Abstract
Since stress both activates the sympathoadrenal axis and profoundly affects inflammation and inflammatory diseases, many of which are sexually dimorphic, we tested whether the effect of stress on neutrophil recruitment, a primary component of the acute inflammatory response, is sexually dimorphic.
The effect of intermittent sound (over 4 days), a nonhabituating stress, on lipopolysaccharide (LPS)-induced recruitment of neutrophils was evaluated in vivo in the rat air pouch model. At 24 h following the last stress exposure, LPS-induced neutrophil recruitment was enhanced in male rats, but not in females.
When gonadectomized prepubertally and tested as adults, stress significantly inhibited the magnitude of LPS-induced neutrophil recruitment in males, while it still had no effect in gonadectomized females. In males, following adrenal denervation, the increase in LPS-induced neutrophil recruitment produced by stress was prevented. Since these data suggest that the effect of stress is dependent on the sympathoadrenal axis, we tested the hypothesis that catecholamines mediate the stress effects.
In male rats, the effect of stress on LPS-induced neutrophil recruitment was significantly attenuated by continuous administration of the β-adrenergic receptor antagonist, propranolol (4 mg kg−1 day−1), during sound stress exposure, and administration of isoproterenol (10 nmoles, i.v.) significantly increased neutrophil recruitment in males, an effect that was qualitatively and quantitatively similar to the effect of stress. Propranolol significantly increased neutrophil recruitment in nonstressed female rats, but did not significantly affect neutrophil recruitment in stressed females.
These findings indicate a marked male sex hormone-dependent sexual dimorphism in the sympathoadrenal-dependent effect of stress on neutrophil migration, a primary component of the inflammatory response, and suggest that the sympathoadrenal axis contributes to this effect via release of epinephrine.
Keywords: Adrenal medulla, sex differences, neutrophils, β-adrenergic receptor
Introduction
Many inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus, have a markedly greater incidence in women. This sexual dimorphism is dependent not only on sex steroids but also on the hypothalamic-pituitary–adrenal (HPA) and sympathoadrenal stress axes (Spinedi et al., 1994; 1997; Gaillard & Spinedi, 1998; Da Silva, 1999; Green et al., 1999; Black, 2002; Cutolo et al., 2003). Immune cells, constitutive components of the inflammatory response, are markedly affected both by sex steroids (Grossman, 1985; Martin, 2000), and by stress axis mediators. For example, estradiol (Puder et al., 2001; Rodriguez et al., 2002; Xing et al., 2004) and glucocorticoids (Pitzalis et al., 2002) inhibit leukocyte adhesion, chemotaxis and cytokine release, while epinephrine inhibits superoxide production in neutrophils (O'Dowd et al., 2004) and at high concentrations, can enhance neutrophil phagocytic activity (Malpica et al., 2002). While the relationship between stress and inflammation is well established (see Black (2002) for a review), the influence of sex on stress-induced effects on inflammation has received little attention despite the fact that stressful events are known to produce sexually dimorphic effects on the immune system, for example, men have an increased risk of major infections following trauma (Offner et al., 1999). We have shown that the sympathoadrenal axis modulates a major component of the inflammatory response (plasma extravasation) in a sexual dimorphic manner (Green et al., 1999) and we have also shown that activation of the sympathoadrenal axis by nonhabituating stress inhibits plasma extravasation in male rats, while enhancing it in females (Green & Levine, 2005). In this study, we tested the hypothesis that neutrophils, which are the primary initial defense against bacterial infection, exhibit a sexually dimorphic chemotactic response in reaction to a nonhabituating chronic stress, and that this effect is mediated by the sympathoadrenal axis.
Methods
Animals
The experiments were performed on male Sprague–Dawley rats (300–400 g). Animal care and use conformed to the NIH guidelines for the care and use of experimental animals. The University of California at San Francisco, Committee on Animal Research, approved all experimental protocols used in these experiments.
Sound stress
Sound stress was performed on days 1, 3 and 4, as previously described (Singh et al., 1990; Strausbaugh et al., 2003). Animals were placed in a 55 × 55 × 70 cm sound-insulated box in a cage 25 cm away from a speaker. The box was closed and animals were exposed to a 105 dB tone of mixed frequencies ranging from 11 to 19 kHz. Over 30 min, rats were exposed to 5- or 10-s sound epochs presented every minute at random intervals during the minute. Rats were then placed back in their home cages and returned to the animal care facility until the neutrophil migration measurements were performed, 24 h later. All stress exposures occurred between 08:00 and 12:00 hours.
Gonadectomy
Gonadectomies were performed prepubertally as we have previously shown that by puberty, sex steroids in males permanently influence inflammatory response (Green et al., 2001). Female rats, 3-week old, were ovariectomized through bilateral flank incisions; fallopian tubes were clamped and ligated with 4-0 silk suture and the ovaries removed by cutting above the ligation. The fascia were closed with 5-0 chromic gut suture and the skin was closed with metal wound clips. Male rats, 3-week old, were castrated through a single scrotal incision. Vascular bundles were clamped and ligated with 4-0 silk was suture and the skin closed with metal wound clips (Waynforth & Flecknell, 1992). Neutrophil recruitment studies were performed in adults, at ∼10–12 weeks of age for males and 12–14 weeks of age for females.
Adrenal denervation
The greater splanchnic nerve innervating the adrenal gland was exposed following a lateral incision in the abdominal wall; the ‘adrenal innervation region' was isolated close to the adrenal gland and cut as previously described (Celler & Schramm, 1981; Miao et al., 1993). Experiments were carried out at least 7 days after adrenal denervation.
