Abstract
We first determined whether the cardioprotection resulting from kappa opioid receptor (κ-OR) stimulation was blocked by the KCa channel inhibitor, paxilline (Pax), administered before or during ischaemic insults in vitro.
In isolated rat hearts, 30 min of ischaemia and 120 min of reperfusion induced infarction and increased lactate dehydrogenase (LDH) release. In isolated ventricular myocytes subjected to 5 min of metabolic inhibition and anoxia followed by 10 min of reperfusion, the percentage of live cells and the amplitude of the electrically induced intracellular Ca2+ ([Ca2+]i) transient decreased, while diastolic [Ca2+]i increased. Pretreatment with 10 μM U50,488H, a κ-OR agonist, attenuated the undesirable effects of ischaemic insults in both preparations. The beneficial effects of κ-OR stimulation, that were abolished by 5 μM nor-BNI, a κ-OR antagonist, were also abolished by 1 μM Pax administered before ischaemic insults or 20 μM atractyloside, an opener of the mitochondrial permeability transition pore.
Activation of protein kinase C (PKC) with 0.1 μM phorbol 12-myristate 13-acetate decreased the infarct size and LDH release in isolated rat hearts subjected to ischaemia/reperfusion, and these effects were abolished by blockade of PKC with its inhibitors, 10 μM GF109203X or 5 μM chelerythrine, and more importantly by 1 μM Pax. On the other hand, the cardioprotective effects of opening the KCa channel with 10 μM NS1619 were not altered by either PKC inhibitor.
In conclusion, the high-conductance KCa channel triggers cardioprotection induced by κ-OR stimulation that involves inhibition of MPTP opening. The KCa channel is located downstream of PKC.
Keywords: Kappa opioid receptor, Ca2+-activated potassium channel, ischaemia and reperfusion, metabolic inhibition and anoxia
Introduction
Ischaemic preconditioning (IPC) is a phenomenon in which transient nonlethal periods of ischaemia increase the resistance to a subsequent and more prolonged ischaemic insult (Murry et al., 1986). It has been demonstrated that Gi protein-coupled receptors mediate the cardioprotection of IPC (Liu et al., 1991; Schultz et al., 1998). The kappa opioid receptor (κ-OR) is one of the receptors that mediate the cardioprotection of IPC (Wang et al., 2001a) and the action of its stimulation is mediated via a pertussis toxin-sensitive G-protein (Sheng et al., 1997). We have shown that preconditioning with a κ-OR agonist, U50,488H (U50) (Wang et al., 2001a), reduces the injury induced by myocardial ischaemia and reperfusion, and this effect is abolished by blockade of κ-OR with its antagonist, nor-BNI, indicating cardioprotection by κ-OR stimulation. Recently, we have shown that the administration of paxilline (Pax), an inhibitor of the high-conductance Ca2+-activated potassium (KCa) channel located mainly in the mitochondrial membrane (Xu et al., 2002), before, but not during, ischaemia, abolishes the cardioprotection of IPC (Cao et al., 2005), indicating that the KCa channel acts as a trigger of IPC, defined as the mechanism that puts the heart into a preconditioned state and occurs before index ischaemia (Krieg et al., 2003). We therefore hypothesize that the high-conductance KCa channel also triggers the cardioprotection by κ-OR stimulation.
Protein kinase C (PKC) triggers the cardioprotection of IPC (Speechly-Dick et al., 1994) or by κ-OR stimulation (Wang et al., 2001b). A recent study has shown that the cardioprotection resulting from PKC activation involves the mitochondrial permeability transition pore (referred to below as ‘the pore'); inhibition of its opening triggers the cardioprotection of IPC (Hausenloy et al., 2002) and it is located downstream of PKC (Baines et al., 1997). Interestingly, the pore is also located downstream of the KCa channel (Cao et al., 2005). If the cardioprotection of κ-OR stimulation involves the KCa channel as a trigger as we hypothesized, the KCa channel and PKC might act sequentially. The relationship between PKC and the KCa channel is, however, not known.
Therefore, the aim of this study was to determine: firstly, the roles of the KCa channel in cardioprotection by κ-OR stimulation, and, secondly, the relationship between the KCa channel and PKC. We used two in vitro preparations, namely, the isolated perfused heart and isolated myocytes. In addition to determining injury, viability, and diastolic intracellular Ca2+ ([Ca2+]i) and its transient, we also determined the opening/inhibiting of the pore. We determined the effects of κ-OR stimulation with either U50, or activation of PKC, or closing of the pore on cardiac injury and [Ca2+]i in response to ischaemic insults in both preparations. We also determined whether activation of PKC would confer cardioprotection following blockade of the KCa channel and vice versa in the isolated perfused heart. The results showed that the high-conductance KCa channel triggers cardioprotection by κ-OR stimulation, which involves the mitochondrial permeability transition pore. PKC is located upstream of the KCa channel.
