Abstract
In this study, we have examined cellular responses of neuroblastoma SH-SY5Y cells after chronic treatment with galantamine, a drug used to treat Alzheimer's disease that has a dual mechanism of action: inhibition of acetylcholinesterase and allosteric potentiation of nicotinic acetylcholine receptors (nAChR). Acute experiments confirmed that maximum potentiation of nicotinic responses occurs at 1 μM galantamine; hence this concentration was chosen for chronic treatment.
Exposure to 1 μM galantamine for 4 days decreased Ca2+ responses (by 19.8±3.6%) or [3H]noradrenaline ([3H]NA) release (by 23.9±3.3%) elicited by acute application of nicotine. KCl-evoked increases in intracellular Ca2+ were also inhibited by 10.0±1.9% after 4 days' treatment with galantamine. These diminished responses are consistent with the downregulation of downstream cellular processes.
Ca2+ responses evoked by activation of muscarinic acetylcholine receptors were unaffected by chronic galantamine treatment. Exposure to the more potent acetylcholinesterase inhibitor rivastigmine (1 μM) for 4 days failed to alter nicotine-, KCl-, or muscarinic receptor-evoked increases in intracellular Ca2+. These observations support the hypothesis that chronic galantamine exerts its effects through interaction with nAChR in this cell line.
Exposure to 10 μM nicotine for 4 days produced decreases in acute nicotine- (18.0±3.5%) and KCl-evoked Ca2+ responses (10.6±2.5%) and nicotine-evoked [3H]NA release (26.0±3.3%) that are comparable to the effects of a corresponding exposure to galantamine.
Treatment with 1 μM galantamine did not alter numbers of [3H]epibatidine-binding sites in SH-SY5Y cells, in contrast to 62% upregulation of these sites in response to 10 μM nicotine.
Thus, chronic galantamine acts at nAChR to decrease subsequent functional responses to acute stimulation with nicotine or KCl. This effect appears to be independent of the upregulation of nAChR-binding sites.
Keywords: Neuronal nicotinic acetylcholine receptor, acetylcholinesterase inhibitor, galantamine, rivastigmine, SH-SY5Y neuroblastoma cell line, noradrenaline release, intracellular Ca2+, [3H]epibatidine-binding sites, upregulation, muscarinic receptor
Introduction
The increasing longevity of the human population is accompanied by a rise in the incidence of neurodegenerative conditions such as Alzheimer's disease (AD). AD, which accounts for 70% of dementia cases in most industrialised countries, is characterised by cell atrophy and extensive neurone loss, with a marked reduction in cholinergic transmission in the cortex and hippocampus (Coyle et al., 1983; Ladner & Lee, 1998). Analysis of brain tissue from AD patients reveals a decreased number of nicotinic acetylcholine receptors (nAChR) that display high affinity for agonist binding (Flynn & Mash, 1986; Gotti & Clementi, 2004). In the CNS, these nAChR exert a modulatory influence through diverse mechanisms, such as presynaptic facilitation of transmitter release, activation of intracellular signalling cascades, or the more conventional mediation of synaptic transmission at certain synapses (Dajas-Bailador & Wonnacott, 2004). Nicotine improves attention and cognitive performance in animals (Levin & Simon, 1998) and in AD patients (Newhouse et al., 2004), while chronic treatment with nicotine has neuroprotective effects in models of neurodegenerative diseases (O'Neill et al., 2002). Although these factors have encouraged the quest for subtype-selective nAChR agonists as a therapeutic approach, no suitable compounds have yet been described (Hogg & Bertrand, 2004).
At present, approved therapeutic strategies in AD are aimed, predominantly, at enhancing residual cholinergic transmission, through the administration of acetylcholinesterase inhibitors (AChEI), which have shown some efficacy (Wilcock et al., 2000; Winblad & Jelic, 2004). Of the currently prescribed AChEI, galantamine is unique in also acting as an allosteric potentiating ligand (APL) of nAChR, in addition to inhibiting AChE (Pereira et al., 2002). Acutely, galantamine potentiates agonist-induced nicotinic currents (Schrattenholz et al., 1996) and downstream processes modulated by nAChR activation, such as Ca2+ signalling and transmitter release (Dajas-Bailador et al., 2003). APLs such as galantamine and physostigmine bind to a site that is close to, but distinct from, the agonist-binding site of the nAChR. When applied alone, galantamine does not give rise to macroscopic nicotinic currents, although it does increase single-channel activity. Hence, it is proposed that APLs potentiate nAChR agonist responses by increasing the probability of channel opening and slowing nAChR desensitisation (Pereira et al., 2002).
Clinically, cognitive improvements are seen after 8 weeks of treatment with galantamine and treatment typically continues for 3–6 months (Loy & Schneider, 2004). Therefore, it is important to examine the cellular consequences of long-term galantamine exposure. Several studies (Barnes et al., 2000; Woodruff-Pak et al., 2001; Svedberg et al., 2004) have reported an upregulation by chronic galantamine of nAChR-binding sites in mammalian brain. However, these studies do not distinguish between the AChEI and APL activities of galantamine, and no information on the functional status of nAChR after such treatment is available. Therefore, the aim of this study was to determine the effects of sustained exposure to galantamine on cellular functions initiated by nAChR activation. The human neuroblastoma cell line SH-SY5Y expresses various nAChR subtypes (Lukas et al., 1993), making it a good model for investigating the cellular consequences of nAChR activation (Vaughan et al., 1993; Dajas-Bailador et al., 2002; 2003; Ridley et al., 2002; Dunckley & Lukas, 2003). The effects of chronic exposure to galantamine or nicotine on subsequent nicotine-evoked increases in intracellular Ca2+ and nicotine-induced [3H]noradrenaline ([3H]NA) release were measured, and chronic treatment with rivastigmine, a potent AChEI lacking APL activity, was compared. The results indicate that chronic galantamine treatment specifically modulates nAChR-mediated responses and this is independent of AChE inhibition. The decreased functional cellular responses after chronic galantamine resemble the effects of chronic nicotine treatment.