Propranolol and isoproterenol administration
A day prior to the first exposure to sound stress, rats were anesthetized with isoflurane and osmotic mini-pumps (Alzet, Model Number 1007D, Durect, CA, U.S.A.) were implanted subcutaneously in the dorsal interscapular region. These pumps were loaded with the β-adrenoceptor antagonist dl-propranolol (Sigma, St Louis, MO, U.S.A.) to deliver 4 mg kg−1 day−1, a dose that is in the range that can maintain β-adrenergic receptor blockade in rats (Greenberg & Wilborn, 1982; Steinle & Smith, 2002). Isoproterenol was administered intravenously at 10 nmol rat−1 immediately prior to the administration of lipopolysaccharide (LPS).
Measurement of neutrophil migration
Air pouch
Rats were anesthetized with 2–3% isoflurane in oxygen, the back shaved, swabbed with 70% ethanol, and 20 ml of sterile air (passed through a 0.2 μm filter) was injected subcutaneously so as to form an air pouch. After 3 days, this procedure was repeated, except that only 10 ml of sterile air was injected. Rats were used for assessment of neutrophil recruitment 3 days after the second air injection. The air pouch model is a well-established model of joint synovium that possesses histological (Edwards et al., 1981) and biochemical (Claxson et al., 1999) features that are analogous to the synovium in inflamed arthritic joints (Edwards et al., 1981).
Neutrophil harvesting
Rats were briefly anesthetized with isoflurane and 300 μl LPS (30 ng) or sterile phosphate-buffered saline injected into the 7-day old air pouch. Beginning 3–3.5 h later, rats were anesthetized with pentobarbital (65 mg kg−1), and cells collected 3.5–4 h after LPS by injecting 5 ml of sterile phosphate-buffered saline into the pouch. After gentle massaging of the pouch, cell-containing fluid were aspirated and placed into sterile culture tubes, and centrifuged at 1500 r.p.m. for 10 min (25°C). Supernatant was aspirated and cells resuspended in 1 ml at phosphate-buffered saline containing 1% bovine serum albumin room temperature. A measure of 10 μl of 1 mg ml−1 Hoechst 33342 (bisbenzimide), to distinguish nucleated cells from red blood cells, was added to flow cytometry tubes, in subdued lighting, and each sample was added to one of the tubes. For antibody labeling (see below), 100 μl of sample was used. Bisbenzimide was incubated with sample for at least 40 min in subdued lighting. A measure of 1 μl of 1 mg ml−1 propidium iodide, to assess nucleated cell viability, was added immediately before flow cytometry was performed.
Antibody labeling
Cell population was also determined by specific antibody labeling. In all, 100 μl of sample was labeled with rabbit anti-rat polymorphonuclear leukocyte fluorescein-5-isothiocyanate ((FITC) labeled) and monoclonal anti-B-cell/monocyte antibody R-phycoerythrin ((RPE) labeled). Labeled antibodies were added to each 100 μl sample (separate sample for FITC and RPE label) and incubated on ice for 40 min, centrifuged and resuspended in 1 ml ice-cold phosphate-buffered saline (in subdued lighting).
Flow cytometry
Neutrophil quantification was performed using nuclear content, forward scatter and side scatter patterns obtained from excitation at 488 and 354/63 nm wavelengths. Populations were verified by specific antibody labeling; 94±4% of leukocytes in the air pouch were neutrophils. Log fluorescence was measured for 30 s at constant pressure for each sample, using a triple laser Vantage SE cell sorter (Becton Dickinson, San Jose, CA, U.S.A.). Data acquisition was performed using CellQuest Pro software, version 4.01 (Becton Dickinson), and off-line analysis was performed using FlowJo, version 4.5 (Tree Star, Inc., Ashland, OR, U.S.A.).
Results
Sexual dimorphism in the effect of sound stress on LPS-induced neutrophil recruitment
There was no significant sex difference in LPS-induced neutrophil recruitment into the air pouch (Male LPS vs Female LPS, P>0.05). However, following sound stress, LPS-induced recruitment of neutrophils was significantly enhanced in male rats (Male Naïve vs Male Stress, P<0.05), while in the females the number of LPS-recruited neutrophils was not significantly different, even tending towards a decrease (Female Naïve vs Female Stress, P>0.05).
Sympathoadrenal axis in the effect of sound stress on LPS-induced neutrophil recruitment
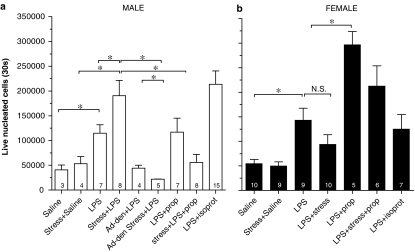
We tested the hypothesis that the effect of sound stress on LPS-induced neutrophil recruitment seen in male rats is dependent on the sympathoadrenal system (Figure 1). Following adrenal denervation, the effect of stress on LPS-induced neutrophil recruitment was completely abolished (Male Naïve vs Male Stress Ad-Den, P>0.05.; Male Stress vs Male Stress Ad-Den, P<0.05). We next tested the hypothesis that stress-induced release of catecholamines could account for the effect of stress on LPS-induced neutrophil recruitment. The β-adrenergic receptor antagonist, propranolol (4 mg kg−1 day−1) was administered beginning 1 day prior to first exposure to sound stress, and continuing throughout the 4-day period stress exposure. Antagonizing the β-adrenergic receptor with propranolol completely prevented the increase in LPS-induced neutrophil recruitment following sound stress in males (Male Stress vs Male Stress+Prop, P<0.05); propranolol had no effect of neutrophil recruitment in nonstressed males (Male Naïve vs Male Prop, P>0.05). The β-adrenergic receptor agonist, isoproterenol (10 nmol/rat, i.v.), significantly increased LPS-induced neutrophil recruitment in male rats (Male Naïve vs Male Isoprot, P<0.05), mimicking the effect of sound stress.
Figure 1.
