Abstract
Activation, or the altered perception of activation, of trigeminal nerves that innervate the cranial vasculature is considered to be a pivotal component of the pathophysiology of acute migraine.
Calcitonin gene-related peptide (CGRP) levels are increased during migraine and after trigeminal nerve stimulation in the cat. Both CGRP and nitric oxide (NO) infusion causes headache and delayed migraine in migraineurs. Neurogenic stimulation of a cranial window, CGRP and NO injection all cause meningeal artery dilation in the rat when viewed using intravital microscopy.
Topiramate is an antiepileptic drug with established efficacy as a migraine preventive, and has recently been shown to inhibit neurons of the trigeminocervical complex after superior sagittal sinus stimulation.
In this study, we used intravital microscopy with neurogenic dural vasodilation, and CGRP- and NO-induced dilation to examine whether intravenous topiramate has effects on the trigeminovascular system.
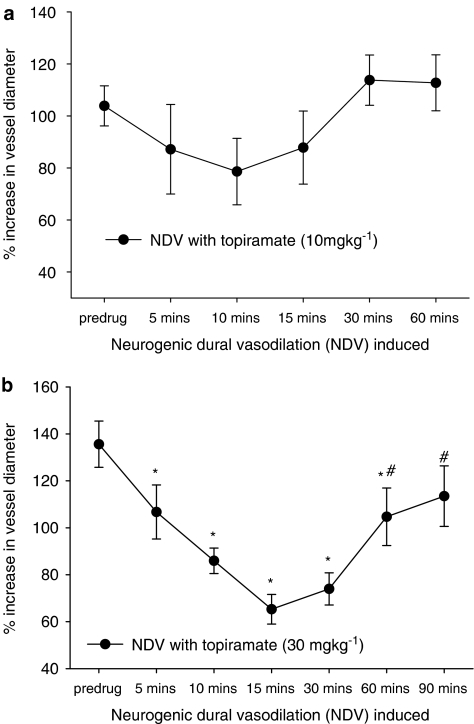
Topiramate was able to attentuate neurogenic dural vasodilation maximally after 15 min by 52% at 30 mg kg−1 (t5=6.78, n=6); there was no significant inhibition at 10 mg kg−1.
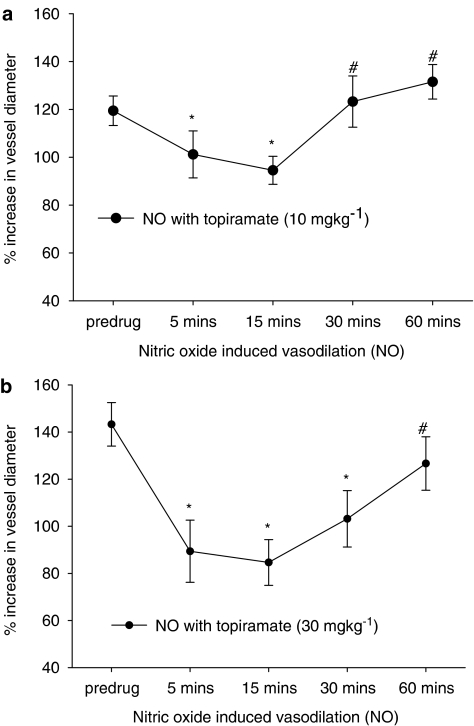
There was also significant attenuation of the NO-induced dilation maximally after 15 min, at both 10 and 30 mg kg−1 by 21% (t6=6.09, n=7) and 41% (t6=5.3, n=7), respectively.
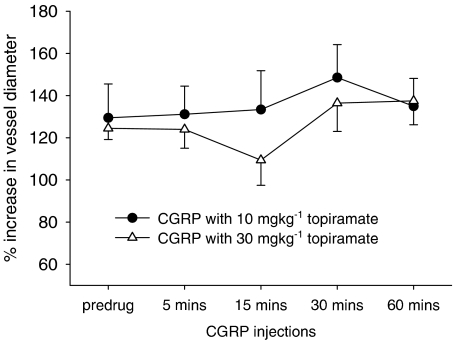
CGRP-induced dilation was not inhibited at either dose of topiramate.
The study demonstrates that topiramate is likely to inhibit neurogenic dural vasodilation by inhibiting the release of CGRP from prejunctional trigeminal neurons, thus attenuating the dural vasodilation. Topiramate is not able to act postsynaptically at the blood vessels themselves as the CGRP-induced dilation was not attenuated.
The data are consistent with an effect of topiramate on trigeminovascular activation which may form part of its preventive antimigraine mechanisms of action.
Keywords: Topiramate, calcitonin gene-related peptide, nitric oxide, migraines
Introduction
The pathophysiology of migraine is still to be fully understood, but it is thought to involve activation of trigeminal afferents (Goadsby et al., 2002). Trigeminal sensory nerve fibres that innervate cranial structures contain the neuropeptides calcitonin gene-related peptide (CGRP), substance P and neurokinin A (Uddman & Edvinsson, 1989). Activation of Aδ-trigeminal nerve fibres causes the release of CGRP and dilation of dural arteries in animals (Goadsby et al., 1988; Williamson et al., 1997a), while CGRP levels in the blood plasma of migraineurs are increased during migraine (Goadsby et al., 1990). Intravenous injection of CGRP causes a dull headache and subsequent migraine in humans (Lassen et al., 2002) and dural blood vessel dilation in rats (Williamson et al., 1997a). This all seems to indicate that CGRP may be a key transmitter in the pathogenesis of migraine, especially given that recent clinical evidence suggests that blockade of CGRP has a potent acute antimigraine effect (Olesen et al., 2004).