Methods
Cell culture
SH-SY5Y human neuroblastoma cells, passage 16–19 (ECACC, Salisbury, U.K.), were cultured as described previously (Ridley et al., 2001). Briefly, cultures were maintained in Dulbecco's modified Eagle's medium/Ham's F12, supplemented with 15% foetal bovine serum, 2 mM L-glutamine, 1% nonessential aminoacids and 190 U ml−1 of penicillin and 0.2 mg ml−1 of streptomycin. Cells were plated (1 : 5 dilution) into 96-well Primaria plates (Falcon, Franklin Lakes, NJ, U.S.A.) for Ca2+ assays or into 96-well Nunc plates (Fisher Scientific UK, Leicestershire, U.K.) for [3H]NA release experiments, and incubated in fresh medium with or without test drugs. Experiments were performed 4 days later (unless otherwise stated) with confluent cultures. Before assay, the cells were washed six times over 3 h with fresh medium (Ridley et al., 2001), to remove drugs accumulated in the cells and media. For binding experiments cells were subcultured in 175 cm2 flasks (Sharples et al., 2000), in the presence or absence of test drugs (nicotine 10, 50 μM, galantamine 1, 10 μM), and incubated for 4 days.
Calcium fluorimetry
Increases in intracellular Ca2+ in confluent cultures of SH-SY5Y cells in 96-well plates were monitored as described by Dajas-Bailador et al. (2002). In brief, cells were washed twice with Tyrode's salt solution (TSS: NaCl 137 mM; KCl 2.7 mM; MgCl2 1 mM; CaCl2 1.8 mM; NaH2PO4 0.2 mM; NaHCO3 12 mM; glucose 5.5 mM, pH 7.4) and incubated for 1 h at room temperature with 10 μM fluo-3 AM and 0.02% pluronic F127 in the dark. Two further washes with TSS were performed before adding 80 μl TSS with or without antagonist per well. After 10 min incubation in the dark, cells were stimulated by addition of 20 μl nicotine, acetylcholine, or KCl. To examine the acute potentiation of nicotine-evoked responses by galantamine, this drug (0.5–10 μM) was added 5 min prior to stimulation with nicotine. Changes in fluorescence (excitation 485 nm, emission 538 nm) were measured using a Fluoroskan Ascent fluorescent plate reader (Labsystems, Helsinki, Finland). Basal fluorescence was monitored for 5 s before addition of stimulus and changes in fluorescence were monitored for a further 20 s, or in some cases for 10 min. Basal fluorescence values were unchanged from control in nicotine- or galantamine-treated cells, respectively, 0.24±0.03, 0.23±0.09 and 0.24±0.06 arbitrary fluorescence units (n=7). At the end of each experiment, calibration was performed by the sequential addition of 0.2% Triton X-100, followed by 70 mM MnCl2 to obtain Fmax and Fmin values, respectively. Data were calculated as a percentage of (Fmax–Fmin). Unless otherwise stated, values were expressed as a % of the response of control cells (no chronic drug treatment) to stimulation. Each experiment was conducted at least three times, with four replicates per experiment.
Binding assays
SH-SY5Y cell membrane preparation
Membranes were prepared from SH-SY5Y cells seeded in 175 cm2 flasks as described previously (Sharples et al., 2000). Cells were scraped in 15 ml ice-cold PBS containing 1 mM EDTA and 1 mM PMSF, and centrifuged for 10 min at 1500 × g. Pellets were resuspended in 10 ml supplemented PBS and sonicated three times for 10 s (amplitude: 9 μm). The homogenate was then centrifuged for 30 min at 45,000 × g. Another round of resuspension and centrifugation was performed before final resuspension in 50 mM phosphate buffer (40 mM K2HPO4, 10 mM KH2PO4, 1 mM EDTA, 0.1 mM PMSF and 0.01% sodium azide, pH 7.4). Membrane preparations were frozen until use. Estimation of protein concentration was by (Bradford, 1976) assay using BSA as a standard.
[3H]epibatidine binding assay
[3H]epibatidine binding was performed on 50 μg of SH-SY5Y membranes, in a final volume of 1 ml (NaCl 118 mM, KCl 4.8 mM, CaCl2 2.5 mM, MgSO4 2 mM, Hepes 20 mM, Tris 20 mM, PMSF 0.1 mM and 0.01% sodium azide, pH 7.4). The final concentration of [3H]epibatidine was 500 pM. Nonspecific binding was determined in the presence of 1 mM nicotine. Samples were incubated for 1 h at room temperature, followed by 30 min at 4°C. Then samples were filtered through Gelman GFA filters, presoaked overnight in 0.3% polyethylene immine using a Brandel cell harvester. Filters were washed three times with ice-cold PBS, and counted for radioactivity using a Packard 1600 Tricarb scintillation counter (counting efficiency 45%). Each assay was conducted in triplicate and repeated on three independently treated sets of cells.
[3H]NA release
[3H]NA release was performed as described by Dajas-Bailador et al. (2003). Cells were cultured for 4 days in the presence or absence of drugs, followed by extensive washing over 3 h as described above. Cells were then washed twice with warm oxygenated Krebs buffer (NaCl 118 mM, KCl 2.4 mM, CaCl2 2.4 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 25 mM, D-glucose 10 mM, ascorbic acid 1 mM, pH 7.4). [3H]NA (0.07 μM, 37 MBq ml−1) in Krebs buffer containing 10 μM pargyline (to prevent degradation of [3H]NA by monoamine oxidase) was added (60 μl well−1), and the cells were incubated for 1 h at 37°C. The cells were then washed twice with Krebs buffer containing pargyline and 0.5 μM nomifensine (to prevent re-uptake of released [3H]NA), and incubated for 5 min in the same buffer (60 μl). Buffer was removed and SH-SY5Y cells were stimulated for 5 min with buffer alone (basal release) or with buffer containing 30 μM nicotine. After 5 min, the medium containing released [3H]NA was transferred to a 96-well Packard Optiplate™ (Perkin-Elmer, Zaventem, Belgium). Microscint-40 (170 μl; Packard Bioscience, Groeningen, The Netherlands) was added to each well and radioactivity counted using a microbeta liquid scintillation counter (Wallac 1450 Microbeta Trilux, Perkin-Elmer, Finland; counting efficiency 31%). Radioactivity remaining in the cultures was determined by addition of 80 μl 0.5 M perchloric acid and incubation for 1 h at 37°C, followed by scintillation counting. The total amount of [3H]NA present in the cells at the point of stimulation was equivalent to the tritium released plus tritium remaining. Released [3H]NA was calculated as a percentage of total radioactivity in the corresponding wells, and results were then expressed as a percentage of basal release (buffer stimulation). Each condition was examined at least four times with different cultures, each assayed with eight replicates per condition.
