Abstract
Experimental data implicate calpain activation in the pathways involved in neuronal apoptosis. Indeed, calpain inhibitors confer neuroprotection in response to various neurotoxic stimuli. However, the pathways involved in calpain activation-induced apoptosis are not well known.
We demonstrate that apoptosis (40%) induced by serum/potassium (S/K) withdrawal on cerebellar granule cells (CGNs) is inhibited by selective calpain inhibitors PD150606 (up to 15%) and PD151746 (up to 29%), but not PD145305 in CGNs. zVAD-fmk, a broad spectrum inhibitor of caspases, attenuates apoptosis (up to 20%) mediated by S/K deprivation and protects against cell death, as measured by MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium]) assay.
PD150606 and PD151746 prevented apoptosis mediated by S/K withdrawal through inhibition of calpain. Furthermore, PD151746 was able to inhibit caspase-3 activity.
After S/K withdrawal, we observed an increase in cdk5/p25 formation and MEF2 phosphorylation that was prevented by 40 μM PD150606 and PD151746. This indicates that calpain inhibition may be an upstream molecular target that prevents neuronal apoptosis in vitro.
Taken together, these data suggest an apoptotic route in S/K withdrawal in CGNs mediated by calpain activation, cdk5/p25 formation and MEF2 inhibition. Calpain inhibitors may attenuate S/K withdrawal-induced apoptosis and may provide a potential therapeutic target for drug treatment in a neurodegenerative process.
Keywords: Apoptosis, cdk5/p25, calpain, MEF2
Introduction
Apoptoaracterized by nuclear condensation, DNA fragmentation, cell shrinkage and formation of apoptotic bodies (Honig & Rosenberg, 2000; Maccioni et al., 2001; Friedlander, 2003). In recent years, considerable progress has been made towards elucidating the intracellular signal transduction pathways that can lead to neuronal cell death (D'Mello et al., 2000). Accumulated evidence suggests that there are two main apoptotic routes, the intrinsic and the extrinsic, receptor mediated, apoptotic pathways. Although the initial phase of both routes is different, they converge on mitochondrial alteration, which plays a prominent role through the release of several proteins, such as cytochrome c and AIF, thus amplifying the apoptotic response (Honig & Rosenberg, 2000; McCollum et al., 2002). Moreover, several authors suggest that mitochondria constitute the point of no return in apoptosis (Liu et al., 2004). Extensive literature describes the implication of caspases in neuronal cell death, and furthermore, several studies performed in postmortem human brain tissues suggest the participation of these enzymes in neurodegenerative processes (Battaglia et al., 2003). In the last phase of the apoptosis, the executive phase, caspases are activated, mainly caspase-3 (Canu & Calissano, 2003; Friedlander, 2003). Another class of cysteine proteases involved in neuronal cell death is the calcium-activated protease calpain. Calpain has two isoenzyme forms, μ-calpain and m-calpain, that are classified on the basis of the calcium concentrations necessary for their activity in vitro (Nixon, 2003; Rami, 2003; Ray & Banik, 2003; Ray et al., 2003). Several studies propose that calpain activation is involved in both necrotic and apoptotic cell death, but the complete pathway of these processes is unknown (Neumar et al., 2003). Furthermore, calpain activation is involved in the modulation of several intracellular substrates, for example, protein kinase C, cytoskeletal alteration, etc. (Wang, 2000; Di Rosa et al., 2002). Likewise, a potential route proposed is the breakdown of cdk5/p35 to cdk5/p25 mediated or modulated by calpains and implicated in neuron apoptosis (Lee et al., 2000). Although the main function of cyclin-dependent kinases (Cdks) is cell cycle regulation, cdk5 is not associated with the cell cycle and is modulated by p35 and p39. When cdk5/p35 is activated, it is converted into cdk5/p25, a neurotoxic fragment that changes its cellular localization and relocalizes to the cytoplasm and nucleus (Alvarez et al., 1999; Smith & Tsai, 2002; Lee & Tsai, 2003; Weishaupt et al., 2003). Therefore, a potential route proposed is the breakdown cdk5/p35 to cdk5/p25 mediated or modulated by calpains and implicated in neuron apoptosis (Lee et al., 2000). Moreover, increased activation of calpain could be implicated in the neurodegenerative pathway process in several neurodegenerative disorders such as Parkinson's, Alzheimer's and Huntington's diseases (Lee et al., 2000). Recently, a new potential nuclear pathway has been suggested to be involved in cdk5-induced apoptosis, namely phosphorylation of the myocyte enhancer factor (MEF-2). cdk5, once localized in the nucleus, may phosphorylate MEF2 and thus inhibit its activity. Since MEF-2 is necessary for neuronal survival, its phosphorylation may induce neuronal apoptosis through the inhibition of prosurvival activity (Li et al., 2001; Linseman et al., 2003; Heidenreich & Linseman, 2004).
Here, we use potassium and serum withdrawal in cerebellar granule cells (CGNs) to examine the antiapoptotic effects of PD150606 and other cell-permeable selective calpain inhibitors directed against its calcium-binding sites (Wang et al., 1996).