(a) Stress enhances neutrophil recruitment in male rats: sympathoadrenal- and β-adrenergic receptor-dependence. Basal (saline) levels of neutrophils in the air pouch were not affected by stress. LPS increase recruitment of neutrophils compared to saline, and compared to nonstressed rats (LPS), sound stress enhanced LPS-induced neutrophil recruitment (LPS+stress). The effect of stress was abolished by adrenal denervation (LPS+stress+Ad-Den) or by chronic administration of the β-adrenergic receptor antagonist, propranolol (LPS+stress+prop.). Administration of the β-adrenergic receptor agonist isoproterenol (LPS+isoprot.) mimicked the effect of stress. Results are expressed as mean±s.e.m. (n, indicated on the graph). Significance differences (P<0.05) are indicated by *; NS indicates no significant difference. (b) Stress has no effect on neutrophil recruitment in female rat. Basal (saline) levels of neutrophils in the air pouch were not affected by stress. LPS increase recruitment of neutrophils compared to saline, but compared to nonstressed rats (LPS), sound stress had no effect. Chronic administration of the β-adrenergic receptor antagonist, propranolol in naïve females (LPS+prop.) significantly enhanced the recruitment of neutrophils compared to naïve rats, but neutrophil recruitment in stressed rats was not affected by chronic propranolol. Administration of the β-adrenergic receptor agonist isoproterenol (LPS+isoprot.) was not significantly different from LPS alone. Results are expressed as mean±s.e.m. (n, indicated on the graph). Significance differences (P<0.05) are indicated by *; N.S. indicates no significant difference.
Role of sex steroids in the effect of sound stress on LPS-induced neutrophil recruitment
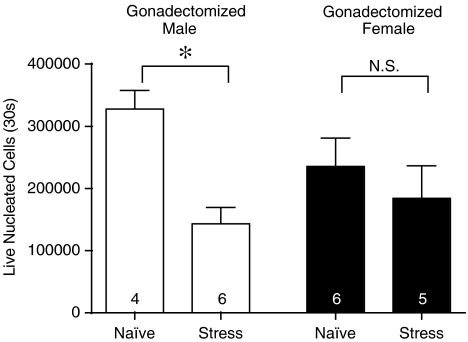
To determine the influence of sex steroids on the sexual dimorphism for the effect of sound stress on LPS-induced neutrophil recruitment, gonadectomies were performed on prepubertal male and female rats (Figure 2). LPS-induced neutrophil recruitment was significantly increased in adult males gonadectomized prepubertally compared to intact males (Naïve male in Figure 1 vs Figure 2; P<0.05), and in contrast to intact males, sound stress now significantly decreased LPS-induced neutrophil recruitment (Naïve vs Stress, P<0.05). Of note, we did not compare gonadectomized with sham surgery males, since the gonadectomy surgeries were performed 7–9 weeks prior to evaluation of LPS-induced neutrophil recruitment – a persistent effect of surgery over such a long period is questionable. Furthermore, we also performed gonadectomies in adult males, and when evaluated 2 weeks later, LPS-induced neutrophil recruitment was not significantly different from that in intact, naïve rats (data not shown). This is consistent with our previous observations that prepubertal, but not adult, gonadectomy in males increased the magnitude of another measure of the inflammatory response (plasma protein extravasation) (Green et al., 2001). Gonadectomy in females did not significantly affect LPS-induced neutrophil recruitment compared to intact females, and sound stress was still without significant effect on LPS-induced neutrophil recruitment (Naïve vs Stress in female, P>0.05).
Figure 2.
Effect of stress on neutrophil recruitment: male sex steroid–dependence. In adult males, gonadectomized prepubertally, stress significantly reduced the recruitment of neutrophils. In contrast, in adult females, gonadectomized prepubertally, stress had no effect on the recruitment of neutrophils. Results are expressed as mean±s.e.m. (n, indicated on the graph).
Discussion
In this study, we have shown that while the magnitude of neutrophil chemotaxis in response to LPS is not significantly different in adult male and female rats, following chronic stress there is a significant enhancement in neutrophil recruitment, but only in males. This stress-induced enhancement of neutrophil attraction in males was abolished after gonadectomy (implicating a role for androgens), and after adrenal medulla denervation (implicating a role for epinephrine). Adrenal denervation or continuous administration of the β-adrenergic receptor antagonist, propranolol, during the sound stress exposure period prevented the stress effects, and administration of the β-adrenergic receptor agonist, isoproterenol mimicked the effect of stress on neutrophil recruitment. These data show that stress has significant effects on neutrophil recruitment that are sexually dimorphic, and specifically that the effects of nonhabituating sound stress are modulated in males by activation of the sympathoadrenal axis, in a male gonadal sex steroid-dependent manner.
While these data provide evidence for an important role of the adrenergic system in the modulation of neutrophil function, recently we evaluated adrenergic regulation chemotaxis of human neutrophils in vitro (de Coupade et al., 2004). In that study, we showed that β-adrenergic stimulates chemokinesis (but not chemotaxis) in neutrophils from women, but had no effect on neutrophils from men. The fact that in that study we observed a β-adrenergic sensitivity in neutrophils from females may be due to species differences, or that LPS-induced recruitment in vivo is not directly comparable to chemokinesis in vitro.
There are some interesting contrasts between the findings from this study and those from our earlier work evaluating the role of the sympathoadrenal axis (Green et al., 1999) and, more recently, the effect of intermittent stress on the magnitude of another component of the inflammatory response, plasma extravasation (Green & Levine, 2005). The magnitude of bradykinin-induced plasma extravasation is lower in female compared to male rats, and is increased by adrenal denervation or by repeated sound stress, while in males adrenal denervation was without effect in naïve rats, but blocked the marked inhibition of plasma extravasation produced by repeated sound stress. As with neutrophil recruitment, the magnitude of plasma extravasation is affected by stress in a markedly sexual dimorphic manner, but the direction of the change differs between these two components of the inflammatory response. Of note, plasma extravasation is inversely correlated with the severity of experimental arthritis in rats (Coderre et al., 1990; 1991; Green et al., 1991; Miao et al., 1992), while recruitment of neutrophils is positively associated with the severity of experimental arthritis in mice (Wipke & Allen, 2001). Further study is needed to determine how in male vs female rats stress affects the integration of these, and other, inflammatory processes.