Drugs and reagents
Tissue culture media, serum and plasticware were obtained from Gibco BRL (Paisley, U.K.). (−)-Nicotine hydrogen tartrate and mecamylamine hydrochloride were purchased from Sigma Chemical (Poole, Dorset, U.K.). [3H]epibatidine (2 TBq mmol−1) and [3H]NA; (1.22 TBq mmol−1) were purchased from Amersham Biosciences (Buckinghamshire, U.K.) and α-conotoxin-MII was from Tocris Cookson Ltd (Avonmouth, U.K.). Galantamine was provided by Sanochemia Pharmazeutika AG (Austria) and rivastigmine was donated by Intelligen Corp. (Cold Spring Harbour, NY, U.S.A.). Fluo-3 AM and pluronic F127 were from Molecular Probes (The Netherlands).
Data analysis
All data are presented as mean±s.e.m. of at least four independent experiments. Statistical significance was determined using Student's paired or unpaired t-test or one-way ANOVA plus post hoc Tukey's test, as stated in the figure legends. Values of P<0.05 were taken to be statistically significant.
Results
Effects of galantamine on nicotine-evoked increases in Ca2+ in SH-SY5Y cells
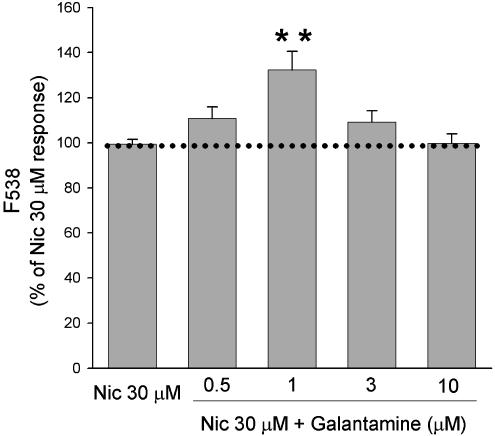
The ability of acute application of galantamine to potentiate nicotinic responses was confirmed in SH-SY5Y cells loaded with the Ca2+ indicator fluo-3 AM and stimulated with 30 μM nicotine (approximate EC50 value for this response; Dajas-Bailador et al., 2002; Ridley et al., 2002). In agreement with our previous studies (Dajas-Bailador et al., 2003), galantamine (1 μM) significantly potentiated nicotine-induced increases in fluorescence by 32.2±8.4% (Figure 1). The concentration dependence of this effect showed a bell-shaped profile, with lower (0.5 μM) and higher (3 μM) concentrations of galantamine resulting in nonsignificant increases in nicotine-induced fluorescences of 10.7±5.2 and 9.2±5.0%, respectively.
Figure 1.
Galantamine potentiates nicotine-evoked Ca2+ increases in SH-SY5Y cells. SH-SY5Y cells loaded with fluo-3 AM were incubated for 5 min with a range of concentrations of galantamine and then stimulated by addition of 30 μM nicotine (Nic). Responses were monitored by determining the change in fluorescence after 20 s. Data are expressed as a percentage of the response to nicotine in the absence of galantamine. Values represent the mean±s.e.m. of seven experiments, each performed in quadruplicate. Significantly different from control, **P<0.01, one-way ANOVA with post hoc Tukey's test.
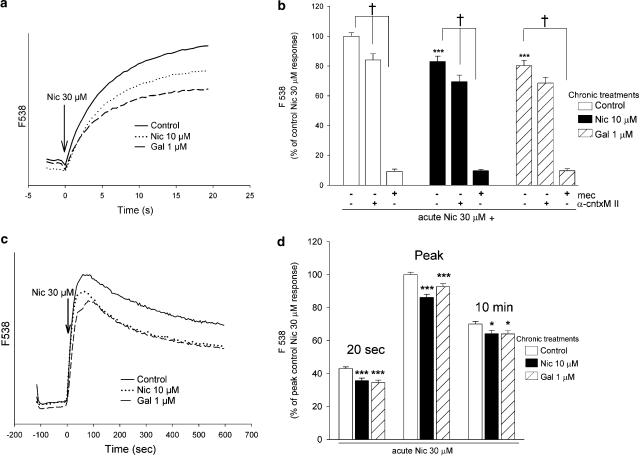
We then compared the effects of chronic exposure of cells (4 days) to the maximally effective concentration of galantamine (1 μM) or 10 μM nicotine (previously shown to modulate subsequent responses to acute nicotinic stimulation; Ridley et al., 2002), or control without drug, on acute nicotine-evoked increases in Ca2+. The drug treatments did not affect the basal levels of fluorescence, see Methods. Although both control and treated cells displayed rapid increases in fluorescence in response to acute nicotine stimulation (see traces, Figure 2a), cells treated for 4 days with nicotine or galantamine gave lower responses than control: Ca2+ signals at 20 s were significantly decreased, by 18.0±3.5% (n=13) and 19.8±3.6% (n=13), respectively (Figure 2b). These acute responses were mediated by stimulation of nAChR, as preincubation with mecamylamine (10 μM; 10 min), a nonselective, noncompetitive nicotinic antagonist, virtually abolished the nicotine-induced increase in fluorescence. The α3/α6β2*-selective antagonist α-conotoxin MII (200 nM) only partially blocked responses to 30 μM nicotine in untreated cells, by 16.0±4.1% (n=5). After treatment of the SH-SY5Y cells for 4 days with nicotine or galantamine, α-conotoxin MII produced a similar level of inhibition of nicotine-evoked increases in fluorescence, of 14.3±5.4 and 10.0±5.4%, respectively (n=6; Figure 2b).
Figure 2.
Effect of chronic drug treatment on nAChR-mediated increases in Ca2+ in SH-SY5Y cells. SH-SY5Y cells were cultured for 4 days in presence or absence of 10 μM nicotine (Nic) or 1 μM galantamine (Gal). After extended washing over 3 h with fresh medium, SH-SY5Y cells were loaded with fluo-3 AM and stimulated with 30 μM nicotine. (a) Representative traces showing the time course of increases in fluorescence over 20 s in cells treated for 4 days with 10 μM nicotine (dotted line), 1 μM galantamine (dashed line) or no drug (control; solid line). (b) Cells were stimulated in the presence or absence of 10 μM mecamylamine (mec) or 200 nM α-conotoxin MII (α-cntxMII). Fluorescence increases were monitored for 20 s (see panel a). Values at 20 s were expressed as a percentage of response of control cells. Data are the mean±s.e.m. of at least five experiments, each carried out in quadruplicate. (c) Representative traces showing the time course of increases in fluorescence over an extended time course (10 min) in cells treated for 4 days with 10 μM nicotine (dotted line), 1 μM galantamine (dashed line) or no drug (control; solid line). (d) Comparison of responses at 20 s, peak and 10 min. Values are expressed as a percentage of peak responses and are the mean±s.e.m. of eight experiments, each performed in quadruplicate. Significantly different from control, *P<0.05, ***P<0.001 one-way ANOVA with post hoc Tukey's test; †P<0.05 paired t-test.