Another trigger of headache and migraine in migraineurs is nitric oxide (NO). NO is a potent endogenous vasodilator with an impressive array of biological actions (Moncada et al., 1991). Intravenous infusion of glycerol trinitrate (GTN, an exogenous NO donor) causes an immediate headache in migraineurs and some control subjects, and it is also able to induce a delayed migraine attack, that fulfilled the International Headache Society criteria for migraine (Headache Classification Committee of The International Headache Society, 1988) several hours after the infusion of GTN had ceased (Iversen et al., 1989; Olesen et al., 1993; 1994). Similarly, it was found that NO infusion was able to cause a reproducible vasodilation of middle meningeal arteries in the rat (Akerman et al., 2002b). It is believed that NO activates guanylate cyclase, catalysing the formation of cyclic guanosine monophosphate (cGMP); the activation of this pathway leads to the relaxation of the smooth muscle and vasodilation of the blood vessel (Moncada et al., 1991). The mechanism by which NO causes a delayed migraine response is still unclear, but it is likely to involve activation at central sites that triggers a cascade of events in the brain that lead to migraine in susceptible persons.
Topiramate is a derivative of the naturally occurring monosaccharide D-fructose. It has antiepileptic activity, and established efficacy as a migraine preventive (Brandes et al., 2004; Diener et al., 2004; Silberstein et al., 2004). It is not known by what mechanism topiramate acts as a migraine preventive. Topiramate has five potential mechanisms for its antiepileptic effects: through a modulation of voltage-gated sodium ion channels and voltage-gated calcium ion channels, blockade of excitatory glutamate transmission and potentiation of GABA inhibition, and by inhibition of carbonic anhydrase (Shank et al., 2000). These mechanisms can influence the excitatory state of a cell by influences on receptor/channel protein complexes, including changes to their phosphorylation states. Such mechanisms are known to influence trigeminovascular activity.
Inhibition of neurogenic dural vasodilation using intravital microscopy is highly predictive of acute antimigraine efficacy, examples in the ‘triptans', 5-HT1B/1D receptor agonists, opioids and CGRP receptor antagonists (Shepheard et al., 1997; Williamson et al., 1997b; 2001a, 2001b). Also, given that both CGRP and NO are able to cause headache and migraine in patients (Iversen et al., 1989; Olesen et al., 1993; 1994; Lassen et al., 2002), and that CGRP and NO are capable of causing reproducible meningeal vasodilation in rats (Williamson et al., 1997a; Akerman et al., 2002b), compounds that inhibit the response in rats may have potential at inhibiting the induced headache and subsequent migraine in patients. In this series of experiments, we wanted to examine the effect of topiramate on neurogenic dural vasodilation using intravital microscopy. We tested topiramate at both 10 and 30 mg kg−1 doses against neurogenic dural vasodilation, CGRP- and NO-induced dilation of meningeal blood vessels. This method uses dilation of dural blood vessel as a measure of trigeminal nerve activation, and thus compounds that can inhibit the vasodilatory response have the potential to inhibit trigeminovascular nociceptive transmission and may explain topiramate's antimigraine mode of action.
Methods
Surgical preparation
All experiments were conducted under U.K. Home Office Animals (Scientific Procedures) Act (1986). Male Sprague–Dawley rats (260–400 g) were anaesthetised throughout the experiments with sodium pentobarbitone (60 mg kg−1 i.p. and then 18 mg kg−1 h−1 i.v. infusion). The left femoral artery and vein were cannulated for blood pressure recording and intravenous infusion of anaesthetic and test compounds, respectively. Temperature was maintained throughout using a homeothermic blanket system. The rats were placed in a stereotaxic frame and ventilated with oxygen-enriched air, 3–5 ml, 60–80 strokes min−1 (Small Rodent Ventilator – Model 683, Harvard Instruments, U.K.). End-tidal CO2 was monitored (Capstar-100, CWE Inc., U.S.A.) and kept between 3.5 and 4.5% and blood pressure was monitored continually. This allows one to monitor for changes to respiration and blood pressure due to long-term anaesthetic maintenance. The rats were placed in a stereotaxic frame, the skull exposed and the right or left parietal bone thinned by drilling with a saline-cooled drill until the blood vessels of the dura were clearly visible through the intact skull.
Intravital microscopy
The cranial window was covered with mineral oil (37°C) and a branch of the middle meningeal artery viewed using an intravital microscope (Microvision MV2100, U.K.) and the image displayed on a television monitor. Dural blood vessel diameter was continuously measured using a video dimension analyzer (Living Systems Instrumentation, U.S.A.) and displayed with blood pressure on an online data analysis system (CED spike5v1 software).