The sympathetic nervous system and the sympathoadrenal axis are closely associated with the immune system and the control of the inflammatory response; the sympathetic nervous system innervates lymphoid organs and directly contacts lymphocytes and macrophages (Felten et al., 1984; Felten & Olschowka, 1987), and many immune cells (e.g. lymphocytes, natural killer (NK) cells, neutrophils and macrophages) express α- and β-adrenergic receptors. The β2-adrenergic receptor subtype mediates several of the observed effects of catecholamines on neutrophils (Benschop et al., 1996), such as generation of reactive oxygen intermediates in granulocytes in response to activation with formyl-Met-Leu-Phe (fMLP) (Opdahl et al., 1993) and interleukin (IL)-8 production in macrophages (Kavelaars et al., 1997). Of relevance to our findings, epinephrine acts via β1-adrenergic receptors to increase neutrophil migration (Jain et al., 2003) and antagonizing α2-adrenergic receptors inhibits LPS-induced neutrophil recruitment into pleura (Altenburg et al., 2000). Since nonhabituating sound stress produces a chronic increase in plasma epinephrine (P.G. Green, unpublished observations), it is likely that the functioning of adrenergic receptor-bearing immune cells will be altered in stressed rats. In fact, normal circulating levels of catecholamines may affect neutrophil function since propranolol has been shown to increase neutrophil chemotaxis in response to fMLP (Anderson & van Rensburg, 1979; Weisdorf & Jacob, 1987). In fact, although we found that propranolol had no significant effect in naïve males, it significantly enhanced migration in naïve females (Figure 1b), suggesting that tonic β-adrenergic stimulation in females suppresses migration of neutrophils to inflammatory stimuli. Interestingly, acute stress (Landmann et al., 1984) and acute isoproterenol infusion (Van Tits et al., 1990) produce lymphocytosis in humans, and isoproterenol also increases the number of T and NK cells (Mills et al., 2000), favoring enhanced neutrophil adhesion and migration to nonlymphoid tissue (Mills et al., 2000). However, 9-day spaceflight stress in astronauts, with concomitant raised plasma catecholamines, also increased plasma neutrophil, monocyte and T cell numbers (but decreased NK cells) (Stowe et al., 2003), which is compatible with the hypothesis that stress enhances immune response in a sympathoadrenal-dependent manner. In addition to enhancing some immune functions, the adrenergic system also suppresses some other functions. For example, epinephrine, acting via the β2-adrenergic receptor increases intracellular cAMP levels and inhibits the production of superoxide by neutrophils (Tintinger et al., 2001; O'Dowd et al., 2004), while isoproterenol increases intracellular cAMP concentration and inhibits adhesion of equine PMN to immune complexes (Chilcoat et al., 2002). However, while increased intracellular cAMP levels also suppress neutrophil chemotaxis (Tyagi et al., 1991), the role of intracellular levels of cAMP on neutrophil immune function is not clear since increased plasma levels of norepinephrine (produced by exercise stress) increases neutrophil phagocytosis in parallel with increased intracellular levels of cAMP (Ortega et al., 2005). It is important to note that these studies evaluating the relationship between adrenergic stimulation, cAMP levels and immune cell function have focused on acute effects of adrenergic stimulation; it is important, therefore, to evaluate the effect of chronic stress and/or chronic exposure to adrenergic stimulation. It has been shown that in humans, chronic stress (over months) down regulates both lymphocyte β2-adrenergic receptor density and isoproterenol cAMP response (Mills et al., 2004), but in contrast chronic stress in mice (8 weeks) upregulates β2-adrenergic receptor density and isoproterenol cAMP response in mice (Edgar et al., 2003).
While a stress- or isoproterenol-induced alteration in the circulating neutrophil population may have contributed to the observed changes in neutrophil migration into the air pouch, there does not appear to be a linear relationship between circulating levels of leukocytes and recruitment into the air pouch. For example, in rats, NG-nitro-L-arginine methyl ester treatment increases circulating leukocyte levels by 100%, but it actually decreases migration into the air pouch by 50% (Farsky et al., 2004). However, a decrease neutrophil recruitment into the rat air pouch can be produced when marked leukopenia is induced with cyclophosphamide treatment (Omata et al., 1991). Of note, social stress increases the circulating neutrophil population in rats ∼1.8-fold (Engler et al., 2004). Therefore, while it is possible that extremes of leukopenia/leukophilia may affect recruitment of leukocytes into the air pouch, existing literature argues against such a pronounced effect occurring with stress or isoproterenol.
Our results contrast with some previous reports evaluating the effect on inflammation of acute stress, such as foot shock, hemorrhagic shock, surgery and trauma. For example, 3 h foot-shock stress reduces zymosan-induced neutrophil accumulation into the air pouch of male rats (Harmsen & Turney, 1985) and hemorrhagic shock produces a depressed cell-mediated immune response in males, but an unchanged or enhanced response in females (Wichmann et al., 1996; Angele et al., 2000); similarly, women exhibit a greater immediate IL-6 response postsurgically, compared to men (Wichmann et al., 2003) and infection is much greater in men following trauma (Offner et al., 1999). However, the fact that the duration of exposure to a stressor is a major determinant of the effect on the inflammatory response (Strausbaugh et al., 1999; 2003) may account, at least in part, for the differences in the literature regarding the effects of stress on immune responses. Consistent with this notion, it has been shown recently that 3-day restraint stress in ovalbumin-sensitized and challenged male mice decreased bronchoalveolar neutrophil recruitment, while 7-day restraint stress increased bronchoalveolar neutrophil recruitment, in this case via a corticosterone-independent mechanism (Forsythe et al., 2004). Furthermore, while acute (5 h) stress enhanced delayed-type hypersensitivity reactions, which are cell-mediated immune responses, chronic (3-week) stress suppressed it (Dhabhar & McEwen, 1997).
