Abstract
The muscarinic heteroreceptors modulating noradrenaline release in atria, urinary bladder and vas deferens were previously studied in mice in which the M2 or the M4 muscarinic receptor genes had been disrupted. These experiments showed that these tissues possessed both M2 and non-M2 heteroreceptors. The analysis was now extended to mice in which either the M3, both the M2 and the M3, or both the M2 and the M4 genes had been disrupted (M3-knockout, M2/3-knockout and M2/4-knockout). Tissues were preincubated with 3H-noradrenaline and then stimulated electrically (20 pulses per 50 Hz).
In wild-type atria, carbachol (0.01–100 μM) decreased the electrically evoked tritium overflow by maximally 60–78%. The maximum inhibition of carbachol was reduced to 57% in M3-knockout and to 23% in M2/4-knockout atria. Strikingly, the effect of carbachol was abolished in M2/3-knockout atria.
In wild-type bladder, carbachol (0.01–100 μM) reduced the evoked tritium overflow by maximally 57–71%. This effect remained unchanged in the M3-knockout, but was abolished in the M2/4-knockout bladder.
In wild-type vas deferens, carbachol (0.01–100 μM) reduced the evoked tritium overflow by maximally 34–48%. The maximum inhibition of carbachol was reduced to 40% in the M3-knockout and to 18% in the M2/4-knockout vas deferens.
We conclude that the postganglionic sympathetic axons of mouse atria possess M2 and M3, those of the urinary bladder M2 and M4, and those of the vas deferens M2, M3 and M4 release-inhibiting muscarinic receptors.
Keywords: Presynaptic muscarinic receptors, sympathetic axon terminals, 3H-noradrenaline, M3-knockout, M2/3-knockout, M2/4-knockout, mouse atria, mouse urinary bladder, mouse vas deferens, heterogeneity
Introduction
Ever since the discovery of the M1–M5 muscarinic receptor subtypes, their role in the function of specific cells has been the subject of intense research. Among these cells are postganglionic sympathetic neurons where presynaptic muscarinic receptors (often called muscarinic heteroreceptors) either facilitate or inhibit action potential-evoked release of noradrenaline (see Fuder & Muscholl, 1995). Initial studies based on the use of antagonists endowed with a limited degree of receptor subtype selectivity suggested that the noradrenaline release-facilitating receptors may represent M1 receptors (Fuder & Muscholl, 1995). The more prominent muscarinic receptors mediating inhibition of transmitter release, however, often escaped clear identification, although M2 receptors seemed to play a predominant role (Fuder & Muscholl, 1995). The main reason for this uncertainty was the lack of muscarinic agonists or antagonists endowed with a high degree of receptor subtype selectivity (Caulfield & Birdsall, 1998; Wess, 2004).
Genetically engineered mice in which specific muscarinic receptor genes (M1–M5) have been disrupted represent valuable novel tools for identifying the muscarinic receptor subtype(s) involved in specific physiological functions (see Wess, 2004). We have recently used a set of muscarinic receptor mutant mice to identify the presynaptic muscarinic heteroreceptors present on postganglionic sympathetic neurons (Trendelenburg et al., 2003a). Using mice that lacked either the M2 or the M4 receptor gene (M2-knockout and M4-knockout, respectively), we found that the muscarinic heteroreceptors displayed an unexpected heterogeneity in the heart atria, urinary bladder and vas deferens. In all the three tissues, the sympathetic axons possessed both M2 and non-M2 release-inhibiting muscarinic receptors. The non-M2 heteroreceptors in the atria and bladder remained unknown, whereas those in the vas deferens were tentatively identified as M4 (Trendelenburg et al., 2003a).
We have now extended this analysis to mice lacking either M3 (M3-knockout; Yamada et al., 2001), both M2 and M3 (M2/3-knockout; Struckmann et al., 2003) or both M2 and M4 muscarinic receptors (M2/4-knockout; Zhang et al., 2002a). The new findings derived from the present study allow a more accurate identification of the muscarinic heteroreceptors involved in inhibiting noradrenaline release in different peripheral tissues of the mouse.
Methods
Tissues and superfusion
The generation of M3-knockout mice (genetic background: 129SvEv × CF1) as well as M2/3- and M2/4-knockout mice (genetic background: 129J1 × 129SvEv × CF1) has been described previously (Yamada et al., 2001; Zhang et al., 2002a; Struckmann et al., 2003). Age- and sex-matched wild-type mice of the corresponding genetic background were used as controls. Genotyping was carried out by PCR analysis of mouse tail DNA. The mice (male) were killed by cervical dislocation when aged >2 months. From each animal 6–8 pieces of the atria, 12–15 pieces of the urinary bladder or 8–12 pieces of the vas deferens were obtained. Tissue pieces were preincubated in 1 ml medium (see below) containing 0.2 μM 3H-noradrenaline for 30 min at 37°C and then placed in 12 superfusion chambers between platinum electrodes, one piece per chamber, where they were superfused with 3H-noradrenaline-free medium at a rate of 1.2 ml min−1. Successive 2-min samples of the superfusate were collected from t=50 min onwards (t=0 min being the start of superfusion). At the end of experiments, tissues were dissolved and tritium was determined in superfusate samples and tissues.