Despite the rapid desensitisation rates of individual nAChR, the stimulation of a cell population of nAChR can generate Ca2+ signals that are sustained for several minutes in SH-SY5Y cells (Dajas-Bailador et al., 2002). Therefore, nicotine-evoked Ca2+ increases were also monitored for an extended period in cells that had been exposed to nicotine (10 μM) or galantamine (1 μM) for 4 days. The rapid rise in fluorescence in response to acute nicotine reached a peak in about 60 s in each case, followed by a slow decline (see Figure 2c). However, cells treated with nicotine or galantamine continued to exhibit significantly decreased responses, both at the peak (86.1±1.9 and 92.7±1.8%) and after 10 min (91.6±2.9 and 91.5±2.8% of control, respectively; Figure 2d).
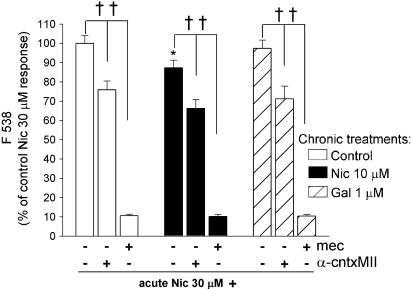
We then examined the effects of nicotine and galantamine applied to SH-SY5Y cells for only 24 h before stimulation with acute nicotine (Figure 3). Treatment with nicotine significantly decreased the subsequent response to 30 μM nicotine, by 12.7±3.9%, compared to untreated cells cultured in parallel, whereas responses were unaffected by 24 h exposure to galantamine (97.3±4.3% of control). Sensitivity to 10 μM mecamylamine and 200 nM α-conotoxin MII (Figure 3) was comparable to that of control cells cultured in parallel without chronic drug treatment.
Figure 3.
Effect of 24 h drug treatment on nAChR-mediated Ca2+ responses in SH-SY5Y cells. SH-SY5Y cells were cultured for 4 days in presence of 10 μM nicotine (Nic; black bars), 1 μM galantamine (Gal; hatched bars) or no drug (control; open bars). After extended washing over 3 h with fresh medium, SH-SY5Y cells were loaded with fluo-3 AM and stimulated with 30 μM nicotine in the presence or absence of 10 μM mecamylamine (mec) or 200 nM α-conotoxin MII (α-cntxMII). Changes in fluorescence were monitored for 20 s. Values are expressed as a percentage of control responses. Bars represent the mean±s.e.m. of at least four independent experiments. Significantly different from control *P<0.05, one-way ANOVA with post hoc Tukey's test; ††P<0.01 paired t-test.
Comparison of chronic treatment of SH-SY5Y cells with nicotine or AChEI on Ca2+ responses evoked by muscarinic or nicotinic agonists, or by KCl depolarisation
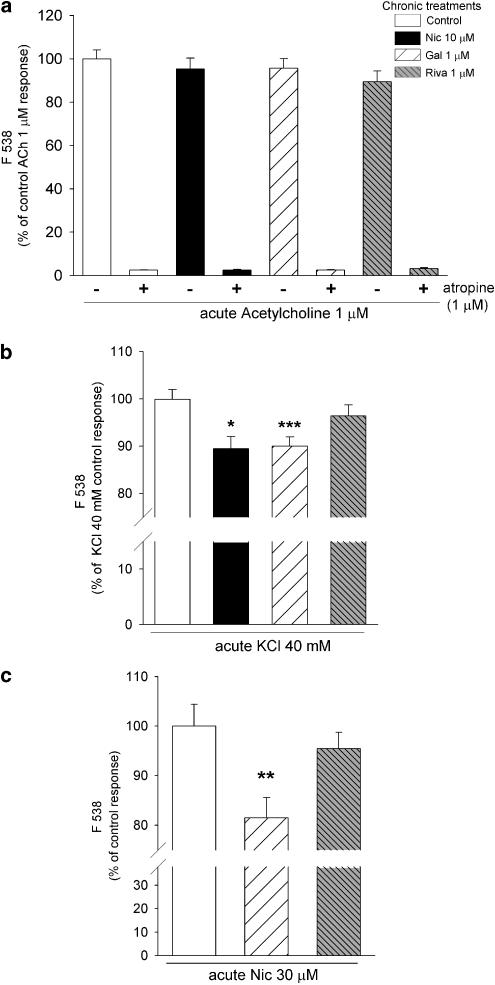
SH-SY5Y cells are endowed with muscarinic AChR (mAChR; Vaughan et al., 1997). A low concentration of ACh (1 μM) was employed to stimulate mAChR-mediated increases in Ca2+, in order to determine if chronic treatment with 10 μM nicotine or 1 μM galantamine had any effect on this receptor system. At this concentration, ACh-evoked increases in fluorescence were completely abolished by 1 μM atropine (Figure 4a), confirming the selective activation of mAChR. Higher concentrations of acetylcholine (50 μM) were required to activate nAChR: at this concentration responses were only partially blocked by atropine (1 μM) by 53.3±4.1% (data not shown). Neither of the chronic drug treatments had any effect on the magnitude of 1 μM ACh-evoked increases in Ca2+ (Figure 4a), consistent with a lack of effect on either mAChR themselves or Ca2+ sources dependent on mAChR activation.
Figure 4.
Comparison of chronic drug treatments on mAChR-, nicotine- and KCl-evoked responses. SH-SY5Y cells were cultured for 4 days in presence of 10 μM nicotine (Nic; black bars), 1 μM galantamine (Gal; hatched bars); 1 μM rivastigmine (Riva; finely hatched bars) or no drug (Control; open bars). After extended washing over 3 h with fresh medium, SH-SY5Y cells were loaded with fluo-3 AM and stimulated with (a) 1 μM ACh, in the presence or absence of 1 μM atropine; (b) 40 mM KCl or (c) 30 μM nicotine. Fluorescence was measured for 20 s and results are expressed as a percentage of control responses; significantly different from control, *P<0.05, **P<0.01, one-way ANOVA with post hoc Tukey's test.