Methods
Isolated perfused heart preparation
Male Sprague–Dawley rats of 250–300 g body weight were anaesthetized with sodium pentobarbitone (60 mg kg−1, i.p.) and given heparin (200 IU, i.v.). Hearts were excised rapidly and placed in ice-cold Krebs–Henseleit (K–H) perfusion buffer before being mounted on a Langendorff apparatus for perfusion at 37°C with K–H buffer at a constant pressure (100 cm H2O). The buffer, which had the following composition (mM): NaCl 118.0, KCl 4.7, CaCl2 1.25, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25.0, and glucose 11.0, was equilibrated with 95% CO2/5% O2. In hearts subjected to regional ischaemia, a silk suture was placed around the left coronary artery to form a snare. The coronary artery was occluded by pulling the snare to produce ischaemia. Reperfusion was achieved by releasing the snare. The protocol of this study was approved by the Committee on the Use of Experimental Animals for Teaching and Research, The University of Hong Kong.
Measurement of the area of risk
For determination of infarct size in hearts subjected to regional ischaemia, the coronary artery was re-occluded at the end of the reperfusion period and a solution with 2.5% Evans blue was perfused to delineate the area of risk. Hearts were then frozen and cut into slices, which were then incubated in a sodium phosphate buffer containing 1% w v−1 2,3,5-triphenyl-tetrazolium chloride for 15 min to visualize the unstained infarcted region. Infarct and risk zone areas were determined by planimetry using Image/J software from NIH, and infarct was expressed as a percentage of the risk zone.
Determination of myocardial injury by lactate dehydrogenase (LDH) efflux
The effluent from the isolated perfused heart was collected at 5 min of reperfusion and LDH was spectrophotometrically assayed using a kit purchased from Sigma-Aldrich. LDH activity was expressed as U l−1.
Preparation of isolated ventricular myocytes
Single ventricular myocytes were prepared from the hearts of male Sprague–Dawley rats by enzymatic dissociation. Immediately after stunning and decapitation, the heart was rapidly removed, rinsed in ice-cold Ca2+-free Tyrode's solution and perfused via a Langendorff apparatus with 100% oxygenated, nonrecirculating, Ca2+-free Tyrode's solution. Then the perfusion solution was switched to a 100% oxygenated, recirculating, low Ca2+ (50 μM) Tyrode's solution containing 0.03% collagenase and 1% bovine serum albumin for 10 min. The ventricles were cut, minced, and gently triturated with a pipette in a low-Ca2+ Tyrode's solution containing bovine serum albumin at 37°C for 10 min. The cells were filtered through a 200-μm nylon mesh, resuspended in the Tyrode's solution in which the Ca2+ concentration was gradually increased to 1.25 mM in 40 min. Only rod-shaped cells with clear cross-striations were used for experiments. For ischaemic insults, myocytes were incubated for 5 min in a solution containing 10 mM 2-deoxy-D-glucose and 10 mM sodium dithionite (Na2S2O4), that induce metabolic inhibition and anoxia (MI/A) (Wu et al., 1999; Ho et al., 2002), two consequences of ischaemia. This was followed by perfusion with normal K–H solution for 10-min reperfusion.
Intracellular calcium recording
[Ca2+]i and its transient were determined by a spectrofluorometric method using the sensitive dye fura-2 as Ca2+ indicator. After stabilization, isolated myocytes were incubated with 1 μM fura-2/AM at room temperature for 30 min. Then, the loaded cells were washed three times with fresh K–H buffer solution containing 1% BSA. Fluorescence was measured on an Olympus inverted microscope equipped with a fluorometer system (TILL, Germany). A small aliquot of fura-2-loaded cells was placed on the glass bottom of a chamber and then perfused with K–H buffer solution with a gas phase of 95% O2/5% CO2 at room temperature. The Ca2+-dependent signal of fura-2 was obtained by illuminating at 340 and 380 nm and recording the emitted light at 510 nm. The [Ca2+]i transient was induced by supra-threshold stimuli at 0.2 Hz delivered through two platinum field-stimulation electrodes in the bathing fluid.
Cell viability
Trypan blue exclusion was used as an index of the viability of the myocytes (Zhou et al., 1996; Hiebert & Ping, 1997). After cells were incubated with 0.4% trypan blue dye for 3 min, they were counted in a haemocytometer chamber under a light microscope. Dead cells are not able to exclude trypan blue and thus appear blue. The cell morphology was determined by microscopic examination (Zhou et al., 1996). Only rod-shaped (length/width ratio, >3 : 1) cells were used for data collection.
Experimental protocol
Two preparations were used: the isolated perfused heart and isolated ventricular myocytes.