The superfusion medium contained (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11, ascorbic acid 0.57, disodium EDTA 0.03 and desipramine 0.001. The medium for preincubation with 3H-noradrenaline contained no desipramine and only 0.2 mM CaCl2 (Limberger et al., 1992).
Protocol
There were seven periods of electrical stimulation. Each stimulation period consisted of rectangular pulses of 1 ms width and 47 V cm−1 voltage drop between the electrodes of each chamber, yielding a current strength of 80 mA. The first stimulation period (180 pulses at 3 Hz) was delivered at t=30 min and was not used for determination of tritium overflow. The subsequent stimulation periods (S1–S6) were applied at t=54, 72, 90, 108, 126 and 144 min and consisted of 20 pulses at 50 Hz. Carbachol was introduced at increasing concentrations after S1, 12 min before S2, S3, S4, S5 and S6. The muscarinic receptor antagonists methoctramine and pirenzepine were present throughout superfusion at a fixed concentration (M3-knockout vas deferens only).
Evaluation
The outflow of tritium was calculated as a fraction of the tritium content of the tissue at the onset of the respective collection period (fractional rate; min−1). The overflow elicited by electrical stimulation was calculated as the difference ‘total tritium outflow during and after stimulation' minus ‘basal outflow', and was then expressed as a percentage of the tritium content of the tissue at the time of stimulation (see Trendelenburg et al., 1997).
For further evaluation, Sn/S1 overflow ratios were calculated. Overflow ratios obtained in the presence of carbachol, added after S1, were also calculated as a percentage of the corresponding ratio in controls in which no drug was added after S1. Effects of drugs on basal tritium outflow were evaluated similarly (Trendelenburg et al., 1997).
Concentration–response data for carbachol given alone were evaluated by sigmoid curve fitting (Equation (25) of Waud, 1976). This yielded the Emax (maximal effect) of carbachol and its EC50 (concentration causing a half-maximal effect) in the absence of antagonist. Sigmoids could not be fitted to concentration–response data for carbachol in the presence of antagonists (M3-knockout vas deferens). Therefore, for determination of apparent antagonist pKd values (negative logarithms of the apparent Kd), carbachol EC50 values in the presence of antagonist were interpolated from the nearest points of the respective concentration–response curves, assuming that the Emax of the agonist had not changed; the pKd value was then calculated from the EC50 increase (Equation (4) of Furchgott, 1972). The pKd values are apparent because only one antagonist concentration was used and the competitive character of the interaction was not verified.
Results are expressed as arithmetic means±s.e.m. (estimates±s.e. defined by Waud (1976), in the case of Emax values of carbachol). Groups were tested for significant differences with the Mann–Whitney test with Bonferroni correction. P<0.05 was taken the as limit of statistical difference. n represents the number of tissue pieces.
Drugs
The following drugs were used: (−)-[ring-2,5,6-3H]noradrenaline, specific activity 51.8–70.7 Ci mmol−1 (NEN, Köln, Germany), carbachol chloride, desipramine HCl, methoctramine 4 HCl and pirenzepine 2 HCl (Sigma, Deisenhofen, Germany). Drugs were dissolved in distilled water.
Results
In a previous study, we used M2 and M4 receptor single-knockout mice to characterize the presynaptic muscarinic heteroreceptors on sympathetic nerve endings of segments from atria, urinary bladder and vas deferens (Trendelenburg et al., 2003a). In the present study, we extended this analysis by carrying out analogous experiments with atria, urinary bladders and vasa deferentia from M3- and M2/4-knockout mice as well as atria from M2/3-knockout mice and their corresponding wild-type controls. Vesicular noradrenaline stores were labelled with 3H-noradrenaline. Short bursts of 20 pulses at 50 Hz were used for stimulation, conditions with little, if any, development of α2-autoinhibition and optimal for the characterization of presynaptic heteroreceptors (see Starke, 1987; Schlicker & Göthert, 1998; Trendelenburg et al., 2003a). In the present study, both basal efflux and evoked tritium overflow (S1) were similar to values obtained previously in NMRI, M2-knockout, M4-knockout and their corresponding wild-type control mice (data not shown; Wahl et al., 1996; Trendelenburg et al., 1999, 2003a). In control experiments without carbachol the overflow peaks were similar from S1 to S6, giving Sn/S1 ratios close to unity (data not shown; compare Wahl et al., 1996; Trendelenburg et al., 1999, 2003a). The antagonists pirenzepine and methoctramine, when present throughout superfusion, did not change the stimulation-evoked overflow of tritium (S1; M3-knockout vas deferens). Neither carbachol nor the antagonists had any effect on basal tritium outflow (data not shown).
