Abstract
Zanapezil (TAK-147 (3-[1benzylpiperdin-4-yl]-1-(2,3,4,5-tetrahydro-1 H-1-benzazepin-8-yl) propan-1-one fumarate)) is a selective acetylcholine (ACh) esterase inhibitor under investigation as a drug for Alzheimer's disease (AD) treatment.
In this study, the effects of TAK-147 at 2 mg kg−1 p.o. for 21 days, compared to donepezil (E2020), on the levels of ACh, catecolamines and indoleamines were investigated in the ventral hippocampus (VH) of freely moving rats by microdialysis-high-performance liquid chromatography.
The results revealed that the VH contains 92.05±21.97 fmol 20 μl−1 ACh and the following monoamines levels (pg 30 μl−1), norepinephrine (NE) 1.92±0.39, epinephrine (Epi) 1.91±0.183, 3-methoxy-4-hydroxyphenylglycol (MHPG) 11.53±3.22, normetanephrine 3.26±0.61, dopamine (DA) 0.77±0.23, 3,4-dihydroxyphenylacetic acid (DOPAC) 3.37±1.01, homovanillic acid (4-hydroxy-3-methoxyphenylacetic acid; HVA) 4.04±0.93, 3-methoxytyramine 0.64±0.13, serotonin (5-HT) 0.73±0.16 and 5-hydroxyindoleacetic acid (5-HIAA) 313.15±18.42.
On the 21st day and prior to the last dose, TAK-147 increased ACh, Epi, DA and 5-HT, whereas E2020 increased MHPG, Epi and DA. Following the last dose, TAK-147 increased NE, whereas E2020 increased NE, ACh and 5-HT in addition to their effects prior to the last dose. TAK-147 decreased HVA : DA ratio, but only marginally decreased DOPAC : DA and 5-HIAA : 5-HT ratios. On the other hand, E2020 decreased ratios of HVA : DA, DOPAC : DA (prior to the last dose), and 5-HIAA : 5-HT (90–180 min after the last dose). Both drugs decreased MHPG : NE only at 180 min after the last dose. The results also showed that TAK-147 increased Epi : NE ratio prior to and for 120 min following the last dose, whereas E2020 increased the ratio only before the last dose. The present results show that TAK-147 at a subthreshold dose could differentially increase ACh and 5-HT, compared to MHPG increased by E2020. The last dose of each drug could extend their effects to other monoamines.
The increase of the monoamines levels, in addition to that on the ACh, and decrease of their oxidation could be of value in the treatment of the AD, other dementic diseases and the cohort neurological disorders depending on the type of the monoamine underlying the disorder.
Keywords: Zanapezil, donepezil, ventral hippocampus, acetylcholine, monoamines, microdialysis, Alzheimer's disease
Introduction
Zanapezil (TAK-147 (3-[1benzylpiperdin-4-yl]-1-(2,3,4,5-tetrahydro-1 H-1-benzazepin-8-yl) propan-1-one fumarate)) has been introduced into Alzheimer's disease (AD) treatment as a novel, potent, reversible and selective acetylcholine (ACh) esterase (AChE) inhibitor (AChEI) with brain/plasma concentration ratio of 7.0–20.6 (Kosasa et al., 2000) and lesser (compared to donepezil, E2020) peripheral and central side effects (Hatip-Al-Khatib et al., 2004) or even without producing peripheral side effects (Ishihara et al., 2000). Additionally, TAK-147 has been shown to inhibit moderately muscarinic M1 and M2 receptor binding, but with very weak or no inhibition of high-affinity choline ([2-hydroxyethyl] trimethylammonium) uptake or binding to nicotinic receptor (Hirai et al., 1997). Moreover, TAK-147 increases cerebral energy metabolism (Nakayama et al., 1996; Ishihara et al., 2000), improves hippocampal metabolic activity (Xu et al., 2002) and, like E2020, enhances the choline acetyltransferase activity more potently than tacrine (Kato et al., 1999). Furthermore, TAK-147, at AChE inhibiting dose, is reported to provide NGF-like neurotrophic activity on central cholinergic neurons not only via AChE inhibition but also possibly via an effect on tau receptors (Kato et al., 1999; Ishihara et al., 2000). Behaviorally, TAK-147 ameliorates the passive avoidance deficit induced by diazepam and the impairment of delayed-matching-to-position performance induced by scopolamine without affecting the general behavior of the rats, and reverses reserpine-induced hypothermia and ptosis in mice (Miyamoto et al., 1996). Moreover, TAK-147 improves memory impairment in the passive avoidance test and Morris water maze induced by scopolamine (Ishihara et al., 2000; Chen et al., 2002), provides neuroprotection and ameliorates ischemia-induced memory deficits (Xu et al., 2002).
E2020 is an other potent, selective and reversible central AChEI (Kosasa et al., 2000; Ogura et al., 2000; 2001; Isoma et al., 2002). E2020 is used in the treatment of AD, where it has been reported to provide significant improvements in cognition and the global function (Wilkinson et al., 2003). It has been reported that chronic treatment with E2020 at 0.1–2 mg kg−1 for 14 days could extend long-term potentiation decay times at the perforant path-granule cell synapse, and elevate the number of nicotinic receptors within the hippocampus and neocortex of aged rats (Barnes et al., 2000). In rat, E2020 increases hippocampal blood flow (Hatip-Al-Khatib et al., 2004). In the AD patients receiving chronic (11±2.6 months) treatment at 5 mg day−1, E2020 preserves brain perfusion (Nobili et al., 2002). Additionally, it has been reported that E2020 at 5 mg given every morning for 7 days could improve sedation and fatigue in patients receiving opioids for cancer pain (Bruera et al., 2003). Moreover, treatment with E2020 at 10 mg day−1 for 1 month dramatically improves sensorimotor function in the hemiplegic lower limb and shoulder (Berthier et al., 2003). Experimentally, E2020 improves learning impairments in some hypocholinergic models in rats (Ogura et al., 2001; Balducci et al., 2003; Misane & Ogren, 2003) and in patients with traumatic brain injury (Morey et al., 2003). Acute and chronic administration of E2020 (0.1 mg kg−1 for 7 or 14 days) significantly reduces the selective serotonin, 5-HT(2A/2C) agonist 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane-induced head twitch response (Hayslett & Tizabi, 2003).
In spite of the fact that treatment of the AD requires chronic drug therapy, most of the experimental studies on the relevant drugs had been conducted using acute administration of a wide range of doses. Previous studies had dealt with acute effects of TAK-147 and E2020 on ACh, of effects of E2020 on dopamine (DA), norepinephrine (NE) and 5-HT, and of chronic effects of E2020 on ACh. In a single-dose study (Hatip-Al-Khatib et al., 2004), we have determined the ED50 value of 4.52 and 4.07 mg kg−1 for TAK-147 and E2020, respectively, and the threshold dose of 5 mg kg−1 p.o. for the ACh-increasing effect of both drugs in the ventral hippocampus (VH). No comparative study of TAK-147 and E2020 or of chronic effects of TAK-147 on ACh and DA, NE and 5-HT, and of E2020 on DA, NE and 5-HT are available in the literature. Accordingly, the present study aimed at determining the effect of the subthreshold dose of TAK-147 administered p.o. for 21 days on the ACh and monoamines levels and their metabolites in the VH. The effect of TAK-147 was compared to E2020.
Methods
Animals
The experiments were performed on 7-week-old (220–230 g) male Wistar rats (Kyudo Co., Saga, Japan). The rats were housed in groups of five per Plexiglas cage (42 × 26 × 15 cm) under standard conditions of temperature (21–24°C), humidity (40–60%) and 12 h light : dark cycle with light period starting at 07:00. Food and water were available ad lib. After arrival of the rats and before the experiments, 1 week was allowed. The experiments were carried out in compliance with the guidelines of Fukuoka University. The body weights were recorded daily. While the groups were homogeneous without intergroup differences, the control (0.5% methylcellulose, MC), TAK-147 and E2020 groups exhibited significant (P<0.01) 16, 20 and 12% increments, respectively, on day 21 compared to the pretreatments.
