Abstract
We have investigated whether the activation of cAMP- and cGMP-dependent pathways modifies the properties of voltage-dependent Ba2+ currents (IBa) recorded from guinea-pig gastric myocytes using patch-clamp techniques. All experiments were carried on single smooth muscle cells, dispersed from the circular layer of the guinea-pig gastric antrum.
Both dibutyryl cAMP (db-cAMP, 0.1–1 mM), a membrane-permeable ester of cAMP, and isoproterenol, a selective β-stimulant, inhibited IBa in a concentration-dependent manner.
Forskolin, but not dideoxy-forskolin, an inactive isomer of forskolin, inhibited the peak amplitude of IBa.
In the presence of either Rp-cAMP or the PKA (cAMP-dependent protein kinase) inhibitor peptide 5-24 (PKA-IP), neither forskolin nor db-cAMP inhibited IBa.
After establishing a conventional whole-cell recording, the peak amplitude of IBa gradually decreased when the catalytic subunit of PKA was included in the pipette. The further application of Rp-cAMP reversibly enhanced IBa.
Sodium nitroprusside (0.1–1 mM) and 8-Br-cGMP (0.1–1 mM) also inhibited IBa in a concentration-dependent manner.
The inhibitory effects of forskolin or db-cAMP on IBa were not significantly changed by pretreatment with a cGMP-dependent protein kinase (PKG) inhibitor. Similarly, the inhibitory actions of 8-Br-cGMP on IBa were not modified by PKA-IP.
The membrane-permeable cyclic nucleotides db-cAMP and 8-Br-cGMP caused little shift of the voltage dependence of the steady-state inactivation and reactivation curves.
Neither of the membrane-permeable cyclic nucleotides db-cAMP or 8-Br-cGMP had additive inhibitory effects on IBa.
These results indicate that two distinct cyclic nucleotide-dependent pathways are present in the guinea-pig gastric antrum, and that both inhibited IBa in an independent manner.
Keywords: Cyclic nucleotide, gastrointestinal smooth muscle, voltage-dependent calcium channels
Introduction
Applied catecholamines relax most regions of the gastrointestinal tract. As an example, in the stomach, adrenergic receptor stimulation causes a relaxation that is associated with a reduction in the amplitude and duration of slow waves (Bülbring & Tomita, 1987). Much of the relaxation results from the stimulation of β-adrenoceptors but which second messenger cascade is involved remains unclear. In cardiac muscle, applied catecholamines activate β-adrenoceptors, which in turn stimulates adenylate cyclase, thus increasing the formation of cAMP. The increased levels of cAMP stimulate cAMP-dependent protein kinase (PKA), leading to the phosphorylation of voltage-dependent Ca2+ channels (Trautwein & Hescheler, 1990; Hartzell et al., 1991). Consequently, the heart rate and force of contraction, associated with each heartbeat, are increased. It is unclear whether a similar cAMP/PKA cascade regulates voltage-dependent Ca2+ channels in gastric smooth muscle and, if so, how the activation of such a pathway leads to inhibition. Surprisingly despite the inhibitory effects of β-adrenoceptor stimulation on many smooth muscles, activation of the cAMP/PKA cascade frequently enhances the opening of voltage-dependent Ca2+ channels in these muscles. Thus in low concentrations of forskolin, which directly stimulates adenylate cyclase, or db-cAMP, a membrane-permeable analogue of cAMP, increase the amplitudes of voltage-dependent Ca2+ currents. However, high concentrations of either agent inhibited voltage-dependent Ca2+ currents (Koh & Sanders, 1996). In cultured rat mesenteric artery cells, cAMP analogues enhanced the activity of L-type Ca2+ channels (Taguchi et al., 1997). Similarly, L-type Ca2+ channels activity in portal vein smooth muscle was enhanced by increased levels of cAMP (Ishikawa et al., 1993).