Methods
Materials
The pharmacological agents used in this study were as follows: PD150606, PD151746 and PD145305 and z-VAD-fmk were from Calbiochem; cell culture media and fetal calf serum (FCS) were obtained from GIBCO (Life Technologies, Paisley, U.K.); roscovitine, cell culture salts, enzymes, Mowiol® 4-88 and Triton X-100 were from Sigma Chemical Co. (St Louis, MO, U.S.A.); other chemical reagents were of analytical quality and purchased from Panreac Química (Barcelona, Spain).
Cell cultures
Primary cultures of CGNs were prepared from 7-day-old Sprague–Dawley rat pups as described elsewhere (Verdaguer et al., 2002). Cerebella, freed of meninges, were trypsinized and treated with DNase. Cell density in solution was adjusted to 8.0 × 105 cells ml−1 and cells were then plated on poly-L-lysine-coated plates at a density of 3.2 × 105 cells cm−2. Cultures were grown in Eagle's medium (Eagle's basal medium, BME) containing 10% FCS, 2 mM L-glutamine, 0.1 mg ml−1 gentamicin and 25 mM KCl. Cytosine arabinoside (10 μM) was added 16–18 h after plating in order to inhibit the growth of non-neuronal cells. Cultures prepared using this method were enriched in granule neurons by more than 95%.
Treatment of CGNs and viability assay
CGNs were used after 7–10 days in vitro. To investigate the effect of PD150606, PD151746, PD145305 and z-VAD-fmk, drugs or vehicle were added to the medium, at defined concentrations, and incubated for 12 h in complete medium. Thereafter, complete medium was replaced by medium without serum and containing 5 mM KCl (this is referred to as S/K-deprived medium) in absence or presence of the testing drugs.
To assess the loss of cell viability, we used the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium] method. MTT was added to the cells at a final concentration of 250 μM and incubated for 1 h to allow the reduction of MTT to produce a dark blue formazan product. Media were removed, and the cells were dissolved in dimethylsulfoxide. The production of formazan was measured by absorbance change at 595 nm using a microplate reader (BioRad Laboratories, CA, U.S.A.). Viability results are expressed as percentages. The absorbance measured from nontreated cells was taken to be 100% cellular viability.
Analysis of DNA fragmentation by flow cytometry
Apoptosis was measured 12 h after S/K withdrawal in the experimental conditions indicated above. In brief, the culture medium was removed, and the cells were collected from culture plates by pipetting and then washed in phosphate-buffered saline solution (PBS). Flow cytometry was performed on Epics XL, adding propidium iodide (PI, 10 μg ml−1) 30 min before analysis. The instrument was set up with a standard configuration: excitation of the sample was performed using a 488 nm air-cooled argon-ion laser at 15 mW. Forward scatter (FSC), side scatter (SSC) and red (620 nm) fluorescence for PI were acquired. Optical alignment was based on the optimized signal from 10 nm fluorescent beads (Immunocheck, Epics Division). Time was used as a control for the stability of the instrument; red fluorescence was projected on a 1024 monoparametrical histogram. Aggregates were excluded, gating single cells by their area vs peak fluorescence signal (Sureda et al., 1999).
Detection of condensed nuclei by PI staining
PI staining was used to evaluate morphological evidence of apoptosis, for exmple, condensed nuclei. CGNs were grown on glass coverslips and incubated for 12 h in S/K-deprived medium in the absence or presence of calpain inhibitors and z-VAD-fmk. After treatment, cells were fixed in 4% paraformaldehyde/PBS, pH 7.4, for 1 h at room temperature. After washing in PBS, they were incubated for 3 min with a solution of PI in PBS (10 μg ml−1). Coverslips were mounted in Mowiol® 4–88. Stained cells were visualized under UV illumination using the × 20 objective of a Nikon Eclipse fluo microscope and digitized images were captured.
Apoptotic cells contained shrunken, brightly fluorescent apoptotic nuclei with condensed chromatin, compared with nonapoptotic cells. Apoptotic cells were scored by counting at least 500 cells for each sample in three different experiments.
Assay of caspases enzymatic activities
We used the colorimetric substrate Ac-DEVD-p-nitroaniline for the determination of caspase-3, as follows: 12 h after S/K withdrawal, CGNs were collected in a lysis buffer (50 mM HEPES, 100 mM NaCl, 0.1% CHAPS, 0.1 mM EDTA, pH 7.4). Protein (50 μg μl−1) was incubated with 200 μM corresponding p-nitroaniline substrate in assay buffer (50 mM HEPES, 100 mM NaCl, 0.1% CHAPS, 10 μM dithiothreitol, 0.1 mM EDTA, pH 7.4) in 96-well plates at 37°C for 24 h. Absorbance of the cleaved product was measured at 405 nm in a microplate reader (BioRad). Results were expressed as percentages of the absorbance measured in vehicle treated cells.