Stereotaxic procedure
The rats were anesthetized with sodium pentobarbital (50 mg kg−1 intraperitoneally (i.p.); Tokyo Kasei, Tokyo, Japan) and placed in a stereotaxic frame (Narishige Scientific Instruments Lab., Tokyo, Japan). Guide cannulae (13 mm long, i.d. 0.75 mm, o.d. 0.85 mm, Eicom, Kyoto, Japan) were implanted into the VH according to Paxinos & Watson (1998). The coordinates (mm), relative to skull surface with the upper incisor bar 3.4 mm below the level of interaural line, were: posterior to bregma, AP=−4.8; lateral from midsagittal line, L=5; and dorsoventral, DV=6. Body temperature was maintained using a heating pad and a lamp during the surgery and upon recovery from anesthesia. The implanted cannulae were then secured with dummy cannulae kept in place by a cap. After surgery, each rat was injected with penicillin in hindquarter muscle (100,000 U) and housed singly after operation.
Microdialysis procedures
The extracellular levels of ACh and monoamines were measured in the VH by microdialysis technique, at least 3 days after surgery, in unanesthetized freely moving rats. The microdialysis probes were 13 mm long (i.d. 0.5 mm, o.d. 0.6 mm; Eicom) with an active dialysis membrane exposed at the tip. The dialysis membrane was U-shaped tube (each arm 3 mm long, i.d. 0.22 mm, o.d. 0.24 mm, total length of both arms 6–7 mm) made of cellulose hollow fiber with a molecular weight cutoff value 50 kDa. The Ringer-primed probes were implanted through the guide cannulae with the U-shaped dialysis membrane parallel to the longitudinal axis of the brain, and protruded 3 mm into the VH through the tip of the guide cannulae and secured by caps. The rats were then placed in a Plexiglas chamber (50 × 35 × 35 cm). The inlet of the probe was connected to the descending limb of spiral two-way tubing, which was in turn connected via pair-ring swivel system to another Teflon tube connected to microsyringes driven by micro syringe pump (SP-64, Eicom). The outlet of the probe was connected to the ascending limb of the swivel that was connected by another Teflon tube to an autoinjector (EAS-20, Eicom). The tubes and each limb of the spiral two-way tubing were 0.1 (i.d.) × 0.4 (o.d.) mm × 50 cm long Teflon tubes (Eicom). The implanted probe was perfused for 3 h with Ringer's solution (NaCl 147.0 mM, KCl 4.0 mM, CaCl2·2H2O 2.2 mM), free from any AChEI, at a flow rate of 1 μl min−1. Dialysate aliquots were collected every 20 min (20 μl, ACh) or 30 min (30 μl, monoamines) and directly injected into high-performance liquid chromatography (HPLC) system and assayed for ACh or monoamines. After a settling period (at least 2–3 h), three samples were collected in basal conditions. The rats were then challenged with each treatment and aliquots were then collected for the following 120 min (ACh) or 180 min (monoamines).
Determination of extracellular ACh and monoamines (catecholamines and indolamines) levels
The levels of ACh in the dialysates were assayed directly by HPLC method (EP-300 Liquid chromatography, Eicom). ACh was separated on a reverse-phase analytical column (Eicompak AC-GEL, 2.0∅ × 150 mm; Eicom). A guard (pre) column (3.0∅ × 4 mm with CH-GEL filter; Eicom) was placed before the analytical column outside the HPLC. An enzyme column (AC-ENZYMPAK, 3.0 mm ∅; Eicom) in which AChE and choline oxidase are immobilized on controlled-pore glass (546 Å diameter of pore and 200/400 mesh glass beads) was placed after the analytical column inside the HPLC. Following its separation on the analytical column, ACh was hydrolyzed by AChE to acetate and choline in a postcolumn enzyme reactor, and choline was oxidized by choline oxidase to produce betaine and hydrogen peroxide. The analytical and enzyme columns were maintained at 33°C by a column oven (ATC-300, Eicom). Detection was performed using an electrochemical detector (ECD-300, Eicom) with a platinum electrode (WE-PT) set at +450 mV vs Ag/AgCl reference electrode (Ag/AgCl RE-100). The mobile phase (Na2HPO4·12H2O 50 mM, H3PO4 50 mM, sodium 1-decanesulfonate 1.23 mM and EDTA 2Na 0.013 mM, pH 8.2) was degassed (DG-300, Eicom) and pumped at a flow rate 0.15 ml min−1. An internal standard (isopropylhemicholine 0.05 pg μl−1) was injected into the autoinjector at a rate of 1 μl min−1 simultaneously with the perfusion Ringer solution. Preliminary studies revealed that 10−6 M tetrodotoxine decreased the release of ACh (80%) and DA (90%). The level of ACh decreased in calcium-free perfusing Ringer by 60%. Pargyline increased DA and 5-HT by 150 and 350%, respectively (data not shown).
The levels of catecholamines and indolamines were determined as mentioned above, except that the analysis conditions were as follows: column – EICOMPAK SC-50 ODS ∅3.0 mm × 150 mm; precolumn – PREPAK ∅4.0 mm × 5.0 mm; ECD +750 mV against Ag/AgCl reference electrode; and oven temperature 25°C. The mobile phase contained 0.1 M acetate-citrate buffer (pH 3.5), 190 mg l−1 sodium 1-octanesulfonate, 5 mg l−1 EDTA and 16% methanol, degassed and pumped at a flow rate 0.25 ml min−1.
The peaks were recorded using Powerchrom integrator (Eicom). To evaluate the amount of ACh and monoamines in the samples, a linear regression curve was constructed for the standards (1 pmol of choline, isopropylhemicholine and ACh standards for ACh; 10 pg μl−1 of each standard material for the monoamines), and the peak heights of the ACh and monoamines in the samples were compared with those of the standards by means of a data processor (EPC-300, Eicom).
At the end of the experiment, the rats were killed by decapitation. Brains were quickly removed, sectioned at 50 μm thickness and placement of the microdialysis probes in the VH was verified. The result obtained from rats in which the dialysis membrane was not positioned exactly in the VH was discarded.
Drugs and chemicals
TAK-147 and E2020 were obtained from Takeda Chemical Industries Ltd, Osaka, Japan, and Eisai Co., Ltd, Tokyo, Japan, respectively. The drugs were dissolved in MC. The solutions were prepared freshly prior to each experiment. The administration volume was 1 ml kg−1 p.o. The HPLC standards ACh perchlorate and choline were purchased from Sigma (MO, U.S.A.). Isopropylhomocholine was from Eicom, Japan. The following HPLC monoamine standards were used: (−) epinephrine (L-adrenaline; Epi) and (−) NE (L-noradrenaline) were from Nacalai tesque Inc., Kyoto, Japan. 5-HT creatinine sulfate (3-[2-aminoethyl]-5-hydroxyindole creatinine sulfate complex) was from Wako Pure Chemical Industries Ltd, Osaka, Japan. The other standards were from Sigma: 3-methoxy-4-hydroxyphenylglycol (MHPG), 3,4-dihydroxyphenylacetic acid (DOPAC), normetanephrine HCl (DL-α-[aminomethyl]-4-hydroxy-3-methoxybenzenemethanol HCl), DA HCl (3,4-dihydroxyphenylethylamine HCl), 5-hydroxyindoleacetic acid (5-HIAA), (±)-isoproterenol HCl (1-[3′,4′-dihydroxyphenyl]-2-isopropylaminoethanol HCl), homovanillic acid (4-hydroxy-3-methoxyphenylacetic acid; HVA) and methoxytyramine HCl (3-methoxy-4-hydroxyphenethylamine).