Experimental protocols
Defining electrical stimulation parameters
Electrical stimulation was used to evoke dilation of the dural blood vessels with a bipolar stimulating electrode (NE 200X, Clark Electromedical) placed on the surface of the cranial window approximately 200 μm from the vessel of interest. The surface of the cranial window was stimulated at 5 Hz, 1 ms for 10 s (Grass Stimulator S88, Grass Instrumentation) with increasing voltage until maximal dilation was observed. Subsequent electrically induced responses in the same animal were then evoked using that voltage (Williamson et al., 1997a; Akerman et al., 2002b).
CGRP- and NO-induced dilation
In the preparations where CGRP was used to dilate dural blood vessels, CGRP was given as an intravenous bolus of 1 μg kg−1, and this has been shown previously to produce a maximal dilation (Williamson et al., 1997a). In preparations where NO was used to dilate the dural blood vessel, sodium nitroprusside, the NO donor, was given as a 5-min infusion at 4–8 μg kg−1 min−1 via the femoral vein, to produce maximal dilation (Akerman et al., 2002b).
The reproducibility of the vasodilator challenges has been demonstrated previously using either three or four consecutive saline-controlled stimuli for electrical stimulation and CGRP bolus (Akerman et al., 2002a) and sodium nitroprusside (Akerman et al., 2002b), in order to test whether there was any systematic effect of test compounds over time in the meningeal circulation. In each case there was no significant effect across the cohort. CGRP and sodium nitroprusside injections caused decreases in blood pressure that were not significantly different across the cohorts.
Effect of the topiramate on evoked dural vessel dilation
A control response to dural electrical stimulation was performed and at least 10 min later topiramate (10 mg kg−1) was administered intravenously, and then the electrical stimulation was repeated 5, 10, 15, 30 and 60 min after the topiramate treatment. A similar protocol was used with a higher (30 mg kg−1) dose of topiramate, except that there was also stimulation at 90 min. In a separate series of experiments a control response to CGRP (1 μg kg−1) was performed and at least 10 min later topiramate (either 10 or 30 mg kg−1) was administered and CGRP bolus repeated after 5, 15, 30 and 60 min.
NO-induced dural vessel dilation was tested with topiramate as well, using a similar protocol. A control response to sodium nitroprusside infusion (4–8 μg kg−1 min−1) for 5 min was followed 10 min later by an intravenous dose of topiramate (10 or 30 mg kg−1) and sodium nitroprusside infusion repeated after 5, 15, 30 and 60 min.
Data analysis
The effects of electrical stimulation, bolus of CGRP and sodium nitroprusside infusion on dural vessel diameter were calculated as a percentage increase from the prestimulation baseline diameter. The nature of the experimental set-up, where the magnification of the dural vessel visualised was different in each set-up by virtue of selecting an appropriate target vessel, made it impossible to standardise the dural vessel measurement; therefore, the dural vessel diameter were measured in arbitrary units. The typical vessel diameter measured ranges from 120 to 150 μM. All data are expressed as mean±s.e.m. Statistical analysis was performed using an ANOVA for repeated measures with Bonferroni post-hoc correction for multiple comparisons, followed by Student's paired t-test (SPSS v10.0). Significance was assessed at the P<0.05 levels. The experiments were not conducted in a blinded fashion although the data are digitised and the analysis automated to avoid bias.
Drugs
The infusion of anaesthetic and experimental drugs were all via the same femoral catheter; however, the line was always flushed with saline first, several minutes before administering the different compound. Topiramate was provided as a gift by Johnson and Johnson, U.S.A. It was dissolved in water for injection at a maximum concentration of 10 mg kg−1 and heated in an ultrasonic water bath to 55°C until fully dissolved. CGRP (Sigma-Aldrich, U.K.) was dissolved in deoxygenated water, aliquotted and frozen until required and then redissolved in 0.9% NaCl for use. Sodium nitroprusside (Sigma-Aldrich) was dissolved in 0.9% NaCl.
Results
Effects of topiramate on neurogenic dural vasodilation
Topiramate was not able to attenuate significantly neurogenic dural vasodilation at 10 mg kg−1 (F4,24=1.283, P=0.304), but had a significant effect at 30 mg kg−1 (F6,30=10.225, P=0.002, n=6), see Figure 1. At the 30 mg kg−1 dose neurogenic dural vasodilation was attenuated, compared to the control response, at 5, 10, 15, 30 and 60 min post-topiramate injection. Attenuation was greatest after 15 min, 136±10% compared to 65±6% (t5=6.78, P<0.05, n=6; see Table 1).
Figure 1.
Effects of topiramate treatment on neurogenic dural vasodilation. Following control responses to electrical stimulation, rats were injected with topiramate (a) 10 mg kg−1 or (b) 30 mg kg−1, and electrical stimulation repeated after 5, 10, 15, 30, 60 and 90 min. *P<0.05, significance compared to the control response. #P<0.05, significance compared to the response 15 min post topiramate.
Table 1.
