Abstract
Hydrogen sulfide (H2S) is described as a mediator of diverse biological effects, and is known to produce irritation and injury in the lung following inhalation. Recently, H2S has been found to cause contraction in the rat urinary bladder via a neurogenic mechanism. Here, we studied whether sodium hydrogen sulfide (NaHS), used as donor of H2S, produces responses mediated by sensory nerve activation in the guinea-pig airways.
NaHS evoked an increase in neuropeptide release in the airways that was significantly attenuated by capsaicin desensitization and by the transient receptor potential vanilloid 1 (TRPV1) antagonist capsazepine. In addition, NaHS caused an atropine-resistant contraction of isolated airways, which was completely prevented by capsaicin desensitization. Furthermore, NaHS-induced contraction was reduced by TRPV1 antagonism (ruthenium red, capsazepine and SB366791), and was abolished by pretreatment with the combination of tachykinin NK1 (SR140333) and NK2 (SR48968) receptor antagonists.
In anesthetized guinea-pigs, intratracheal instillation of NaHS increased the total lung resistance and airway plasma protein extravasation. These two effects were reduced by TRPV1 antagonism (capsazepine) and tachykinin receptors (SR140333 and SR48968) blockade.
Our results provide the first pharmacological evidence that H2S provokes tachykinin-mediated neurogenic inflammatory responses in guinea-pig airways, and that this effect is mediated by stimulation of TRPV1 receptors on sensory nerves endings. This novel mechanism may contribute to the irritative action of H2S in the respiratory system.
Keywords: Airways, capsaicin, hydrogen sulfide, neurogenic inflammation, transient receptor potential vanilloid receptor 1
Introduction
Hydrogen sulfide (H2S) is a malodorous gas representing a chemical hazard in certain manufacturing industries. Exposure to H2S can produce serious toxic effects especially in the airways where the gas can cause acute respiratory problems which include cough, respiratory tract irritation, dyspnea, chest pain (tightness), pulmonary edema and airway hyperreactivity (Enarson et al., 1987; Burney et al., 1989; Reiffenstein et al., 1992; Hessel et al., 1997). The interest around H2S has increased recently following the demonstration that the gas is endogenously generated in the central nervous system and in peripheral organs via at least two enzymatic pathways, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (Hosoki et al., 1997; Kimura, 2002; Wang, 2003). A number of studies suggest that endogenous H2S is involved in: neuronal excitation via a Ca2+- and calmodulin-mediated pathway (Eto et al., 2002); enhancement of the cAMP-induced NMDA receptor response (Kimura, 2000); modification of long-term potentiation (Abe & Kimura, 1996) and hypothalamo–pituitary–adrenal axis (Dello Russo et al., 2000). Other biological activities of H2S include epithelial deregulation via mitogen-activated protein kinases (MAPK) (Deplancke & Gaskins, 2003) and, in the cardiovascular system, relaxation of blood vessels and production of transient hypotension via activation of ATP-sensitive K+ channels (KATP) (Hosoki et al., 1997; Zhao et al., 2001).
Recently, a previously unrecognized mechanism through which H2S affects smooth muscle tone has been reported in the rat urinary bladder (Patacchini et al., 2004), where H2S contracts the isolated detrusor muscle via the stimulation of a subset of sensory nerve terminals exquisitely sensitive to the excitatory/desensitizing actions of capsaicin. The observation (Patacchini et al., 2004) that the H2S-induced contractile response in the rat detrusor muscle was totally prevented by the combination of the tachykinin NK1 and NK2 receptor antagonists indicates that endogenous tachykinins, presumably released from sensory nerve terminals, are the final mediators of H2S-induced excitatory effects in the rat bladder. The local release from sensory nerve terminals of the neuropeptide calcitonin gene-related peptide (CGRP) and the tachykinins, substance P (SP) and neurokinin A (NKA) causes a series of inflammatory responses collectively referred to as ‘neurogenic inflammation' (Maggi et al., 1995; Geppetti & Holzer, 1996). In the airways, neurogenic inflammatory responses induced by endogenously released SP/NKA include plasma protein extravasation, bronchoconstriction, mucus secretion and recruitment/activation of inflammatory and immune cells (Barnes, 1996; Geppetti & Holzer, 1996; Joos, 2001).
The present study was designed to test the hypothesis that H2S may cause neurogenic inflammation in the respiratory system via the stimulation of sensory nerve endings and the release of endogenous tachykinins, SP and NKA. Capsaicin, as well as other irritant stimuli (low extracellular pH, noxious temperature, xenobiotics, various lipid derivatives) (Szallasi & Blumberg, 1989; Bevan & Geppetti, 1994; Caterina et al., 1997; Zygmunt et al., 1999; Hwang et al., 2000; Trevisani et al., 2002), stimulate sensory nerve terminals via the activation of a recently cloned nonselective cation channel (Caterina et al., 1997) termed transient receptor potential vanilloid 1 (TRPV1) (Gunthorpe et al., 2002). The second objective of the present study was to verify whether in the guinea-pig airways H2S excites sensory nerves by the activation of the TRPV1.