Statistical analysis
The study included three main groups: group 1 – received MC (the vehicle for the drugs) and served as intact control for the following two groups that received 2 mg kg−1 p.o. of either TAK-147 (group 2) or E2020 (group 3). Statistically significant differences in drug effects, compared to the MC, prior to (pre) and following (post) the last dose on the 21st day, pre/post extracellular ACh or amines levels within each group (each two consequent postvalues reduced to one bin) and their variation with repetition were evaluated using multifactor analysis of variance (ANOVA) with repeated measures-unequal group size. The treatments were regarded as between-subject factor and the level at each point as within-subject factor. The data for the three groups in pre- or postperiod were firstly evaluated, and then the effect of each drug was compared to the other groups in case any significance was detected. Post hoc comparisons were performed by Tukey's test whenever ANOVA revealed a significant difference. The significance of the effects denote the main effect of each of TAK-147 and E2020 compared to MC, otherwise the comparison of both drugs with each other is referred to in the text. The level of significance was set at P<0.05. Extracellular ACh levels were expressed as fmol 20 μl−1, whereas the amines as pg 30 μl−1. All data are presented as means±s.e.m. The numbers of rats were seven, 10 and eight in MC, TAK-147 and E2020 groups, respectively, for all HPLC analysis.
Results
Effect of TAK-147 and E2020 on ACh level in the VH
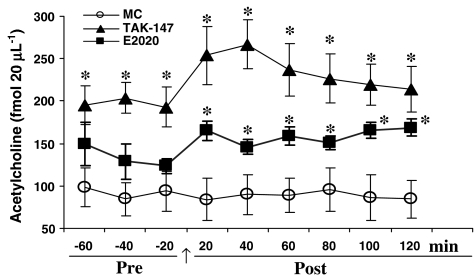
The basal level of ACh detected in the VH of intact rats prior to the last MC administration was 92.05±0.21.97 fmol 20 μl−1. This level was maintained at 88.08±23.76 fmol 20 μl−1 after MC administration throughout analysis.
Figure 1 shows the time course of changes in the level of ACh that were produced by 2 mg kg−1 TAK-147 and E2020. TAK-147 increased the level of ACh in the VH by 114% to 197.33±21.40 fmol 20 μl−1 on the 21st day before the last dose (F1,15=12, P<0.01). TAK-147 further increased (168%) the ACh level after the 21st dose to 236.15±29.18 fmol 20 μl−1 (F1,15=16.29, P<0.01). However, comparison of the post- and prevalues did not show significant change.
Figure 1.
Effect of TAK-147 (n=10) and E2020 (n=8) on acetylcholine level (fmol 20 μl−1) in dialysates from the VH. The drugs were injected at 2 mg kg−1 p.o. for 21 days. After establishment of a stable acetylcholine level (pre) on the 21st day, the last dose of either TAK-147 or E2020 was administered (arrow) and acetylcholine was collected and analyzed by HPLC every 20 min thereafter. Values are means±s.e.m. of each group. Data were analyzed by ANOVA-unequal group size with repeated measures followed by Tukey's post hoc test. Each point is compared to MC (n=7) group. *P<0.05.
Although E2020 increased (46%) the ACh on the 21st day prior to the last dose to 133.96±18.03 fmol 20 μl−1, the effect did not reach significant level. However, the level of ACh was significantly increased (80.7%) after the last dose to 159.13±8.16 fmol 20 μl−1 (F1,13=10.86, P<0.01). However, pre-/post comparison revealed significant change only at the last time bin, 100–120 min (F1,14=7.58, P<0.02). The effect of E2020 was lower than TAK-147 by 47% prior to (F1,16=5.28, P<0.05) and by 48% after the last dose (F1,16=6.32, P<0.05). Moreover, the group × repetition interaction of TAK-147 and E2020 was different following the last dose (F5,80=2.63, P<0.05), indicating different ACh-increasing pattern for both drugs. No significant effect of HPLC repetition was detected for TAK-147 or E2020 within the same group.
Effect of TAK-147 and E2020 on catecholamines level in the VH
Effect on the NE and its metabolites
The present results showed that the level of NE in the VH of intact rats prior to the last MC on the 21st day was 1.92±0.39 pg 30 μl−1. The level of NE was maintained after MC administration throughout analysis at 1.81±0.49 pg 30 μl−1.
Figure 2a shows that the level of NE in TAK-147 group (1.867±0.26 pg 30 μl−1) was not different from that of MC group. However, the level of NE was significantly increased after the last dose to 4.07±1.09 pg 30 μl−1. This value was higher by 125% than MC (F1,15=8.22, P<0.03) and by 118% compared to prevalue within the same group (F1,18=4.44, P<0.05). On the other hand, E2020 increased the level of NE to 3.13±0.49 pg 30 μl−1 prior to the last dose, but the effect was not significant. The last dose of E2020 increased (296%) the level of NE to 7.156±2.08 pg 30 μl−1 (F1,13=5.88, P<0.05). This level was significantly higher than the prevalue of the same group by 129% (F1,14=4.7, P<0.05). However, the effect of E2020 was not significantly different from TAK-147, but the dose × repetition interaction of E2020 was significantly different from MC (F5,65=3.7, P<0.01) and TAK-147 (F5,80=2.98, P<0.05), indicating that its pattern of increase is different from the other two groups.
Figure 2.
Effect of TAK-147 (n=10) and E2020 (n=8) on NE (a), Epi (b), 3-methoxy-4-hydroxy-phenylglycol (c) and normetanephrine (d) level in dialysates from the VH. The drugs were injected at 2 mg kg−1 p.o. for 21 days. After establishment of a stable level (pre) for each monoamine on the 21st day, the last dose of either TAK-147 or E2020 was administered (arrow) and the monoamines were collected and analyzed by HPLC every 30 min thereafter. The values are means±s.e.m. of each group. Data were analyzed by ANOVA-unequal group size with repeated measures followed by Tukey's post hoc test. Each point is compared to MC (n=7) group. *P<0.05.
The present results showed that the level of Epi in the VH was 1.91±0.18 pg 30 μl−1. The level of Epi was maintained at 1.36±0.16 pg 30 μl−1 for 180 min after MC. Figure 2b shows that treatment with TAK-147 increased the Epi to 8.61±2.15 pg 30 μl−1 (F1,15=9.98, P<0.01) and 7.96±1.68 pg 30 μl−1 (F1,15=10.92, P<0.01) before and after the last dose, respectively. The present results also showed that the level of Epi in E2020-treated rats was also increased to 6.73±0.93 pg 30 μl−1 before the last dose (F1,13=27.04, P<0.01) and to 6.38±0.69 pg 30 μl−1 after the last dose (F1,13=55.48, P<0.01). No difference was detected between TAK-147 and E2020 groups.
The level of MHPG in the VH of rats that received MC was 11.53±3.22 pg 30 μl−1, maintained at 10.93±4.09 pg 30 μl−1 for 180 min following MC. TAK-147 did not significantly increase MHPG prior to the last dose, and increased the level to 15.88±1.21 pg 30 μl−1 after the last dose, but the effect was significant only after 90 min (16.35±1150 pg 30 μl−1; F1,15=4.94, P<0.05). On the other hand, the highest level of MHPG was observed in the E2020 group (17.85±1.47 pg 30 μl−1). The effect of E2020 was significantly higher than MC (F1,13=7.4, P<0.05) and TAK-147 (F1,16=6.16, P<0.05). However, the effect of the last dose of E2020 decreased after 120 min and the MHPG value was not different from either MC or TAK-147 thereafter (Figure 2c).
The present results showed that the VH of rats that received MC contains 3.26±0.64 pg 30 μl−1 normetanephrine. The level was maintained at 2.41±0.46 pg 30 μl−1 following MC (Figure 2d). No significant effect was detected for either TAK-147 or E2020 on normetanephrine.