Effects of 30 mg kg−1 topiramate on neurogenic dural vasodilation
| Neurogenic dural vasodilation | Increase in dural vessel diameter (% age change) |
|---|---|
| Control response | 135.6±10% |
| 5 min post 30 mg kg−1 TPM | 106.7±12%a (t5=4.56, n=6) |
| 10 min post | 85.9±5%a (t5=6.23, n=6) |
| 15 min post | 65.3±6%a (t5=6.78, n=6) |
| 30 min post | 74.0±7%a (t5=7.61, n=6) |
| 60 min post | 104.7±12%a (t5=3.05, n=6) |
| b (t5=3.39, n=6) | |
| 90 min post | 113.5±13%b (t5=2.94, n=6) |
TPM=topiramate.
P<0.05, significance compared to control response.
P<0.05, significance compared to vasodilation 15 min post TPM injection.
Effects of topiramate on CGRP-induced dilation
Topiramate was not able to significantly attenuate CGRP-induced dilation at 10 mg kg−1 (F4,24=0.91, n=6, P=0.423) and 30 mg kg−1 doses (F4,24=2.8, n=6, P=0.098; see Figure 2).
Figure 2.
Effects of topiramate treatment on CGRP-induced dilation. Following control responses to CGRP, bolus rats were injected with topiramate (10 or 30 mg kg−1) and CGRP bolus repeated after 5, 15, 30 and 60 min.
Effects of topiramate on NO-induced dilation
Topiramate was able to attenuate significantly NO-induced dilation (caused by sodium nitroprusside infusions) at both 10 mg kg−1 (F4,24=11.55, n=7, P=0.000) and 30 mg kg−1 (F4,24=8.19, n=7, P=0.002; see Figure 3). At the 10 mg kg−1 dose, the NO-induced dilation was attenuated 5 and 15 min post-topiramate injection, at the 30 mg kg−1 dose the NO-induced dilation was attenuated 5, 15 and 30 min post-topiramate injection. At each dose the attenuation was greatest after 15 min, 119±6% compared to 95±6% (10 mg kg−1, t6=5.30, P<0.05) and 143±9% compared to 86±10% (30 mg kg−1, t6=6.09, P<0.05; see Table 2).
Figure 3.
Effects of topiramate treatment on NO-induced dilation. Following control responses to sodium nitroprusside infusion, rats were injected with topiramate (a) 10 mg kg−1 or (b) 30 mg kg−1, and sodium nitroprusside infusion repeated after 5, 15, 30 and 60 min. *P<0.05, significance compared to the control response. #P<0.05 significance compared to the response 15 min post topiramate injection. χP<0.05, significance compared to the response 5 min post topiramate injection.
Table 2.
Effects of 30 mg kg−1 topiramate on NO-induced dilation
| NO-induced dilation | Increase in dural vessel diameter (% age change) |
|---|---|
| Control response | 143.3±9% |
| 5 min post 30 mg kg−1 TPM | 89.4±13%a (t6=4.55, n=7) |
| 15 min post | 84.6±10%a (t6=6.09, n=7) |
| 30 min post | 103.2±12%a (t6=2.58, n=7) |
| 60 min post | 127.7±11%b (t6=2.49, n=7) |
| c (t6=3.35, n=7) |
TPM=topiramate
P<0.05, significance compared to control response.
P<0.05, significance compared to vasodilation 15 min post TPM injection.
P<0.05, significance compared to vasodilation 30 min post TPM injection.
Effect of topiramate on mean arterial blood pressure, and the blood pressure effects caused by CGRP and sodium nitroprusside
Topiramate caused a significant reduction in mean arterial blood pressure at both 10 and 30 mg kg−1 doses, of 4±2 mmHg (10 mg kg−1, t15=2.19, P<0.05, n=16) and 7.7±1 mmHg (30 mg kg−1, t15=5.5, P<0.05, n=16). This blood pressure change returned to preinjection levels before the next vasodilator challenge. There was no significant effect on dural blood vessel diameter.
CGRP caused a significant decrease in mean arterial blood pressure on each occasion it was given; when we looked at the entire cohort this was significantly reduced by administration of 10 mg kg−1 topiramate (F4,24=4.56, P<0.05, n=7), and specifically at 15 min (t6=2.98, P<0.05, n=7) and 45 min (t6=3.34, P<0.05, n=7), but was not significant with 30 mg kg−1 topiramate (F4,24=1.40, P=0.284, n=7); the data are summarised in Table 4. Sodium nitroprusside caused a significant decrease in mean arterial blood pressure on each occasion it was given; when we looked across the entire cohort the mean arterial blood pressure changes were not significantly different after topiramate intervention at both 10 mg kg−1 (F4,24=3.00, P=0.67, n=7) and 30 mg kg−1 (F4,24=0.95, P=0.42, n=7); the data are summarised in Table 4.
Table 4.