The type of stressor employed is also an important variable in how stress affects inflammation and immune responses. For example, acute inescapable electrical foot or tail shock (Harmsen & Turney, 1985; Fleshner et al., 1998; Campisi & Fleshner, 2003; O'Connor et al., 2003) and acute restraint (Dhabhar et al., 1997) facilitate innate immunity, while it is inhibited by acute cold exposure with restraint stress (Cao et al., 2003). These differences are likely to be dependent on differences in stress intensity (Rinner et al., 1992; Hale et al., 2001) producing different levels of glucocorticoids and catecholamines (Dhabhar & McEwen, 1999). Importantly, we have demonstrated that different types of stressor inhibit components of the inflammatory response by different mechanisms; while repeated restraint stress inhibits plasma extravasation immediately after exposure to the stressor, via activation of the HPA axis, repeated sound and ether stress inhibits plasma extravasation 24–48 h poststress, via activation of the sympathoadrenal axis (Strausbaugh et al., 2003). Since sound and ether stress are more intense and nonhabituating, compared to restraint stress, we have hypothesized that this difference may be dependent on the intensity of the stressor; low-intensity stress preferentially activates the HPA axis while high-intensity stress also activates the sympathoadrenal axis (Strausbaugh et al., 1999). Consistent with this hypothesis, we have found that administration of corticosterone (subcutaneously implanted 100 mg pellet) was without effect on LPS-induced neutrophil recruitment in either males or females (P.G. Green, unpublished observations), suggesting that the HPA axis does not participate in the effect of sound stress on neutrophil recruitment.
The data from this study also suggest that androgens may affect neutrophil recruitment, since the stress-induced increase in LPS-induced neutrophil recruitment was absent in gonadectomized males. Although testosterone may modulate the circadian variation in LPS-stimulated neutrophil migration (Bureau & Labrecque, 1996), testosterone does not appear to directly affect neutrophil chemotaxis (Jungi et al., 1977; Miyagi et al., 1992). However, testosterone may have indirect action to affect neutrophil migration. While there is no evidence for a role of physiological variations of testosterone in the regulation of catecholamine action in leukocytes (Rosen et al., 1984), testosterone has been shown to increase TNF-α-induced expression of E-selectin (which regulates the extravasation and migration of neutrophils) (Zhang et al., 2002). Further studies are required to elucidate the relationship between stress, testosterone and neutrophil migration.
In summary, we have shown that chronic intermittent stress enhances LPS-induced leukocyte recruitment in male rats, and that this sexually dimorphic effect is dependent on the sympathoadrenal axis and androgens. Future studies are necessary to clarify the cellular mechanisms underlying the enhanced cell recruitment in males. Understanding how chronic intermittent stress affects inflammatory and immune processes could lead to better management of morbidity in chronic stress.
Acknowledgments
This work was supported by the National Institutes of Health.
Abbreviations
- FITC
fluorescein-5-isothiocyanate
- fMLP
formyl-Met-Leu-Phe
- LPS
lipopolysaccharide
- HPA
hypothalamic–pituitary–adrenal
- RPE
R-phycoerythrin
References
- ALTENBURG S.P., PAIXAO E SILVA S., VENTURA D.G., GOMES R.N., BOZZA P.T., CASTRO-FARIA-NETO H.C. A role for adrenoceptors in the regulation of pleural neutrophilia induced by LPS. J. Neuroimmunol. 2000;111:15–22. doi: 10.1016/s0165-5728(00)00376-3. [DOI] [PubMed] [Google Scholar]
- ANDERSON R., VAN RENSBURG A.J. The in vitro effects of propranolol and atenolol on neutrophil motility and post-phagocytic metabolic activity. Immunology. 1979;37:15–24. [PMC free article] [PubMed] [Google Scholar]
- ANGELE M.K., SCHWACHA M.G., AYALA A., CHAUDRY I.H. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- BENSCHOP R.J., RODRIGUEZ-FEUERHAHN M., SCHEDLOWSKI M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav. Immun. 1996;10:77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- BLACK P.H. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav. Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- BUREAU J.P., LABRECQUE G. Biological rhythm, inflammation and non-steroidal anti-inflammatory agents. Pathol. Biol. (Paris) 1996;44:610–617. [PubMed] [Google Scholar]
- CAMPISI J., FLESHNER M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J. Appl. Physiol. 2003;94:43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- CAO L., HUDSON C.A., LAWRENCE D.A. Immune changes during acute cold/restraint stress-induced inhibition of host resistance to Listeria. Toxicol. Sci. 2003;74:325–334. doi: 10.1093/toxsci/kfg146. [DOI] [PubMed] [Google Scholar]
- CELLER B.G., SCHRAMM L.P. Pre- and postganglionic sympathetic activity in splanchnic nerves of rats. Am. J. Physiol. 1981;241:R55–R61. doi: 10.1152/ajpregu.1981.241.1.R55. [DOI] [PubMed] [Google Scholar]