Methods
Neuropeptide release
Guinea-pigs were killed by cervical dislocation and the airway tissue removed and prepared at 4°C using a tissue slicer (McIlwain Tissue Chopper, U.K.). Slices of airway tissue (approximately 0.4 mm thick and weighing 100 mg) were then placed into 1 ml chambers (37°C) and superfused at a rate of 0.4 ml min−1 with oxygenated (95% O2 and 5% CO2) Krebs–Henseleit solution (composition mM: NaCl 119, NaHCO3 25, KH2PO4 1.2, MgSO4 1.5, KCl 4.7, CaCl2 2.5, glucose 11) containing 0.1% bovine serum albumin. After a 60-min stabilization period, 10-min fractions were collected, freeze-dried, reconstituted with the assay buffer, and analysed by enzyme immunoassays for SP and CGRP like immunoreactivities (LI) (SP-LI and CGRP-LI, respectively) according to the methods reported previously (Ricciardolo et al., 2000). The level of release of SP-LI and CGRP-LI were calculated by subtracting the mean pre-stimulus value from those values obtained during and post-stimulation. The results are expressed as fmol of peptide g−1 tissue 20 min. The highest concentration of capsaicin (10 μM) and the TRPV1 receptor antagonist capsazepine (10 μM) did not show any significant cross-reactivity with SP and CGRP antisera.
Organ bath assay
Guinea-pigs were killed by cervical dislocation, the main bronchi and distal trachea (∼2 mm in width) were removed and suspended under a resting tension of 1.5 and 2 g, respectively. The tissues were bathed and aerated (95% O2 and 5% CO2) with Krebs–Henseleit solution (as described above at 37°C) that contained the neutral endopeptidase inhibitor phosphoramidon (1 μM) and the cyclooxygenase inhibitor indomethacin (5 μM), to minimize peptide degradation and prevent endogenous prostanoid generation, respectively. Tissues were allowed to equilibrate for 60 min prior to the beginning and between each set of experiments (washed every 5 min for the first 15 min). All tissues were first contracted with carbachol (CCh, 1 μM, as reported previously (Trevisani et al., 2004)) to record the maximal contractile response of each preparation. Motor activity was recorded isometrically on a force transducer (Ugo Basile, Milan, Italy).
Cumulative concentration–response curves were performed with sodium hydrogen sulfide (NaHS) or NaOH (10 μM–30 mM), capsaicin (1 nM–3 μM) and SP (1 nM–1 μM) either in the presence of the TRPV1 receptor antagonists ruthenium red (10 μM), capsazepine (10 μM) or SB366791 (1 μM), the combination of the selective tachykinin NK1 (SR140333, 1 μM) and NK2 (SR48968, 1 μM) receptor antagonists, the nonselective muscarinic antagonist atropine (1 μM) or their respective vehicles. In another set of experiments, airway preparations were pre-incubated twice for 20 min with a capsaicin (10 μM) concentration known to desensitize the sensory nerve endings (Szallasi & Blumberg, 1999). The bathing fluid was then changed repeatedly (every 5 min over a period of 30 min) until the contractile response had returned to baseline, and cumulative concentration–response curves were performed with test agents.
Total lung resistance (RL)
Guinea pigs were anesthetized with sodium pentobarbital (60 mg kg−1, i.p.) and then ventilated artificially through a tracheal cannula using a constant-volume ventilator (model 683, Harvard Apparatus Co., Inc., South Natick, U.S.A.) at a frequency of 60 breaths min−1. The tidal volume was adjusted to maintain normal arterial blood gases as described previously (Dusser et al., 1988). Airflow was monitored continuously using a pneumotachograph (A. Fleisch Medical, Inc., Richmond, U.S.A.) connected to a differential pressure transducer (Model DP45, Validyne, Northridge, U.S.A.). The tidal volume was obtained by electrical integration of airflow (Model FV156, Validyne). A fluid-filled polyethylene catheter was introduced into the esophagus to measure the esophageal pressure as an approximation of pleural pressure. Intratracheal pressure was measured at the thoracic outlet by the use of a polyethylene catheter inserted into a short tube connecting the tracheal cannula to the pneumotachograph. The transpulmonary pressure (defined as the pressure difference between the intratracheal and the esophageal pressures) was measured with a differential pressure transducer (Model DP7; Validyne). Output signals representing tidal volume, transpulmonary pressure and airflow were amplified with an amplifier (model CD19; Validyne) and recorded by a polygraph recorder (Model 1508B Visicorder; Honeywell, Inc., Denver, U.S.A.). Total RL was calculated as described previously (Dusser et al., 1988) using the method of Amdur & Mead (1958). The cannulated right carotid artery was used for drug administration. Increase in RL was provoked by a single application of NaHS (50 mM, 200 μl) in the presence of two tachykinin receptor antagonists SR140333 (1.6 μmol kg−1, i.v.) and SR48968 (1.6 μmol kg−1, i.v.), capsazepine (10 μmol kg−1, i.p.) or their vehicles given 10 min prior to the stimulus.
Plasma protein extravasation
Guinea-pigs were anesthetized with sodium pentobarbital (60 mg kg−1, i.p.). Evans blue (30 mg kg−1, i.v.) was injected into the jugular vein and drugs were administered 1 min later. After five additional minutes, animals were transcardially perfused, as described previously (Trevisani et al., 2002). Pretreatment with the NK1 tachykinin receptor antagonists SR140333 (1.6 μmol kg−1, i.v.), CPZ (0.1 mM, 100 μl, i.t.) or their respective vehicles were given 15 min prior to the injection of the dye. Trachea was removed, weighed and incubated in 1 ml of formamide for 24 h in the dark (room temperature). The amount of extravasated dye was measured spectrophotometrically at 620 nm.