Carbachol reduced the evoked overflow of tritium with very similar concentration–response curves in atria from M3-wild-type, M2/4-wild-type and M2/3-wild-type mice (Figure 1), and these curves were also very similar to our previous curves in atria from NMRI, M2-wild-type and M4-wild-type mice (Trendelenburg et al., 2003a) with values from 60 to 78% inhibition of transmitter release (Trendelenburg et al. (2003a) and present study taken together). The same was true for urinary bladders (Emax values from 57 to 71% inhibition) and vasa deferentia (Emax values from 34 to 48% inhibition; Figures 2 and 3; Trendelenburg et al., 2003a).
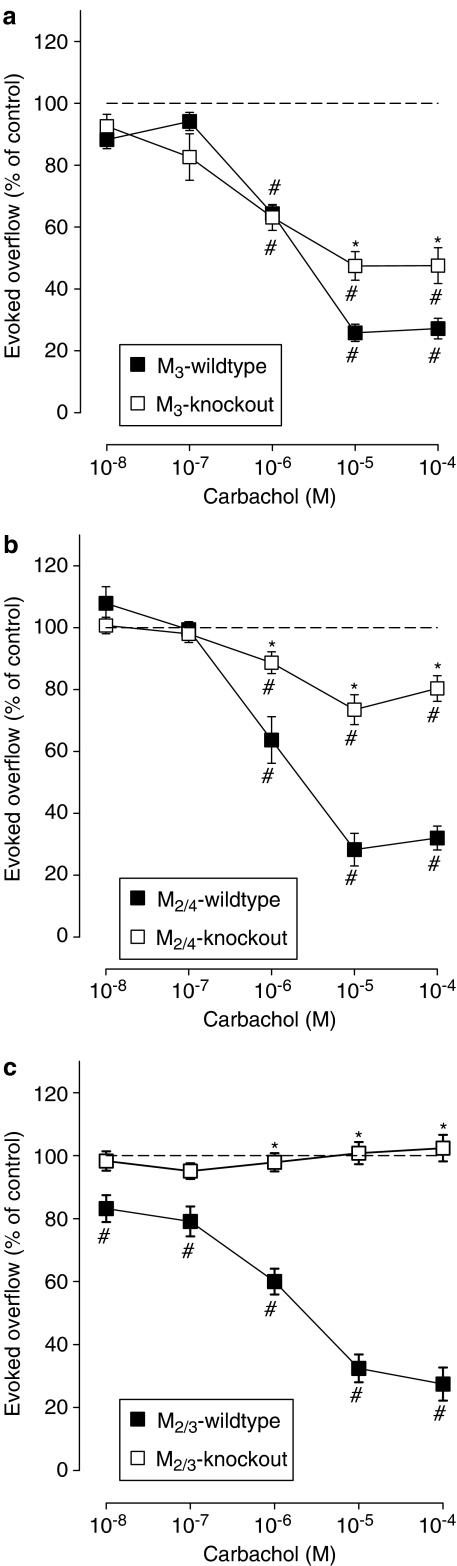
Figure 1.
Effect of carbachol on the evoked overflow of tritium from atria of M3-wild-type or M3-knockout (a), M2/4-wild-type or M2/4-knockout (b), and M2/3-wild-type or M2/3-knockout (c) mice. After preincubation with 3H-noradrenaline, tissues were superfused and stimulated six times by 20 pulses at 50 Hz (S1–S6). Carbachol was added at increasing concentrations (abscissae) before S2–S6. Ordinates, evoked overflow of tritium, calculated from Sn/S1 ratios and expressed as a percentage of the corresponding control (no carbachol). Means±s.e.m. from n=6–14 tissue pieces. Significant differences from corresponding control (no carbachol): #P<0.05. Significant differences from corresponding wild type: *P<0.05.
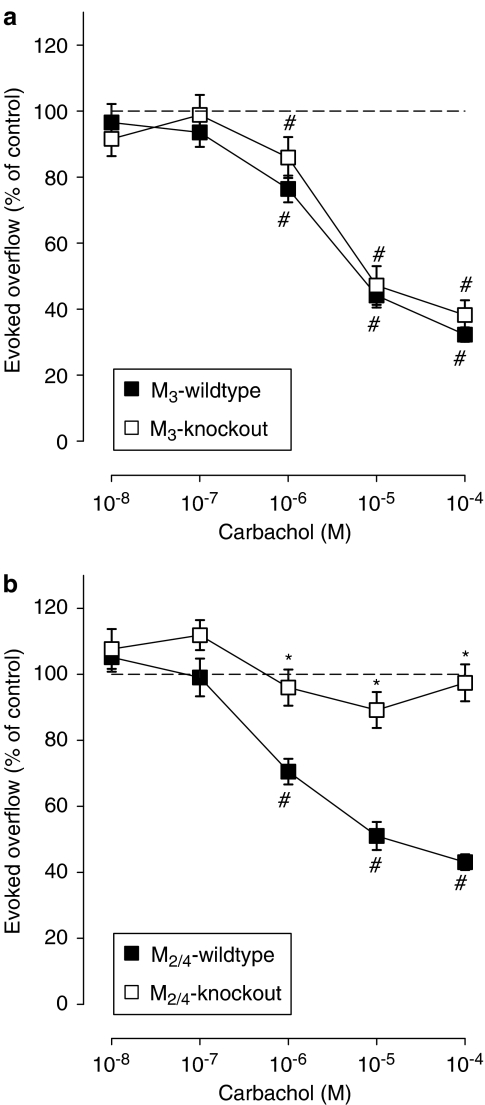
Figure 2.