- CHILCOAT C.D., ROWLINGSON K.A., JONES S.L. The effects of cAMP modulation upon the adhesion and respiratory burst activity of immune complex-stimulated equine neutrophils. Vet. Immunol. Immunopathol. 2002;88:65–77. doi: 10.1016/s0165-2427(02)00137-x. [DOI] [PubMed] [Google Scholar]
- CLAXSON A., GROOTVELD M., CHANDER C., EARL J., HAYCOCK P., MANTLE M., WILLIAMS S.R., SILWOOD C.J., BLAKE D.R. Examination of the metabolic status of rat air pouch inflammatory exudate by high field proton NMR spectroscopy. Biochim. Biophys. Acta. 1999;1454:57–70. doi: 10.1016/s0925-4439(99)00020-4. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., BASBAUM A.I., DALLMAN M.F., HELMS C., LEVINE J.D. Epinephrine exacerbates arthritis by an action at presynaptic B2-adrenoceptors. Neuroscience. 1990;34:521–523. doi: 10.1016/0306-4522(90)90160-6. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., CHAN A.K., HELMS C., BASBAUM A.I., LEVINE J.D. Increasing sympathetic nerve terminal-dependent plasma extravasation correlates with decreased arthritic joint injury in rats. Neuroscience. 1991;40:185–189. doi: 10.1016/0306-4522(91)90184-p. [DOI] [PubMed] [Google Scholar]
- CUTOLO M., SULLI A., PIZZORNI C., CRAVIOTTO C., STRAUB R.H.Hypothalamic–pituitary–adrenocortical and gonadal functions in rheumatoid arthritis Neuroendocrine and Neural Regulation of Autoimmune and Inflammatory Disease: Molecular, Systems, and Clinical Insights 2003New York: New York Academy of Sciences; 107–117.ed. Sternberg, E.M., Haour, F.G. & Smith, C.C. pp [DOI] [PubMed] [Google Scholar]
- DA SILVA J.A.P.Sex hormones and glucocorticoids: interactions with the immune system Neuroendocrine Immune Basis of the Rheumatic Diseases 1999New York: New York Academy of Sciences; 102–118.ed. Cutolo, M. pp [DOI] [PubMed] [Google Scholar]
- DE COUPADE C., GEAR R.W., DAZIN P.F., SROUSSI H.Y., GREEN P.G., LEVINE J.D. Beta2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br. J. Pharmacol. 2004;143:1033–1041. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHABHAR F.S., MCEWEN B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- DHABHAR F.S., MCEWEN B.S. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHABHAR F.S., MCEWEN B.S., SPENCER R.L. Adaptation to prolonged or repeated stress—comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- EDGAR V.A., SILBERMAN D.M., CREMASCHI G.A., ZIEHER L.M., GENARO A.M. Altered lymphocyte catecholamine reactivity in mice subjected to chronic mild stress. Biochem. Pharmacol. 2003;65:15–23. doi: 10.1016/s0006-2952(02)01457-0. [DOI] [PubMed] [Google Scholar]
- EDWARDS J.C., SEDGWICK A.D., WILLOUGHBY D.A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- ENGLER H., DAWILS L., HOVES S., KURTH S., STEVENSON J.R., SCHAUENSTEIN K., STEFANSKI V. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J. Neuroimmunol. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- FARSKY S.H., BORELLI P., FOCK R.A., PROTO S.Z., FERREIRA J.M., Jr., MELLO S.B. Chronic blockade of nitric oxide biosynthesis in rats: effect on leukocyte endothelial interaction and on leukocyte recruitment. Inflamm. Res. 2004;53:442–452. doi: 10.1007/s00011-004-1288-7. [DOI] [PubMed] [Google Scholar]
- FELTEN D.L., LIVNAT S., FELTEN S.Y., CARLSON S.L., BELLINGER D.L., YEH P. Sympathetic innervation of lymph nodes in mice. Brain Res. Bull. 1984;13:693–699. doi: 10.1016/0361-9230(84)90230-2. [DOI] [PubMed] [Google Scholar]
- FELTEN S.Y., OLSCHOWKA J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synaptic like contacts on lymphocytes in the splenic white pulp. J. Neurosci. Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- FLESHNER M., NGUYEN K.T., COTTER C.S., WATKINS L.R., MAIER S.F. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am. J. Physiol. 1998;275:R870–R878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- FORSYTHE P., EBELING C., GORDON J.R., BEFUS A.D., VLIAGOFTIS H. Opposing effects of short- and long-term stress on airway inflammation. Am. J. Resp. Crit. Care Med. 2004;169:220–226. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- GAILLARD R.C., SPINEDI E. Sex- and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest. Anim. Endocrinol. 1998;15:345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- GREEN P.G., BASBAUM A.I., HELMS C., LEVINE J.D. Purinergic regulation of bradykinin-induced plasma extravasation and adjuvant-induced arthritis in the rat. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4162–4165. doi: 10.1073/pnas.88.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN P.G., DAHLQVIST S.R., ISENBERG W.M., MIAO F.J.P., LEVINE J.D. Role of adrenal medulla in development of sexual dimorphism in inflammation. Eur. J. Neurosci. 2001;14:1436–1444. doi: 10.1046/j.0953-816x.2001.01768.x. [DOI] [PubMed] [Google Scholar]
- GREEN P.G., DAHLQVIST S.R., ISENBERG W.M., STRAUSBAUGH H.J., MIAO F.J.P., LEVINE J.D. Sex steroid regulation of the inflammatory response: sympathoadrenal dependence in the female rat. J. Neurosci. 1999;19:4082–4089. doi: 10.1523/JNEUROSCI.19-10-04082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN P.G., LEVINE J.D. Sexual dimorphism in the effect of nonhabituating stress on neurogenic plasma extravasation. Eur. J. Neurosci. 2005;21:486–492. doi: 10.1111/j.1460-9568.2005.03872.x. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., WILBORN W. Effect of clonidine and propranolol on venous smooth muscle from spontaneously hypertensive rats. Arch. Int. Pharmacodyn. Ther. 1982;258:234–259. [PubMed] [Google Scholar]