Animals and reagents
All animals were provided by Charles River (Italy) and all experiments complied with regional and national guidelines. Reagents were obtained from Sigma (Italy) or otherwise stated. SB366791 was obtained from Tocris (U.K.), SR140333 and SR48968 were synthesized at Sanofi-Synthlabo' (France). Drugs were dissolved in saline, with the exception of capsaicin, capsazepine and indomethacin that were stocked at a concentration of 1 mM in DMSO (100%) with further solution dissolved in saline. For the source of H2S, we used NaHS, which was dissolved in distilled water at 1 M concentration and then diluted with Krebs–Henseleit solution. NaHS quickly dissociates into Na+ and HS− ions which, in turn, react with H2O to give H2S and OH− in a proportion of about 30% in a buffered (pH=7.4) solution (Hosoki et al., 1997). For this reason, we checked whether NaHS in addition to the Krebs solution changed significantly the pH. However, NaHS at the highest concentrations employed (30–50 mM) produced a minimal increase in Krebs' pH (0.1–0.2 U). Nevertheless, to test whether changes in pH could influence our experiments, NaOH, at a concentration identical to NaHS (10 μM–30 mM), was tested in the organ bath assay, where it did not produce any effect. Thus, as a vehicle control of NaHS, we used either distilled water or physiological salt solution, in in vitro and in vivo experiments, respectively.
Statistical analysis
Data are expressed as mean±standard error of the mean (s.e.m.). Emax denotes the maximal response achieved and EC50 the concentration of test agent required to elicit 50% contractile response. Statistical analysis was performed by means of the Student's t-test and Dunnett's test when required, a P<0.05 was considered significant.
Results
Neuropeptide release
Capsaicin (0.1 μM) produced a significant increase in SP-LI (21.5±1.7 fmol g−1 20 min, n=6) and CGRP-LI outflow (246±60 fmol g−1 20 min, n=6) when compared to that produced by the vehicle control (1±0.6 fmol g−1 20 min and 11±5 fmol g−1 20 min, respectively, n=6). Similar to capsaicin, NaHS (10 mM) also produced a significant increase in SP-LI (6.7±0.6 fmol g−1 20 min, n=6) and CGRP-LI outflow (61±4 fmol g−1 20 min, n=6) when compared to that produced by the vehicle of NaHS (0.6±0.4 fmol g−1 20 min and 7±1 fmol g−1 20 min, respectively, n=6) (Figure 1). In addition, NaHS-induced SP-LI and CGRP-LI outflow was completely abolished in experiments performed with tissues pretreated with capsaicin (10 μM for 60 min before NaHS administration) and significantly reduced when in the presence of the TRPV1 antagonist, capsazepine (10 μM) (Figure 1).
Figure 1.
The NaHS-induced release of SP and CGRP LI was significantly reduced following capsaicin desensitization (Caps Des, 10 μM for 60 min) or in the presence of capsazepine (CPZ, 10 μM). Each entry is the mean±s.e.m. of at least five experiments. *P<0.05 vs veh, Dunnett's t-test.
Organ bath assay
Effect of NaHS in comparison to other known airway spasmogens
Administration of NaHS (10 μM–30 mM) to guinea-pig isolated bronchial (Figure 2) or tracheal rings caused a concentration-related contraction. In contrast, NaOH failed to significantly contract guinea-pig isolated airways (Figure 2c, d and Table 1). The EC50 of the contractile response induced by NaHS in the bronchus was 1.3±0.13 mM (n=6). The potency of NaHS in guinea-pig isolated bronchus was about four orders of magnitude lower than that of capsaicin (EC50 47.7±0.07 nM, n=6) and SP (EC50 20.5±0.05 nM, n=6) (Figure 2d, data were also collected in the trachea and are reported in Table 1). Although, the maximal contractile response produced by NaHS (85±11% of CCh, n=6) was comparable to that produced by capsaicin (89±19% of CCh, n=6) (Figures 1d, 2a). Similar findings were also obtained in tracheal rings (refer to Table 1).
Figure 2.
Typical traces taken from guinea-pig isolated bronchus representing cumulative concentration-response curves (CRC) to (b) NaHS following capsaicin desensitization (10 μM twice for 20 min, 30 min before the stimulus) or its vehicle (a) and CRC to NaOH (c); (d) pooled data of CRC to capsaicin (Caps), SP, NaHS and NaOH. Each point represents the mean±s.e.m. value of at least six experiments.
Table 1.