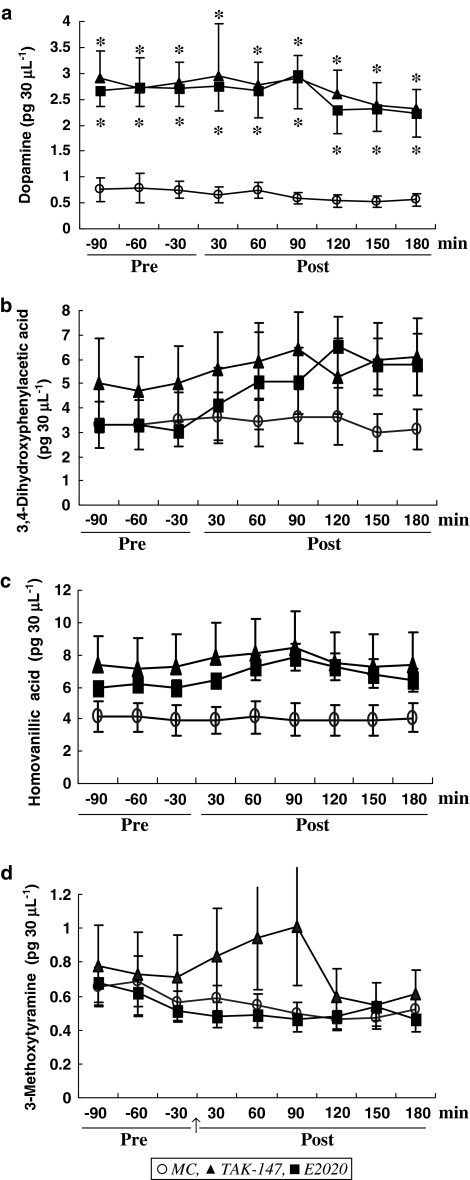
Effect on level of DA and its metabolites level in the VH
The level of DA in the VH was 0.77±0.23 pg 30 μl−1, maintained throughout the experiment period at 0.61±0.13 pg 30 μl−1 after MC. Figure 3a shows that TAK-147 increased DA before the last dose to 2.63±0.51 pg 30 μl−1 (F1,15=9.09, P<0.01) and after the last dose to 2.31±0.42 pg 30 μl−1 (F1,15=8.46, P<0.05). The results also showed that E2020 increased the level of DA to 2.632±0.341 pg 30 μl−1 on the 21st day (F1,13=20.59, P<0.01), which was further increased to 5.39±0.51 pg 30 μl−1 after the last dose (F1,13=12.76, P<0.01). No difference was detected between pre and post within the same group or TAK-147 and E2020.
Figure 3.
Effect of TAK-147 (n=10) and E2020 (n=8) on DA (a), DOPAC (b), methoxytyramine (c) and HVA (d) level in dialysates from the VH. *P<0.05 compared to MC (n=7). Other details are as in Figure 2.
The present results showed that the VH contains 3.37±1.01 pg 30 μl−1 DOPAC, maintained at 3.39±0.99 pg 30 μl−1 after MC on the 21st day. Although the level was slightly elevated by both TAK-147 and E2020, statistical analysis showed that the effects of both drugs were not significant (Figure 3b).
The level of HVA in the VH was 4.04±0.93 pg 30 μl−1. This level was maintained at 3.99±0.93 pg 30 μl−1 for 180 min after MC. The level of HVA was not significantly changed by either TAK-147 or E2020 in spite of an apparent increase by the last dose of TAK-147 for 30–90 min (Figure 3c).
The level of 3-methoxytyramine in the VH of MC-treated rats was 0.635±0.127 pg 30 μl−1 prior to MC, and maintained at 0.51±0.07 pg 30 μl−1 for 180 min thereafter. No significant effect was produced by either TAK-147 or E2020, in spite of the increase by TAK-147 at the 30–90 min period following the last dose (Figure 3d).
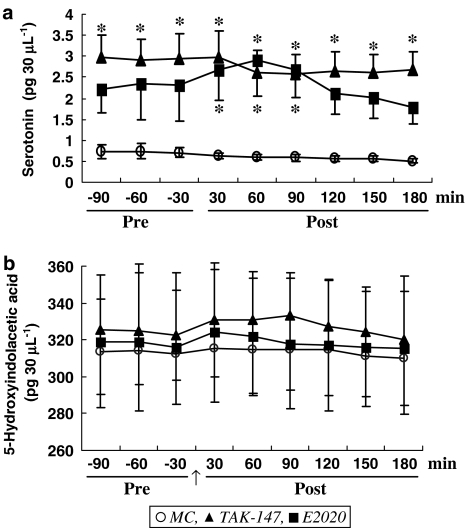
Effect of TAK-147 and E2020 on indoleamines level in the VH
The mean level of 5-HT detected in the VH was 0.73±0.16 pg 30 μl−1 before MC, and maintained at 0.58±0.07 pg 30 μl−1 after MC for 180 min. Figure 4a shows that TAK-147 significantly increased (F1,15=6.38, P<0.05) the level of 5-HT in the VH to 2.94±0.74 pg 30 μl−1. The level of 5-HT was not increased further after the last dose, but remained significantly higher than the corresponding level detected in MC (F1,15=4.74, P<0.05). Figure 4a also shows that E2020 nonsignificantly increased 5-HT prior to the last dose, but significantly increased it following the last dose to 2.41±0.61 pg 30 μl−1 (F1,13=9.14, P<0.01). The effect is possibly due to the increment obtained at 30–90 min.
Figure 4.
Effect of TAK-147 (n=10) and E2020 (n=8) on 5-HT (a) and 5-hydroxyindolacetic acid (b) level in dialysates from the VH. *P<0.05 compared to MC (n=7). Other details are as in Figure 2.
The present results also showed that the VH of control rats contains 313.15±18.42 pg 30 μl−1 5-HIAA. Neither TAK-147 nor E2020 did significantly change the level of 5-HIAA (Figure 4b).
Effect on metabolite/amine ratios
The respective ratios of DOPAC : DA, HVA : DA, MHPG : NE, 5-HIAA : 5-HT and Epi : NE in the VH of MC-treated rats were 5.7, 6.6, 8.0, 556.1 and 1.2 prior to the last dose, and 6.4, 7.4, 8.8, 577.7 and 1.1 following the last dose. TAK-147 decreased HVA : DA to 3.5 and 3.9 prior to (P<0.03) and following (P<0.05) the last dose. On the other hand, E2020 significantly decreased DOPAC : DA ratio to 1.5 prior to the last dose (P<0,02). Moreover, E2020 decreased the HVA : DA ratio to 2.9 and 3.7 prior to and following the last dose, respectively (P<0.03). TAK-147 and E2020 decreased MHPG : NE ratio only 180 min after the last dose to 5.7 and 4.0, respectively (P<0.05). E2020 decreased 5-HIAA : 5-HT ratio only at 90–180 min following the last dose to 237 (P<0.004). The present results revealed that prior to the last dose, TAK-147 and E2020 increased Epi : NE ratio to 6.5 (P<0.02) and 2.7 (P<0.05), respectively. Only TAK-147 increased the ratio after the last dose for 120 min to 5.5 (P<0.05).
Discussion
The management of the AD patients encompasses chronic prescription of the relevant drugs. AChEIs are the major drug class used in AD treatment. In our previous study (Hatip-Al-Khatib et al., 2004), we compared the effect of single administration of TAK-147 and E2020, and determined the p.o. threshold (5 mg kg−1) and subthreshold doses (2 mg kg−1) for the effect of both drugs on ACh level in the VH. However, we could not find in the literature any study of the effect of chronic or subchronic TAK-147 on the levels of ACh and monoamines in the VH. In this study, we investigated the effect of 21-day administration (subchronic) of the subthreshold dose of TAK-147, compared to E2020, on the ACh and monoamines levels in the VH.