A second cyclic nucleotide pathway is also present in many smooth muscles. This pathway is activated by NO and leads to the formation of cGMP. L-type Ca2+ channel activity is inhibited by cGMP/cGMP-dependent protein kinase (PKG) stimulation in a wide variety of smooth muscles (canine colon, Koh & Sanders, 1996; human coronary artery, Quignard et al., 1997; rabbit portal vein, Ruiz-Velasco et al., 1998). This is thought to result from PKG causing the phosphorylation of an inhibitory binding site associated with L-type Ca2+ channels (Ruiz-Velasco et al., 1998). It has been suggested that cAMP may also modulate the cGMP-dependent pathway, thus explaining the inhibitory effects of β-adrenoceptor activation. This interaction is referred to as the ‘crossactivation of kinase hypothesis' (Koh & Sanders, 1996; Ruiz-Velasco et al., 1998).
In the present experiments, we have obtained cells from circular layer of guinea-pig gastric antrum in which the cAMP/PKA and cGMP/PKG cascades inhibited IBa recorded with patch-clamp techniques. We have further studied whether or not cyclic nucleotides (cAMP and cGMP) cause a crossaction mediated by the opposing kinases for the inhibitory effects on IBa, by determining channel kinetics.
Methods
Cell dispersion
Guinea-pigs of either sex were stunned, exsanguinated, and the stomach was removed. Briefly, the antral region was isolated and immersed in nominally Ca2+-free solution (mM): Na+ 140, K+ 5, Mg2+ 0.5, Cl− 146, HEPES 10/Tris, titrated to pH 7.35–7.40. After removing the longitudinal muscle layer and mucosa, the circular muscle layer was dissected free. Guinea-pig gastric antrum myocytes were freshly isolated by the gentle tapping method after treatment with collagenase (Sigma Chemical K.K., Tokyo, Japan, Type I, 1 mg ml−1), as described previously (Teramoto & Brading, 1996). Relaxed spindle-shaped cells were isolated and stored at 4°C. The dispersed cells were used within 4–5 h for experiments.
Recording procedure
Patch-clamp experiments were performed at room temperature (21–23°C), as described previously (Teramoto et al., 2001). Junction potentials between bath and pipette solutions were measured with a 3 M KCl reference electrode and were <2 mV, so that correction for these potentials was not necessary. Capacitance noise was kept to a minimum by maintaining the test solution in the electrode as low as possible.
Drugs and solutions
To record IBa in whole-cell configuration, pipettes containing a high concentration of caesium were used, the composition of the pipette solutions was (mM): Cs+ 130, tetraethylammonium (TEA+) 10, Mg2+ 2, Cl− 144, glucose 5, EGTA 5, ATP 5, HEPES 10/Tris (pH 7.35–7.40). The bath solution contained (mM): Ba2+ 10, TEA+ 135, Cl− 155, glucose 10, HEPES 10/Tris (pH 7.35–7.40). The bath solution was superfused by gravity throughout the experiments at a rate of 2 ml min−1. All drugs were obtained from Sigma Chemical (Sigma Chemical K.K., Tokyo, Japan). Nifedipine, forskolin and dideoxy-forskolin were prepared as 10 mM stock solutions in dimethyl sulphoxide (DMSO). The final concentration of DMSO was less than 0.3%, and this concentration was shown not to affect IBa in guinea-pig gastric myocytes.
Data analysis
The whole-cell current data were low-pass filtered at 1 kHz by an eight-pole Bessel filter, sampled at 1 ms and analysed on a computer (PowerMac G4, Tokyo, Japan) by the commercial software ‘MacLab 3.5.6' (ADInstruments Pty Ltd, Castle Hill, Australia). In order to obtain precise component of inward IBa, the method for subtraction of the leak and capacitive currents was performed to subtract IBa in the presence of 100 μM Cd2+ from IBa (Teramoto et al., 2001).
Conditioning pulses of various amplitudes were applied (up to +30 mV, 10 s duration) before application of the test pulse (to +10 mV, 1 s duration). An interval of 20 ms was allowed between these two pulses to eliminate possible contamination by the capacitive current. The peak amplitude of IBa evoked by each test pulse was measured before and after application of drugs. The peak amplitude of IBa in the absence and presence of drugs without application of any conditioning pulse was normalized as one. The lines were draw by fitting the data to the following equation in the least-squares method:
where I, Imax, V, Vhalf, k and C are the relative amplitude of IBa observed at various amplitude of the conditioning pulse (I) and observed with application of the conditioning pulse of −70 mV (Imax), amplitude of the conditioning pulse (V), and that where the amplitude of IBa was reduced to half (Vhalf), slope factor (k) and fraction of the noninactivating component of IBa (C).