The effect of NaHS in guinea-pig isolated tracheal rings
| Drug protocol | EC50 | Emax (% ofCCh) |
|---|---|---|
| NaHS | ||
| Vehicle | 0.9±0.20 mM | 43±11 |
| Capsaicin desensitization | N/D | 2±2* |
| SR140333 & SR48968 (1 μM) | N/D | 2±1* |
| Ruthenium Red (10 μM) | N/D | 7±5* |
| Capsazepine (10 μM) | N/D | 4±4* |
| SB366791 (1 μM) | N/D | 6±3* |
| NaOH | N/D | 2±1* |
| Capsaicin | ||
| Vehicle | 150±0.07 nM | 43±10 |
| Ruthenium Red (10 μM) | N/D | 10±4† |
| Capsazepine (10 μM) | N/D | 12±3† |
| SB366791 (1 μM) | N/D | 9±4† |
| Substance P | ||
| Vehicle | 39.0±0.19 nM | 94±15 |
| Capsazepine (10 μM) | 46.9±0.04 nM | 113±18 |
| Carbachol | ||
| Vehicle | 10.0±0.21 nM | 105±5 |
| SR140333 & SR48968 (1 μM) | 9.8±0.18 nM | 101±15 |
| Ruthenium Red (10 μM) | 10.5±0.22 nM | 95±18 |
| Capsazepine (10 μM) | 9.9±0.20 nM | 97±8 |
| SB366791 (1 μM) | 10.3±0.13 nM | 99±16 |
All pretreatments were given 15 min prior to the administration of NaHS, except for capsaicin desensitization (Caps, 10 μM twice for 20 min, 30 min before the stimulus). N/D=not detectable. Each entry represents the mean±s.e.m. value of at least six experiments.
P<0.05 vs NaHS vehicle treated.
P<0.05 vs capsaicin vehicle treated.
Influence of pretreatment with high capsaicin concentration on NaHS-induced effects
The contractile effect of NaHS in the guinea-pig isolated bronchial rings was converted into a mild relaxant response by in vitro pretreatment with a high capsaicin concentration (10 μM pre-incubated twice for 20 min, 30 min prior to NaHS stimulus) (Figures 2b and 3a), a procedure that causes a complete desensitization of sensory nerve terminals, that become unresponsive to the excitatory action of further administration of capsaicin itself (Szallasi & Blumberg, 1999) (Figure 3b). On the other hand, the SP (1 μM)-induced airway contractile response was unchanged in capsaicin-pretreated tissues (Figure 3c) as compared to that produced in control preparations, thus indicating the selectivity of NaHS on sensory nerve terminals.
Figure 3.
Cumulative CRC to (a) NaHS, (b) capsaicin (Caps) and (c) SP following capsaicin desensitization (Caps Des, 10 μM twice for 20 min, 30 min before the stimulus) or vehicle (veh) pretreatment. Each entry represents the mean±s.e.m. value of at least six experiments (*P<0.05 vs veh).
Blockade of receptors and channels in NaHS-induced airway contraction
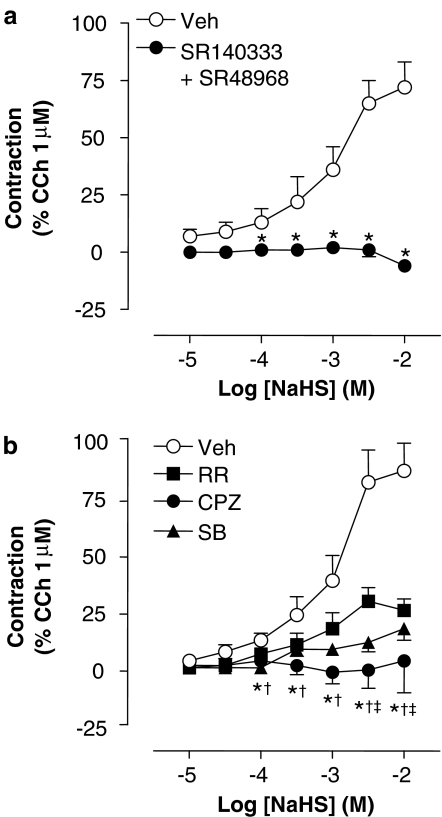
The possible role of a cholinergic mechanism in the contractile effect produced by NaHS was ruled out, as the nonselective muscarinic antagonist atropine (1 μM) failed to reduce NaHS (10 mM)-induced bronchial contraction (72±14% of CCh in vehicle-pretreated vs 80±16% of CCh in atropine-pretreated preparations, respectively; n=6 each). On the contrary, the contractile effect of NaHS in the guinea-pig bronchus was totally prevented by a combination of the tachykinin NK1 and NK2 (SR140333 and SR48968 both at 1 μM, respectively) receptor antagonists (Figure 4a). The contractile response to NaHS was also greatly reduced by the TRPV1 receptor antagonists ruthenium red (10 μM), capsazepine (10 μM) or SB366791 (1 μM) (Figure 4b). In contrast, the contraction evoked by CCh (1 μM) was totally unaffected by any of these antagonists (SR140333, SR48968, ruthenium red, capsazepine, SB366791) compounds (data for guinea-pig isolated trachea are shown in Table 1), thus showing that they do not modify smooth muscle contractility at the concentrations employed, and suggesting selectivity. All data concerning isolated tracheal preparations, similar to those obtained in bronchial rings, are reported in Table 1.
Figure 4.
Cumulative CRC to NaHS (a) in the presence of the combination of the tachykinin NK1 (SR140333 1 μM) and NK2 (SR48968, 1 μM) receptor antagonists, (b) the TRPV1 receptor antagonists, ruthenium red (RR, 10 μM), capsazepine (CPZ, 10 μM), SB366791 (1 μM) or the combination of their respective vehicles (veh). Each entry represents the mean±s.e.m. value of at least six experiments (*P<0.05 tachykinin antagonists or CPZ vs veh; †P<0.05 SB366791 vs veh; ‡P<0.05 RR vs veh).