- GROSSMAN C.J. Interactions between the gonadal-steroids and the immune system. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- HALE K.D., GHANTA V.K., GAUTHIER D.K., HIRAMOTO R.N. Effects of rotational stress of different duration on NK cell activity, proinflammatory cytokines, and POMC-derived peptides in mice. Neuroimmunomodulation. 2001;9:34–40. doi: 10.1159/000049005. [DOI] [PubMed] [Google Scholar]
- HARMSEN A.G., TURNEY T.H. Inhibition of in vivo neutrophil accumulation by stress. Possible role of neutrophil adherence. Inflammation. 1985;9:9–20. doi: 10.1007/BF00915407. [DOI] [PubMed] [Google Scholar]
- JAIN F.A., ZHAO L.H., SELIG M.K., KRADIN R.L. Epinephrine promotes pulmonary angiitis: evidence for a beta1-adrenoreceptor-mediated mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L232–L239. doi: 10.1152/ajplung.00248.2002. [DOI] [PubMed] [Google Scholar]
- JUNGI T.W., BESEDOVSKY H.O., SORKIN E., SCHARDT M. Hormonal control of neutrophil chemotactic activity in the rat vagina. Am. J. Physiol. 1977;233:R59–R65. doi: 10.1152/ajpregu.1977.233.1.R59. [DOI] [PubMed] [Google Scholar]
- KAVELAARS A., VAN DE POL M., ZIJLSTRA J., HEIJNEN C.J. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J. Neuroimmunol. 1997;77:211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- LANDMANN R.M., MULLER F.B., PERINI C., WESP M., ERNE P., BUHLER F.R. Changes of immunoregulatory cells induced by psychological and physical stress: relationship to plasma catecholamines. Clin. Exp. Immunol. 1984;58:127–135. [PMC free article] [PubMed] [Google Scholar]
- MALPICA M.I., RODRIGUEZ A.B., SAEZ M.C., GARCIA J.J., BARRIGA C., ORTEGA E. In-vitro study of the effect of adrenaline on the functional capacity of human neutrophils: role during exercise. J. Neuroendocrinol. 2002;14:824–828. doi: 10.1046/j.1365-2826.2002.00847.x. [DOI] [PubMed] [Google Scholar]
- MARTIN J.T. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur. J. Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. [DOI] [PubMed] [Google Scholar]
- MIAO F.J., DALLMAN M.F., BENOWITZ N.L., BASBAUM A.I., LEVINE J.D. Adrenal medullary modulation of the inhibition of bradykinin-induced plasma extravasation by intrathecal nicotine. J. Pharmacol. Exp. Ther. 1993;264:839–844. [PubMed] [Google Scholar]
- MIAO F.J., HELMS C., BENOWITZ N.L., BASBAUM A.I., HELLER P.H., LEVINE J.D. Chronically administered nicotine attenuates bradykinin-induced plasma extravasation and aggravates arthritis-induced joint injury in the rat. Neuroscience. 1992;51:649–655. doi: 10.1016/0306-4522(92)90304-k. [DOI] [PubMed] [Google Scholar]
- MILLS P.J., ADLER K.A., DIMSDALE J.E., PEREZ C.J., ZIEGLER M.G., ANCOLI-ISRAEL S., PATTERSON T.L., GRANT I. Vulnerable caregivers of Alzheimer disease patients have a deficit in beta 2-adrenergic receptor sensitivity and density. Am. J. Geriatr. Psychiatry. 2004;12:281–286. [PubMed] [Google Scholar]
- MILLS P.J., GOEBEL M., REHMAN J., IRWIN M.R., MAISEL A.S. Leukocyte adhesion molecule expression and T cell naïve/memory status following isoproterenol infusion. J. Neuroimmunol. 2000;102:137–144. doi: 10.1016/s0165-5728(99)00180-0. [DOI] [PubMed] [Google Scholar]
- MIYAGI M., AOYAMA H., MORISHITA M., IWAMOTO Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J. Periodontol. 1992;63:28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- O'CONNOR K.A., JOHNSON J.D., HANSEN M.K., WIESELER FRANK J.L., MAKSIMOVA E., WATKINS L.R., MAIER S.F. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- O'DOWD Y.M., EL-BENNA J., PERIANIN A., NEWSHOLME P. Inhibition of formyl-methionyl-leucyl-phenylalanine-stimulated respiratory burst in human neutrophils by adrenaline: inhibition of Phospholipase A2 activity but not p47phox phosphorylation and translocation. Biochem. Pharmacol. 2004;67:183–190. doi: 10.1016/j.bcp.2003.08.026. [DOI] [PubMed] [Google Scholar]
- OFFNER P.J., MOORE E.E., BIFFL W.L.Male gender is a risk factor for major infections after surgery Arch. Surg. 1999134935–938.discussion 938–940 [DOI] [PubMed] [Google Scholar]
- OMATA M., WATANABE M., HIRASAWA N., TSURUFUJI S., MUE S., OHUCHI K. A role of peripheral leukocytes in vascular permeability and edema formation in air pouch type allergic inflammation in rats. J. Pharmacobiodyn. 1991;14:267–275. doi: 10.1248/bpb1978.14.267. [DOI] [PubMed] [Google Scholar]
- OPDAHL H., BENESTAD H.B., NICOLAYSEN G. Effect of beta-adrenergic agents on human neutrophil granulocyte activation with N-formyl-methionyl-leucyl-phenylalanine and phorbol myristate acetate. Pharmacol. Toxicol. 1993;72:221–228. doi: 10.1111/j.1600-0773.1993.tb01640.x. [DOI] [PubMed] [Google Scholar]
- ORTEGA E., MARCHENA J.M., GARCIA J.J., BARRIGA C., RODRIGUEZ A.B. Norepinephrine as mediator in the stimulation of phagocytosis induced by moderate exercise. Eur. J. Appl. Physiol. 2005;93:714–718. doi: 10.1007/s00421-004-1245-8. [DOI] [PubMed] [Google Scholar]
- PITZALIS C., PIPITONE N., PERRETTI M. Regulation of leukocyte-endothelial interactions by glucocorticoids. Ann. N.Y. Acad. Sci. 2002;966:108–118. doi: 10.1111/j.1749-6632.2002.tb04208.x. [DOI] [PubMed] [Google Scholar]