The elevation of ACh level in the brain regions that are associated with memory is still one of the goals in AD treatment. The present results showed that both TAK-147 and E2020 increased the ACh. However, the effect of TAK-147 was higher than E2020 both prior to and following the last dose. The effect on ACh level could either be due to modification of the drugs activity on AChE by subchronic administration or possibly involves activities other than AChE inhibition, since TAK-147 : E2020 potency ratio for the effect of single administration on ACh level is 0.773 (Hatip-Al-Khatib et al., 2004) and the AChE inhibitory levels are 12 and 6.7 nM (Ogura et al., 2000) or 26.8 and 6.3 μmol kg−1 (Kosasa et al., 2000) for TAK-147 and E2020, respectively. The effect of TAK-147 possibly does not involve inhibition of high-affinity choline uptake or binding to nicotinic receptor, because TAK-147 has weak or no activity in this regard (Hirai et al., 1997). Several studies had investigated the effect of long duration administration of E2020. Our results showed that E2020 induced 46% increase of ACh level. The maximal increase obtained 20 min after the last dose was 97%. This rate is lower than the 2100% reported for the same dose administered for the same duration (Giacobini et al., 1996), possibly due to the different methods of calculation or analysis. The effect of chronic E2020 could involve 36–39% inhibition of AChE due to complete blockade of phosphorylation (Giacobini et al., 1996; Scali et al., 2002) or involvement of M2–M4 blockade. Moreover, the effects could also involve increased level of hippocampal non-α7-nAChRs nicotinic ACh receptors (Reid & Sabbagh, 2003). The present results suggest that long-term administration of a subthreshold dose of TAK-147 produced a greater increase of the ACh, a result different from that of single dose.
There is an abundance of evidence indicating that monoamines, in addition to ACh, are also involved in the pathogenesis of the AD and other dementic disorders. In the AD, amyloid deposition and Alzheimer neurofibrillary tangles occur not only in the cholinergic areas but also in the aminergic nuclei (Yang & Schmitt, 2001). In patients with AD, multiple changes indicate disturbed metabolism in the ACh, DA, NE and 5-HT systems (Gottfries, 1990). It has been reported that the monoaminergic presynaptic terminal density decreases in the dementic diseases (Gilman et al., 2004). Moreover, AD is a neurodegenerative disorder associated with a decline of not only the cognitive abilities but also with frequent manifestation of noncognitive symptoms (such as anxiety, depression, apathy, psychosis) and other conduct disorders that impair daily living and require treatment with antidepressants with serotonergic and noradrenergic properties (Schrag, 2004) alone or in combination with AD drugs like that of E2020 and sertraline combination (Finkel et al., 2004). In addition to the cognition, the noncognitive aspects of dementia, however, are also linked to 5-HT and DA rather than ACh, because those neurotransmitter systems most directly influence mood, emotional balance and psychosis, indicating a role of the aminergic system directly or by modulating the cholinergic activity. Moreover, it has been proposed that impairment of the cholinergic activity alone may not be sufficient to cause the marked changes in cognition and cortical activity that are typical for AD. In rats, lowered 5-HT or NE activity alone often produces only minor impairments in learning/memory tasks and does not block EEG activation. The same monoaminergic deficits, however, result in severe behavioral impairments, and reduce or abolish EEG activation when they occur in a brain already affected by lowered cholinergic activity. On the other hand, combined cholinergic–monoaminergic stimulation had been reported to be more effective in reversing behavioral impairments and EEG slowing in rats with multiple neurotransmitter deficiencies than cholinergic enhancement alone (Dringenberg, 2000). Accordingly, the effects of the AD drugs on the aminergic systems in addition to that on the cholinergic system are of value in affecting multiple deficits forming the hallmark of the AD pathology or appear concurrently with it.
Of the monoamines, NE activity is important for neuroprotection, and its deficiency could be involved in the mechanisms predisposing to the AD, especially in depressed AD patients (Hoogendijk et al., 1999). In the AD, there is a significant reduction in NE and HVA levels in the cerebrospinal fluid (Martignoni et al., 1991) and neuronal loss in the locus ceruleus. The reduction in NE concentration is correlated with cognitive impairment (Matthews et al., 2002). Inflammation and reduced forebrain NE are features of the AD that may interact to contribute to the degeneration of specific neural systems. It has been reported that NE deficiency eventually augments inflammatory responses to Abeta (Heneka et al., 2002). Experimental evidence suggests that cortical NE depletion due to degeneration of the locus ceruleus, a pathological hallmark of the AD, plays a permissive role in the development of inflammation in the AD (Galea et al., 2003). This is because NE decreases the inflammatory reaction observed in the AD by increasing peroxisome proliferator-activated receptor gamma (Klotz et al., 2003) and reducing cytokine expression in microglial, astroglial and brain endothelial cells (Feinstein et al., 2002). Moreover, ACh can stimulate the hippocampal NA release via muscarinic receptors (Kiss et al., 1999). The present results reveal that the VH content of NE is greater than DA and 5-H. Moreover, our results showed that TAK-147 and E2020 significantly increased NE after the last dose, a result in line with that reported for neostigmine (Kiss et al., 1999), suggesting a possible trigger of NE turnover rate and release by the last dose on multiple dosing. In addition to the ACh, TAK-147 activates the monoaminergic systems (Ishihara et al., 2000), and inhibits ligand binding at α-1, α-2 receptors (Hirai et al., 1997). The NE-increasing effect of TAK-147 could be via acceleration of the turnover rate and stimulation of hippocampal M1 and M2 muscarinic receptors (Hirai et al., 1997). The effect of at least E2020 on the NE is a central one because E2020 does not change the plasma concentration of NE (Masuda & Kawamura, 2003), and could be attributed to an increase of hippocampal non-α7 nAChR (Rogers & Hagan, 2001) leading to NE release (Dajas-Bailador et al., 2003). The increase by E2020 of NE agrees with the results reported for E2020 (Giacobini et al., 1996). However, the percentage increase of NE reported in this study produced by the last dose of E2020 (296%) is higher than the 100% NE increase reported for single subcutaneous 2 mg kg−1 E2020 (Giacobini et al., 1996). This difference is possibly due to the multiple oral administration of E2020 in our study compared to single subcutaneous route in the latter study, and could indicate increase of the effect by repetition of the doses. Moreover, the present results revealed that E2020-induced NE release is higher than that produced by TAK-147 prior to (63% vs no increase) and following the last dose (296 vs 125% increase). Moreover, the E2020-produced NE increase at the peak effect time 150 min after the last dose (304%) is also greater than TAK-147 (82%).
Epi is another monoamine that could also be involved in the AD, as Epi system interacts with the cholinergic one. It has been reported that stimulation of the hippocampal cholinergic system results in the elevation of plasma catecholamines and glucose in rats. Moreover, the plasma level of Epi during a fasting state in patients with dementia of Alzheimer type is significantly lower than that of nondementic subjects (Umegaki et al., 2000). The present results showed that the level of Epi in the VH is close to that detected for NE, and the mean Epi : NE ratio is one in MC group. TAK-147 and E2020 increased the Epi level. The effect of E2020 on Epi is probably produced centrally, because E2020 does not change the plasma concentration of Epi (Masuda & Kawamura, 2003). Moreover, our results showed that TAK-147 increased Epi : NE ratio more than E2020. The elevation of Epi and Epi : NE ratio by TAK-147 and E2020 could be important in the activity of both drug.
The dopaminergic activity is another candidate involved in the memory regulation. It has been reported that the marginal division (at the caudomedial border of the neostriatum in the brain of the rat, and links the limbic system and the basal nucleus of Meynert) plays an important role in the execution of digital working memory, and involved in AD pathology (Lehericy et al., 1994; Shu et al., 2002). Moreover, the dopaminergic activity is modified by the ACh. It has been reported that the ACh and DA act convergently on the nucleus accumbens circuit (Hikida et al., 2003). On the other hand, AChEIs are effective in the treatment of disorders other than the AD. The AChEIs have been reported to suppress both cocaine- and morphine-induced conditioned place preference and block the induction and persistence of cocaine-evoked hyperlocomotion (Hikida et al., 2003). Moreover, AChEIs are effective in treatment of attention-deficit/hyperactivity disorder (Spencer & Biederman, 2002) and management of symptoms of dementia accompanying patients with comorbid schizophrenia and dementia (Stryjer et al., 2003). In the neostriatum (caudate nucleus), loss of DA and increased HVA : DA ratio is correlated with the reduction in substantia nigra neurons in Lewy body dementia but not Parkinson's disease (Perry et al., 1993). In this study, TAK-147 increased DA to a similar extent produced by E2020. The effect of TAK-147 could mainly be due to inhibition of DA reuptake and/or acceleration of DA turnover, but not to activity on DA1 or DA2 receptors (Hirai et al., 1997). On the other hand, the effect of E2020 on DA is due to increase of DA release (Zhang et al., 2004). The dopaminergic properties of both TAK-147 and E2020 may be useful in the treatment of disorders involving dopaminergic systems in specific patients subgroup.