Isolated perfused heart
After an initial stabilization for 20 min, all hearts were subjected to 30 min of regional ischaemia and 120 min of reperfusion. For pretreatment with U50, hearts were treated for two periods of 5 min each of U50 (10 μM) with 5 min of intervening reperfusion before ischaemia (Chen et al., 2003; Cao et al., 2004). To activate PKC, phorbol 12-myristate 13-acetate (PMA, 0.1 μM), an activator of PKC, was administered for 10 min (Wang et al., 2001a). To activate the KCa channel, 10 μM NS1619 was perfused for 10 min, 15 min before ischaemia. At 5 min after administration of U50 or PMA, the heart was subjected to ischaemic insult for 30 min followed by 2 h reperfusion. Blockade of κ-OR or PKC or the KCa channel was achieved by administration of the selective blocker nor-BNI (5 μM) or the inhibitors GF109203X (GF, 10 μM), chelerythrine (Che, 5 μM), or Pax (1 μM) for 30 min before the ischaemic insult. The pore opener, atractyloside (Atr, 20 μM), and inhibitor, cyclosporine A (CsA, 0.2 μM), was administered for 25 min, from the final 10 min of ischaemia to 15 min into reperfusion (Hausenloy et al., 2002). LDH was measured at 5 min into reperfusion, when its release reaches the peak level (Chen et al., 2003; Cao et al., 2004). Infarct size was determined at the end of reperfusion.
Isolated ventricular myocytes
Isolated ventricular myocytes were allowed to equilibrate for 5 min with continuous perfusion of K–H solution. In the metabolic inhibition and MI/AR group, after stabilization, myocytes were perfused with K–H solutions for 25 min and then for 5 min with MI/A solution containing 10 mM 2-deoxy-D-glucose and 10 mM sodium dithionite, that produced anoxia and metabolic inhibition (Wu et al., 1999; Ho et al., 2002), followed by perfusion with normal K–H solution for 10-min reperfusion. In the groups pretreated with U50, myocytes were perfused with two periods of 5 min each with U50 (10 μM) with a 5-min intervening reperfusion before MI/A. The pore opener, Atr (20 μM), the inhibitor of the KCa channel, Pax (1 μM), and the κ-OR antagonist, nor-BNI (5 μM), were administered for 25 min before MI/A (Wu et al., 1999; Xu et al., 2002). Determination of diastolic [Ca2+]i, its transient and cell viabilities were performed at the end of 10-min reperfusion.
Chemicals
Collagenase (type I), bovine serum albumin, nor-BNI, PMA, U50, Che, GF, NS1619, 2-deoxy-D-glucose, sodium dithionite, Atr, CsA, Pax, 2,3,5-triphenyl-tetrazolium chloride, and fura-2-acetoxymethyl ester were purchased from Sigma-Aldrich (St Louis, U.S.A.).
Nor-BNI (Portoghese et al., 1994), a selective antagonist of κ-ORs, was used to block the effect of κ-OR stimulation at 5 μM (Schoffelmeer et al., 1997; Wang et al., 2001a). Pax (1 μM) and NS1619 (10 μM), which have been shown to inhibit and open the KCa channel, respectively (Olesen et al., 1994; Gribkoff et al., 1996; Xu et al., 2002; Shintani et al., 2004; Cao et al., 2005; Sato et al., 2005), were used to block and activate the KCa channel, at doses used in a previous study (Xu et al., 2002; Cao et al., 2005). PMA (0.1 μM) and Che or GF (10 μM), PKC activator and inhibitors, respectively, were used at the doses used by Liang (1997) and Ahmet et al. (2000). Atr (20 μM) is a pore opener, and the dose used was based on previous studies (Hausenloy et al., 2002).
Statistical analysis
Values presented here are means±standard errors of means. Statistical comparisons were performed by one-way analysis of variance and the Newman–Keuls test. Differences of P<0.05 were regarded as significant.
Results
Effects of U50 on myocardial injury induced by ischaemia and reperfusion – blockade of the KCa channel with Pax
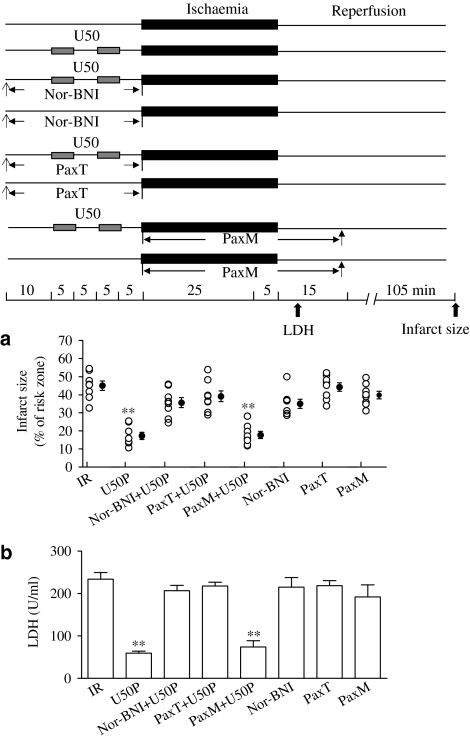
There were no significant differences between control and treatment groups in risk zone size (data not shown). Myocardial ischaemia for 30 min followed by reperfusion for 120 min induced an infarct of 43.1±7.7%. The infarct size was significantly reduced when 10 μM U50 was administered for two cycles of 5 min each, interspersed with 5 min of drug-free perfusion before ischaemia (Figure 1a). Pretreatment of the heart with 10 μM U50 also significantly reduced the release of LDH (Figure 1b).