α-Spectrin digestion immunoblotting
Caspase-3 and calpain activities were measured using α-spectrin breakdown. Western blot analysis were carried out using treated or control cells protein aliquots, containing 5 μg of protein per sample. In brief, samples were placed in sample buffer (0.5 M Tris-HCl, pH 6.8, 10% glycerol, 2% (w v−1) SDS, 5% (v v−1) 2-β-mercaptoethanol, 0.05% bromophenol blue) and denatured by boiling at 95–100°C for 5 min. Samples were separated by electrophoresis on 10% acrylamide gels. Thereafter, proteins were transferred to polyvinylidene fluoride (PVDF) sheets (ImmobilonTM-P, Millipore Corp., Bedford, MA, U.S.A.) using a transblot apparatus (BioRad). Membranes were blocked overnight with 5% nonfat milk dissolved in TBS-T buffer (Tris 50 mM; NaCl 1.5%; Tween 20, 0.05%, pH 7.5). They were then incubated with a primary antibody against α-spectrin (1 : 1000, Oncogene). After 90 min, blots were washed thoroughly in TBS-T buffer and incubated for 1 h with a peroxidase-conjugated IgG antibody (Amersham Corp., Arlington Heights, IL, U.S.A.). Immunoreactive protein was visualized using a chemiluminescence-based detection kit following the manufacturer's protocol (ECL kit; Amersham Corp.). Routinely, protein load was monitored using phenol red staining of the blot membrane or immunodetection of α-tubulin.
Immunodetection of cdk5, p35/p25 and MEF-2
Aliquots of cell homogenate containing 30 μg of protein per sample were analyzed by Western blot, as described above. Membranes were incubated with primary monoclonal antibodies against cdk5 (sc-173), p35/p25 (sc-820) and MEF2 (sc-13919-R) (1 : 1000, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). After 90 min, blots were washed thoroughly in TBS-T buffer and incubated for 1 h with a peroxidase-conjugated IgG secondary antibody (Amersham Corp., Arlington Heights, IL, U.S.A.). The immunoreactive protein was visualized as described above. Routinely, protein load was monitored using phenol red staining of the blot membrane or immunodetection of α-tubulin.
For immunocytochemistry experiments, CGNs were grown on sterile coverslips. After stimuli, cells were washed twice in PBS and fixed in 4% paraformaldehyde/PBS, pH 7.4 for 1 h at room temperature. They were preincubated for 30 min in PBS containing 0.3% Triton X-100 and 30% normal horse serum at room temperature. The cultures were immunostained with antibodies specific for cdk5 (1 : 400 dilution; sc-173), MEF2 (1:400; sc-13919-R) followed by rhodamine-conjugated anti-rabbit IgG or anti-mouse IgG (1 : 200). Subsequently, coverslips were thoroughly washed and mounted in Mowiol® 4–88 and cells were then imaged using fluorescence microscopy at × 100 oil immersion objective (Nikon Eclipse).
Statistical analysis
Data are given as mean±s.e.m. from at least quadruplicate experiments across 4–6 independent cultures. Data were analyzed by two-tailed Student's t-test or ANOVA followed by Tukey–Kramer multiple comparisons test.
Results
Evaluation of neuroprotective effects of calpain inhibitors
Cell survival decreased by up to 49.5±4% after 12 h of S/K withdrawal. When CGNs were pre-incubated with 20–40 μM PD150606 or PD151746 for 24 h, neurons were partially protected against the effect of S/K withdrawal, restoring MTT-assessed survival (P<0.05, Figure 1). In contrast, PD145305, the negative control of calpain inhibitors, was not neuroprotective. Morphological analysis, by phase-contrast microscopy, indicated that S/K withdrawal destroyed the neurites and caused severe neuronal damage and morphological changes in CGNs associated with apoptosis or necrosis. When cultures were treated with calpain inhibitors PD150606 or PD151746, neurites appeared intact, like control cells (Figure 1). Since calpain inhibitors at 40 μM provided neuroprotection, this concentration was used in our subsequent experiments.
Figure 1.
Antineurotoxic effect of PD150606, PD151746 and PD145305 on S/K withdrawal in CGNs. MTT quantification and representative phase-contrast images of CGN cultures exposed to different drug treatments. Calibration bar, 10 μM. Statistical analysis was carried out by one-way ANOVA followed by Tukey's test, *P<0.05 vs S/K withdrawal values.
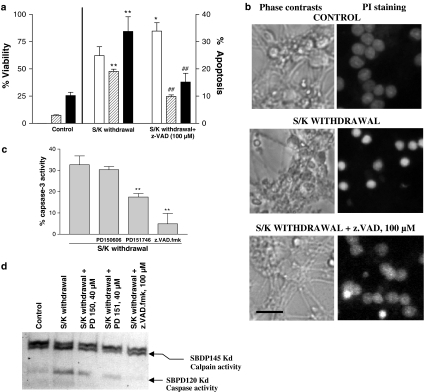
z-VAD-fmk protected CGNs from S/K withdrawal-induced apoptosis (Figure 3) and prevented the drop in MTT values induced by S/K withdrawal.
Figure 3.