Total RL
After a stabilization period of 30 min, basal level of total RL of anaesthetized guinea-pigs remained stable for at least 2 h. Intratracheal instillation of NaHS (50 mM, 200 μl) produced a significant increase in baseline value of RL (51±11%, n=13) when compared to that of the vehicle (4±1%, n=9) (Figure 5a). Furthermore, NaHS-induced increase in RL was significantly reduced in the presence of the two tachykinin receptor antagonists SR140333 (1.6 μmol kg−1, i.v.) and SR48968 (1.6 μmol kg−1, i.v.) (10±1.5%, n=6; P<0.05 vs vehicle pretreated), given 10 min prior to the stimuli (Figure 5a). In addition, the bronchoconstrictor response induced by NaHS was significantly attenuated by the TRPV1 receptor antagonist capsazepine (10 μM) (17±1.8%, n=7; P<0.05 vs vehicle pretreated) (Figure 5a).
Figure 5.
(a) Total RL and (b) plasma protein extravasation in guinea-pig trachea after the intratracheal instillation of NaHS (50 mM, 200 μl; black column) or its vehicle (white columns). The effects of NaHS were analyzed in the presence or absence of the tachykinin NK1 (SR140333) and/or NK2 (SR48968) receptor antagonist (both at 1.6 μmol kg−1, i.v.), and the TRPV1 receptor antagonists, capsazepine (CPZ, 10 μM). Each entry is the mean±s.e.m. of at least six experiments (*P<0.05 vs veh of NaHS; #P<0.05 vs veh).
Plasma protein extravasation
Intratracheal instillation of NaHS (50 mM, 200 μl) produced an increase in the Evans blue dye extravasation in guinea-pig trachea (46.1±3.8 ng mg−1 of tissue, n=6) that was statistically greater (P<0.05) as compared to the extravasation produced by the instillation of the vehicle alone (26.2±7.5 ng mg−1 of tissue, n=6) (Figure 5b). Furthermore, pretreatment with SR140333 (1.6 μmol kg−1, i.v.), given 20 min prior to the administration of Evans blue, significantly inhibited NaHS-induced plasma protein extravasation in the guinea-pig trachea (27.0±4.7 ng mg−1 of tissue, n=6; P<0.05 vs vehicle pretreated) (Figure 5b). Finally, the plasma-protein-induced extravasation induced by NaHS was significantly reduced by the TRPV1 receptor antagonists capsazepine (10 μM) (33±4.0 ng mg−1 of tissue, n=6; P<0.05 vs vehicle pretreated) (Figure 5b).
Discussion
The odourous gas H2S, as for nitric oxide, has a role as mediator of important biological functions, including long-term potentiation (Abe & Kimura, 1996), neuronal excitation via a Ca2+- and calmodulin-mediated pathway (Eto et al., 2002), enhancement of the camp-induced NMDA receptor response (Kimura, 2000), hypothalamo–pituitary–adrenal axis (Dello Russo et al., 2000), epithelial deregulation via MAPK (Deplancke & Gaskins, 2003) and, in the cardiovascular system, relaxation of blood vessels and production of transient hypotension via activation of KATP (Hosoki et al., 1997; Zhao et al., 2001). In contrast with these homeostatic actions, exposure to H2S, especially in the respiratory system, represents a chemical hazard resulting from the irritant properties of the gas. Toxicity following exposure to high levels of H2S has been well documented in different professional conditions (Costigan, 2003; Hendrickson et al., 2004), including life-stock farmers (Pickrell, 1991) and personnel working in sewage works (Thorn & Beijer, 2004). However, the mechanism by which H2S produces toxic effects and the consequent plethora of symptoms in the airways (Enarson et al., 1987; Burney et al., 1989; Reiffenstein et al., 1992; Hessel et al., 1997) remains to be elucidated. Recent findings obtained in the rat urinary bladder indicate that H2S contracts the smooth muscle cell by stimulating sensory nerve endings and by releasing tachykinins, as the effect of the gas was inhibited by desensitization of sensory nerves with capsaicin and by the blockade of tachykinin NK1 and NK2 receptors (Patacchini et al., 2004). Thus, in the rat urinary bladder H2S causes a typical neurogenic inflammatory response which suggests that the gas may induce irritation of this subset of nociceptive neurons (Patacchini et al., 2004).
The present study confirms and extends these previous findings by showing that H2S possesses the ability to stimulate sensory neurons and cause the release of neuropeptides also in the guinea-pig airways. This conclusion is derived from the following three observations. Firstly, H2S-induced release of both SP-LI and CGRP-LI in guinea-pig airways was totally prevented by capsaicin desensitization. Secondly, H2S-induced contraction of the isolated guinea-pig bronchus or trachea was unaffected by muscarinic receptor blockade, but it was abolished or, for certain H2S concentrations, converted into relaxation by capsaicin desensitization. Thirdly, H2S-induced contraction was abolished by blockade of tachykinin NK1 and NK2 receptors. This finding clearly indicates that SP/NKA released from sensory nerve terminals are the final mediators of the excitatory effect of H2S on the airway smooth muscle. The role of neuropeptide-containing sensory nerves in the irritating effect of H2S, indicated by in vitro experiments, was corroborated by in vivo findings, showing that H2S increases total RL and tracheal plasma protein extravasation, two major neurogenic inflammatory responses typically occurring in the guinea-pig airways. The additional observations that both responses were abated by pretreatment with the combination of the tachykinin NK1 and NK2 receptor antagonists (bronchoconstriction) or by an NK1 receptor antagonist (plasma extravasation) strengthen our conclusion that H2S produces responses in guinea-pig airways by stimulating the sensory nerve terminals.
