data not shown). OTMK (100 μM) also reduced the peaks due to VDM11 metabolism, whereas no obvious inhibition of VDM11 metabolism was seen with either 3 or 30 μM arachidonoyl serotonin, or with 1 or 3 μM OTMK (data not shown). None of the compounds affected, at the highest concentrations used, the peaks produced by 4-amino-m-cresol (data not shown).
Discussion
The present study has three conceptually simple aims, namely to determine (a) whether the difference in sensitivity of FAAH towards VDM11 seen in different laboratories is related to the presence or absence of fatty acid-free BSA in the assay; (b) whether VDM11, like AM404, is a substrate for FAAH and (c) whether VDM11 inhibits MAGL. From the data presented here, it is clear that the presence of fatty acid-free BSA gives a lower sensititivity of FAAH (and MAGL) towards VDM11, which may be to a certain extent due to the higher assay AEA concentration when binding of this very sticky molecule to pipette tips, etc. is avoided. However, this problem did not appear to occur for 2-OG, but the same shift in potency was seen for MAGL, so presumably the change in the absolute assay substrate concentration is not the whole explanation for the effect of fatty acid-free BSA. Given that BSA binds very potently to arachidonate groups (Bojesen & Bojesen, 1994; Bojesen & Hansen, 2003), it is not unreasonable to suppose that the BSA can also bind to the arachidonoyl moiety of VDM11 and thereby reduce its free concentration, although further experiments would be needed to prove this point. In any case, since the assays reported in the literature that give the highest sensitivity of FAAH to inhibition by VDM11 and AM404 were those that contained fatty acid-free BSA (see Introduction), the absence or presence of this agent in the assay is most clearly not the explanation for the interassay variation. The finding that the potency of VDM11 as an inhibitor of FAAH is reduced when the substrate concentration is increased also can be ruled out as a major explanantion of the variation (although a competitive mode of inhibition would be consistent with VDM11 acting as a competing substrate, see below) since the substrate concentrations used relative to the Km values reported previously by the same laboratories were not that different (see Maurelli et al., 1995; Jonsson et al., 2001). The variation therefore remains an unexplained phenomenon.
On a more positive note, the present study has provided data on the interaction of VDM11 and AM404 with MAGL, and provides evidence that VDM11 indeed is a substrate for FAAH. With respect to the latter, the present study demonstrates that incubation of VDM11 with cerebellar membranes results in a pattern of HPLC peaks that are (a) dependent upon the activity of FAAH, since they are prevented by URB597 and CAY10401 and (b) have the same retention times as the pattern of HPLC seen with the putative VDM11 breakdown product 4-amino-m-cresol. 4-Amino-m-cresol is well known to be easily oxidated and to produce a number of metabolites when incubated with biological material (Eggenreich et al., 2004), so the multiplicity of peaks is not surprising. It can be argued that the long incubation time and the high VDM11 concentration used render the data of limited relevance. However, these were a necessity due to the relatively high detection limit for 4-amino-m-cresol, and there is measurable metabolism of VDM11 that can be seen by 60 min. From incubations of 120 and 180 min, the rate of breakdown of 400 μM VDM11 was estimated at 160 pmol mg protein−1 min−1. This value can be compared with the rate of hydrolysis of 2 μM [3H]AEA seen in the preparations (560 pmol mg protein−1 min−1). Given that the Km for AEA in our hands is ∼1 μM (see e.g. Jonsson et al., 2001), and assuming that at such a high VDM11 concentration (relative to its affinity towards FAAH) the Vmax value is measured, the present data would suggest that the Vmax for VDM11 metabolism is about 15–20% of that for AEA. By comparison, myristic-, palmitic- and oleic-amides (100 μM) are metabolised by rat FAAH expressed in COS-7 cells at rates of 5.8, 9.9 and 24%, respectively, of that of 100 μM AEA (Cravatt et al., 1996). This would suggest that the rate of VDM11 metabolism by FAAH, although clearly not as good as for AEA, is within the range seen for other alternative substrates for this enzyme.