Effect of carbachol on the evoked overflow of tritium from urinary bladder of M3-wild-type or M3-knockout (a) and M2/4-wild-type or M2/4-knockout (b) mice. After preincubation with 3H-noradrenaline, tissues were superfused and stimulated six times by 20 pulses at 50 Hz (S1–S6). Carbachol was added at increasing concentrations (abscissae) before S2–S6. Ordinates, evoked overflow of tritium, calculated from Sn/S1 ratios and expressed as a percentage of the corresponding control (no carbachol). Means±s.e.m. from n=9–14 tissue pieces. Significant differences from corresponding control (no carbachol): #P<0.05. Significant differences from corresponding wild type: *P<0.05.
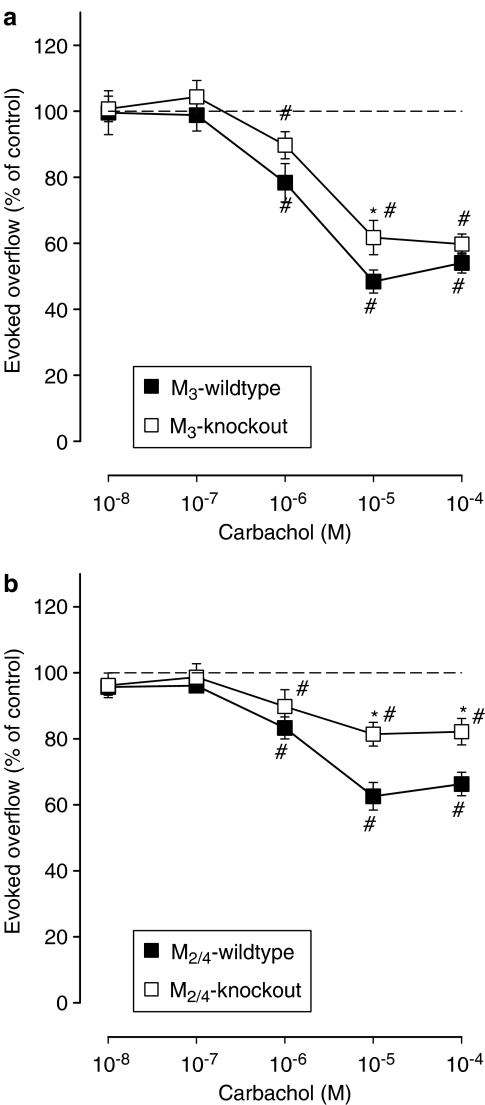
Figure 3.
Effect of carbachol on the evoked overflow of tritium from vas deferens of M3-wild-type or M3-knockout (a) and M2/4-wild-type or M2/4-knockout (b) mice. After preincubation with 3H-noradrenaline, tissues were superfused and stimulated six times by 20 pulses at 50 Hz (S1–S6). Carbachol was added at increasing concentrations (abscissae) before S2–S6. Ordinates, evoked overflow of tritium, calculated from Sn/S1 ratios and expressed as a percentage of the corresponding control (no carbachol). Means±s.e.m. from n=6–15 tissue pieces. Significant differences from corresponding control (no carbachol): *P<0.05. Significant differences from corresponding wild type: #P<0.05.
Atria
We showed previously that deletion of the M4 receptor had no significant effect on the release-inhibitory activity of carbachol in mouse atria, whereas deletion of the M2 receptor reduced the maximum inhibitory effect of carbachol from 68 to 26%, indicating the coexistence of predominant M2 and non-M2 muscarinic heteroreceptors in mouse atria (Trendelenburg et al., 2003a). In the present study, combined knockout of both the M2 and the M4 genes (M2/4-knockout) attenuated the maximum inhibition by carbachol from 69±3% (M2/4-wild type) to 23±4% (M2/4-knockout; Figure 1b), an effect similar to that observed after disruption of the M2 receptor gene alone. Deletion of the M3 receptor also attenuated the maximum inhibitory effect of carbachol from 76±9% (M3-wild type) to 57±4% (M3-knockout; Figure 1a). Strikingly, deletion of both the M2 and M3 receptor genes (M2/3-knockout) abolished the release-inhibiting effect of carbachol (Figure 1c), indicating that the non-M2 presynaptic muscarinic heteroreceptors present in mouse atria represent M3 receptors.
Urinary bladder
We demonstrated previously that deletion of the M4 receptor had no significant effect on the release-inhibiting effects of carbachol in mouse urinary bladder segments, whereas deletion of the M2 receptor reduced the maximum inhibitory effect of carbachol from 69 to 19%, indicating the coexistence of predominant M2 and non-M2 muscarinic heteroreceptors on sympathetic nerve endings in the urinary bladder (Trendelenburg et al., 2003a). In the present study, disruption of the M3 receptor gene had no significant effect on the release-inhibitory activity of carbachol in the urinary bladder (Figure 2a). In contrast, combined deletion of the M4 and M2 receptors abolished this activity. As shown in Figure 2b, no carbachol-mediated inhibition of transmitter release was observed in bladder preparations from M2/4-knockout mice, indicating that the non-M2 muscarinic heteroreceptors present in this tissue represent M4 receptors.