Next, we attempted to determine the molecular mechanism by which H2S causes the activation of sensory nerve terminals. TRPV1 is a nonselective ligand-gated ion channel (Caterina et al., 1997), expressed in a subpopulation of sensory neurons and gated by an heterogeneous series of chemical and physical agents, including vanilloid compounds, such as capsaicin and resiniferatoxin (Szallasi & Blumberg, 1989), noxious heat (Caterina et al., 1997), low extracellular pH (Bevan & Geppetti, 1994), anandamide (Zygmunt et al., 1999), N-arachidonoyl-dopamine (Harrison et al., 2003), 12-HPETE and other lipid derivatives (Hwang et al., 2000). It could be possible that the contractile response observed with H2S is via an indirect activation of TRPV1. As stated previously, TRPV1 can be activated by a number of physiological agents, for example, lipoxygenase metabolites. Thus, the possibility that H2S could evoke the release of such products or other endogenous mediators and in turn these agents cause direct activation of TRPV1. TRPV1 is not apparently involved in acute nociceptive sensation, but is essential for thermal hyperalgesia (Davis et al., 2000). Antagonists of TRPV1 with an increasing degree of selectivity are ruthenium red (Amann & Maggi, 1991), capsazepine (Bevan et al., 1992; Lou & Lundberg, 1992; McIntyre et al., 2001) and SB366791 (Gunthorpe et al., 2004). The present finding that TRPV1 antagonists were able to inhibit the in vitro neuropeptide release and bronchial contraction induced by H2S strongly suggests that the gas excites sensory nerve endings by the stimulation of TRPV1. In addition, our in vivo results confirm these original findings that H2S evokes both bronchoconstriction and protein plasma extravasation via a TRPV1-dependent pathway. These observations are of particular relevance because TRPV1 undergoes remarkable sensitization/upregulation by a large variety of exogenous agents (ethanol) (Trevisani et al., 2002) or endogenous stimuli, including activation of certain G-protein-coupled receptors (bradykinin B2 receptor and proteinase activated receptor-2) (Amadesi et al., 2004) or tyrosine kinase receptor (nerve growth factor) via protein kinase C (Premkumar & Ahern, 2000) or phospholipase C (Chuang et al., 2001) stimulation. Thus, mediators whose expression is increased by airway acute and chronic inflammation may synergize with endogenous H2S to exaggerate TRPV1 excitation on afferent and efferent discharge of sensory nerve terminals, thus aggravating the symptoms produced by sensory nerve stimulation.
It is worth mentioning that the first effective concentrations of H2S producing contractile responses in the guinea-pig bronchus (∼100 μM) are very close to the endogenous levels (10–150 μM) of H2S found under physiological conditions in blood and other tissues (Warenycia et al., 1989; Zhao et al., 2001; Wang, 2003). Although there is no direct confirmation of a role of endogenous H2S in airway neurogenic inflammation, it is worth noting that a pro-inflammatory role of endogenously generated H2S is emerging in different tissues and experimental conditions. DL-propargylglycine (PAG), an inhibitor of the H2S-synthesizing enzyme CSE, was found to inhibit carrageenan-induced oedema (Bhatia et al., 2005a). In addition, PAG inhibited both pancreatic and lung injury following caerulein-induced rat pancreatitis (Bhatia et al., 2005b), a model of injury mediated by sensory neuron activation (Nathan et al., 2001). In addition, endogenous H2S may exert a regulatory activity on sensory neurons innervating the airways, contributing to the maintenance of smooth muscle tone. To this regard, it should be noted that H2S is able to inhibit smooth muscle tone in the guinea-pig airways, an effect that is unmasked by ablation of sensory neuron-mediated excitatory effects by this gas (e.g. see Figures 2b and 3a). Similar inhibitory effects of H2S had been reported in gastrointestinal (Hosoki et al., 1997; Teague et al., 2002) and reproductive (Hayden et al., 1989; Teague et al., 2002) organs and in the urinary bladder (Patacchini et al., 2004). As found in the latter study, our present results demonstrate that H2S is endowed with smooth muscle-relaxing activity of sensory neuron-independent unknown origin.
In conclusion, our present findings suggest that H2S should be added to the list of the known environmental irritant compounds whose inhalation elicits inflammatory responses by activating sensory nerve terminals, like cigarette smoke, toluene diisocyanate, ozone and others (Joos et al., 2000; Evangelista et al., 2003). Should our hypothesis be confirmed in human beings, the use of tachykinin receptor antagonists and/or TRPV1 receptor antagonists in the emergency treatment of H2S intoxication could be taken into consideration. It must be noted, however, that human airway tissue is 2–3 orders of magnitude less sensitive to capsaicin in producing airway smooth muscle contraction than that of the guinea-pig (Spina et al., 1998). Thus, it is possible that H2S-induced effects in human respiratory system likely result from stimulation of sensory nerve terminals that activate reflex responses, including rapid shallow breathing and cough (Belvisi, 2003; Page et al., 2004). Finally, the present study provides matter for speculation on the role of endogenous H2S in the respiratory system under physiological and pathophysiological conditions.