Analysis of calpain and caspase-3 activities in 12 h S/K withdrawal in CGNs in the absence or presence of PD150606 (40 μM), PD151746 (40 μM) and z.VAD.fmk (100 μM). (a) Effect of z.VAD.fmk (100 μM) on S/K withdrawal-induced changes in the cell viability (open bars) or in the percentage of cells rated as apoptotic (hypodiploid cells/dashed bars; condensed nuclei/black bars). (b) Representative phase contrasts and fluorescence photomicrographs of CGNs in the different experimental conditions. Calibration bar, 10 μm. (c) Caspase-3 activity in CGNs after 12 h S/K withdrawal in the presence or absence of tested compounds. Results are the mean±s.e.m. of three cultures. The statistical analysis was carried out with the one-way ANOVA followed by Tukey's test, **P<0.05 vs S/K withdrawal values. (d) Representative Western blot of α-spectrin digestion showing calpain activity (SBPD145kD fragment) and caspase 3 activity (SBPD120kD fragment), influence of preincubation with 40 μM PD150606 or PD151746 and 100 μM z.VAD.fmk.
Antiapoptotic properties of calpain inhibitors
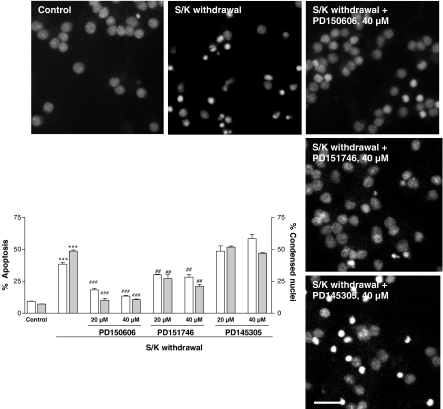
Flow cytometric results showed that the percentage of fragmented nuclei was 6.1±0.3% in control samples (n=6). When CGNs were deprived, the percentage of fragmented nuclei increased to 38±2.4% (P<0.001, n=6). At the higher concentration of PD150606 and PD151746 (40 μM), the percentage of fragmented nuclei decreased significantly (Figure 2).
Figure 2.
Antiapoptotic effect of PD150606, PD151746 and PD145305 on S/K withdrawal-induced changes in the percentage of cells rated as apoptotic by means of flow cytometric (hypodiploid cells, open bars) or morphological analysis (gray bars). Results are show as mean±s.e.m. of 4–6 independent cultures. Statistical analysis was carried out by one-way ANOVA followed by Tukey's test; ***P<0.001 vs control values; ###P<0.001 vs S/K withdrawal values. Representative fluorescence photomicrographs showing chromatin condensation in permeabilized CGNs in the different experimental conditions. The nuclei were counted at the fluorescence microscope, distinguishing the normal from the condensed nuclei with the criteria stated in Methods. Calibration bar, 10 μm.
The nuclear morphology of neurons after S/K withdrawal was analyzed using the fluorescent PI dye. A small number of condensed apoptotic nuclei, 6.9±0.5% (n=5) of total nuclei present in each microscopic field, were always found in control cultures (Figure 2). Most nuclei showed homogenous staining. When CGNs were exposed to S/K withdrawal for 12 h, the number of condensed nuclei increased 48.65±2.5% (P<0.001, n=5) and the nuclei were smaller (Figure 2). In contrast, when CGNs were pretreated with PD150606 and PD151746, the number of condensed apoptotic nuclei decreased significantly, P<0.001 and P<0.01, respectively (Figure 2).
However, PD145305 did not exert any antiapoptotic action in either experiment. So, additional experiments were carried out with only neuroprotective calpain inhibitors. On the other hand, z-VAD-fmk (100 μM) attenuated both the DNA fragmentation measured by flow cytometry and the number of condensed nuclei measured by cell counting (Figure 3).
S/K withdrawal induced caspase-3 activation as well as calpain activation in CGNs. Analysis of Western blot for α-spectrin digestion demonstrated that both catalytic enzymes were activated after 12 h S/K withdrawal, as demonstrated by the bands at 145 kDa (calpain-derived fragment) and 120 kDa (caspase-3-derived fragment) that appeared in the blot. The presence of PD150606, PD151746 or z-VAD-fmk reduced the levels of SBPD120kD indicating the participation of caspase-3 activity in neurotoxic stimuli. Moreover, both PDs reduced the band at 145 kDa, indicative of calpain inhibition (Figure 4). These results are corroborated by the measurements of caspase-3 activity, which revealed that all drugs tested attenuated the effects of the S/K withdrawal (Figure 3).
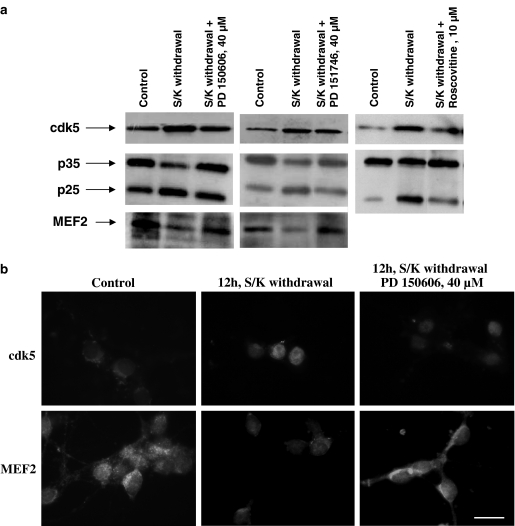
Figure 4.