The serotonergic system, directly or indirectly, could affect the mental state. It has been reported that the cholinergic and serotonergic systems are involved in maintaining brain arousal (Abe et al., 2003). The serotonergic raphe nuclei with ascending projections to the forebrain is involved in frontotemporal dementia both with and without motor neuron disease (Yang & Schmitt, 2001). Moreover, it has been reported that blockade of 5-HT(1A) receptors attenuates the anterograde amnesia produced by muscarinic-receptor blockade (Misane & Ogren, 2003), and may be useful in the treatment of some aspects of attentional dysfunction in the AD (Balducci et al., 2003). 5-HT6 antagonism improves retention of water maze task in the rat (Rogers & Hagan, 2001). In patients with AD, 5-HT and melatonin levels are decreased in the cerebrospinal fluid during the progression of the AD neuropathology (Xu et al., 2002), and 5-HIAA is reduced in the temporal cortex (Perry et al., 1993). In this study, TAK-147 increased 5-HT, but nonsignificantly increased 5-HIAA, indicating that TAK-147 accelerates 5-HT turnover rate, but at an extent exceeding its metabolism. The effect of TAK-147 could also be due to inhibition of uptake of 5-HT (Hirai et al., 1997). We do not think the effect of TAK-147 is produced on 5-HT1 receptor because TAK-147 displays only weak activity on these receptors (Hirai et al., 1997). On the other hand, E2020 increased the 5-HT only for 90 min after the last dose, suggesting a lesser effect of E2020 on the 5-HT than TAK-147. The dual effect, especially by TAK-147, on 5-HT and NE in addition to the DA could be of great value in the treatment of diseases where these amines are decreased, like AD (Abe et al., 2003) and diffuse Lewy body (Ohara et al., 1998).
The present results showed a higher level of MHPG (NE), DOPAC, HVA (DA) and 5-HIAA (5-HT) than the corresponding amine, indicating a high metabolic rate for these amines in the VH of intact rats that received MC. Moreover, the results also showed high metabolic rate of NE (high MHPG level and higher mean MHPG : NE ratio) that is higher than another catecholamine, that is, DA (DOPAC : DA and HVA : DA). However, the results also showed that the 5-HIAA : 5-HT ratio is greater than the ratio values for catecholamines, indicating high metabolic rate of 5-HT in the VH. The metabolism of various monoamines is impaired to a different extent according to the type of dementia. 5-HIAA and HVA were proposed important in certain circadian symptoms, for example, the changes in sleep/wake rhythms that are rather often observed in dementia (Wallin et al., 1991). Moreover, 5-HIAA and HVA are decreased in the patients with vascular dementia and AD (Parnetti et al., 1992; Sjogren et al., 1998). In this study, TAK-147 and E2020 increased the level of the metabolites (especially MHPG). This activity may add to the aminergic effects of TAK-147 and E2020.
In the AD, not only an impairment of the monoaminergic systems but an overactivity of cerebral monoamineoxidase B has frequently been reported as well (Martignoni et al., 1991). In patients with Lewy body dementia, there is lower neuron counts, loss of DA and increased HVA : DA ratio in the neostriatum, possibly due to higher turnover ratios in the surviving neurons. Moreover, it has been reported that a subgroup of the Lewy body dementia patients experiencing hallucinations could be distinguished from the other categories by an increase in the 5-HIAA : 5-HT and serotonergic : cholinergic ratio measured in the frontal cortex (Perry et al., 1993). Moreover, it has been reported that 5-HT (precursor of melatonin) is stepwise depleted during the course of the AD, as indicated by the upregulated monoamine oxidase A mRNA activity and increased 5-HIAA : 5-HT ratio (Wu et al., 2003). In this study, TAK-147 decreased mainly the HVA : DA ratio. E2020 additionally decreased DOPAC : DA and 5-HIAA : 5-HT ratio. Both drugs decreased MHPG : NE ratio only at 180 min. The reduction in the ratio of monoamine metabolite to the relevant transmitter and subsequent reduction of free radical production by either TAK-147 or E2020 could be involved in the neuroprotective activity displayed by both drugs, treatment of cohort psychiatric disorders and may suggest extension of the therapeutic property of both drugs to include dementia other than the AD, for example, Lewy body dementia. The present results showed that Epi : NE ratio was increased by both drugs, especially the TAK-147. The implication of this effect in the clinical effects of TAK-147 and E2020, if any, remains to be clarified.
In conclusion, the present results showed that 21-day treatment with 2 mg kg−1 p.o. TAK-147 increased ACh, Epi and DA. TAK-147 additionally increased 5-HT, whereas E2020 increased MHPG. The last dose triggered the NE-increasing effect of both drugs, and 5-HT-increasing effect of E2020 on 5-HT. Moreover, TAK-147 and E2020 decreased HVA : DA ratio, but increased Epi : NE ratio. E2020 additionally decreased DOPAC : DA prior to, and 5-HIAA : 5-HT ratio following, the last dose. TAK-147 could differentially be more useful in cases where ACh or 5-HT are reduced, whereas E2020 in cases involving reduced MHPG. Both drugs could be of value in cases involving reduced DA, NE and Epi. Stimulation of monoaminergic activity in conjunction with the AChE activity may provide an effective treatment option for AD and the accompanying psychiatric disorders.
Acknowledgments
We thank Takeda Chemical Industries Ltd, Osaka, Japan and Eisai Co., Ltd, Tokyo, Japan for generous supply of TAK-147 and E2020, respectively. The present work was supported by the Advanced Material Institute of Fukuoka University, Japan.