In their study, Fegley et al. (2004) measured the concentrations of AM404 itself by HPLC/MS and could show that low added amounts of AM404 (0.1–1 nmol) were effectively removed following incubation for 30 min with membranes from wild-type, but not from FAAH−/− mice. Their study showing FAAH-dependent loss of substrate and our study showing URB597 and CAY10401-sensitive appearance of postulated product thus complement each other rather well. Of course, it is not possible to compare the relative rates of VDM11 and AM404 as substrates for FAAH, since different methodologies were used. Nevertheless, regardless of their absolute kcat values, the fact that both compounds act in a manner consistent with their being FAAH substrates means that they will by definition interact with FAAH and would thereby be expected to reduce the metabolism of AEA as a result of substrate competition. Whether or not this explains completely their mode of inhibition awaits elucidation.
With respect to the interaction of AM404 and VDM11 with MAGL, Saario et al. (2004) recently reported that 1 mM AM404 did not inhibit the hydrolysis of 50 μM 2-AG (assayed in the presence of 0.5% BSA) by rat cerebellar membrane fractions assayed at 25°C. In contrast, we found here a complete inhibition of 2 μM 2-OG metabolism by cytosolic MAGL following incubation with 100 μM AM404. A similar divergency in sensitivity was seen with arachidonoyltrifluoromethylketone, which in their hands inhibited membrane-bound 2-AG metabolism with an IC50 value of 66 μM (Saario et al., 2004), whereas we found it to be more potent in our assay (IC50 value of 2.9 μM towards cytosolic 2-OG metabolism) (Ghafouri et al., 2004), as did Dinh et al. (2002) (IC50 value of 2.9 μM towards cytosolic 2-OG metabolism). This would suggest that the variations seen in assay sensitivity for FAAH are also apparent with MAGL, underlining the requirement that comparisons between compounds are made in the same laboratory.
The ability of AM404 and VDM11 to interact with MAGL is worthy of comment. The fact that they can interact does not mean that they are substrates, unlike the situation for FAAH – indeed, in our hands, AEA (which is not metabolised by MAGL, Dinh et al., 2002) inhibits 2-OG metabolism by soluble fractions from rat cerebella with an IC50 value of 60 μM (i.e. considerably lower than the affinity towards FAAH), and a similar value was seen for arachidonic acid (Ghafouri et al., 2004). A similar situation is presumably operative for AM404 and VDM11. Of course, it is possible that, by reducing the rate of 2-AG metabolism, these compounds may interefere indirectly with 2-AG reuptake, but the sensitivities of 2-AG uptake and/or 2-AG levels to these compounds (Bifulco et al., 2004; Hájos et al., 2004; Melis et al., 2004) are far more likely to reflect an action upon the uptake process itself.
A final note concerns the relevance of the present data to the vexed question of the mechanisms of AEA uptake. It was not the intention of the paper to shed light on this issue, merely to determine whether or not VDM11 interacts with endocannabinoid-metabolising enzymes. It is clear that while FAAH is important in the uptake process (Day et al., 2001; Deutsch et al., 2001), it is by no means the only mechanism involved, since AEA uptake and in vivo actions of AEA uptake inhibitors can be demonstrated in FAAH−/− mice (Fegley et al., 2004; Ligresti et al., 2004; Ortega-Gutiérrez et al., 2004), and since compounds like UCM707 and OMDM-2, which only weakly interact with FAAH (regardless of the assay used) (López-Rodríguez et al., 2003; Ortar et al., 2003; Fowler et al., 2004), can potentiate the effects of AEA in vivo (de Lago et al., 2002; 2004). Clearly, the debate concerning this elusive transporter will continue.
Acknowledgments
We are grateful to Britt Jacobsson and Ingrid Persson for expert technical assistance with the FAAH/MAGL assays shown in Figure 2. We are also indebted to Emma Söderström for her help in the revitalisation of our somewhat antiquated HPLC apparatus. SV was supported by a post-doctoral fellowship from the foundation Wenner-Grenska Samfundet and an FSR (Fonds spécial de recherche) from the catholic university of Louvain. The research was also supported by grants from the Swedish Research Council (Grant no. 12158, medicine), Konung Gustav V's and Drottning Victorias Foundation, Stiftelsen för Gamla Tjänarinnor, Gun and Bertil Stohne's Foundation and the Research Funds of the Medical Faculty, Umeå University.