(a) Western blot analysis of cdk5, p35/p25 and p-MEF2. Evidence that cdk5 overexpression in CGNs after 12 h S/K withdrawal is blocked in the presence of 40 μM PD150606 or PD151746. The p35/p25 ratio was also evaluated, showing an increase in p25 band intensity after S/K deprivation and a return to control levels in the presence of PD150606 or PD151746. Representative results for 10 μM roscovitine are showed. Finally, MEF2 band intensity decreased when CGNs were S/K deprived, indicating inactivation of MEF2, and increased in the presence of PD150606 or PD151746. All experiments were carried out at least in duplicate on three different cultures. (b) Immunostaining against cdk5 and MEF2 of CGNs after 12 h S/K withdrawal in the absence or presence of 40 μM PD150606 demonstrated an increase in cdk5 expression after S/K withdrawal and an inactivation of prosurvival MEF. Similar results were obtained with PD151746 (data not shown) (scale bar, 25 μm).
Role of cdk5/p35 pathway in S/K withdrawal-induced apoptosis in CGNs
Figure 4 shows the increase in expression of cdk5 seen after S/K withdrawal measured by Western blot analysis and immunocytochemistry experiments. This increase was blocked by PD150606 or PD151746 (Figure 4a). Moreover, S/K withdrawal induced the breakdown of p35 to p25. Calpain inhibitors prevented this transformation (Figure 4a). Roscovitine, a specific inhibitor of cdk1,-2,-5, has been used to provide evidence that S/K withdrawal-induced apoptosis is mediated by cdk5 pathway. Roscovitine (10 μM) reduced the activation of cdk5/p25 after 12 h S/K deprivation (Figure 4a).
Recently, it has been demonstrated that Cdk5 promotes neuronal apoptosis by phosphorylation of MEF2 and thus inhibits MEF2 prosurvival activity (Li et al., 2001; Heidenreich & Linseman, 2004). In this way, S/K withdrawal increased MEF2 phosphorylation, seen as a reduction in inmunostaining, an effect that was abolished in the presence of 40 μM PD150606 or PD151746 (Figure 4a and b).
Discussion
The aim of the present study was to elucidate a possible neuroprotective pathway regulated by calpain inhibition on S/K withdrawal-induced apoptosis in CGNs. Nath et al. (1996a, 1996b) described the neuroprotective properties of calpain inhibitors against potassium deprivation-induced apoptosis in CGNs. A prominent role for calpain activation has recently been proposed in experimental models of neurodegenerative diseases (Bizat et al., 2003; Crocker et al., 2003). All these results suggested that calpain activation is a key component of the biochemical pathway involved in neuronal cell death (Volbracht et al., 2001). Likewise, several studies proposed a participation of calpains in both necrotic and apoptotic processes in ischemia, but the mechanism involved in their regulation of the apoptotic pathway remains to be elucidated (Knoblach et al., 2004). Indeed, in vitro data indicate that calpain is activated by β-amyloid, in an in vitro model of Alzheimer's disease (Jordan et al., 1997; Boland & Campbell, 2003; Liu et al., 2004), MPP+, an in vitro model of Parkinson's disease (Crocker et al., 2003), and after stimulation of ionotropic glutamate receptors (Rami et al., 1997; Zhao et al., 2000; Newcomb-Fernandez et al., 2001; Moore et al., 2002).
Most experiments performed to study the apoptotic process after S/K withdrawal in CGNs are focused on the caspase activation pathway (Villalba et al., 1997). Here, we have demonstrated that zVAD-fmk prevented the decrease in MTT values induced by S/K withdrawal. Furthermore, the pan-caspase inhibitor reduced caspase-3 activation and prevented DNA fragmentation and nuclear cell condensation. This result confirms that caspase inhibitors attenuate and delay apoptosis, probably because they operate downstream of mitochondrial alteration (Taylor et al., 1997; Harada & Sugimoto, 1998). Furthermore, it has been demonstrated using neuronal cell cultures lacking caspase-3 that this enzyme is necessary for DNA fragmentation but not for neuronal cell death (D'Mello et al., 2000).
Calpain and caspase activation degrade the cytoskelal protein α-spectrin (280 kDa) into three main fragments of 145 kDa (calpains), 120 kDa (caspase-3) and 150 kDa (calpains-caspases) (Vanderklish & Bahr, 2000). In the present study, S/K withdrawal increased the levels of 145/120 kDa bands. Our results indicated that PD151746 and PD150606, apart from the inhibition of calpain, prevented the activation of caspase-3.
There are two main obstacles to our understanding of the neuroprotective properties of calpain/caspase inhibitors. Firstly, there is crosstalk between the two pathways. Inhibition of calpain system could increase or decrease the activity of the caspase pathway, thus both cysteine-proteases take part in the apoptotic process (McCollum et al., 2002; Neumar et al., 2003; Rami, 2003). The other obstacle is that although tetrapeptide-based synthetic caspase inhibitors have been considered selective for caspases, they could inhibit other cysteine proteases, including calpains (Waterhouse et al., 1998; Knoblach et al., 2004).