Stimulation with KCl increases intracellular Ca2+ by activation of VOCC and release of Ca2+ from stores, responses that are also evoked by nAChR stimulation (Dajas-Bailador et al., 2002). Therefore, we also compared the effects of 4 days' exposure to nicotine or galantamine on KCl-evoked increases in fluorescence. Acute stimulation with 40 mM KCl increased fluorescence to 175.4±8.3% of the response to 30 μM nicotine (n=15). In contrast to the lack of effect on muscarinic responses, KCl-evoked increases in fluorescence were significantly smaller after galantamine treatment than in untreated control cells (90.0±1.9% of control, Figure 4b). This was mimicked by nicotine treatment (89.4±2.5% of control, Figure 4b).
To address the possibility that AChE inhibition by galantamine could be responsible for its effects, by preventing the breakdown of any endogenous ACh, cells were also exposed for 4 days to another, more potent AChEI, rivastigmine, that is devoid of nicotinic potentiating activity (Dajas-Bailador et al., 2003). This treatment had no effect on nicotine-evoked responses (95.5±3.29% of control; Figure 4b), in contrast to the significant decrease in nicotine-evoked responses after chronic galantamine treatment (81.5±4.05% of control). Similarly, chronic treatment with rivastigmine had no effect on responses evoked by ACh (89.8±5.0% of control; Figure 4a) or by KCl (96.4±2.1% of control; Figure 4b). Together, these results indicate that chronic treatment with galantamine exerts an effect that is specific to nAChR-mediated responses and is not due to the AChEI activity of the drug.
Nicotine-evoked [3H]NA release
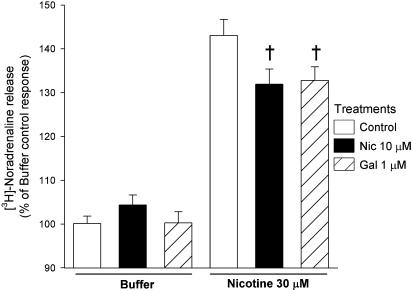
Nicotine stimulates Ca2+-dependent NA release from SH-SY5Y cells (Vaughan et al., 1993; Dajas-Bailador et al., 2003). As nAChR-mediated Ca2+ responses were decreased after chronic drug exposure (Figure 2), we examined the effect of 4 days' treatment with 10 μM nicotine or 1 μM galantamine on nicotine-evoked [3H]NA release from SH-SY5Y cells. Basal release was unchanged from control by either drug treatment (1300.0±83, 1236.4±72 and 1292.1±42 c.p.m. respectively, for control-, nicotine- and galantamine-treated cells, n=5). In control cells, stimulation with nicotine (30 μM) for 5 min increased the release of tritium by 43.1±3.6% above basal (Figure 5). This response was significantly reduced in cells that had been exposed to galantamine or nicotine (32.8±3.1 and 31.9±3.6% above basal, respectively; Figure 5). In parallel, nicotine-evoked Ca2+ assays confirmed the decreases in fluorescence readings observed after treatment with 10 μM nicotine or 1 μM galantamine (83.2±3.3 and 87.2±2.6% of control, respectively, n=6; significantly different from control, one-way ANOVA plus post hoc Tukey's test, P<0.01).
Figure 5.
Nicotine-evoked [3H]NA release from SH-SY5Y cells after chronic drug treatment. SH-SY5Y cells were cultured for 4 days in presence of 10 μM nicotine (Nic; black bars), 1 μM galantamine (Gal; hatched bars) or no drug (control; open bars). After extended washing over 3 h with fresh medium, SH-SY5Y cells were loaded with [3H]NA and the release of tritium was induced by 5 min incubation with buffer or 30 μM nicotine. Data are expressed as a percentage of buffer-evoked release and represent the mean±s.e.m. of five independent experiments, each conducted with eight replicates. †P<0.001, significantly different from control response to nicotine stimulation (one-way ANOVA with post hoc Tukey's test).
Upregulation of [3H]epibatidine binding sites
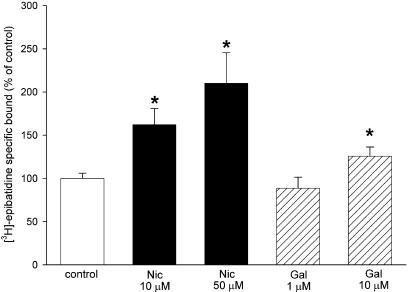
Chronic exposure to nicotine upregulates numbers of nAChR (Gentry & Lukas, 2002). SH-SY5Y cells expressed 154.8±9.2 fmol mg−1 of [3H]epibatidine-binding sites (n=12). Treatment for 4 days with 10 or 50 μM nicotine concentration-dependently upregulated the number of sites labelled with [3H]epibatidine, by 61.9±18.7 and 110.1±35.4%, respectively (Figure 6). In contrast, treatment with 1 μM galantamine had no significant effect on the number of [3H]epibatidine-binding sites, compared with control (88.5±12.6%; Figure 6). However, a higher concentration of galantamine (10 μM) resulted in a modest but significant upregulation of [3H]epibatidine sites of 25.7±10.6% (Figure 6).
Figure 6.
Upregulation of [3H]epibatidine-binding sites in SH-SY5Y cells. SH-SY5Y cells were cultured for 4 days in presence of 10 or 50 μM nicotine (Nic; black bars), 1 or 10 μM galantamine (Gal; hatched bars) or no drug (control; open bar). The cells were washed and membranes prepared as described in Methods. The density of nAChR-binding sites was determined by incubation with 500 pM [3H]epibatidine in the absence or presence of 1 mM nicotine, to determine total and nonspecific binding, respectively. Results are expressed as a percentage of control and are the mean±s.e.m. of four experiments, each performed in triplicate. Significantly different from control, *P<0.05, Student's paired t-test.
SH-SY5Y cells chronically treated with 1 or 10 μM galantamine for 4 days were compared with respect to nicotine-evoked Ca2+ responses. In parallel, cells exposed for 4 days to 10 or 50 μM nicotine were also examined. Nicotine treatment resulted in concentration-dependent decreases in subsequent responses to acute application of nicotine, by 16.3±3.7 and 28.3±4.4% of control (SH-SY5Y cells incubated without drugs, in parallel), respectively. However, an inverse concentration dependence was observed for galantamine treatment: after 1 or 10 μM galantamine, nicotine-evoked increases in fluorescence were reduced by 19.6±2.6 and 12.6±4.1% (significantly different from control, P<0.05, Student's t-test, n=4), respectively.