Acknowledgments
Funding came from MURST (Cofin 2000) and ARCA (Padua). SR140333 and SR48968 were kindly given as gifts from Sanofi-Synthlabo', Montpellier, France.
Abbreviations
- CCh
carbachol
- CBS
cystathionine β-synthase
- CGRP
calcitonin gene-related peptide
- CSE
cystathionine γ-lyase
- H2S
hydrogen sulfide
- KATP
ATP-sensitive K+ channels
- MAPK
mitogen activated protein kinases
- NaHS
sodium hydrogen sulfide
- NaOH
sodium hydroxide
- NKA
neurokinin A
- RL
lung resistance
- SP
substance P
- TRPV1
transient receptor potential vanilloid receptor-1
References
- ABE K., KIMURA H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMADESI S., NIE J., VERGNOLLE N., COTTRELL G.S., GRADY E.F., TREVISANI M., MANNI C., GEPPETTI P., MC ROBERTS J.A., ENNES H., DAVIS J.B., MAYER E.A., BUNNETT N.W. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMANN R., MAGGI C.A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- AMDUR M.O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am. J. Physiol. 1958;192:364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Neuroeffector mechanisms: the interface between inflammation and neuronal responses. J. Allergy Clin. Immunol. 1996;98:S73–S81. [PubMed] [Google Scholar]
- BELVISI M.G. Sensory nerves and airway inflammation: role of A delta and C-fibres. Pulmon. Pharmacol. Ther. 2003;16:1–7. doi: 10.1016/S1094-5539(02)00180-3. [DOI] [PubMed] [Google Scholar]
- BEVAN S., GEPPETTI P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATIA M., SIDHAPURIWALA J., MOOCHHALA S.M., MOORE P.K. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005a;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATIA M., WONG F.L., FU D., LAU H.Y., MOOCHHALA S.M., MOORE P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005b;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- BURNEY P.G., LAITINEN L.A., PERDRIZET S., HUCKAUF H., TATTERSFIELD A.E., CHINN S., POISSON N., HEEREN A., BRITTON J.R., JONES T. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. Eur. Respir. J. 1989;2:940–945. [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHUANG H.H., PRESCOTT E.D., KONG H., SHIELDS S., JORDT S.E., BASBAUM A.I., CHAO M.V., JULIUS D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- COSTIGAN M.G. Hydrogen sulfide: UK occupational exposure limits. Occup. Environ. Med. 2003;60:308–312. doi: 10.1136/oem.60.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DELLO RUSSO C., TRINGALI G., RAGAZZONI E., MAGGIANO N., MENINI E., VAIRANO M., PREZIOSI P., NAVARRA P. Evidence that hydrogen sulphide can modulate hypothalamo–pituitary–adrenal axis function: in vitro and in vivo studies in the rat. J. Neuroendocrinol. 2000;12:225–233. doi: 10.1046/j.1365-2826.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- DEPLANCKE B., GASKINS H.R. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003;17:1310–1312. doi: 10.1096/fj.02-0883fje. [DOI] [PubMed] [Google Scholar]
- DUSSER D.J., UMENO E., GRAF P.D., DJOKIC T., BORSON D.B., NADEL J.A. Airway neutral endopeptidase-like enzyme modulates tachykinin-induced bronchoconstriction in vivo. J. Appl. Physiol. 1988;65:2585–2591. doi: 10.1152/jappl.1988.65.6.2585. [DOI] [PubMed] [Google Scholar]
- ENARSON D.A., VEDAL S., SCHULZER M., DYBUNCIO A., CHAN -YEUNG M. Asthma, asthmalike symptoms, chronic bronchitis, and the degree of bronchial hyperresponsiveness in epidemiologic surveys. Am. Rev. Respir. Dis. 1987;136:613–617. doi: 10.1164/ajrccm/136.3.613. [DOI] [PubMed] [Google Scholar]
- ETO K., OGASAWARA M., UMEMURA K., NAGAI Y., KIMURA H. Hydrogen sulfide is produced in response to neuronal excitation. J. Neurosci. 2002;22:3386–3391. doi: 10.1523/JNEUROSCI.22-09-03386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- EVANGELISTA S., PATACCHINI R., MAGGI C.A. Role of peripheral tachykinin receptors in neurogenic inflammation of the respiratory, genitourinary and gastrointestinal systems. Curr. Med. Chem. Antinflamm. Antiallergy Agents. 2003;2:157–174. [Google Scholar]
- GEPPETTI P., HOLZER P. Neurogenic Inflammation. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., RAMI H.K., JERMAN J.C., SMART D., GILL C.H., SOFFIN E.M., LUIS HANNAN S., LAPPIN S.C., EGERTON J., SMITH G.D., WORBY A., HOWETT L., OWEN D., NASIR S., DAVIES C.H., THOMPSON M., WYMAN P.A., RANDALL A.D., DAVIS J.B. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- HARRISON S., DE PETROCELLIS L., TREVISANI M., BENVENUTI F., BIFULCO M., GEPPETTI P., DI MARZO V. Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. Eur. J. Pharmacol. 2003;475:107–114. doi: 10.1016/s0014-2999(03)02114-9. [DOI] [PubMed] [Google Scholar]