Figure 1.
Effects of U50 in the presence of nor-BNI, 5 μM or Pax on myocardial infarct (a) or LDH release (b) in the isolated perfused rat heart subjected to ischaemia for 30 min and reperfusion for 120 min. Experimental protocol is shown in the upper panel. Hearts were pretreated with 10 μM U50 interspersed with 5 min drug-free perfusion. nor-BNI (5 μM) was administered for 30 min before ischaemia. Pax (1 μM) was either administered for 30 min before ischaemia (PaxT) or throughout the ischaemia and 15 min after start of reperfusion (PaxM). Data are expressed as individual values and mean±s.e.m.; n=10 in each group. **P<0.01 vs the IR group.
The effects of U50 on infarction and LDH release were blocked by a selective κ-OR blocker, 5 μM nor-BNI (Figure 1), and by a KCa channel inhibitor, 1 μM Pax, administered before (PaxT), but not during, ischaemia (PaxM) (Figure 1). Nor-BNI or Pax alone had no effect.
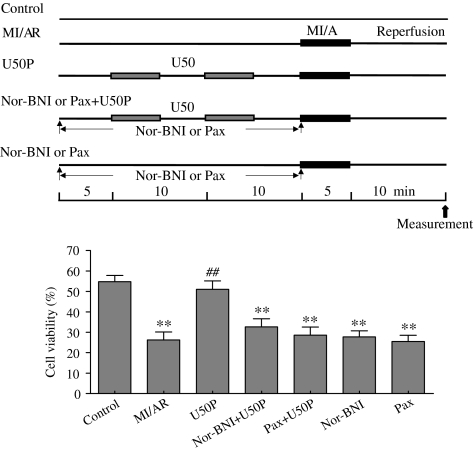
Effect of U50 on viability of ventricular myocytes subjected to MI/A – blockade of KCa channel with Pax
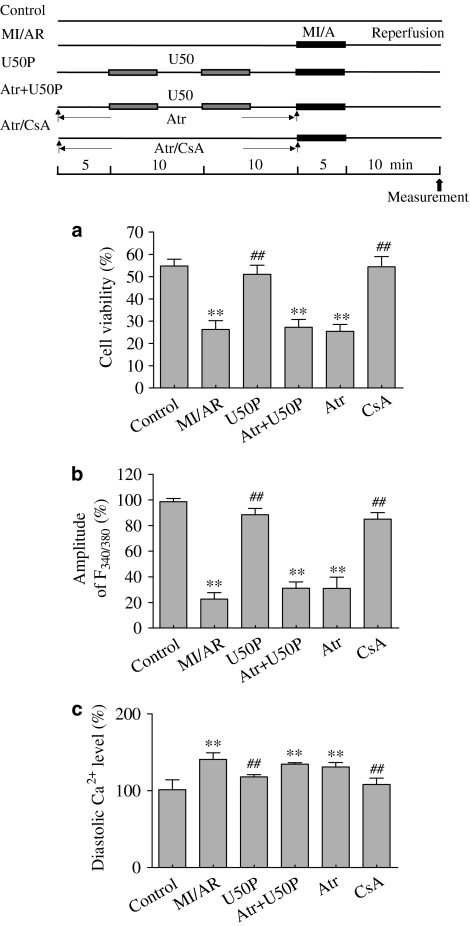
The percentage of rod-shaped unstained cells 10 min after start of reperfusion was significantly decreased in ventricular myocytes subjected to MI/A compared with that in control (Figure 2). In cells pretreated with U50, the percentage of viable cells after MI/A was significantly greater than that in untreated cells. This effect was significantly attenuated in the presence of 5 μM nor-BNI, or 1 μM Pax (Figure 2). Nor-BNI or Pax alone had no effect.
Figure 2.
Effects of U50 in the presence of nor-BNI or Pax on trypan blue exclusion of rat ventricular myocytes subjected to MI/AR. Experimental protocol is shown in the upper panel. Cells were pretreated with 10 μM U50 interspersed with 5 min drug-free perfusion. nor-BNI (5 μM) or 1 μM Pax was administered for 30 min before MI/A. Data are expressed as mean±s.e.m.; n=10 in each group. **P<0.01 compared with the control group; ##P<0.01 compared with the MI/AR group.