Activation curves were derived from the current–voltage relationships. Conductance (G) was calculated from the equation G=IBa/(Em−EBa), where IBa is the peak current elicited by depolarizing test pulses from −60 to 40 mV from holding membrane potential of −70 mV and EBa is the equilibrium potential for Ba2+. Gmax is the maximal Ba2+ conductance (calculated at potentials above 10 mV). The points for G/Gmax were plotted against the membrane potential as relative amplitudes.
Statistics
Data are expressed as mean with the standard deviation (s.d.). Statistical analyses were performed with a two-paired t-test (two-factor with replication). Changes were considered significant at P<0.05.
Results
General observations
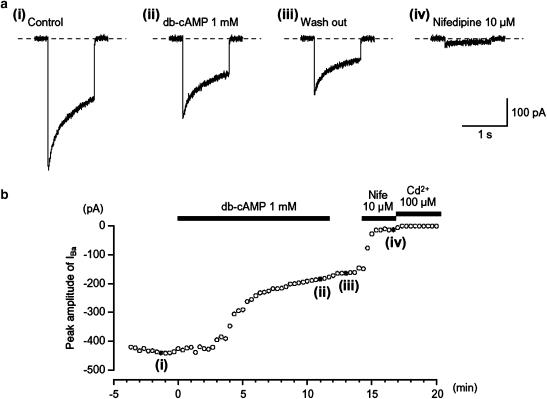
In order to enhance the amplitudes of inward currents for analysis and to isolate voltage-dependent inward currents through Ca2+ channels, other Ca2+-activated mechanisms (such as Ca2+-activated K+ currents and Ca2+-activated Cl− currents, etc.) were inhibited by using 10 mM Ba2+ bath solution containing 135 mM TEA+ and the recording pipette was filled with a Cs+-TEA+ solution containing 5 mM EGTA. Application of depolarizing step to +10 mV from a holding potential of −70 mV produced IBa in guinea-pig gastric myocytes, using a conventional whole-cell configuration (Figure 1a(i)). After establishing a conventional whole-cell configuration, IBa evoked by a depolarizing pulse of +10 mV from −70 mV increased slightly in amplitude until a steady-state level of IBa was reached four minutes after the rapture of the membrane patch (n=10). This peak value was then maintained for 25 min when test depolarizations were applied at 20 s intervals, with the peak amplitude of IBa at 25 min being 0.97±0.04 (n=12) of the value determined at 4 min. Consequently, all experiments were performed within 25 min of the establishment of conventional whole-cell configuration. The voltage-activated current was dominantly carried by L-type Ca2+ channels. Thus, the current was reduced to less than 7% of its control value (0.07±0.03, n=15) by adding nifedipine 10 μM to the bath solution and had an activation potential near −30 mV (Figure 1a(iv) and Figure 3). Similarly, IBa was inactivated when the membrane potential was stepped to voltages positive of −50 mV from a holding membrane potential of −70 mV, with the half inactivation potential (Vhalf) being estimated to be approximately −38 mV. Similar observations have been made on L-type Ca2+ channels in a wide range of gastrointestinal smooth muscle cells (Farrugia, 1999). In each experiment, the small inward current that persisted in the presence of nifedipine also appeared to result from Ca2+ entry since it was readily abolished by adding Cd2+ to the bath solution. Since the peak amplitude of nifedipine-insensitive IBa was too small to estimate precisely in comparison to that of nifedipine-sensitive IBa, the nifedipine-insensitive IBa component was ignored throughout the present study.
Figure 1.
Effects of db-cAMP (1 mM) on IBa using conventional whole-cell recording from an isolated gastric antral myocyte. The upper four traces show (a) inward currents, elicited by voltage steps in control solution ((a) (i)), after the application of db-cAMP ((a) (ii)), following washout of db-cAMP ((a) (iii)) and in the presence of nifedipine ((a) (iv)). The cell capacitance was 51 pF. (b) The time course of inhibition of the peak amplitude of IBa by db-cAMP (1 mM) is shown. Time 0 indicates the time when db-cAMP was applied. The inhibition produced by db-cAMP was not reversed by washing with drug-free solution. The application of 10 μM nifedipine inhibited most of the db-cAMP-resistant current. The nifedipine-resistant current was suppressed by Cd2+ (100 μM). In each experiment, inward currents were elicited by voltage steps (1 s duration) to +10 mV from a holding potential of −70 mV every 20 s.