In spite of these disadvantages, we attempted to clarify the role of calpains in neuronal apoptosis and we demonstrated a role for these cysteine proteases in cdk5 activation. Thus, in the present study, we demonstrated that S/K withdrawal-induced apoptosis in CGNs is mediated by an increase in the expression of p25 (Lee et al., 2000; Tsai et al., 2004). The increase in the expression of this cleaved form of p35 is involved in the apoptotic process; one of the mechanisms proposed is through the hyperphosphorylation of tau. On the other hand, it has been suggested that cdk5/p25 plays a role in increased expression of p53, an oncogene involved in the neuronal apoptotic process (Sederaus et al., 2003). In addition, cdk5/p25 modulates the excitotoxic effects of glutamate via the phosphorylation of NMDA receptors and increased calcium influx (Shelton & Johnson, 2004).
The present study shows an increase in MEF2 phosphorylation, which suggests inactivation of the prosurvival activity of this transcriptional factor (Li et al., 2001; Heidenreich & Linseman, 2004). The calpain inhibition prevents cdk5/p35 cleavage, thus preventing inactivation of myocyte enhancer factor-2. Taken together, these results strongly suggest that the antiapoptotic effects of calpain inhibitors during S/K withdrawal in CGNs occur through inhibition of calpain activation/cdk5 pathway (Hung et al., 2005).
In summary, these results may improve our understanding of the neuroprotective effects of calpain inhibitors and their potential application in the treatment of neurodegenerative diseases (Figure 5). The potential connection between cdk5 and other kinases, such as c-Jun N-terminal kinase (JNK), involved in apoptotic pathways, is a key point to be discerned in further studies (Harris et al., 2002; Li et al., 2002; Cheung & Ip, 2004).
Figure 5.
Representative cartoon of calpain inhibition effect after serum potassium withdrawal on CGNs.
Acknowledgments
We acknowledge Maria Teresa Iglesias for invaluable technical assistance. The excellent secretarial support of Ms Mar Morales is also greatly appreciated. We thank the Language Advice Service of the University of Barcelona for revising the manuscript. This study was supported by grants SAF2002-00790 (FEDER founds) from the Spanish Minister of Science, FISS G03/137, FISS G03/167 and PI041300 from the Spanish Ministry of Health. EV and DA are recipients of fellowships from the University of Barcelona and from FISS, respectively.
Abbreviations
- Cdk
cyclin-dependent kinase
- CGNs
cerebellar granule cells
- FSC
forward scatter
- JNK
c-Jun N-terminal kinase
- MEF-2
myocyte enhancer factor 2
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium]
- PBS
phosphate buffer saline
- PI
propidium iodide
- PVDF
polyvinylidene fluoride
- SSC
side scatter
References
- ALVAREZ A., TORO R., CACERES A., MACCIONI R.B. Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett. 1999;459:421–426. doi: 10.1016/s0014-5793(99)01279-x. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA F., TRINCHESE F., LIU S., WALTER S., NIXON R.A., ARANCIO O. Calpain inhibitors, a treatment for Alzheimer's disease. J. Mol. Neurosci. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- BIZAT N., HERMEL J.M., BOYER F., JACQUARD C., CREMINON C., OUARY S., ESCARTIN C., HANTRAYE P., KRAJEWSKI S., BROUILLET E. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: Implications for Huntington's disease. J. Neurosci. 2003;23:5020–5030. doi: 10.1523/JNEUROSCI.23-12-05020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLAND B., CAMPBELL V. B-amyloid (1–40)-induced apoptosis of cultured cortical neurones involves calpain-mediated cleavage of poly-ADP-ribose polymerase. Neurobiol. Aging. 2003;24:179–186. doi: 10.1016/s0197-4580(02)00060-x. [DOI] [PubMed] [Google Scholar]
- CANU N., CALISSANO P. In vitro cultured neurons for molecular studies correlating apoptosis with events related to Alzheimer disease. Cerebellum. 2003;2:270–278. doi: 10.1080/14734220310004289. [DOI] [PubMed] [Google Scholar]
- CHEUNG Z.H., IP N.Y. cdk5: mediator of neuronal death and survival. Neurosci. Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
- CROCKER S.J., SMITH P.D., JACKSON-LEWIS V., LAMBA W.R., HAYLEY S.P., GRIMM E., CALLAGHAN S.M., SLACK R.S., MELLONI E., PRZEDBORSKI S., ROBERTSON G.S., ANISMAN H., MERALI Z, PARK DS. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI ROSA G., ODRIJIN T., NIXON R.A., ARANCIO O. Calpain inhibitors: a treatment for Alzheimer's disease. J. Mol. Neurosci. 2002;19:135–141. doi: 10.1007/s12031-002-0024-4. [DOI] [PubMed] [Google Scholar]
- D'MELLO S.R., KUAN C., FLAVELL R.A., RAKIC P. Caspase-3 is required for apoptosis-associated DNA fragmentation but not for cell death in neurons deprived of potassium. J. Neurosci. Res. 2000;59:24–31. [PubMed] [Google Scholar]