Effect of topiramate on the hypotensive effects of sodium nitroprusside
| Mean arterial blood pressure and decreases (mmHg) after SNP infusions (4–8 μg kg−1) | ||||
|---|---|---|---|---|
| Pretreatment | 10 mg kg−1 TPM | 30 mg kg−1 TPM | ||
| Baseline BP | BP drop | Baseline BP | BP drop | |
| SNP control | 124.2±7 | 53.4±10 | 108.3±8 | 43.7±7 |
| Post TPM 5 min | 122.4±6 | 50.0±8 | 122.7±7 | 40.5±5 |
| Post TPM 15 min | 121.3±8 | 48.0±8 | 119.3±7 | 42.9±7 |
| Post TPM 30 min | 119.0±8 | 48.9±10 | 118.6±7 | 46.9±8 |
| Post TPM 45 min | 115.3±7 | 41.8±10 | 116.3±6 | 39.9±9 |
BP=blood pressure.
TPM=topiramate.
SNP=sodium nitroprusside (a NO donor).
Discussion
Topiramate was able to significantly inhibit neurogenic dural vasodilation at the 30 mg kg−1 dose and it was able to significantly inhibit NO-induced dural blood vessel dilation at both 10 and 30 mg kg−1 doses. CGRP-induced dural blood vessel dilation was unaffected by pretreatment with topiramate at either dose. Taken together, the data suggest a prejunctional effect for topiramate in the trigeminovascular system that would be consistent with its antimigraine effect as demonstrated in clinical trials. The prospect that preventive agents may influence the trigeminovascular system has not hitherto been widely expected (Edvinsson, 1999), but is an interesting outcome in the context of the development of new agents.
Topiramate has been shown previously to inhibit cell firing in the trigeminocervical complex (TCC) after stimulation of the superior sagittal sinus in cats (Storer & Goadsby, 2004). It was found to be effective at a range of doses, from 5 to 50 mg kg−1, with the 30 mg kg−1 dose proving to be optimal. In the data presented here we show that topiramate is also able to inhibit neurogenic dural vasodilation, but only at the 30 mg kg−1 dose. The dose is in line with effects on seizure activity and in a model of allodynia in rat (Wieczorkiewicz-Plaza et al., 2004; Grabenstatter et al., 2005), and thus is consistent with other biological effects in rat. Neurogenic dural vasodilation is thought to activate perivascular trigeminal sensory nerve fibres, and cause CGRP release that result in dural blood vessel dilation (Williamson et al., 1997a), and thus inhibition of this response, by, for example, topiramate, might be via a pre- or postsynaptic receptor.
CGRP acts directly at CGRP receptors on the smooth muscle of the dural blood vessels; therefore, any inhibition of CGRP-induced dilation is likely to be postsynaptic (Edvinsson et al., 2000). Topiramate is unable to inhibit the CGRP-induced dural blood vessel dilation, and therefore it is unlikely to be acting postsynaptically.
The trigeminal ganglion is a bipolar ganglion whose neural fibres innervate the dural blood vessels in the periphery and project to the trigeminal nucleus caudalis centrally. In general, the pharmacology of the two poles mirror each other, and therefore evidence gained from studying evoked cell firing in the TCC may also apply to neural mechanisms involving the dural blood vessel. The ‘triptans', serotonin 5-HT1B/1D receptor agonists (Storer & Goadsby, 1997; Williamson et al., 1997b, 1997c; Goadsby et al., 2001), ergotamine and dihydroergotamine (Goadsby et al., 2001; Williamson et al., 2001a), opioids (Williamson et al., 2001b; Storer et al., 2003), calcium channel blockers (Akerman et al., 2003a; Shields et al., 2003) and CGRP receptor blockers (Williamson et al., 1997a; Storer et al., 2004) are all able to inhibit the activation of both peripheral trigeminal afferents and neurons of the trigeminal nucleus caudalis. It is, therefore, clear that evidence gleaned from trigeminal neurovascular studies and trigeminal electrophysiological studies may be mutually informative.
It has been shown previously in the trigeminovascular intravital model that L-, N- and P/Q-type voltage-dependent calcium channel blockers are able to inhibit neurogenic dural vasodilation (Akerman et al., 2003b), and they are also able to inhibit superior sagittal sinus-activated neurons of the trigeminal nucleus caudalis (Shields et al., 2003). It is therefore possible that modulation of voltage-gated calcium channels by topiramate is contributing to the inhibition of neurogenic dural vasodilation. It has been argued that inhibition of voltage-gated calcium channels prevents the release of transmitters by preventing calcium influx into cells and thus preventing exocytosis and the mechanism of transmitter release. It is established that GABA agonists do not appear to be able to inhibit neurogenic dural vasodilation (Williamson & Hargreaves, 2001); therefore it appears that this mechanism is not relevant. However, it remains possible that topiramate acts by promoting GABA inhibition in the trigeminal nucleus caudalis to cause inhibition of neurons activated by trigeminovascular nociceptive stimuli (Storer & Goadsby, 2004).
Evidence gained from studying the trigeminal nucleus caudalis indicates that inhibiting both N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoaxoleproprionate (AMPA)/kainate receptors results in an inhibition in neuronal firing (Storer & Goadsby, 1999; Goadsby & Classey, 2000; Classey et al., 2001). No studies have been carried out using neurogenic dural vasodilation, but it is possible that inhibition of glutamatergic transmission will also inhibit peripheral trigeminal afferents. This would provide another avenue for the effect of topiramate.