Abbreviations
- AEA
arachidonoylethanolamide (anandamide)
- 2-AG
2-arachidonoylglycerol
- AM404
N-(4-hydroxyphenyl)arachidonylamide
- BSA
bovine serum albumin
- CAY10401
1-oxazolo[4,5-b]pyridin-2-yl-9-octadecyn-1-one
- FAAH
fatty acid amide hydrolase
- 2-OG
2-oleoylglycerol
- OTMK
oleoyltrifluoromethyl ketone
- URB597, 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate, VDM11
N-(4-hydroxy-2-methylphenyl) arachidonoyl amide
References
- BELTRAMO M., STELLA N., CALIGNANO A., LIN S.Y., MAKRIYANNIS A., PIOMELLI D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- BIFULCO M., LAEZZA C., VALENTI M., LIGRESTI A., PORTELLA G., DI MARZO V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MELCK D., DE PETROCELLIS L., BOBROV M.Y., GRETSKAYA N.M., BEZUGLOV V.V., SITACHITTA N., GERWICK W.H., Di Marzo V. Arachidonoylserotonin and other novel inhibitors of fatty acid amide hydrolase. Biochem. Biophys. Res. Commun. 1998;248:515–522. doi: 10.1006/bbrc.1998.8874. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., HEDRICK M.P., FECIK R.A., MIYAUCHI H., WILKIE G.D., AUSTIN B.J., PATRICELLI M.P., CRAVATT B.F. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOJESEN I.N., BOJESEN E. Binding of arachidonate and oleate to bovine serum albumin. J. Lipid Res. 1994;35:770–778. [PubMed] [Google Scholar]
- BOJESEN I.N., HANSEN H.S. Binding of anandamide to bovine serum albumin. J. Lipid Res. 2003;44:1790–1794. doi: 10.1194/jlr.M300170-JLR200. [DOI] [PubMed] [Google Scholar]
- BRADSHAW H.B., WALKER J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN W.-C., HUANG J.-K., CHENG J.-S., TSAI J.C.-R., CHIANG A.-J., CHOU K.-J., LIU C.-P., JAN C.-R. AM-404 elevates renal intracellular Ca2+, questioning its selectivity as a pharmacological tool for investigating the anandamide transporter. J. Pharmacol. Toxicol. Meth. 2001;45:195–198. doi: 10.1016/s1056-8719(01)00148-4. [DOI] [PubMed] [Google Scholar]
- CRAVATT B.F., GIANG D.K., MAYFIELD S.P., BOGER D.L., LERNER R.A., GILULA N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- DAY T.A., RAKHSHAN F., DEUTSCH D.G., BARKER E.L. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol. Pharmacol. 2001;59:1369–1375. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- DE LAGO E., FERNÁNDEZ-RUIZ J., ORTEGA-GUTIÉRREZ S., VISO A., LÓPEZ-RODRÍGUEZ M.L., RAMOS J.A. UCM707, a potent and selective inhibitor of endocannabinoid uptake, potentiates hypokinetic and antinociceptive effects of anandamide. Eur. J. Pharmacol. 2002;449:99–103. doi: 10.1016/s0014-2999(02)01996-9. [DOI] [PubMed] [Google Scholar]
- DE LAGO E., LIGRESTI A., ORTAR G., MORERA E., CABRANES A., PRYCE G., BIFULCO M., BAKER D., FERNANDEZ-RUIZ J.J., DI MARZO V. In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur. J. Pharmacol. 2004;484:249–257. doi: 10.1016/j.ejphar.2003.11.027. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DI MARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., CASCIO M.G., DI MARZO V. The endocannabinoid system: a general view and latest additions. Br. J. Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH D.G., CHIN S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GLASER S.T., HOWELL J.M., KUNZ J.S., PUFFENBARGER R.A., HILLARD C.J., ABUMRAD N. The cellular uptake of anandamide is coupled to its breakdown by fatty acid amide hydrolase (FAAH) J. Biol. Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.G., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- DINH T.P., CARPENTER D., LESLIE F.M., FREUND T.F., KATONA I., SENSI S.L., KATHURIA S., PIOMELLI D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGENREICH K., GOLOUCH S., TÖSCHER B., BECK H., KUEHNELT D., WINTERSTEIGER R. Determination of 4-amino-m-cresol and 5-amino-o-cresol and metabolites in human keratinocytes (HaCaT) by high performance liquid chromatography with DAD and MS detection. J. Biochem. Biophys. Meth. 2004;61:23–34. doi: 10.1016/j.jbbm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- FEGLEY D., KATHURIA S., MERCIER R., LI C., GOUTOPOULOS A., MAKRIYANNIS A., PIOMELLI D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEZZA F., GASPERI V., MAZZEI C., MACCARRONE M. Radiochromatographic assay of N-acyl-phosphatidylethanolamine-specific phospholipase D activity. Anal. Biochem. 2005;339:113–120. doi: 10.1016/j.ab.2004.12.005. [DOI] [PubMed] [Google Scholar]
- FOWLER C.J., HOLT S., NILSSON O., JONSSON K.-O., TIGER G., JACOBSSON S.O.P.The endocannabinoid signaling system: pharmacological and therapeutic aspects Pharmacol. Biochem. Behav. 2005. in press [DOI] [PubMed]
- FOWLER C.J., TIGER G., LIGRESTI A., LÓPEZ-RODRÍGUEZ M.L., DI MARZO V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur. J. Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- GHAFOURI N., TIGER G., RAZDAN R.K., MAHADEVAN A., PERTWEE R.G., MARTIN B.R., FOWLER C.J. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br. J. Pharmacol. 2004;143:774–784. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER S., ABUMRAD N., FATADE F., KACZOCHA M., STUDHOLME K., DEUTSCH D. Evidence against the presence of an anandamide transporter. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOPARAJU S.K., UEDA N., YAMAGUCHI H., YAMAMOTO S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., KATHURIA S., DINH T., PIOMELLI D., FREUND T.F. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur. J. Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- HARRINGTON C.R. Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determination on fractions from brain tissue. Anal. Biochem. 1990;186:285–287. doi: 10.1016/0003-2697(90)90081-j. [DOI] [PubMed] [Google Scholar]
- HILLARD C.J., EDGEMOND W.S., JARRAHIAN A., CAMPBELL W.B. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J. Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- HILLARD C.J., JARRAHIAN A. Cellular accumulation of anandamide: consensus and controversy. Br. J. Pharmacol. 2003;140:802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARRAHIAN A., MANNA S., EDGEMOND W.S., CAMPBELL W.B., HILLARD C.J. Structure–activity relationships among N-arachidonoylethanolamine (anandamide) head group analogues for the anandamide transporter. J. Neurochem. 2000;74:2597–2606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- JONSSON K.-O., ANDERSSON A., JACOBSSON S.O.P., VANDEVOORDE S., LAMBERT D.M., FOWLER C.J. AM404 and VDM 11 non-specifically inhibit C6 glioma cell proliferation at concentrations used to block the cellular accumulation of the endocannabinoid anandamide. Arch. Toxicol. 2003;77:201–207. doi: 10.1007/s00204-002-0435-6. [DOI] [PubMed] [Google Scholar]
- JONSSON K.-O., VANDEVOORDE S., LAMBERT D.M., TIGER G., FOWLER C.J. Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide. Br. J. Pharmacol. 2001;133:1263–1275. doi: 10.1038/sj.bjp.0704199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSSON M., PÅHLSSON C., FOWLER C.J. Reversible, temperature-dependent, and AM404-inhibitable adsorption of anandamide to cell culture wells as a confounding factor in release experiments. Eur. J. Pharm. Sci. 2004;22:181–189. doi: 10.1016/j.ejps.2004.03.009. [DOI] [PubMed] [Google Scholar]