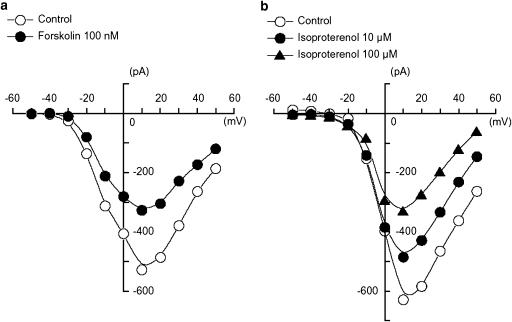
Figure 3.
Effects of forskolin (100 nM) and isoproterenol (10 and 100 μM) on the peak amplitude of IBa using conventional whole-cell recording. (a) Effects of forskolin (100 nM) on the peak amplitude of IBa. The current–voltage relationships were obtained in the absence (control) or presence of 100 nM forskolin. The current amplitude was measured as the peak amplitude of IBa in each condition. The lines were drawn by eye. The cell capacitance was 43 pF. (b) Effects of isoproterenol on the peak amplitude of IBa. The current–voltage relationships were shown in the absence and presence of isoproterenol (10 and 100 μM). Isoproterenol also inhibited the peak amplitude of IBa evoked by depolarizing pulses (1 s duration) from −70 mV at levels more positive than −30 mV and the inhibitory effects of isoproterenol on IBa showed a voltage- and concentration dependency. The lines were drawn by eye. The cell capacitance was 52 pF.
Effects of dibutyryl-cAMP and forskolin on IBa in guinea-pig gastric myocytes
Application of 1 mM dibutyryl-cAMP (db-cAMP), a cell membrane permeant analogue of cAMP, gradually reduced the amplitude of IBa, without changing either the threshold or kinetics of the current. After approximately 6 min, the inhibitory effects of db-cAMP reached a steady-state value with the peak amplitude of IBa being 0.61±0.09 (n=6) of the control value (Figure 1a(ii)). The time course of the effects of 1 mM db-cAMP is shown in Figure 1b, where IBa was evoked by a depolarizing pulse of +10 mV from −70 mV. The inhibitory effect of db-cAMP on IBa was not reversed by washing with drug-free solution (Figure 1a(iii)).
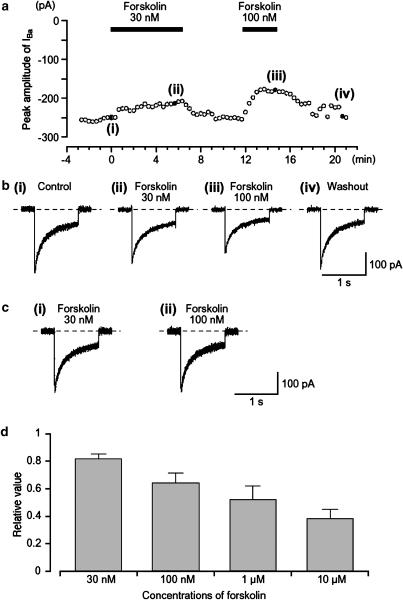
Forskolin, a direct activator of adenylate cyclase, caused a concentration-dependent reversible reduction of IBa. Figure 2a shows the time course of the effects of forskolin (30–100 nM) on IBa evoked by a depolarizing pulse of +10 mV from −70 mV. The inactive isomer of forskolin, dideoxy-forskolin (1 μM), which does not stimulate adenylate cyclase, had no effect on IBa, with the peak amplitude of IBa being 0.98±0.02 (n=5) of the control value although forskolin 1 μM was active in each preparation (0.52±0.10, n=5). Even a high concentration of dideoxy-forskolin, 10 μM, failed to inhibit IBa (data not shown).
Figure 2.