Discussion
The present study shows that chronic treatment of SH-SY5Y cells with galantamine or nicotine produces decrements in functional responses similar to a subsequent acute nicotine stimulation. Indeed, both nicotine- and KCl-evoked increases in intracellular Ca2+ were diminished by prior treatment with galantamine or nicotine, in contrast to the lack of effect of chronic treatment with the AChEI rivastigmine. Ca2+ increases provoked by activation of mAChR were unaffected by any drug treatment, supporting a specific nAChR interaction to mediate the effects of both galantamine and nicotine. Nicotine-evoked [3H]NA release was also decreased after 4 days' exposure to either drug. However, chronic treatment with 1 μM galantamine failed to upregulate numbers of [3H]epibatidine-binding sites (in contrast to chronic treatment with 10 μM nicotine), suggesting that nAChR upregulation and the observed functional changes are independent.
It is well established that galantamine acts as an APL at nAChR (Pereira et al., 2002), and that this interaction translates to a potentiation of downstream cellular responses (Santos et al., 2002; Dajas-Bailador et al., 2003). Here we first confirmed that galantamine potentiates nicotine-evoked Ca2+ increases in SH-SY5Y cells, with a familiar bell-shaped dose response profile (Figure 1). Acutely, maximum potentiation of nAChR responses occurred at 1 μM galantamine, consistent with literature values (Santos et al., 2002; Dajas-Bailador et al., 2003). Therefore, we compared the effects of chronic treatment of SH-SY5Y cells with this concentration of galantamine and with 10 μM nicotine (a concentration previously shown to exert effects on Ca2+ responses after long-term treatment, Ridley et al., 2002) on subsequent nicotine-evoked responses. In agreement with our previous findings (Ridley et al., 2002), 4 days' exposure to nicotine produced a significant decrease in the subsequent Ca2+ response elicited by acute nicotine stimulation. This is unlikely to reflect acute nAChR desensitisation arising from residual nicotine, as the cells were subjected to an extensive washing regime over a long period (see Methods) to completely remove nicotine that had entered the cells (Ridley et al., 2002; Jia et al., 2003). However, this does not exclude a persistent desensitisation or inactivation of nAChR that might result from the 4 days' treatment (Quick & Lester, 2002), although this phenomenon is more pronounced for the α4β2 nAChR subtype that is not present in SH-SY5Y cells due to the lack of expression of the α4 subunit (Lukas et al., 1993). Moreover, a direct depression of nAChR activity is difficult to reconcile with the observed reduction in KCl-evoked responses after chronic nicotine treatment (Figure 4b).
Interestingly, the presence of galantamine for 4 days prior to acute stimulation with nicotine produced a comparable decrease in the nicotine-evoked response (Figure 2). Nicotine stimulation provokes long-lasting increases in intracellular Ca2+ (Dajas-Bailador & Wonnacott, 2004); under the present experimental paradigm, increases in fluo3 fluorescence were maintained for at least 10 min after nicotine application. The diminished response following chronic treatment with nicotine or galantamine was observed throughout this period (Figure 2d). However, nicotine may exert its effect more rapidly than galantamine: after exposure to nicotine for 24 h there was a significant decrease in the subsequent nicotine-evoked Ca2+ response (although not as great an effect as observed after 4 days), whereas treatment with galantamine for 24 h resulted in responses that were indistinguishable from control (Figure 3).
SH-SY5Y cells express several nAChR subunits (α3, α5, α7, β2, β4), giving rise to multiple nAChR subtypes, including those containing α3 and β2 subunits (Lukas et al., 1993; Wang et al., 1996). This is consistent with the partial inhibition of the nicotine-evoked Ca2+ increase by α-Ctx-MII (Figure 2; Ridley et al., 2002), a toxin selective for α3/α6β2-containing (α3/α6β2*) nAChR (Dowell et al., 2003). After chronic drug treatment this toxin would be expected to block a lower proportion of the response if α3β2* nAChR were selectively inactivated as a result of the exposure to drug, whereas an increase in the α-Ctx-MII-sensitive component would imply compensation for the inactivation of another nAChR subtype. The proportion of the response that was sensitive to α-Ctx-MII was preserved after chronic treatment with nicotine or galantamine for 24 h (Figure 3) or 4 days (Figure 2b). These data suggest that the contribution of the α3β2* nAChR subtype in SH-SY5Y cells is not significantly modified by chronic drug treatment.
It is now well established that stimulation of nAChR initiates a complex cascade, including activation of VOCC and release of Ca2+ from intracellular stores (Dajas-Bailador et al., 2002; see Dajas-Bailador & Wonnacott, 2004) that shares similarities with the response initiated by other depolarising stimuli, including KCl. Indeed, the small but significant (∼10%) decrease in KCl-evoked fluorescence (Figure 4b) after both nicotine or galantamine chronic treatments suggests that the sustained activation (or desensitisation) of nAChR during chronic treatment may lead to the functional downregulation of one or more downstream components in this cascade that also respond to acute KCl depolarisation. As 40 mM KCl evokes a bigger response than 30 μM nicotine, the absolute magnitude of the decrease in response to chronic drug treatment is similar for both stimuli, consistent with the downregulation of a common downstream component. VOCC constitutes a plausible candidate (Agis-Torres et al., 2002; Katsura et al., 2002; Ridley et al., 2002). An indirectly mediated reduction in VOCC activity could be important for the neuroprotective actions ascribed to nicotine (see O'Neill et al., 2002), and more recently to galantamine (Arias et al., 2004; Kihara et al., 2004).
Galantamine has been reported to be devoid of direct functional interaction with heterologously expressed mAChR (Samochocki et al., 2003). In agreement with this, the absence of any change in Ca2+ responses initiated by mAChR activation following chronic treatment with nicotine or galantamine (Figure 4a) demonstrates the specificity of the drug effects, and the lack of cross-talk between nAChR and mAChR, at least with respect to the sources of Ca2+ modified by these treatments. The failure of rivastigmine, a more potent AChEI than galantamine, but devoid of APL activity (Santos et al., 2002; Dajas-Bailador et al., 2003), to reproduce the effects of chronic galantamine treatment (Figure 4b and c) is critically important in ruling out the AChEI properties of galantamine as a determinant of the drug's long-term effects in this cell culture model.
What is the mechanism whereby 4 days' treatment with 10 μM nicotine or 1 μM galantamine produces similar decreases in subsequent nicotine-evoked Ca2+ responses or neurotransmitter release? Having excluded a contribution from the AChEI activity of galantamine, or a muscarinic component, it is the APL activity, even in the absence of agonist, which is presumably sufficient for achieving this effect. Although 1 μM galantamine alone cannot generate macroscopic currents, it can induce single-channel activity at nAChR in a variety of clonal, neuronal and transfected cell types (Pereira et al., 2002). This activity may be sufficient over 4 days (but not after only 24 h) to achieve the same cellular changes as stimulation with 10 μM nicotine.