Vas deferens
As shown previously, deletion of the M4 receptor reduced the maximum inhibitory effect of carbachol on transmitter release from 46 to 33%, and deletion of the M2 receptor decreased this effect from 34 to 20% inhibition, indicating the coexistence of M2 and M4 heteroreceptors on sympathetic nerve terminal in the mouse vas deferens (Trendelenburg et al., 2003a). In the present study, combined knockout of both the M2 and M4 receptor genes attenuated the maximum inhibition by carbachol from 37±5% (M2/4-wild type) to 18±2% (M2/4-knockout; Figure 3b), an effect similar to that seen after disruption of the M2 or M4 receptor genes alone. The carbachol-mediated inhibition remaining in the M2/4-knockout preparations is consistent with the presence of at least one additional muscarinic heteroreceptor in the mouse vas deferens. Interestingly, deletion of the M3 receptor also attenuated the maximum inhibitory response of carbachol in the vas deferens, from 48±2% (M3-wild type) to 40±2% (M3-knockout; Figure 3a), suggesting that M3 receptors, in addition to the M2 and M4 receptor subtypes, may function as muscarinic heteroreceptors in the mouse vas deferens.
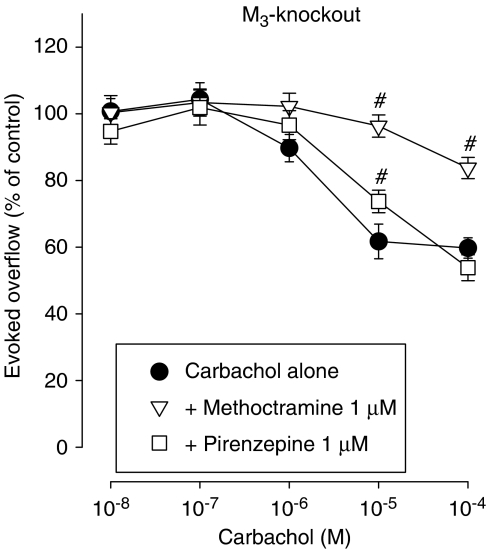
To further test the hypothesis that M3 heteroreceptors are present on sympathetic nerves innervating the mouse vas deferens, we carried out additional experiments using the two muscarinic antagonists, methoctramine and pirenzepine. A previous study using vas deferens preparations from NMRI, M2-wild-type and M4-wild-type mice showed that carbachol-mediated inhibition of noradrenaline release was antagonized with a potency order pirenzepine (pKd 6.3–6.8) ⩾methoctramine (pKd<6–6.1), which is the characteristic affinity profile of these two antagonists at M3, M4 and M5 receptors (see Introduction of Trendelenburg et al., 2003a). In vas deferens preparations from M4-knockout mice, this order was reversed to methoctramine (pKd 7.2) >pirenzepine (pKd<6), a pattern characteristic for M2 receptors (see Introduction of Trendelenburg et al., 2003a), suggesting that the pharmacology of the mixed population of muscarinic heteroreceptors present in the vas deferens of wild-type mice is dominated by the presence of M4 receptors. In the present study, deletion of the M3 receptor resulted in an antagonist affinity order similar to that observed with vas deferens preparations from M4-knockout mice (methoctramine (pKd 7.8)>pirenzepine (pKd 6.4)) (Figure 4), indicating the presence of M3 heteroreceptors which, like the M4 receptors, also appear to be required for the observation that the mixed population of muscarinic heteroreceptors present in the vas deferens of wild-type mice displays an M3/4/5 pharmacology.
Figure 4.
Interaction of the muscarinic antagonists methoctramine and pirenzepine with carbachol on the evoked overflow of tritium from vas deferens of M3-knockout mice. After preincubation with 3H-noradrenaline, tissues were superfused and stimulated six times by 20 pulses at 50 Hz (S1–S6). Carbachol was added at increasing concentrations (abscissae) before S2–S6. Carbachol was given either alone or combined with the indicated antagonists which were present throughout superfusion. Ordinates, evoked overflow of tritium, calculated from Sn/S1 ratios and expressed as a percentage of the corresponding control (no carbachol). Means±s.e.m. from n=15–19 tissue pieces. Significant differences from carbachol alone: #P<0.05.
Discussion
The present results confirm the heterogeneity of presynaptic muscarinic heteroreceptors at postganglionic sympathetic axons. Moreover, our findings indicate that different peripheral tissues are endowed with distinct populations (mixtures) of presynaptic muscarinic heteroreceptors.