- PUDER J.J., FREDA P.U., GOLAND R.S., WARDLAW S.L. Estrogen modulates the hypothalamic–pituitary–adrenal and inflammatory cytokine responses to endotoxin in women. J. Clin. Endocrinol. Metab. 2001;86:2403–2408. doi: 10.1210/jcem.86.6.7528. [DOI] [PubMed] [Google Scholar]
- RINNER I., SCHAUENSTEIN K., MANGGE H., PORTA S., KVETNANSKY R. Opposite effects of mild and severe stress on in vitro activation of rat peripheral-blood lymphocytes. Brain Behav. Immun. 1992;6:130–140. doi: 10.1016/0889-1591(92)90013-e. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ E., LOPEZ R., PAEZ A., MASSO F., MONTANO L.F. 17Beta-estradiol inhibits the adhesion of leukocytes in TNF-alpha stimulated human endothelial cells by blocking IL-8 and MCP-1 secretion, but not its transcription. Life Sci. 2002;71:2181–2193. doi: 10.1016/s0024-3205(02)01999-9. [DOI] [PubMed] [Google Scholar]
- ROSEN S.G., BERK M.A., POPP D.A., SERUSCLAT P., SMITH E.B., SHAH S.D., GINSBERG A.M., CLUTTER W.E., CRYER P.E. Beta 2- and alpha 2-adrenergic receptors and receptor coupling to adenylate cyclase in human mononuclear leukocytes and platelets in relation to physiological variations of sex steroids. J. Clin. Endocrinol. Metab. 1984;58:1068–1076. doi: 10.1210/jcem-58-6-1068. [DOI] [PubMed] [Google Scholar]
- SINGH V.B., ONAIVI E.S., PHAN T.H., BOADLE-BIBER M.C. The increases in rat cortical and midbrain tryptophan hydroxylase activity in response to acute or repeated sound stress are blocked by bilateral lesions to the central nucleus of the amygdala. Brain Res. 1990;530:49–53. doi: 10.1016/0006-8993(90)90656-v. [DOI] [PubMed] [Google Scholar]
- SPINEDI E., CHISARI A., PRALONG F., GAILLARD R.C. Sexual dimorphism in the mouse hypothalamic–pituitary–adrenal axis function after endotoxin and insulin stresses during development. Neuroimmunomodulation. 1997;4:77–83. doi: 10.1159/000097324. [DOI] [PubMed] [Google Scholar]
- SPINEDI E., SALAS M., Chisari A., PERONE M., CARINO M., GAILLARD R.C. Sex-differences in the hypothalamo–pituitary–adrenal axis response to inflammatory and neuroendocrine stressors–evidence for a pituitary defect in the autoimmune disease-susceptible female Lewis rat. Neuroendocrinology. 1994;60:609–617. doi: 10.1159/000126804. [DOI] [PubMed] [Google Scholar]
- STEINLE J.J., SMITH P.G. Role of adrenergic receptors in vascular remodelling of the rat choroid. Br. J. Pharmacol. 2002;136:730–734. doi: 10.1038/sj.bjp.0704771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOWE R.P., SAMS C.F., PIERSON D.L. Effects of mission duration on neuroimmune responses in astronauts. Aviat. Space Environ. Med. 2003;74:1281–1284. [PubMed] [Google Scholar]
- STRAUSBAUGH H.J., GREEN P.G., DALLMAN M.F., LEVINE J.D. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur. J. Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- STRAUSBAUGH H.J., GREEN P.G., LO E., TANGEMANN K., REICHLING D.B., ROSEN S.D., LEVINE J.D. Painful stimulation suppresses joint inflammation by inducing shedding of L-selectin from neutrophils. Nat. Med. 1999;5:1057–1061. doi: 10.1038/12497. [DOI] [PubMed] [Google Scholar]
- TINTINGER G.R., THERON A.J., ANDERSON R., KER J.A. The anti-inflammatory interactions of epinephrine with human neutrophils in vitro are achieved by cyclic AMP-mediated accelerated resequestration of cytosolic calcium. Biochem. Pharmacol. 2001;61:1319–1328. doi: 10.1016/s0006-2952(01)00588-3. [DOI] [PubMed] [Google Scholar]
- TYAGI S.R., OLSON S.C., BURNHAM D.N., LAMBETH J.D. Cyclic AMP-elevating agents block chemoattractant activation of diradylglycerol generation by inhibiting phospholipase D activation. J. Biol. Chem. 1991;266:3498–3504. [PubMed] [Google Scholar]
- VAN TITS L.J., MICHEL M.C., GROSSE-WILDE H., HAPPEL M., EIGLER F.W., SOLIMAN A., BRODDE O.E. Catecholamines increase lymphocyte beta 2-adrenergic receptors via a beta 2-adrenergic, spleen-dependent process. Am. J. Physiol. 1990;258:E191–E202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- WAYNFORTH H.B., FLECKNELL P.A. Experimental and Surgical Technique in the Rat. London, San Diego: Academic Press; 1992. [Google Scholar]
- WEISDORF D.J., JACOB H.S. Beta-adrenergic blockade: augmentation of neutrophil-mediated inflammation. J. Lab. Clin. Med. 1987;109:120–126. [PubMed] [Google Scholar]
- WICHMANN M.W., MULLER C., MEYER G., ADAM M., ANGELE M.K., EISENMENGER S.J., SCHILDBERG F.W. Different immune responses to abdominal surgery in men and women. Langenbecks Arch. Surg. 2003;387:397–401. doi: 10.1007/s00423-002-0346-2. [DOI] [PubMed] [Google Scholar]
- WICHMANN M.W., ZELLWEGER R., Demaso C.M., AYALA A., CHAUDRY I.H. Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine. 1996;8:853–863. doi: 10.1006/cyto.1996.0114. [DOI] [PubMed] [Google Scholar]
- WIPKE B.T., ALLEN P.M. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- XING D., MILLER A., NOVAK L., ROCHA R., CHEN Y.F., OPARIL S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- ZHANG X., WANG L., DOU Y., ZHAO J., JIANG T., QIAO Z., QIAO J. Testosterone and estradiol modulate TNF-alpha-Induced expression of adhesion molecules in endothelial cells. Methods Find. Exp. Clin. Pharmacol. 2002;24:125–130. doi: 10.1358/mf.2002.24.3.802295. [DOI] [PubMed] [Google Scholar]