Abbreviations
- ACh
acetylcholine
- DA
dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- Epi
epinephrine
- E2020
donepezil
- 5-HT
serotonin
- 5-HIAA
5-hydroxyindoleacetic acid
- HVA
homovanillic acid
- MHPG
3-methoxy-4-hydroxyphenylglycol
- NE
norepinephrine
- zanapezil
TAK-147 (3-[1benzylpiperdin-4-yl]-1-(2,3,4,5-tetrahydro-1 H-1-benzazepin-8-yl)
References
- ABE Y., AOYAGI A., HARA T., ABE K., YAMAZAKI R., KUMAGAE Y., NARUTO S., KOYAMA K., MARUMOTO S., TAGO K., TODA N., TAKAMI K., YAMADA N., ORI M., KOGEN H., KANEKO T. Pharmacological characterization of RS-1259, an orally active dual inhibitor of acetylcholinesterase and serotonin transporter, in rodents: possible treatment of Alzheimer's disease. J. Pharmacol. Sci. 2003;93:95–105. doi: 10.1254/jphs.93.95. [DOI] [PubMed] [Google Scholar]
- BALDUCCI C., NURRA M., PIETROPOLI A., SAMANIN R., CARLI M. Reversal of visual attention dysfunction after AMPA lesions of the nucleus basalis magnocellularis (NBM) by the cholinesterase inhibitor donepezil and by a 5-HT1A receptor antagonist WAY 100635. Psychopharmacology (Berlin) 2003;167:28–36. doi: 10.1007/s00213-002-1385-7. [DOI] [PubMed] [Google Scholar]
- BARNES C.A., MELTZER J., HOUSTON F., ORR G., MCGANN K., WENK G.L. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- BERTHIER M.L., PUJOL J., GIRONELL A., KULISEVSKY J., DEUS J., HINOJOSA J., SORIANO-MAS C. Beneficial effect of donepezil on sensorimotor function after stroke. Am. J. Phys. Med. Rehabil. 2003;82:725–729. doi: 10.1097/01.PHM.0000083668.48396.84. [DOI] [PubMed] [Google Scholar]
- BRUERA E., STRASSER F., SHEN L., PALMER J.L., WILLEY J., DRIVER L.C., BURTON A.W. The effect of donepezil on sedation and other symptoms in patients receiving opioids for cancer pain: a pilot stud. J. Pain Symptom Manage. 2003;26:1049–1054. doi: 10.1016/s0885-3924(03)00332-4. [DOI] [PubMed] [Google Scholar]
- CHEN Z., XU A.J., LI R., WEI E.Q. Reversal of scopolamine-induced spatial memory deficits in Morris water maze by TAK-147. Acta Pharmacol. Sin. 2002;19:27–30. [PubMed] [Google Scholar]
- DAJAS-BAILADOR F.A., HEIMALA K., WONNACOTT S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol. Pharmacol. 2003;64:1217–1226. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- DRINGENBERG H.C. Alzheimer's disease: more than a ‘cholinergic disorder' – evidence that cholinergic–monoaminergic interactions contribute to EEG slowing and dementia. Behav. Brain Res. 2000;115:235–249. doi: 10.1016/s0166-4328(00)00261-8. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN D.L., HENEKA M.T., GAVRILYUK V., DELLO RUSSO C., WEINBERG G., GALEA E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- FINKEL S.I., MINTZER J.E., DYSKEN M., KRISHNAN K.R., BURT T., MCRAE T. A randomized, placebo controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer's disease in outpatients treated with donepezil. Int. J. Geriatr. Psychiatry. 2004;19:9–18. doi: 10.1002/gps.998. [DOI] [PubMed] [Google Scholar]
- GALEA E., HENEKA M.T., RUSSO D.C., FEINSTEIN D.L. Intrinsic regulation of brain inflammatory responses. Cell Mol. Neurobiol. 2003;23:625–635. doi: 10.1023/A:1025084415833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIACOBINI E., ZHU X.D., WILLIAMS E., SHERMAN K.A. The effect of the selective reversible acetylcholinesterase inhibitor E2020 on extracellular acetylcholine and biogenic amine levels in rat cortex. Neuropharmacology. 1996;35:205–211. doi: 10.1016/0028-3908(95)00157-3. [DOI] [PubMed] [Google Scholar]
- GILMAN S., KOEPPE R.A., LITTLE R., AN H., JUNCK L., GIORDANI B., PERSAD C., HEUMANN M., WERNETTE K. Striatal monoamine terminals in Lewy body dementia and Alzheimer's disease. Ann. Neurol. 2004;55:774–780. doi: 10.1002/ana.20088. [DOI] [PubMed] [Google Scholar]
- GOTTFRIES C.G. Neurochemical aspects on aging and diseases with cognitive impairment. J. Neurosci. Res. 1990;27:541–547. doi: 10.1002/jnr.490270415. [DOI] [PubMed] [Google Scholar]
- HATIP-AL-KHATIB I., ARAI T., EGASHIRA N., IWASAKI K., FUJIWARA M. Comparison of the effect of TAK-147 (Zanapezil) and E-2020 (Donepezil) on extracellular acetylcholine level and blood flow in the ventral hippocampus of freely moving rats. Brain Res. 2004;1012:169–176. doi: 10.1016/j.brainres.2004.03.067. [DOI] [PubMed] [Google Scholar]
- HAYSLETT R.L., TIZABI Y. Effects of donepezil on DOI-induced head twitch response in mice: implications for Tourette syndrome. Pharmacol. Biochem. Behav. 2003;76:409–415. doi: 10.1016/j.pbb.2003.08.015. [DOI] [PubMed] [Google Scholar]
- HENEKA M.T., GALEA E., GAVRILUYK V., DUMITRESCU-OZIMEK L., DAESCHNER J., O'BANION M.K., WEINBERG G., KLOCKGETHER T., FEINSTEIN D.L. Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer's disease. J. Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIKIDA T., KITABATAKE Y., PASTAN I., NAKANISHI S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAI K., KATO K., NAKAYAMA T., HAYAKO H., ISHIHARA Y., GOTO G., MIYAMOTO M. Neurochemical effects of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1H-1-benzazepin-8-yl)-1-propanone fumarate (TAK-147), a novel acetylcholinesterase inhibitor in rats. J. Pharmacol. Exp. Ther. 1997;280:1261–1269. [PubMed] [Google Scholar]
- HOOGENDIJK W.J., FEENSTRA M.G., BOTTERBLOM M.H., GILHUIS J., SOMMER I.E., KAMPHORST W., EIKELENBOOM P., SWAAB D.F. Increased activity of surviving locus ceruleus neurons in Alzheimer's disease. Ann. Neurol. 1999;45:82–91. doi: 10.1002/1531-8249(199901)45:1<82::aid-art14>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- ISHIHARA Y., GOTO G., MIYAMOTO M. Central selective acetylcholinesterase inhibitor with neurotrophic activity: structure–activity relationships of TAK-147 and related compounds. Curr. Med. Chem. 2000;7:341–354. doi: 10.2174/0929867003375272. [DOI] [PubMed] [Google Scholar]
- ISOMA K., ISHIKAWA M., OHTA M., OGAWA Y., HASEGAWA H., KOHDA T., KAMEI J. Effects of T-82, a new quinoline derivative, on cholinesterase activity and extracellular acetylcholine concentration in rat brain. Jpn. J. Pharmacol. 2002;88:206–212. doi: 10.1254/jjp.88.206. [DOI] [PubMed] [Google Scholar]
- KATO K., HAYAKO H., ISHIHARA H., MARUI Y., IWANE M., MIYAMOTO M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci. Lett. 1999;260:5–8. doi: 10.1016/s0304-3940(98)00943-4. [DOI] [PubMed] [Google Scholar]
- KISS J.P., VIZI E.S., WESTERINK B.H. Effect of neostigmine on the hippocampal noradrenaline release: role of cholinergic receptors. Neuroreport. 1999;10:81–86. doi: 10.1097/00001756-199901180-00016. [DOI] [PubMed] [Google Scholar]
- KLOTZ L., SASTRE M., KREUTZ A., GAVRILYUK V., KLOCKGETHER T., FEINSTEIN D.L., HENEKA M.T. Noradrenaline induces expression of peroxisome proliferator activated receptor gamma (PPARgamma) in murine primary astrocytes and neurons. J. Neurochem. 2003;86:907–916. doi: 10.1046/j.1471-4159.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- KOSASA T., KURIYA Y., MATSUI K., YAMANISHI Y. Inhibitory effect of orally administered donepezil hydrochloride (E2020), a novel treatment for Alzheimer's disease, on cholinesterase activity in rats. Eur. J. Pharmacol. 2000;389:173–179. doi: 10.1016/s0014-2999(99)00876-6. [DOI] [PubMed] [Google Scholar]