The only sodium channel modulator used against neurogenic dural vasodilation is flunarizine (Akerman et al., 2002a), which has been used as a migraine preventive, and it was unsuccessful in causing any inhibition. Flunarizine acts on a large number of systems, including sodium/calcium channels, but it is not clear how it exacts its preventive action. Given the large array of sodium channels present in the brain and the variety of blockers and modulators that can be directed at them, it is difficult to discount an action of topiramate at sodium channels to inhibit neurogenic dural vasodilation. Perhaps the use of tetrodotoxin, a sodium channel blocker that defines the subtype of sodium channel, may be a useful starting point in the established models of trigeminovascular nociception. It is, however, worth noting that medicines with actions at sodium channels do not have convincing antimigraine effects, such as lamotrigine (Steiner et al., 1997) and carbamazepine (Rompel & Bauermeister, 1970; Anthony & Lance, 1972).
Topiramate was also able to inhibit NO-induced dural blood vessel dilation. It is thought that NO activates trigeminovascular neurons and causes the release of CGRP from the nerve endings, which contributes to the dural blood vessel dilation, as well as the NO acting directly on the dural blood vessels themselves. NO-induced dural and cerebral vasodilation is inhibited by application of a CGRP receptor blocker (Wei et al., 1992; Akerman et al., 2004). Indeed, it is thought that CGRP and NO behave synergistically in promoting trigeminovascular activation, as NO synthase inhibitors are able to inhibit CGRP-induced dilation (Wei et al., 1992; Akerman et al., 2002b). The evidence from this study would seem to indicate the same: topiramate is able to attenuate the NO response, presumably inhibiting the release of CGRP from trigeminal neurons, but there is still the NO–cGMP interaction that produces dilation of blood vessels. It seems unlikely that topiramate is acting directly on the dural blood vessels, as it is unable to inhibit CGRP-induced dilation.
Topiramate also affected the mean arterial blood pressure of the rats with a minor but significant reduction in blood pressure at both 10 and 30 mg kg−1 doses. This was not accompanied by any change in blood vessel diameter, and the blood pressure returned to its baseline level before vasodilator challenges were repeated. Topiramate was also unable to affect the changes in mean arterial blood pressure caused by the NO donor. Taken together, the data indicate that topiramate does not affect the dural blood vessel diameter per se, nor the cardiovascular effects of the NO donor, but only the response of the activated trigeminal neurons. Curiously, topiramate (10 mg kg−1) was found to inhibit the blood pressure changes caused by CGRP after 15 and 45 min, despite no effects on the dural blood vessel diameter, and no effect of the higher dose of topiramate. Considering Table 3 in toto, this result seems anomalous and bears repeating before firm conclusions can be drawn.
Table 3.
Effect of topiramate on the hypotensive effects of CGRP
| Mean arterial blood pressure and decreases (mmHg) after CGRP injection (1 μg kg−1) | ||||
|---|---|---|---|---|
| Pretreatment | 10 mg kg−1 TPM | 30 mg kg−1 TPM | ||
| Baseline BP | BP drop | Baseline BP | BP drop | |
| CGRP control | 123.5±5 | 41.2±3 | 125.8±4 | 41.1±3 |
| Post TPM 5 min | 119.5±5 | 38.4±4 | 121.6±5 | 34.1±5 |
| Post TPM 15 min | 118.8±8 | 29.5±3a | 118.5±4 | 32.7±3 |
| (t6=2.98) | ||||
| Post TPM 30 min | 117.5±3 | 36.4±4 | 121.5±4 | 34.4±4 |
| Post TPM 45 min | 123.6±4 | 33.4±3a | 123.5±5 | 33.2±4 |
| (t6=3.34) | ||||
BP=blood pressure.
TPM=topiramate.
CGRP=calcitonin gene-related peptide.
P<0.05 significance compared to the control response.
A potential limitation of the study is that the doses used in this study and that of Storer & Goadsby (2004) are not comparable with that used in the clinic, where a standard dose is 100 mg day−1 (Silberstein et al., 2004). The dosing for this study was based on Storer's results (2004) and also in response to the observations made during experimentation, where similar levels of attenuation, 50%, experiment were found in each study. We also considered that we were measuring in the periphery while previous work studied the trigeminal nucleus caudalis (Storer & Goadsby, 2004) that is behind the blood–brain barrier. We have also shown previously that a measure of 30 mg kg−1 was sufficient to get into the brain and inhibit cortical spreading depression, and it was more effective in the rat than the cat (Akerman & Goadsby, 2004). Other factors that need to be considered are that topiramate is used as a migraine preventive, which uses a dosing schedule over several weeks to succeed in reducing the number of headaches (Brandes et al., 2004; Silberstein et al., 2004). We only applied a single dose of topiramate to observe whether there is an effect on trigeminovascular neurons. This was achieved with the doses we used, and it is unlikely that we would learn any more from increasing the dose, particularly with respect to previous experiments. Therefore, it seems inappropriate to compare directly the dose in the clinic with the doses in these experiments. It would certainly be interesting to observe the effects of chronic topiramate treatment in the rat, although it would be more difficult to obtain good control data.