We have recently shown that sympathetic axons of mouse atria and urinary bladder possess both M2 and non-M2 muscarinic heteroreceptors (Trendelenburg et al., 2003a). In the present study, we observed that carbachol had no significant inhibitory effect on transmitter release from sympathetic neurons innervating the atria of M2/3-double knockout mice, indicating that the non-M2 atrial heteroreceptors represent M3 receptors (Figure 1c). Analogously, carbachol had no significant effect on noradrenaline release from urinary bladder segments from M2/4-double-knockout mice, strongly suggesting that the non-M2 heteroreceptors represent M4 receptors in the urinary bladder (Figure 2b).
Previously, we detected both M2 and M4 presynaptic muscarinic heteroreceptors in the mouse vas deferens (Trendelenburg et al., 2003a). We therefore considered it likely that the release-inhibitory activity of carbachol would be absent in vas deferens preparations from M2/4-double-knockout mice. However, carbachol still retained some activity in vasa deferentia from M2/4-double-knockout mice (Figure 3b), indicating the presence of at least one additional muscarinic heteroreceptor in this tissue. Two observations suggest that M3 heteroreceptors are present in the mouse vas deferens. First, deletion of the M3 receptor led to a small but significant reduction of the release-inhibitory effect of carbachol (Figure 3a). Second, the lack of M3 receptors changed the pharmacology of the overall presynaptic muscarinic receptor population in the mouse vas deferens. In all wild-type strains, the order of antagonist potencies in blocking carbachol-mediated inhibition of transmitter release was pirenzepine⩾methoctramine, typical for the pharmacology of M3, M4 and M5 receptors. Similar to the pharmacology of vas deferens preparations from M4 receptor knockout mice (Trendelenburg et al., 2003a), deletion of the M3 receptor changed the order of antagonist potencies in blocking carbachol-mediated inhibition of noradrenaline release to methoctramine>pirenzepine, typical of M2 receptors (Trendelenburg et al., 2003a). The presence of both M3 and M4 receptors is therefore required to confer an M3/4/5 receptor-like antagonist pharmacology on the mixed population of muscarinic heteroreceptors in the wild-type vas deferens.
The identification of the release-inhibiting muscarinic heteroreceptors in mouse atria and urinary bladder can be considered clear-cut, because carbachol was devoid of any effect on sympathetic transmitter release in preparations from M2/3- and M2/4-double-knockout-mice, respectively. On the other hand, our data do not rule out the possibility that additional muscarinic receptor subtypes, besides the M2, M3 and M4 receptors, contribute to the mixture of muscarinic heteroreceptors present in the mouse vas deferens.
As is generally the case with gene knockout mouse strains, we cannot completely rule out the possibility that compensatory changes in the expression levels or subcellular distribution of individual muscarinic receptor subtypes may have occurred in the different mutant mouse strains used in the present study. However, all studies that have addressed this issue so far suggest that such changes are unlikely to occur (Gautam et al., 2004, 2005; Wess, 2004). Moreover, we cannot completely rule out the possibility that formation of muscarinic heterodimers (Novi et al., 2005) may have affected the outcome of the functional studies.
It has been suggested that postganglionic sympathetic axons in several tissues including mouse atria (Costa & Majewski, 1991) possess release-facilitating M1 receptors. However, under our experimental conditions, we have never observed facilitation of noradrenaline release by carbachol in any of the three tissues investigated, even after genetic deletion of the release-inhibitory muscarinic heteroreceptors.
Studies on genetically modified mice have revealed heterogeneous receptor populations also for other presynaptic receptor systems. In vitro neurotransmitter release studies showed that the muscarinic autoreceptors inhibiting the release of acetylcholine in mouse brain cortex and hippocampus are predominantly M2, whereas in the corpus striatum they are predominantly M4 (Zhang et al., 2002a). However, in vivo microdialysis studies have shown that M4 autoreceptors may also contribute to the regulation of acetylcholine release in the hippocampus (Tzavara et al., 2003). As to the peripheral parasympathetic system, mouse atria possess both M4 and non-M4 (probably M2) muscarinic autoreceptors, whereas the muscarinic autoreceptors exclusively represent M4 receptors in the mouse urinary bladder (Zhou et al., 2002). In the corpus striatum, presynaptic M5 heteroreceptors enhance the release of dopamine (Zhang et al., 2002b). Early work suggested that the α2 autoreceptors of noradrenergic neurons mainly consist of α2A receptors (see Starke, 2001). However, recent studies with adrenergic receptor knockout mice indicated that the α2C and α2B adrenoceptors also function as autoreceptors (see Hein et al., 1999; Trendelenburg et al., 2003b). In the corpus striatum, both presynaptic α2A and α2B heteroreceptors depress the release of dopamine (Bücheler et al., 2002).
In neurons possessing multiple presynaptic muscarinic receptors or α2 adrenoceptors, deletion of only one receptor subtype gene often has no significant effect on agonist responses. Disruption of only the α2C adrenoceptor gene, for example, did not diminish the inhibitory effect of α2 adrenoceptor agonists on the release of noradrenaline in the vas deferens (Altman et al., 1999), and disruption of only the M4 muscarinic receptor gene did not diminish the inhibitory effect of carbachol on the release of noradrenaline in the urinary bladder (Trendelenburg et al., 2003a; see also Bücheler et al., 2002; Trendelenburg et al., 2003b; Tzavara et al., 2003). It is likely that in these cases the remaining receptor subtypes are able to induce a signal that is sufficiently large to mediate full agonist responses.