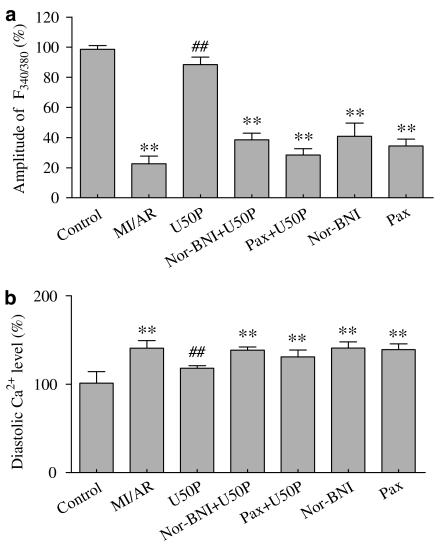
Effect of U50 on intracellular calcium in isolated ventricular myocytes subjected to MI/A – blockade of KCa channel with Pax
The amplitude of the electrically stimulated [Ca2+]i transient was markedly reduced at the end of reperfusion, while the diastolic Ca2+ level was elevated (Figure 3b). Pretreatment with 10 μM U50 attenuated the effects of MI/A and reperfusion on the [Ca2+]i transient and diastolic Ca2+. The attenuating effects of U50 were blocked by 5 μM nor-BNI (Figure 3) or 1 μM Pax (Figure 3). Neither nor-BNI nor Pax given alone had any effect on intracellular calcium.
Figure 3.
Effects of U50 in the presence of nor-BNI (5 μM) or Pax on the amplitude of the electrically induced intracellular [Ca2+]i transient (a) and diastolic [Ca2+]i (b) of rat ventricular myocytes subjected to MI/AR. Experimental protocol is shown in the upper panel of Figure 2. Cells were pretreated with 10 μM U50 interspersed with 5 min drug-free perfusion. nor-BNI (5 μM) or 1 μM Pax was administered for 30 min before MI/A. Data are expressed as mean±s.e.m.; n=10 in each group. **P<0.01 compared with the control group; ##P<0.01 compared with the MI/AR group.
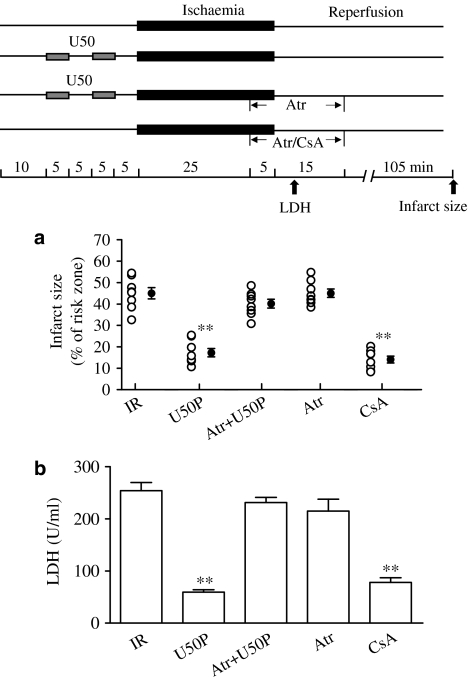
Effects of U50 on hearts subjected to ischaemic insult – opening of the mitochondrial permeability transition pore
End- and postischaemic treatment of the heart with 20 μM Atr blocked the beneficial effects of 10 μM U50 on infarct size (Figure 4a) and LDH release (Figure 4b). Similarly, 20 μM Atr administered for 25 min before MI/A also abolished the beneficial effects of U50 on cell viability, the [Ca2+]i transient and diastolic Ca2+ (Figure 5). Atr (20 μM) alone had no effect in agreement with previous findings (Xu et al., 2001; Hausenloy et al., 2002; Cao et al., 2005).
Figure 4.
Effects of CsA or U50 in the presence of Atr on myocardial infarct (a) and LDH release (b) in the isolated perfused rat heart subjected to 30 min of ischaemia and 120 min reperfusion. Experimental protocol is shown in the upper panel. CsA (0.2 μM) or 20 μM Atr was administered from the last 5 min of ischaemia to 15 min into reperfusion. Data are expressed as individual values and mean±s.e.m.; n=10 in each group. **P<0.01 compared with IR group.
Figure 5.
Effects of CsA or U50 in the presence of Atr on trypan blue exclusion (a) and intracellular calcium (b, c) of rat ventricular myocytes subjected to MI/AR. Experimental protocol is shown in the upper panel. Cells were pretreated with 10 μM U50 interspersed with 5 min drug-free perfusion. CsA (0.2 μM) or 20 μM Atr was administered 30 min before MI/A. Data are expressed as mean±s.e.m.; n=10 in each group. **P<0.01 compared with the control group; ##P<0.01 compared with the MI/AR group.
Administration of 0.2 μM CsA reduced the infarct size and LDH release in the isolated perfused rat heart subjected to ischaemia/reperfusion (Figure 4), and attenuated the effects induced by MI/AR in isolated myocytes (Figure 5). These are in agreement with previous findings (Cao et al., 2005).