- KATHURIA S., GAETANI S., FEGLEY D., VALIÑO F., DURANTI A., TONTINI A., MOR M., TARZIA G., LA RANA G., CALIGNANO A., GIUSTINO A., TATTOLI M., PALMERY M., CUOMO V., PIOMELLI D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- KELLEY B.G., THAYER S.A. Anandamide transport inhibitor AM404 and structurally related compounds inhibit synaptic transmission between rat hippocampal neurons in culture independent of cannabinoid CB1 receptors. Eur. J. Pharmacol. 2004;496:33–39. doi: 10.1016/j.ejphar.2004.06.011. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., LEUNG D., SHELTON C.C., SAGHATELIAN A., HARDOUIN C., BOGER D.L., CRAVATT B.F. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- LIGRESTI A., MORERA E., VAN DER STELT M., MONORY K., LUTZ B., ORTAR G., DI MARZO V. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem. J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÓPEZ-RODRÍGUEZ M.L., VISO A., ORTEGA-GUTIÉRREZ S., FOWLER C.J., TIGER G., DE LAGO E., FERNÁNDEZ-RUIZ J., RAMOS J.A. Design, synthesis and biological evaluation of new endocannabinoid transporter inhibitors: comparison with effects upon fatty acid amidohydrolase. J. Med. Chem. 2003;46:1512–1522. doi: 10.1021/jm0210818. [DOI] [PubMed] [Google Scholar]
- MAURELLI S., BISOGNO T., DE PETROCELLIS L., DI LUCCIA A., MARINO G., DI MARZO V. Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma ‘anandamide amidohydrolase'. FEBS Lett. 1995;377:82–86. doi: 10.1016/0014-5793(95)01311-3. [DOI] [PubMed] [Google Scholar]
- MCFARLAND M.J., PORTER A.C., RAKHSHAN F.R., RAWAT D.S., GIBBS R.A., BARKER E.L. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J. Biol. Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWEE R.G., GRIFFIN G., BAYEWITCH M., BARG J., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MELIS N., PERRA S., MUNTONI A.L., PILLOLLA G., LUTZ B., MARSICANO G., DI MARZO V., GESSA G.L., PISTIS M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J. Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMEIR R.L., CHIN S., HONG Y., AHERN D.G., DEUTSCH D.G. Arachidonoyl ethanolamide-[1,2-14C] as a substrate for anandamide amidase. Life Sci. 1995;56:1999–2005. doi: 10.1016/0024-3205(95)00181-5. [DOI] [PubMed] [Google Scholar]
- ORTAR G., LIGRESTI A., DE PETROCELLIS L., MORERA E., DI MARZO V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem. Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- ORTEGA-GUTIÉRREZ S., HAWKINS E.G., VISO A., LÓPEZ-RODRÍGUEZ M.L., CRAVATT B.F. Comparison of anandamide transport in FAAH wild-type and knockout neurons: evidence for contributions by both FAAH and the CB1 receptor to anandamide uptake. Biochemistry. 2004;43:8184–8190. doi: 10.1021/bi049395f. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D. The endogenous cannabinoid system and the treatment of marijuana dependence. Neuropharmacology. 2004;47:359–367. doi: 10.1016/j.neuropharm.2004.07.018. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ DE FONSECA F., DEL ARCO I., BERMUDEZ-SILVA F.J., BILBAO A., CIPPITELLI A., NAVARRO M. The endocanabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- SAARIO S.M., SAVINAINEN J.R., LAITINEN J.T., JÄRVINEN T., NIEMI R. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem. Pharmacol. 2004;67:1381–1387. doi: 10.1016/j.bcp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUGUKAWA A., NAKANE S., SHINODA A., ITOH K., YAMASHITA A., WAKU K. 2-Arachidonylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., CHUANG H.-H., MOVAHED P., JULIUS D., HÖGESTÄTT E.D. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]