- LEHERICY S., BRANDEL J.P., HIRSCH E.C., ANGLADE P., VILLARES J., SCHERMAN D., DUYCKAERTS C., JAVOY-AGID F., AGID Y. Monoamine vesicular uptake sites in patients with Parkinson's disease and Alzheimer's disease, as measured by tritiated dihydrotetrabenazine autoradiography. Brain Res. 1994;659:1–9. doi: 10.1016/0006-8993(94)90856-7. [DOI] [PubMed] [Google Scholar]
- MARTIGNONI E., BONO G., BLANDINI F., SINFORIANI E., MERLO P., NAPPI G. Monoamines and related metabolite levels in the cerebrospinal fluid of patients with dementia of Alzheimer type. Influence of treatment with L-deprenyl. J. Neural Transm-Park. 1991;3:15–25. doi: 10.1007/BF02251133. [DOI] [PubMed] [Google Scholar]
- MASUDA Y., KAWAMURA A. Acetylcholinesterase inhibitor (donepezil hydrochloride) reduces heart rate variability. J. Cardiovasc. Pharmacol. 2003;41 Suppl 1:S67–S71. [PubMed] [Google Scholar]
- MATTHEWS K.L., CHEN C.P., ESIRI M.M., KEENE J., MINGER S.L., FRANCIS P.T. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry. 2002;51:407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- MISANE I., OGREN S.O. Selective 5-HT1A antagonists WAY 100635 and NAD-299 attenuate the impairment of passive avoidance caused by scopolamine in the rat. Neuropsychopharmacology. 2003;28:253–264. doi: 10.1038/sj.npp.1300024. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO M., TAKAHASHI H., KATO K., HIRAI K., ISHIHARA Y., GOTO G. Effects of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1-H-1-benzazepin-8-yl)-1-propanone fumarate (TAK-147), a novel acetylcholinesterase inhibitor, on impaired learning and memory in animal models. J. Pharmacol. Exp. Ther. 1996;277:1292–1304. [PubMed] [Google Scholar]
- MOREY C.E., CILO M., BERRY J., CUSICK C. The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Injury. 2003;17:809–815. doi: 10.1080/0269905031000088586. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA T., TAKAHASHI H., MIYAMOTO M., GOTO G., NAGAI Y. Effect of TAK-147, a novel AChE inhibitor, on cerebral energy metabolism. Neurobiol. Aging. 1996;17:849–857. doi: 10.1016/s0197-4580(96)00077-2. [DOI] [PubMed] [Google Scholar]
- NOBILI F., VITALI P., CANFORA M., GIRTLER N., DE LEO C., MARIANI G., PUPI A., RODRIGUEZ G. Effects of long-term Donepezil therapy on rCBF of Alzheimer's patients. Clin. Neurophysiol. 2002;113:1241–1248. doi: 10.1016/s1388-2457(02)00110-4. [DOI] [PubMed] [Google Scholar]
- OGURA H., KOSASA T., KURIYA Y., YAMANISHI Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find. Exp. Clin. Pharmacol. 2000;22:609–613. doi: 10.1358/mf.2000.22.8.701373. [DOI] [PubMed] [Google Scholar]
- OGURA H., KOSASA T., KURIYA Y., YAMANISHI Y. Central and peripheral activity of cholinesterase inhibitors as revealed by yawning and fasciculation in rats. Eur. J. Pharmacol. 2001;415:157–164. doi: 10.1016/s0014-2999(01)00824-x. [DOI] [PubMed] [Google Scholar]
- OHARA K., KONDO N., OHARA K. Changes of monoamines in post-mortem brains from patients with diffuse Lewy body disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:311–317. doi: 10.1016/s0278-5846(98)00006-2. [DOI] [PubMed] [Google Scholar]
- PARNETTI L., GAITI A., REBOLDI G.P., SANTUCCI C., MECOCCI P., BRUNETTI M., CADINI D., SENIN U. CSF monoamine metabolites in old age dementias. Mol. Chem. Neuropathol. 1992;16:143–157. doi: 10.1007/BF03159966. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain Stereotaxic Coordinates 1998New York: Academic Press; 4th edn [Google Scholar]
- PERRY E.K., MARSHALL E., THOMPSON P., MCKEITH I.G., COLLERTON D., FAIRBAIRN A.F., FERRIER I.N., IRVING D., PERRY R.H. Monoaminergic activities in Lewy body dementia: relation to hallucinosis and extrapyramidal features. J. Neural Transm.-Park. 1993;6:167–177. doi: 10.1007/BF02260919. [DOI] [PubMed] [Google Scholar]
- REID R.T., SABBAGH M.N. Effects of donepezil treatment on rat nicotinic acetylcholine receptor levels in vivo and in vitro. J. Alzheimer's Dis. 2003;5:429–436. doi: 10.3233/jad-2003-5602. [DOI] [PubMed] [Google Scholar]
- ROGERS D.C., HAGAN J.J. 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology (Berlin) 2001;158:114–119. doi: 10.1007/s002130100840. [DOI] [PubMed] [Google Scholar]
- SCALI C., CASAMENTI F., BELLUCCI A., COSTAGLI C., SCHMIDT B., PEPEU G. Effect of subchronic administration of metrifonate, rivastigmine and donepezil on brain acetylcholine in aged F344 rats. J. Neural Transm. 2002;109:1067–1080. doi: 10.1007/s007020200090. [DOI] [PubMed] [Google Scholar]
- SCHRAG A. Psychiatric aspects of Parkinson's disease-an update. J. Neurol. 2004;251:795–804. doi: 10.1007/s00415-004-0483-3. [DOI] [PubMed] [Google Scholar]
- SHU S.Y., WU Y.M., BAO X.M., WEN Z.B., HUANG F.H., LI S.X., FU Q.Z., NING Q. A new area in the human brain associated with learning and memory: immunohistochemical and functional MRI analysis. Mol. Psychiatry. 2002;7:1018–1022. doi: 10.1038/sj.mp.4001155. [DOI] [PubMed] [Google Scholar]
- SJOGREN M., MINTHON L., PASSANT U., BLENNOW K., WALLIN A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer's disease. Neurobiol. Aging. 1998;19:379–384. doi: 10.1016/s0197-4580(98)00086-4. [DOI] [PubMed] [Google Scholar]
- SPENCER T., BIEDERMAN J. Non-stimulant treatment for attention-deficit/hyperactivity disorder. J. Atten. Disord. 2002;6 Suppl 1:S109–S119. doi: 10.1177/070674370200601s13. [DOI] [PubMed] [Google Scholar]
- STRYJER R., STROUS R.D., BAR F., WERBER E., SHAKED G., BUHIRI Y., KOTLER M., WEIZMAN A., RABEY J.M. Beneficial effect of donepezil augmentation for the management of comorbid schizophrenia and dementia. Clin. Neuropharmacol. 2003;26:12–17. doi: 10.1097/00002826-200301000-00004. [DOI] [PubMed] [Google Scholar]
- UMEGAKI H., IKARI H., NAKAHATA H., YOSHIMURA J., ENDO H., YAMAMOTO T., IGUCHI A. Low plasma epinephrine in elderly female subjects of dementia of Alzheimer type. Brain Res. 2000;858:67–70. doi: 10.1016/s0006-8993(99)02440-3. [DOI] [PubMed] [Google Scholar]
- WALLIN A., CARLSSON A., EKMAN R., GOTTFRIES C.G., KARLSSON I., SVENNERHOLM L., WIDERLOV E. Hypothalamic monoamines and neuropeptides in dementia. Eur. Neuropsychopharmacol. 1991;1:165–168. doi: 10.1016/0924-977x(91)90718-a. [DOI] [PubMed] [Google Scholar]
- WILKINSON D., DOODY R., HELME R., TAUBMAN K., MINTZER J., KERTESZ A., PRATT R.D. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61:479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- WU Y.H., FEENSTRA M.G., ZHOU J.N., LIU R.Y., TORANO J.S., VAN KAN H.J., FISCHER D.F., RAVID R., SWAAB D.F. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab. 2003;88:5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- XU A., CHEN Z., YANAI K., HUANG Y., WEI E. Effect of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2,3,4,5-tetrahydro-1H-1-benzazepin-8-yl)-1-propanone fumarate, a novel acetylcholinesterase inhibitor, on spatial cognitive impairment induced by chronic cerebral hypoperfusion in rats. Neurosci. Lett. 2002;331:33–36. doi: 10.1016/s0304-3940(02)00830-3. [DOI] [PubMed] [Google Scholar]
- YANG Y., SCHMITT H.P. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. (Berlin) 2001;101:256–270. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- ZHANG L., ZHOU F.M., DANI J.A. Cholinergic drugs for Alzheimer's disease enhance in vitro dopamine release. Mol. Pharmacol. 2004;66:538–544. doi: 10.1124/mol.104.000299. [DOI] [PubMed] [Google Scholar]