Effects of PKC activation on myocardial injury induced by ischaemia and reperfusion – blockade of the KCa channel with Pax
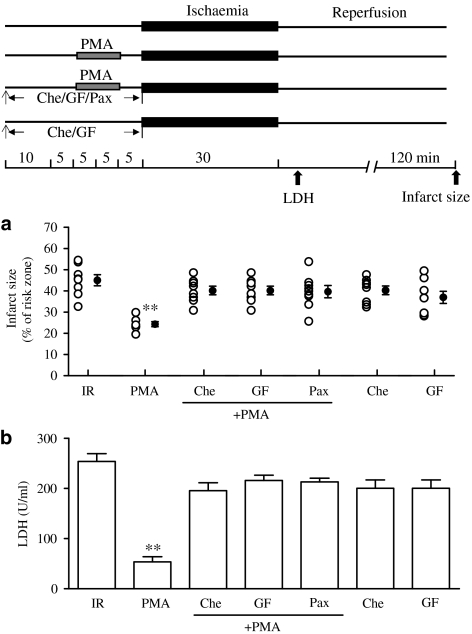
Pretreatment with 0.1 μM PMA before ischaemic insult significantly reduced the infarct size and LDH release compared with the IR group. This is in agreement with our previous finding that PKC-ɛ is a trigger of IPC (Wang et al., 2001b). The effects of PKC activation on infarction and LDH release were blocked by blockade of PKC with 10 μM GF or 5 μM Che. More interestingly, the effects were abolished when Pax was administered for 30 min before ischaemia (Figure 6).
Figure 6.
Effects of PMA on myocardial infarct (a) and LDH release (b) in isolated perfused rat heart subjected to 30 min ischaemia and 120 min reperfusion. Experimental protocol is shown in the upper panel. PMA (0.1 μM) was administered for 10 min before 15 min of ischaemia. Che (5 μM) and 10 μM GF and 1 μM Pax were administered for 30 min before ischaemia. Data are expressed as individual values and mean±s.e.m.; n=10 in each group. **P<0.01 compared with the IR group.
Activation of KCa channel with 10 μM NS1619 also reduced infarct size and LDH release, in agreement with our previous observation (Cao et al., 2004). The effects were not altered by blockade of PKC with either 10 μM GF or 5 μM Che (Figure 7).
Figure 7.
Effects of NS1619 on myocardial infarct (a) and LDH release (b) in isolated perfused rat hearts subjected to 30 min ischaemia and 120 min reperfusion. Experimental protocol is shown in the upper panel. NS1619 (10 μM) was administered 15 min before ischaemia for 10 min. Che (5 μM) and 10 μM GF and 1 μM Pax were administered for 30 min before ischaemia. Data are expressed as individual values and mean±s.e.m.; n=10 in each group. **P<0.01 compared with the IR group.
GF or Che given alone prior to the ischaemic period had no effect on infarct size and LDH release (Figure 6).
Discussion and conclusions
The first important observation of the present study was that the cardioprotective effects conferred by κ-OR stimulation with U50 were abolished when a KCa channel blocker, Pax, was administered before preconditioning, but not during ischaemic insult in the isolated perfused heart. This observation indicates that the KCa channel triggers the cardioprotection of κ-OR stimulation as it does of IPC (Cao et al., 2005). Furthermore, we observed that the beneficial effects of κ-OR stimulation on viability and alterations of [Ca2+]i in isolated ventricular myocytes subsequently subjected to MI/A were also abolished by Pax. This observation not only confirms the role of the KCa channel in κ-OR-stimulated cardioprotection, but also suggests that the KCa channel may mediate cardioprotection by attenuating Ca2+ overload.
Another important observation is that an opener of the mitochondrial permeability transition pore reversed the beneficial effects of κ-OR stimulation. This is also the first evidence that κ-OR cardioprotection involves inhibition of pore opening, well known to play an important role in the cardioprotection of IPC (Hausenloy et al., 2002).
The most interesting observation of the present study is that the cardioprotective effect of PKC activation was abolished by blockade of the KCa channel, while the cardioprotective effect of opening the KCa channel was not altered by blockade of PKC. This is the first ever demonstration that PKC is located upstream of the KCa channel.
In the present study, we observed that inhibition of pore opening by κ-OR stimulation or by the inhibitor of the pore, that conferred cardioprotection, also attenuated the [Ca2+]i overload, particularly during reperfusion, a period when severe cardiac infarction and arrhythmia occur (Piper et al., 2003). In addition, pore opening abolished the cardioprotective effect of κ-OR stimulation on [Ca2+]i overload. These observations support the notion that [Ca2+]i overload leads to mitochondrial Ca2+ accumulation, which leads to pore opening and cardiac injury/death (Weiss et al., 2003).
In addition, we observed that inhibition of pore opening by κ-OR stimulation or CsA restored the electrically induced [Ca2+]i transient that was reduced by ischaemic insult and reperfusion, while opening the pore abolished the beneficial effect of κ-OR. Since the electrically induced [Ca2+]i transient represents influx of Ca2+ through the L-type Ca2+ channel and release of Ca2+ from the sarcoplasmic reticulum during excitation–contraction coupling (Bers, 2000), and is directly correlated to contraction (Yu et al., 1998), these observations indicate that inhibition of pore opening restores cardiac contractility by restoring Ca2+ homeostasis that was impaired by ischaemia/reperfusion (Weiss et al., 2003).