- FRIEDLANDER R.M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- HARADA J., SUGIMOTO M. Inhibitors of interleukin-1 beta-converting enzyme-family proteases (caspases) prevent apoptosis without affecting decreased cellular ability to reduce 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide in cerebellar granule neurons. Brain Res. 1998;793:231–243. doi: 10.1016/s0006-8993(98)00156-5. [DOI] [PubMed] [Google Scholar]
- HARRIS C., MARONEY A.C., JOHNSON E.M. Identification of JNK-dependent and -independent components of cerebellar granule neuron apoptosis. J. Neurochem. 2002;83:992–1001. doi: 10.1046/j.1471-4159.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- HEIDENREICH K.A., LINSEMAN D.A. Myocyte enhancer factor 2 transcription factors in neuronal differentiation and survival. Mol. Neurobiol. 2004;29:155–165. doi: 10.1385/MN:29:2:155. [DOI] [PubMed] [Google Scholar]
- HONIG L.S., ROSENBERG R.N. Apoptosis and neurologic disease. Am. J. Med. 2000;108:300–317. doi: 10.1016/s0002-9343(00)00291-6. [DOI] [PubMed] [Google Scholar]
- HUNG K.S., HWANG S.L., LIANG C.L., CHEN Y.J., LEE T.H., LIU J.K., HOWNG S.L., WANG C.H. Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J. Neuropathol. Exp. Neurol. 2005;64:15–26. doi: 10.1093/jnen/64.1.15. [DOI] [PubMed] [Google Scholar]
- JORDAN J., GALINDO M.F., MILLER R.J. Role of calpain- and interleukin-1β converting enzyme-like proteases in the β-amyloid-induced death of rat hippocampal neurons in culture. J. Neurochem. 1997;68:1612–1621. doi: 10.1046/j.1471-4159.1997.68041612.x. [DOI] [PubMed] [Google Scholar]
- KNOBLACH S.M., ALROY D.A., NIKOLAEVA M., CERNAK I., STOICA B.A., FADEN I. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J. Cerebr. Blood Flow. Metab. 2004;24:1119–1132. doi: 10.1097/01.WCB.0000138664.17682.32. [DOI] [PubMed] [Google Scholar]
- LEE M., KWON Y.T., LI M., PENG J., FRIEDLANDER R.M., TSAI L. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- LEE M.S., TSAI L.H. Cdk5: one of the links between senile plaques and neurofibrillary tangles. J. Alzheimers. Dis. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]
- LI B.S., ZHANG L., TAKAHASHI S., MA W., JAFFE H., KULKARNI A.B., PANT H.C. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 2002;21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI M., LINSEMAN D.A., ALLEN M.P., MEINTZER M.K., WANG X., TRACEY LAESSIG T., WIERMAN M.E., HEIDENREICH K.A. Myocyte enhancer factor 2A and 2D undergo phosphorylation and caspase-mediated degradation during apoptosis of rat cerebellar granule neurons. J. Neurosci. 2001;21:6544–6552. doi: 10.1523/JNEUROSCI.21-17-06544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSEMAN D.A., CORNEJO B.J., LE S.S., MEINTZER M.K., LAESSIG T.A., BOUCHARD R.J., HEIDENREICH K.A. A myocyte enhancer factor 2D (MEF2D) kinase activated during neuronal apoptosis is a novel target inhibited by lithium. J. Neurochem. 2003;85:1488–1499. doi: 10.1046/j.1471-4159.2003.09799.x. [DOI] [PubMed] [Google Scholar]
- LIU T., PERRY G., CHAN H.W., VERDILE G., MARTINS R.N., SMITH M.A., ATWOOD C.S. Amyloid-β-induced toxicity of primary neurons is dependent upon differentiation-associated increases in tau and cyclin-dependent kinase 5 expression. J. Neurochem. 2004;88:554–563. doi: 10.1046/j.1471-4159.2003.02196.x. [DOI] [PubMed] [Google Scholar]
- MACCIONI R.B., MUÑOZ J.P., BARBEITO L. The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch. Med. Res. 2001;32:367–381. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- MCCOLLUM A.T., NASR P., ESTUS S. Calpain activates caspase-3 during UV-induced neuronal death but only calpain is necessary for death. J. Neurochem. 2002;82:1208–1220. doi: 10.1046/j.1471-4159.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- MOORE J.D., ROTHWELL N.J., GIBSON R.M. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. Br. J. Pharmacol. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATH R., RASER K.J., MCGINNIS K., NADIMPALLI R., STAFFORD D., WANG K.K.W. Effects of ICE-like protease and calpain inhibitors on neuronal apoptosis. NeuroReport. 1996a;8:249–255. doi: 10.1097/00001756-199612200-00050. [DOI] [PubMed] [Google Scholar]
- NATH R., RASER K.J., STAFFORD D., HAJIMOHAMMADREZA I., POSNER A., ALLEN H., TALANIAN R.V., YUEN P., GILBERTSEN R.B., WANG K.K.W. Non-erythroid α-spectrin breakdown by calpain and interleukin 1β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem. J. 1996b;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMAR R.W., XU Y.A., GADA H., GUTTMANN R.P., SIMAN R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J. Biol. Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]