The current study demonstrates that topiramate is capable of inhibiting neurogenic dural vasodilation in an animal model. The results complement an electrophysiological study that demonstrates inhibition of trigeminocervical neurons activated by a nociceptive stimulus at comparable doses (Storer & Goadsby, 2004). It is feasible that trigeminovascular inhibition plays a role in the antimigraine effect of topiramate, although it is unlikely to be the full explanation for its effects. Considering well-established preventives like flunarizine are not effective in inhibiting neurogenic dural vasodilation (Akerman et al., 2002a), and mechanisms at other parts of the neuraxis are also likely to be important, such as those in the thalamus (Shields & Goadsby, 2005). Broadly speaking, understanding the extent to which preventive and acute attack treatments can alter trigeminovascular activation provides insights into the underlying mechanisms of both the medicines and into migraine itself.
Acknowledgments
We thank Philip Holland, Kevin Shields and Paul Hammond of the Headache Group at the Institute of Neurology for both assistance and technical support during these experiments. The work has been supported by the Wellcome Trust. P.J.G. is a Wellcome Senior Research Fellow.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoaxoleproprionate
- ANOVA
analysis of variance
- cGMP
cyclic guanosine monophosphate
- CGRP
calcitonin gene-related peptide
- GABA
γ-aminobutyric acid
- GTN
glycerol trinitrate
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- TCC
trigeminocervical complex
References
- AKERMAN S., GOADSBY P.J. Topiramate inhibits cortical spreading depression in rat and cat: a possible contribution to its preventive effect in migraine. Cephalalgia. 2004;24:783–784. [Google Scholar]
- AKERMAN S., KAUBE H., GOADSBY P.J. The effect of anti-migraine compounds on nitric oxide induced dilation of dural meningeal vessels. Eur. J. Pharmacol. 2002a;452:223–228. doi: 10.1016/s0014-2999(02)02307-5. [DOI] [PubMed] [Google Scholar]
- AKERMAN S., KAUBE H., GOADSBY P.J. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J. Pharmacol. Exp. Therap. 2004;309:56–63. doi: 10.1124/jpet.103.059808. [DOI] [PubMed] [Google Scholar]
- AKERMAN S., WILLIAMSON D., GOADSBY P.J. Calcium channels may be involved in CGRP release in trigeminal neurons and modulate subsequent dural dilation. Cephalalgia. 2003a;23:648. [Google Scholar]
- AKERMAN S., WILLIAMSON D., GOADSBY P.J. Voltage-dependent calcium channels are involved in neurogenic dural vasodilation via a pre-synaptic transmitter release mechanism. Br. J. Pharmacol. 2003b;140:558–566. doi: 10.1038/sj.bjp.0705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKERMAN S., WILLIAMSON D.J., KAUBE H., GOADSBY P.J. Nitric oxide synthase inhibitors can antagonise neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002b;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTHONY M., LANCE J.W. A comparative trial of pindolol, clonidine and carbamazepine in the interval therapy of migraine. Med. J. Austr. 1972;1:1343–1346. doi: 10.5694/j.1326-5377.1972.tb116454.x. [DOI] [PubMed] [Google Scholar]
- BRANDES J.L., SAPER J.R., DIAMOND M., COUCH J.R., LEWIS D.W., SCHMITT J., NETO W., SCHWABE S., JACOBS D. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291:965–973. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- CLASSEY J.D., KNIGHT Y.E., GOADSBY P.J. The NMDA receptor antagonist MK-801 reduces Fos-like immunoreactivity within the trigeminocervical complex following superior sagittal sinus stimulation in the cat. Brain Res. 2001;907:117–124. doi: 10.1016/s0006-8993(01)02550-1. [DOI] [PubMed] [Google Scholar]
- DIENER H.C., TFELT-HANSEN P., DAHLOF C., LAINEZ M.J., SANDRINI G., WANG S.J., NETO W., VIJAPURKAR U., DOYLE A., JACOBS D. Topiramate in migraine prophylaxis – results from a placebo-controlled trial with propranolol as an active control. J. Neurol. 2004;251:943–950. doi: 10.1007/s00415-004-0464-6. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L. Experimental Headache Models in Animals and Man. London: Martin Dunitz; 1999. [Google Scholar]
- EDVINSSON L., GOADSBY P.J., OLESEN I.L., UDDMAN R.CGRP, CGRP mRNA and CGRP1 receptor mRNA and release from the human trigeminovascular system The CGRP Family: Calcitonin Gene-Related Peptide (CGRP), Amylin, and Adrenomedullin 2000Georgetown, TX: Landes Bioscience; 167–171.ed. Poyner, D., Marshall, I. & Brain, S. pp [Google Scholar]
- GOADSBY P.J., AKERMAN S., STORER R.J. Evidence for postjunctional serotonin (5-HT1) receptors in the trigeminocervical complex. Ann. Neurol. 2001;50:804–807. doi: 10.1002/ana.10066. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., CLASSEY J.D. Glutamatergic transmission in the trigeminal nucleus assessed with local blood flow. Brain Res. 2000;875:119–124. doi: 10.1016/s0006-8993(00)02630-5. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B., FERRARI M.D. Migraine – current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- GRABENSTATTER H.L., FERRARO D.J., WILLIAMS P.A., CHAPMAN P.L., DUDEK F.E. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- HEADACHE CLASSIFICATION COMMITTEE OF THE INTERNATIONAL HEADACHE SOCIETY Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1–96. [PubMed] [Google Scholar]