Previous work showed that PKC is upstream of the pore (Baines et al., 2003). In support of this finding, we showed that the cardioprotective effects of PKC activation were blocked by opening the pore, while the effects of inhibiting pore opening were not affected by inhibiting PKC (data not shown). So, after κ-OR activation, the signal transduction pathway is PKC–KCa channel–pore.
Electrophysiological studies using the patch-clamp technique showed that NS1619 is an opener (Olesen et al., 1994; Xu et al., 2002), while Pax is an inhibitor (Gribkoff et al., 1996), of the KCa channels. Their selectivity for KCa, but not KATP, was supported by the fact that the effects of NS1619 are not affected by the mitochondrial channel blocker 5-HD (O'Rourke, 2004; Cao et al., 2005; Sato et al., 2005) and that Pax fails to inhibit the effects of diazoxide, a KATP channel opener (Cao et al., 2005; Sato et al., 2005). However, precautions should be taken that these two agents may have nonselective actions.
In conclusion, the present study has provided the first evidence that the high-conductance KCa channel triggers the cardioprotection of κ-OR stimulation, as it does that of IPC. The mechanism involves inhibition of mitochondrial permeability transition pore opening and is located downstream of PKC.
Acknowledgments
We thank Dr I.C. Bruce for reading the manuscript, Dr G.H. Borchert for advice on the viability study and Mr C.P. Mok for technical assistance.
Abbreviations
- Atr
atractyloside
- [Ca2+]i
intracellular Ca2+
- Che
chelerythrine
- CsA
cyclosporine A
- GF
GF109203X
- IPC
ischaemic preconditioning
- IR
ischaemia reperfusion
- κ-OR
kappa opioid receptor
- LDH
lactate dehydrogenase
- MI/A
metabolic inhibition and anoxia
- MI/AR
metabolic inhibition and anoxia/reperfusion
- Nor-BNI
nor-binaltorphimine
- NS1619
1,3-dihydro-1-[2-hydroxy-5 (trifluoromethyl)phenyl]-5-trifluoromethyl-2Hbenzimidazol-2-one
- Pax
paxilline
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- U50
U50,488H
References
- AHMET I., SAWA Y., NISHIMURA M., ICHIKAWA H., MATSUDA H. Diadenosine tetraphosphate (AP4A) mimics cardioprotective effect of ischemic preconditioning in the rat heart: contribution of KATP channel and PKC. Basic Res. Cardiol. 2000;95:235–242. doi: 10.1007/s003950050186. [DOI] [PubMed] [Google Scholar]
- BAINES C.P., GOTO M., DOWNEY J.M. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J. Mol. Cell. Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- BAINES C.P., SONG C.X., ZHENG Y.T., WANG G.W., ZHANG J., WANG O.L., GUO Y., BOLLI R., CARDWELL E.M., PING P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERS D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- CAO C.M., XIA Q., GAO Q., CHEN M., WONG T.M. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J. Pharmacol. Exp. Ther. 2005;312:644–650. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- CAO C.M., XIA Q., TU J., CHEN M., WU S., WONG T.M. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2004;309:560–567. doi: 10.1124/jpet.103.061135. [DOI] [PubMed] [Google Scholar]
- CHEN M., ZHOU J.J., KAM K.W., QI J.S., YAN W.Y., WU S., WONG T.M. Roles of KATP channels in delayed cardioprotection and intracellular Ca(2+) in the rat heart as revealed by kappa-opioid receptor stimulation with U50488H. Br. J. Pharmacol. 2003;140:750–758. doi: 10.1038/sj.bjp.0705475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBKOFF V.K., LUM-RAGAN J.T., BOISSARD C.G., POST-MUNSON D.J., MEANWELL N.A., STARRETT J.E., JR, KOZLOWSKI E.S., ROMINE J.L., TROJNACKI J.T., MCKAY M.C., ZHONG J., DWORETZKY S.I. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol. Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- HAUSENLOY D.J., MADDOCK H.L., BAXTER G.F., YELLON D.M. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning. Cardiovasc. Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- HIEBERT L., PING T. Protective effect of dextran sulfate and heparin on adult rat cardiomyocytes damaged by free radicals. J. Mol. Cell. Cardiol. 1997;29:229–235. doi: 10.1006/jmcc.1996.0267. [DOI] [PubMed] [Google Scholar]
- HO J.C., WU S., KAM K.W., SHAM J.S., WONG T.M. Effects of pharmacological preconditioning with U50488H on calcium homeostasis in rat ventricular myocytes subjected to metabolic inhibition and anoxia. Br. J. Pharmacol. 2002;137:739–748. doi: 10.1038/sj.bjp.0704945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRIEG T., COHEN M.V., DOWNEY J.M. Mitochondria and their role in preconditioning's trigger phase. Basic Res. Cardiol. 2003;98:228–234. doi: 10.1007/s00395-003-0422-y. [DOI] [PubMed] [Google Scholar]