- NEWCOMB-FERNANDEZ J.K., ZHAO X., PIKE B.R., WANG K.K.W., KAMPFL A., BEER R., DEFORD S.M., HAYES R.L. Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J. Cerebr. Blood Flow Metab. 2001;21:1281–1294. doi: 10.1097/00004647-200111000-00004. [DOI] [PubMed] [Google Scholar]
- NIXON R.A. The calpains in aging-related diseases. Ageing Res. Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- RAMI A. Ischemic neuronal death in the rat hippocampus: the calpain-calpastatin-hypothesis. Neurobiol. Dis. 2003;13:75–88. doi: 10.1016/s0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- RAMI A., FERGER D., KRIEGLSTEIN J. Blockade of calpain proteolytic activity rescues neurons from glutamate excitotoxicity. Neurosci. Res. 1997;27:93–97. doi: 10.1016/s0168-0102(96)01123-6. [DOI] [PubMed] [Google Scholar]
- RAY S.K., BANIK N.L. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr. Drug Targets. CNS Neurol. Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- RAY S.K., HOGAN E.L., BANIK N.L. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res. Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- SEDERAUS M., KERAMARIS E., O'HARE M., MELLANI E., SLACK R.S., ELCE J.S., GREER P.A., PARK D.S. Calpains mediate p53 activation and neuronal death evoked by DNA damage. J. Biol. Chem. 2003;278:26031–26038. doi: 10.1074/jbc.M302833200. [DOI] [PubMed] [Google Scholar]
- SHELTON S.B., JOHNSON G.V.W. Cyclin-dependent kinase-5 in neurodegeneration. J. Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- SMITH D.S., TSAI L.H. Cdk5 behind the wheel: a role in trafficking and transport. Trends Cell. Biol. 2002;12:28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- SUREDA F.X., GABRIEL C., COMAS J., PALLAS M., ESCUBEDO E., CAMARASA J., CAMINS A. Evaluation of free radical production, mitochondrial membrane potential and cytoplasmic calcium in mammalian neurons by flow cytometry. Brain Res. Brain, Res. Protoc. 1999;4:280–287. doi: 10.1016/s1385-299x(99)00030-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR J., GATCHALIAN C.L., KEEN G., RUBIN L.L. Apoptosis in cerebellar granule neurones: involvement of interleukin-1β converting enzyme-like proteases. J. Neurochem. 1997;68:1598–1605. doi: 10.1046/j.1471-4159.1997.68041598.x. [DOI] [PubMed] [Google Scholar]
- TSAI L.H., LEE M.S., CRUZ J. cdk5, a therapeutic target for Alzheimer's disease. Biochem. Biophys. Acta. 2004;1697:137–142. doi: 10.1016/j.bbapap.2003.11.019. [DOI] [PubMed] [Google Scholar]
- VANDERKLISH P.W., BAHR B.A. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and diseases states. Int. J. Exp. Path. 2000;81:323–339. doi: 10.1111/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDAGUER E., GARCÍA-JORDÀ E., JIMÉNEZ A., STRANGES A., SUREDA F.X., CANUDAS A.M., ESCUBEDO E., CAMARASA J., PALLÀS M., CAMINS A. Kainic acid-induced neuronal cell death in cerebellar granule cells is not prevented by caspase inhibitors. Br. J. Pharmacol. 2002;135:1297–1307. doi: 10.1038/sj.bjp.0704581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLALBA M., BOCKAERT J., JOURNOT L. Concomitant induction of apoptosis and necrosis in cerebellar granule cells following serum and potassium withdrawal. NeuroReport. 1997;8:981–985. doi: 10.1097/00001756-199703030-00032. [DOI] [PubMed] [Google Scholar]
- VOLBRACHT C., FAVA E., LESIST M., NICOTERA P. Calpain inhibitors prevent nitric oxide-triggered excitotoxic apoptosis. NeuroReport. 2001;12:3645–3648. doi: 10.1097/00001756-200112040-00008. [DOI] [PubMed] [Google Scholar]
- WANG K.K. Calpain and caspase: can you tell the difference. Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- WANG K.K., NATH R., POSNER A., RASER K.J., BUROKER-KILGORE M., I HAJIMOHAMMADREZA I., PROBERT A.W., MARCOUX F.W., YE Q., TAKANO E., HATANAKA M., MAKI M., CANER H., COLLINS J.L., FERGUS A., LEE K.S., LUNNEY E.A., HAYS S.J., YUEN P.-W. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad Sci. U.S.A. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERHOUSE N.J., FINUCANE D.M., GREEN D.R., ELCE J.S., KUMAR S., ALNEMRI E.S., LITWACK G., KHANNA K., LAVIN M.F., Watters D.J. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 1998;5:1051–1061. doi: 10.1038/sj.cdd.4400425. [DOI] [PubMed] [Google Scholar]
- WEISHAUPT J.H., NEUSCH C., BAHR M. Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res. 2003;312:1–8. doi: 10.1007/s00441-003-0703-7. [DOI] [PubMed] [Google Scholar]
- ZHAO X., NEWCOMB J.K., PIKE B.R., WANG K.W., D'AVELLA D., HAYES R.L. Novel characteristics of glutamate-induced cell death in primary septohippocampal cultures: relationship to calpain and caspase-3 protease activation. J. Cerebr. Blood Flow Metab. 2000;20:500–562. doi: 10.1097/00004647-200003000-00014. [DOI] [PubMed] [Google Scholar]