The M2 and M4 muscarinic receptor subtypes are preferentially coupled to G proteins of the Gi/o family (Caulfield & Birdsall, 1998), similar to many other presynaptic inhibitory receptors such as the α2 adrenoceptors, opioid receptors and cannabinoid CB1 receptors. The activation of this class of G proteins has been shown to mediate the inhibition of fast, voltage-sensitive N- and P/Q-type Ca2+ channels, leading to reduced transmitter release (see Shapiro et al., 1999; Starke, 2001; Zhang et al., 2002a). In contrast, the M1, M3 and M5 receptor subtypes selectively couple to G proteins of the Gq family (Caulfield & Birdsall, 1998). It has been suggested, based on the use of subtype-‘preferring' muscarinic antagonists, that M3 receptors mediate the inhibition of noradrenaline release in guinea-pig atria (Olmez et al., 1995) and cat femoral artery (Fernandes et al., 1991) or acetylcholine release in rat stomach (Yokotani et al., 1993). However, the present study provides the first piece of evidence for the existence of release-inhibiting M3 receptors that does not rely on the use of pharmacological tools of limited selectivity. Electrophysiological studies on muscarinic receptors expressed in mouse superior cervical ganglia suggest that Gq-mediated presynaptic inhibition of neurotransmitter release may involve voltage-independent inhibition of Ca2+ channels (Shapiro et al., 1999).
Immunologic and mRNA expression studies have shown that sympathetic neurons express M1, M2 and M4 muscarinic receptors (see Doerje et al., 1991; Ludlam et al., 1994; Shapiro et al., 1999). In contrast, neither M3 nor M5 receptors could be detected by the use of these techniques, most likely due to very low receptor expression levels.
In conclusion, our study involving the use of genetically modified mice clearly shows that postganglionic sympathetic neurons use different mixtures of muscarinic receptor subtypes as presynaptic release-inhibiting heteroreceptors. In atria, this mixture is composed of M2 and M3, in the urinary bladder of M2 and M4, and in vasa deferentia of at least three receptor subtypes, M2, M3 and M4. The mechanism by which Gq-coupled M3 receptors can mediate presynaptic inhibition of neurotransmitter release remains to be investigated.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft Grant SFB 505 (to A.U. Trendelenburg, K. Starke).
Abbreviations
- PCR
polymerase chain reaction
References
- ALTMAN J.D., TRENDELENBURG A.U., MACMILLAN L., BERNSTEIN D., LIMBIRD L., STARKE K., KOBILKA B.K., HEIN L. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- BÜCHELER M.M., HADAMEK K., HEIN L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P., BIRDSALL N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- COSTA M., MAJEWSKI H. Evidence for facilitatory and inhibitory muscarinic receptors on postganglionic sympathetic nerves in mouse isolated atria. Br. J. Pharmacol. 1991;102:855–860. doi: 10.1111/j.1476-5381.1991.tb12266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOERJE F., LEVEY A.I., BRANN M.R. Immunological detection of muscarinic receptor subtype proteins (m1–m5) in rabbit peripheral tissues. Mol. Pharmacol. 1991;40:459–462. [PubMed] [Google Scholar]
- FERNANDES F.A., ALONSO M.J., MARIN J., SALAICES M. M3-muscarinic receptor mediates prejunctional inhibition of noradrenaline release and the relaxation in cat femoral artery. J. Pharm. Pharmcol. 1991;43:644–649. doi: 10.1111/j.2042-7158.1991.tb03555.x. [DOI] [PubMed] [Google Scholar]
- FUDER H., MUSCHOLL E. Heteroreceptor-mediated modulation of noradrenaline and acetylcholine release from peripheral nerves. Rev. Physiol. Biochem. Pharmacol. 1995;126:265–412. doi: 10.1007/BFb0049778. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines. Handbook of Experimental Pharmacology 1972Berlin, Heidelberg, New York: Springer; 283–335.ed. Blaschko H. & Muscholl E. Vol. 33, pp [Google Scholar]
- GAUTAM D., HAN S.J., HEARD T.S., CUI Y., MILLER G., BLOODWORTH L., WESS J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J. Pharmacol. Exp. Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- GAUTAM D., HEARD T.S., CUI Y., MILLER G., BLOODWORTH L., WESS J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- HEIN L., ALTMAN J.D., KOBILKA B.K. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., TRENDELENBURG A.U., STARKE K. Pharmacological characterization of presynaptic α2-autoreceptors in rat submaxillary gland and heart atrium. Br. J. Pharmacol. 1992;107:246–255. doi: 10.1111/j.1476-5381.1992.tb14494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDLAM W.H., ZANG Z., McCARSON K.E., KRAUSE J.E., SPRAY D.C., KESSLER J.A. mRNAs encoding muscarinic and substance P receptors in cultured sympathetic neurons are differentially regulated by LIF or CNTF. Dev. Biol. 1994;164:528–539. doi: 10.1006/dbio.1994.1221. [DOI] [PubMed] [Google Scholar]