The functional consequences of chronic nicotine treatment differ between studies, depending on the parameter measured. Generally, where nAChR channel activity has been monitored, an increase in response is observed after a few days of chronic nicotine exposure, providing that residual nicotine is effectively removed (Buisson & Bertrand, 2001; Nashmi et al., 2003). Here we have monitored two downstream responses, nicotine-evoked increases in intracellular Ca2+ and [3H]NA release, and report a decrease in response. It is plausible to suggest that an increase in nAChR activity leads to a downregulation of some subsequent step, such as VOCC activity. The mechanism for this is not known, but is likely to result from a nAChR-activated signalling cascade (Dajas-Bailador & Wonnacott, 2004).
Very well documented is the ability of chronic nicotine to upregulate numbers of nAChR-binding sites, in cell culture, in vivo and in the brains of human tobacco smokers (Gentry & Lukas, 2002). nAChR desensitisation has been widely accepted as a plausible trigger for this phenomenon, although a recent examination proposes an intracellular action that promotes nAChR assembly and maturation (Sallette et al., 2004). It has been assumed that nAChR upregulation must correlate with functional outcomes. However, it is evident, in heterologous expression systems at least, that the majority of upregulated nicotinic binding sites remain intracellular and so have limited impact on functional responses (Whiteaker et al., 1998). In the present study, 4 days' exposure to 10 μM nicotine upregulated the number of [3H]epibatidine-binding sites (at a radioligand concentration predicted to label both α3β2* and α3β4* populations of nAChR in SH-SY5Y cells), whereas 1 μM galantamine had no effect on binding site density. Using a different APL, Gopalakrishnan et al. (1997) did not find any changes in [3H]cytisine binding in transfected HEK293 cells, following chronic exposure to physostigmine (0.01–100 μM). Hence, numbers of binding sites do not correlate with the observed decreases in nicotine-evoked Ca2+ changes and [3H]NA release following chronic treatment with galantamine in the present study. In vivo, galantamine administered for 35 days has been reported to upregulate the numbers of [3H]nicotine-binding sites in the brains of rats (Barnes et al., 2000), but this effect can be ascribed to the AChEI properties of the drug, as a similar response was achieved with other AChEI (Barnes et al., 2000; Reid & Sabbagh 2003).
The SH-SY5Y cell line has been useful for dissecting the effects of the nicotinic APL action of galantamine, and this drug may have utility for exploring the relationship between nAChR upregulation and functional consequences. Clinically galantamine is given twice daily to achieve a sustained plasma concentration estimated to be ∼0.4 μM (Scott & Goa, 2000), within the range of concentrations that potentiates nAChR activity. The functional consequences of long-term treatment arising from the activation of nAChR due to its APL action, as well as from inhibition of AChE, will contribute to its clinical profile.
Acknowledgments
This research was supported financially by a grant from Sanochemia Pharmazeutika AG and a Research Training Network award from the Commission of the European Community.
Abbreviations
- AChEI
acetylcholinesterase inhibitor
- AD
Alzheimer's disease
- APL
allosteric potentiating ligand
- mAChR
muscarinic acetylcholine receptors
- NA
noradrenaline
- nAChR
nicotinic acetylcholine receptor
- VOCC
voltage-operated calcium channel
References
- AGIS-TORRES A., BALL S.G., VAUGHAN P.F. Chronic treatment with nicotine or potassium attenuates depolarisation-evoked noradrenaline release from the human neuroblastoma SH-SY5Y. Neurosci. Lett. 2002;331:167–170. doi: 10.1016/s0304-3940(02)00881-9. [DOI] [PubMed] [Google Scholar]
- ARIAS E., ALES E., GABILAN N.H., CANO-ABAD M.F., VILLARROYA M., GARCIA A.G., LOPEZ M.G. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–114. doi: 10.1016/s0028-3908(03)00317-4. [DOI] [PubMed] [Google Scholar]
- BARNES C.A., MELTZER J., HOUSTON F., ORR G., MCGANN K., WENK G.L. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUISSON B., BERTRAND D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COYLE J.T., PRICE D.L., DELONG M.R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- DAJAS-BAILADOR F., WONNACOTT S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- DAJAS-BAILADOR F.A., HEIMALA K., WONNACOTT S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol. Pharmacol. 2003;64:1217–1226. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- DAJAS-BAILADOR F.A., MOGG A.J., WONNACOTT S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J. Neurochem. 2002;81:606–614. doi: 10.1046/j.1471-4159.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- DOWELL C., OLIVERA B.M., GARRETT J.E., STAHELI S.T., WATKINS M., KURYATOV A., YOSHIKAMI D., LINDSTROM J.M., MCINTOSH J.M. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J. Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCKLEY T., LUKAS R.J. Nicotine modulates the expression of a diverse set of genes in the neuronal SH-SY5Y cell line. J. Biol. Chem. 2003;278:15633–15640. doi: 10.1074/jbc.M210389200. [DOI] [PubMed] [Google Scholar]
- FLYNN D.D., MASH D.C. Characterization of L-[3H]nicotine binding in human cerebral cortex: comparison between Alzheimer's disease and the normal. J. Neurochem. 1986;47:1948–1954. doi: 10.1111/j.1471-4159.1986.tb13113.x. [DOI] [PubMed] [Google Scholar]
- GENTRY C.L., LUKAS R.J. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Targets CNS Neurol. Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- GOPALAKRISHNAN M., MOLINARI E.J., SULLIVAN J.P. Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol. Pharmacol. 1997;52:524–534. [PubMed] [Google Scholar]
- GOTTI C., CLEMENTI F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- HOGG R.C., BERTRAND D. Nicotinic acetylcholine receptors as drug targets. Curr. Drug Targets CNS Neurol. Disord. 2004;3:123–130. doi: 10.2174/1568007043482507. [DOI] [PubMed] [Google Scholar]