- LIANG B.T. Protein kinase C-mediated preconditioning of cardiac myocytes: role of adenosine receptor and KATP channel. Am. J. Physiol. 1997;273:H847–H853. doi: 10.1152/ajpheart.1997.273.2.H847. [DOI] [PubMed] [Google Scholar]
- LIU G.S., THORNTON J., VAN WINKLE D.M., STANLEY A.W., OLSSON R.A., DOWNEY J.M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- O'ROURKE B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ. Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLESEN S.P., MUNCH E., MOLDT P., DREJER J. Selective activation of Ca(2+)-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- PIPER H.M., MEUTER K., SCHAFER C. Cellular mechanisms of ischemia–reperfusion injury. Ann. Thorac. Surg. 2003;75:S644–S648. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- PORTOGHESE P.S., LIN C.E., FAROUZ-GRANT F., TAKEMORI A.E. Structure-activity relationship of N17′-substituted norbinaltorphimine congeners. Role of the N17′ basic group in the interaction with a putative address subsite on the kappa opioid receptor. J. Med. Chem. 1994;37:1495–1500. doi: 10.1021/jm00036a015. [DOI] [PubMed] [Google Scholar]
- SATO T., SAITO T., SAEGUSA N., NAKAYA H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., HOGENBOOM F., MULDER A.H. Kappa1- and kappa2-opioid receptors mediating presynaptic inhibition of dopamine and acetylcholine release in rat neostriatum. Br. J. Pharmacol. 1997;122:520–524. doi: 10.1038/sj.bjp.0701394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ J.E., HSU A.K., BARBIERI J.T., LI P.L., GROSS G.J. Pertussis toxin abolishes the cardioprotective effect of ischemic preconditioning in intact rat heart. Am. J. Physiol. 1998;275:H495–H500. doi: 10.1152/ajpheart.1998.275.2.H495. [DOI] [PubMed] [Google Scholar]
- SHENG J.Z., WONG N.S., WANG H.X., WONG T.M. Pertussis toxin, but not tyrosine kinase inhibitors, abolishes effects of U-50488H on [Ca2+]i in myocytes. Am. J. Physiol. 1997;272:C560–C564. doi: 10.1152/ajpcell.1997.272.2.C560. [DOI] [PubMed] [Google Scholar]
- SHINTANI Y., NODE K., ASANUMA H., SANADA S., TAKASHIMA S., ASANO Y., LIAO Y., FUJITA M., HIRATA A., SHINOZAKI Y., FUKUSHIMA T., NAGAMACHI Y., OKUDA H., KIM J., TOMOIKE H., HORI M., KITAKAZE M. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J. Mol. Cell. Cardiol. 2004;37:1213–1218. doi: 10.1016/j.yjmcc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- SPEECHLY-DICK M.E., MOCANU M.M., YELLON D.M. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ. Res. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- WANG G.Y., WU S., PEI J.M., YU X.C., WONG T.M. Kappa- but not delta-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmia in rats. Am. J. Physiol. Heart Circ. Physiol. 2001a;280:H384–H391. doi: 10.1152/ajpheart.2001.280.1.H384. [DOI] [PubMed] [Google Scholar]
- WANG G.Y., ZHOU J.J., SHAN J., WONG T.M. Protein kinase C-epsilon is a trigger of delayed cardioprotection against myocardial ischemia of kappa-opioid receptor stimulation in rat ventricular myocytes. J. Pharmacol. Exp. Ther. 2001b;299:603–610. [PubMed] [Google Scholar]
- WEISS J.N., KORGE P., HONDA H.M., PING P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- WU S., LI H.Y., WONG T.M. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of kappa-opioid receptor. Circ. Res. 1999;84:1388–1395. doi: 10.1161/01.res.84.12.1388. [DOI] [PubMed] [Google Scholar]
- XU M., WANG Y., HIRAI K., AYUB A., ASHRAF M. Calcium preconditioning inhibits mitochondrial permeability transition and apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H899–H908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- XU W., LIU Y., WANG S., MCDONALD T., VAN EYK J.E., SIDOR A., O'ROURKE B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- YU X.C., LI H.Y., WANG H.X., WONG T.M. U50,488H inhibits effects of norepinephrine in rat cardiomyocytes-cross-talk between kappa-opioid and beta-adrenergic receptors. J. Mol. Cell. Cardiol. 1998;30:405–413. doi: 10.1006/jmcc.1997.0604. [DOI] [PubMed] [Google Scholar]
- ZHOU X., ZHAI X., ASHRAF M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation. 1996;93:1177–1184. doi: 10.1161/01.cir.93.6.1177. [DOI] [PubMed] [Google Scholar]