- NOVI F., STANASILA L., GIORGI F., CORSINI G.U., COTECCHIA S., MAGGIO R. Paired activation of the two components within muscarinic M3 receptor dimers is required for recruitment of β-arrestin-1 to the plasma membrane. J. Biol. Chem. 2005;280:19768–19776. doi: 10.1074/jbc.M411281200. [DOI] [PubMed] [Google Scholar]
- OLMEZ E., OGUZ GUC M., ILHAN M. Inhibitory muscarinic cholinoceptors on postganglionic sympathetic nerves in the guinea pig isolated atrium are of the M3 subtype. Pharmacology. 1995;51:112–117. doi: 10.1159/000139323. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., GÖTHERT M. Interactions between the presynaptic α2-autoreceptor and presynaptic inhibitory heteroreceptors on noradrenergic neurons. Brain Res. Bull. 1998;47:129–132. doi: 10.1016/s0361-9230(98)00068-9. [DOI] [PubMed] [Google Scholar]
- SHAPIRO M.S., LOOSE M.D., HAMILTON S.E., NATHANSON N.M., GOMEZA J., WESS J., HILLE B. Assignment of muscarinic receptor subtypes mediating G-protein modulation of Ca2+ channels by using knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10899–10904. doi: 10.1073/pnas.96.19.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARKE K. Presynaptic α-autoreceptors. Rev. Physiol. Biochem. Pharmacol. 1987;107:73–146. [PubMed] [Google Scholar]
- STARKE K. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J. Neurochem. 2001;78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- STRUCKMANN N., SCHWERING S., WIEGAND S., GSCHNELL A., YAMADA M., KUMMER W., WESS J., HABERBERGER R.V. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol. Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., GOMEZA J., KLEBROFF W., ZHOU H., WESS J. Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor-deficient mice. Br. J. Pharmacol. 2003a;138:469–480. doi: 10.1038/sj.bjp.0705053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., HEIN L., GAISER E.G., STARKE K. Occurrence, pharmacology and function of presynaptic α2-autoreceptors in α2A/D-adrenoceptor-deficient mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:540–551. doi: 10.1007/s002109900093. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., PHILLIP M., MEYER A., KLEBROFF W., HEIN L., STARKE K. All three α2-adrenoceptor types serve as autoreceptors in postganglionic sympathetic neurons. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003b;368:504–512. doi: 10.1007/s00210-003-0829-x. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., SUTEJ I., WAHL C.A., MOLDERINGS G.J., RUMP L.C., STARKE K. A re-investigation of questionable subclassifications of presynaptic α2-autoreceptors: rat vena cava, rat atria, human kidney and guinea-pig urethra. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:721–737. doi: 10.1007/pl00005111. [DOI] [PubMed] [Google Scholar]
- TZAVARA E.T., BYMASTER F.P., FELDER C.C., WADE M., GOMEZA J., WESS J., MCKINZIE D.L., NOMIKOS G.G. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry. 2003;8:673–679. doi: 10.1038/sj.mp.4001270. [DOI] [PubMed] [Google Scholar]
- WAHL C.A., TRENDELENBURG A.U., STARKE K. Presynaptic α2-autoreceptors in mouse heart atria: evidence for the α2D subtype. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:253–261. doi: 10.1007/BF00171055. [DOI] [PubMed] [Google Scholar]
- WAUD D.R.Analysis of dose–response relationships Advances in General and Cellular Pharmacology 1976New York, London: Plenum; 145–178.ed. Narahshi T. & Bianchi C.P. Vol. 1, pp [Google Scholar]
- WESS J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- YAMADA M, MIYAKAWA T., DUTTAROY A., AMANAKA A., MORIGUCHI T., MAKITA R., OGAWA M., CHOU C.J., XIA B., CRAWLEY J.N., FELDER C.C., DENG C.X., WESS J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- YOKOTANI K., OKUMA Y., NAKAMURA K., OSUMI Y. Release of endogenous acetylcholine from vascularly perfused rat stomach in vitro; inhibition by M3 muscarinic autoreceptors and alpha-2 adrenoceptors. J. Pharmacol. Exp. Ther. 1993;266:1190–1195. [PubMed] [Google Scholar]
- ZHANG W., BASILE A.S., GOMEZA J., VOLPICELLI L.A., LEVEY A.I., WESS J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J. Neurosci. 2002a;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG W., YAMADA M., GOMEZA J., BASILE A.S., WESS J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1–M5 muscarinic receptor knock-out mice. J. Neurosci. 2002b;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU H., MEYER A., STARKE K., GOMEZA J., WESS J., TRENDELENBURG A.U. Heterogeneity of release-inhibiting muscarinic autoreceptors in heart atria and urinary bladder: a study with M2- and M4-receptor-deficient mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:112–122. doi: 10.1007/s00210-001-0517-7. [DOI] [PubMed] [Google Scholar]