- JIA L., FLOTILDES K., LI M., COHEN B.N. Nicotine trapping causes the persistent desensitization of alpha4beta2 nicotinic receptors expressed in oocytes. J. Neurochem. 2003;84:753–766. doi: 10.1046/j.1471-4159.2003.01578.x. [DOI] [PubMed] [Google Scholar]
- KATSURA M., MOHRI Y., SHUTO K., HAI-DU Y., AMANO T., TSUJIMURA A., SASA M., OHKUMA S. Up-regulation of L-type voltage-dependent calcium channels after long term exposure to nicotine in cerebral cortical neurons. J. Biol. Chem. 2002;277:7979–7988. doi: 10.1074/jbc.M109466200. [DOI] [PubMed] [Google Scholar]
- KIHARA T., SAWADA H., NAKAMIZO T., KANKI R., YAMASHITA H., MAELICKE A., SHIMOHAMA S. Galantamine modulates nicotinic receptor and blocks Abeta-enhanced glutamate toxicity. Biochem. Biophys. Res. Commun. 2004;325:976–982. doi: 10.1016/j.bbrc.2004.10.132. [DOI] [PubMed] [Google Scholar]
- LADNER C.J., LEE J.M. Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J. Neuropathol. Exp. Neurol. 1998;57:719–731. doi: 10.1097/00005072-199808000-00001. [DOI] [PubMed] [Google Scholar]
- LEVIN E.D., SIMON B.B. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berlin) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- LOY C., SCHNEIDER L.Galantamine for Alzheimer's disease Cochrane Database. Syst. Rev. 2004(CD001747) [DOI] [PubMed]
- LUKAS R.J., NORMAN S.A., LUCERO L. Characterisation of nicotinic acetylcholine receptors expressed by cells of the SH-SY5Y human neuroblastoma clonal line. Mol. Cell. Biol. 1993;4:1–12. doi: 10.1006/mcne.1993.1001. [DOI] [PubMed] [Google Scholar]
- NASHMI R., DICKINSON M.E., MCKINNEY S., JAREB M., LABARCA C., FRASER S.E., LESTER H.A. Assembly of alpha4beta2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J. Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWHOUSE P.A., POTTER A., SINGH A. Effects of nicotinic stimulation on cognitive performance. Curr. Opin. Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- O'NEILL M.J., MURRAY T.K., LAKICS V., VISANJI N.P., DUTY S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr. Drug Targets CNS Neurol. Disord. 2002;1:399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- PEREIRA E.F., HILMAS C., SANTOS M.D., ALKONDON M., MAELICKE A., ALBUQUERQUE E.X. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- QUICK M.W., LESTER R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- REID R.T., SABBAGH M.N. Effects of donepezil treatment on rat nicotinic acetylcholine receptor levels in vivo and in vitro. J. Alzheimers Dis. 2003;5:429–436. doi: 10.3233/jad-2003-5602. [DOI] [PubMed] [Google Scholar]
- RIDLEY D.L., PAKKANEN J., WONNACOTT S. Effects of chronic drug treatments on increases in intracellular calcium mediated by nicotinic acetylcholine receptors in SH-SY5Y cells. Br. J. Pharmacol. 2002;135:1051–1059. doi: 10.1038/sj.bjp.0704508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDLEY D.L., ROGERS A., WONNACOTT S. Differential effects of chronic drug treatment on alpha3* and alpha7 nicotinic receptor binding sites, in hippocampal neurones and SH-SY5Y cells. Br. J. Pharmacol. 2001;133:1286–1295. doi: 10.1038/sj.bjp.0704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALLETTE J., BOHLER S., BENOIT P., SOUDANT M., PONS S., LE NOVERE N., CHANGEUX J.P., CORRINGER P.J. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J. Biol. Chem. 2004;279:18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- SAMOCHOCKI M., HOFFLE A., FEHRENBACHER A., JOSTOCK R., LUDWIG J., CHRISTNER C., RADINA M., ZERLIN M., ULLMER C., PEREIRA E.F., LUBBERT H., ALBUQUERQUE E.X., MAELICKE A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- SANTOS M.D., ALKONDON M., PEREIRA E.F., ARACAVA Y., EISENBERG H.M., MAELICKE A., ALBUQUERQUE E.X. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Mol. Pharmacol. 2002;61:1222–1234. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- SCHRATTENHOLZ A., PEREIRA E.F., ROTH U., WEBER K.H., ALBUQUERQUE E.X., MAELICKE A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol. Pharmacol. 1996;49:1–6. [PubMed] [Google Scholar]
- SCOTT L.J., GOA K.L. Galantamine: a review of its use in Alzheimer's disease. Drugs. 2000;60:1095–1122. doi: 10.2165/00003495-200060050-00008. [DOI] [PubMed] [Google Scholar]
- SHARPLES C.G., KAISER S., SOLIAKOV L., MARKS M.J., COLLINS A.C., WASHBURN M., WRIGHT E., SPENCER J.A., GALLAGHER T., WHITEAKER P., WONNACOTT S. UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes. J. Neurosci. 2000;20:2783–2791. doi: 10.1523/JNEUROSCI.20-08-02783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEDBERG M.M., BEDNAR I., NORDBERG A. Effect of subchronic galantamine treatment on neuronal nicotinic and muscarinic receptor subtypes in transgenic mice overexpressing human acetylcholinesterase. Neuropharmacology. 2004;47:558–571. doi: 10.1016/j.neuropharm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- VAUGHAN P., PEERS C., WALKER J.H. The regulation of noradrenaline release by receptors and second messengers in the human neuroblastoma clone SH-SY5Y. Curr. Top. Neurochem. 1997;1:195–209. [Google Scholar]
- VAUGHAN P.F., KAYE D.F., REEVE H.L., BALL S.G., PEERS C. Nicotinic receptor-mediated release of noradrenaline in the human neuroblastoma SH-SY5Y. J. Neurochem. 1993;6:2159–2166. doi: 10.1111/j.1471-4159.1993.tb03501.x. [DOI] [PubMed] [Google Scholar]
- WANG F., GERZANICH V., WELLS G.B., ANAND R., PENG X., KEYSER K., LINDSTROM J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J. Biol. Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- WHITEAKER P., SHARPLES C.G., WONNACOTT S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol. Pharmacol. 1998;53:950–962. [PubMed] [Google Scholar]
- WILCOCK G.K., LILIENFELD S., GAENS E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321:1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINBLAD B., JELIC V. Long-term treatment of Alzheimer disease: efficacy and safety of acetylcholinesterase inhibitors. Alzheimer Dis. Assoc. Disord. 2004;18 Suppl 1:S2–S8. doi: 10.1097/01.wad.0000127495.10774.a4. [DOI] [PubMed] [Google Scholar]
- WOODRUFF-PAK D.S., VOGEL R.W., III, WENK G.L. Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2089–2094. doi: 10.1073/pnas.031584398. [DOI] [PMC free article] [PubMed] [Google Scholar]