- HAYDEN L.J., FRANKLIN K.J., ROTH S.H., MOORE G.J. Inhibition of oxytocin-induced but not angiotensin-induced rat uterine contractions following exposure to sodium sulfide. Life Sci. 1989;45:2557–2560. doi: 10.1016/0024-3205(89)90239-7. [DOI] [PubMed] [Google Scholar]
- HENDRICKSON R.G., CHANG A., HAMILTON R.J. Co-worker fatalities from hydrogen sulfide. Am. J. Ind. Med. 2004;45:346–350. doi: 10.1002/ajim.10355. [DOI] [PubMed] [Google Scholar]
- HESSEL P.A., HERBERT F.A., MELENKA L.S., YOSHIDA K., NAKAZA M. Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. Am. J. Ind. Med. 1997;31:554–557. doi: 10.1002/(sici)1097-0274(199705)31:5<554::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- HOSOKI R., MATSUKI N., KIMURA H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOOS G.F. The role of neuroeffector mechanisms in the pathogenesis of asthma. Curr. Allergy Asthma Rep. 2001;1:134–143. doi: 10.1007/s11882-001-0081-8. [DOI] [PubMed] [Google Scholar]
- JOOS G.F., GERMONPRE P.R., PAUWELS R.A. Neural mechanisms in asthma. Clin. Exp. Allergy. 2000;30:60–65. doi: 10.1046/j.1365-2222.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- KIMURA H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem. Biophys. Res. Commun. 2000;267:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- KIMURA H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- LOU Y.P., LUNDBERG J.M. Inhibition of low pH evoked activation of airway sensory nerves by capsazepine, a novel capsaicin-receptor antagonist. Biochem. Biophys. Res. Commun. 1992;189:537–544. doi: 10.1016/0006-291x(92)91591-d. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., MEINI S., SANTICIOLI P. Calcitonin gene related peptide as inhibitory neurotransmitter in the ureter. Can. J. Physiol. Pharmacol. 1995;73:986–990. doi: 10.1139/y95-137. [DOI] [PubMed] [Google Scholar]
- MCINTYRE P., MC LATCHIE L.M., CHAMBERS A., PHILLIPS E., CLARKE M., SAVIDGE J., TOMS C., PEACOCK M., SHAH K., WINTER J., WEERASAKERA N., WEBB M., RANG H.P., BEVAN S., JAMES I.F. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br. J. Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHAN J.D., PATEL A.A., MC VEY D.C., THOMAS J.E., PRPIC V., VIGNA S.R., LIDDLE R.A. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- PAGE C., REYNOLDS S.M., MACKENZIE A.J., GEPPETTI P. Mechanisms of acute cough. Pulmon. Pharmacol. Ther. 2004;17:389–391. doi: 10.1016/j.pupt.2004.09.028. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., SANTICIOLI P., GIULIANI S., MAGGI C.A. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br. J. Pharmacol. 2004;42:31–34. doi: 10.1038/sj.bjp.0705764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKRELL J. Hazards in confinement housing – gases and dusts in confined animal houses for swine, poultry, horses and humans. Vet. Hum. Toxicol. 1991;33:32–39. [PubMed] [Google Scholar]
- PREMKUMAR L.S., AHERN G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- REIFFENSTEIN R.J., HULBERT W.C., ROTH S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- RICCIARDOLO F.L., STEINHOFF M., AMADESI S., GUERRINI R., TOGNETTO M., TREVISANI M., CREMINON C., BERTRAND C., BUNNETT N.W., FABBRI L.M., SALVADORI S., GEPPETTI P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am. J. Respir. Crit. Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- SPINA D., MATERA G.M., RICCIO M.M., PAGE C.P. A comparison of sensory nerve function in human, guinea-pig, rabbit and marmoset airways. Life Sci. 1998;63:1629–1642. doi: 10.1016/s0024-3205(98)00432-9. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- TEAGUE B., ASIEDU S., MOORE P.K. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J. Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORN J., BEIJER L. Work-related symptoms and inflammation among sewage plant operatives. Int. J. Occup. Environ. Health. 2004;10:84–89. doi: 10.1179/oeh.2004.10.1.84. [DOI] [PubMed] [Google Scholar]
- TREVISANI M., GAZZIERI D., BENVENUTI F., CAMPI B., DINH Q.T., GRONEBERG D.A., RIGONI M., EMONDS-ALT X., CREMINON C., FISCHER A., GEPPETTI P., HARRISON S. Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J. Pharmacol. Exp. Ther. 2004;309:1167–1173. doi: 10.1124/jpet.103.064162. [DOI] [PubMed] [Google Scholar]
- TREVISANI M., SMART D., GUNTHORPE M.J., TOGNETTO M., BARBIERI M., CAMPI B., AMADESI S., GRAY J., JERMAN J.C., BROUGH S.J., OWEN D., SMITH G.D., RANDALL A.D., HARRISON S., BIANCHI A., DAVIS J.B., GEPPETTI P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- WANG R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox. Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- WARENYCIA M.W., GOODWIN L.R., BENISHIN C.G., REIFFENSTEIN R.J., FRANCOM D.M., TAYLOR J.D., DIEKEN F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- ZHAO W., ZHANG J., LU Y., WANG R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]