- IVERSEN H.K., OLESEN J., TFELT-HANSEN P. Intravenous nitroglycerin as an experimental headache model. Basic characteristics. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- LASSEN L.H., HADERSLEV P.A., JACOBSEN V.B., IVERSEN H.K., SPERLING B., OLESEN J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- OLESEN J., DIENER H.-C., HUSSTEDT I.-W., GOADSBY P.J., HALL D., MEIER U., POLLENTIER S., LESKO L.M., BIBN 4096 BS CLINICAL PROOF OF CONCEPT STUDY GROUP Calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS is effective in the treatment of migraine attacks. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- OLESEN J., IVERSEN H.K., THOMSEN L.L. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- OLESEN J., THOMSEN L.L., IVERSEN H.K. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol. Sci. 1994;15:149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- ROMPEL H., BAUERMEISTER P.W. Aetiology of migraine and prevention with carbamazepine (Tegretol): results of a double-blind, cross-over study. South Afr. Med. J. 1970;44:75–80. [PubMed] [Google Scholar]
- SHANK R.P., GARDOCKI J.F., STREETER A.J., MARYANOFF B.E. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41:S3–S9. [PubMed] [Google Scholar]
- SHEPHEARD S.L., WILLIAMSON D.J., BEER M.S., HILL R.G., HARGREAVES R.J. Differential effects of 5-HT1B/1D receptor agonists on neurogenic dural plasma extravasation and vasodilation in anaesthetized rats. Neuropharmacology. 1997;36:525–533. doi: 10.1016/s0028-3908(97)00057-9. [DOI] [PubMed] [Google Scholar]
- SHIELDS K.G., GOADSBY P.J. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine. Brain. 2005;128:86–97. doi: 10.1093/brain/awh298. [DOI] [PubMed] [Google Scholar]
- SHIELDS K.G., STORER R.J., AKERMAN S., GOADSBY P.J. Post-synaptic high threshold voltage dependent calcium channels (VDCC) modulate trigeminovascular nociceptive transmission in the trigeminocervical complex (TCC) Cephalalgia. 2003;23:726. [Google Scholar]
- SILBERSTEIN S.D., NETO W., SCHMITT J., JACOBS D. Topiramate in migraine prevention: results of a large controlled trial. Arch. Neurol. 2004;61:490–495. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- STEINER T.J., FINDLEY L.J., YUEN A.W.C. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia. 1997;17:109–112. doi: 10.1046/j.1468-2982.1997.1702109.x. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., GOADSBY P.J. Characterization of opioid receptors that modulate nociceptive neurotransmission in the trigeminocervical complex. Br. J. Pharmacol. 2003;138:317–324. doi: 10.1038/sj.bjp.0705034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., GOADSBY P.J. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br. J. Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120:2171–2177. doi: 10.1093/brain/120.12.2171. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience. 1999;90:1371–1376. doi: 10.1016/s0306-4522(98)00536-3. [DOI] [PubMed] [Google Scholar]
- STORER R.J., GOADSBY P.J. Topiramate inhibits trigeminovascular neurons in the cat. Cephalalgia. 2004;24:1049–1056. doi: 10.1111/j.1468-2982.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., EDVINSSON L. Neuropeptides in the cerebral circulation. Cerebrovasc. Brain Metab. Rev. 1989;1:230–252. [PubMed] [Google Scholar]
- WEI E.P., MOSKOWITZ M.A., BOCCALINI P., KONTOS H.A. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ. Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- WIECZORKIEWICZ-PLAZA A., PLAZA P., MACIEJEWSKI R., CZUCZWAR M., PRZESMYCKI K. Effect of topiramate on mechanical allodynia in neuropathic pain model in rats. Pol. J. Pharmacol. 2004;56:275–278. [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J. Neurogenic inflammation in the context of migraine. Microsc. Res. Tech. 2001;53:167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural blood vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HILL R.G., SHEPHEARD S.L., HARGREAVES R.J. The anti-migraine 5-HT1B/1D agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br. J. Pharmacol. 2001a;133:1029–1034. doi: 10.1038/sj.bjp.0704162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., COOK D.A., HARGREAVES R.J., HILL R.G., CUMBERBATCH M.J. Role of opioid receptors in neurogenic dural vasodilation and sensitization of trigeminal neurones in anaesthetized rats. Br. J. Pharmacol. 2001b;133:807–814. doi: 10.1038/sj.bjp.0704136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D.J., SHEPHEARD S.L., HILL R.G., HARGREAVES R.J. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur. J. Pharmacol. 1997c;328:61–64. doi: 10.1016/s0014-2999(97)83028-2. [DOI] [PubMed] [Google Scholar]