Effects of forskolin (30 and 100 nM) on IBa in a conventional whole-cell recording. (a) The time course of inhibition of the peak amplitude of IBa produced by the application of forskolin (30 and 100 nM) is shown. Time 0 indicates the time when 30 nM forskolin was applied. Inward current were elicited by voltage steps (1 s duration) to +10 mV from a holding potential of −70 mV every 20 s. The cell capacitance was 39 pF. (b) Examples of inward current traces recorded at the indicated points in (a) are shown in ((b) (i)–(iv)). (c) The time course of the current decay IBa was identical when the current traces of IBa in the absence and presence of forskolin (30 and 100 nM) were superimposed. (d) It shows the relationship between different concentrations of forskolin and the normalized peak amplitude of IBa when the peak amplitude of IBa was taken as one just before application of each concentration of forskolin; each column indicates the mean of 4–9 observations with +s.d. shown by vertical lines.
Depolarizing step pulses of duration 1 s were applied in 10 mV increments from −50 to +50 mV from a holding potential of −70 mV. At potentials more positive than −30 mV, an inward IBa was evoked (Figure 3). The maximum peak amplitude was obtained at approximately +10 mV and the amplitude was reduced at more positive potentials. The inward IBa was reduced by forskolin (100 nM) at all values of membrane potential. An experiment is illustrated in Figure 3a, which compares the current–voltage relationships in control solution and 5 min after forskolin was applied to the bath. In the presence of 100 nM forskolin, the peak amplitude was reduced. These observations suggest cAMP analogues and cAMP produced within the cells directly inhibit IBa in guinea-pig gastric antrum myocytes. Figure 3b shows the current–voltage relationships in the absence and presence of isoproterenol (10 and 100 μM). Isoproterenol reduced the peak amplitude of IBa evoked by depolarizing pulses (1 s duration) from a holding potential of −70 mV at levels more positive than −30 mV. The inhibitory effects of isoproterenol on IBa showed a voltage- and concentration-dependency.
Effects of forskolin on IBa in the presence of specific cAMP-dependent protein kinase inhibitors
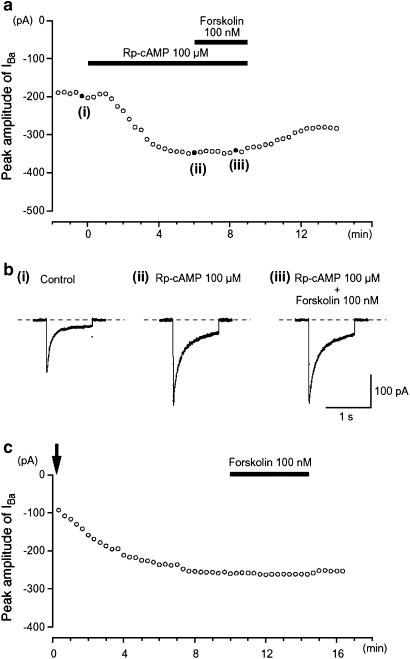
To further investigate the effects of activating the cAMP/PKA pathway on IBa, two different types of specific PKA inhibitors (i.e. Rp-cAMP or PKA inhibitor peptide 5-24 (PKA-IP)) were used. Extracellular application of 100 μM Rp-cAMP, a cAMP membrane-permeable analogue that inhibits PKA by binding to its regulatory subunit (Rothermel & Parker Botelho 1988), caused a gradual enhancement of IBa evoked by a depolarizing pulse of +10 mV from −70 mV (Figure 4a). After the peak amplitude of IBa became stable in the presence of Rp-cAMP, 100 nM forskolin had little effect on IBa. Similarly, 1 mM db-cAMP had no inhibitory effect on IBa in the presence of 100 μM Rp-cAMP (Figure 4b).
Figure 4.
Effects of forskolin on IBa in the presence of PKA inhibitors (Rp-cAMP and PKA-IP). The PKA inhibitor Rp-cAMP (100 μM) potentiated the amplitude of IBa and abolished the inhibitory effect of 100 nM forskolin. (a) Inward current were again elicited by voltage steps (1 s duration) to +10 mV from a holding potential of −70 mV every 20 s. Typical current traces are shown in (b), at the points ((i)–(iii)) indicated in (a). The cell capacitance was 36 pF. The change in the peak amplitude of IBa as a function of time caused by 100 nM forskolin when PKA-IP (1 μM) was included in the pipette solution is shown in (c). Time 0 indicates the time when a conventional whole-cell configuration was established. The cell capacitance was 34 pF.
When PKA-IP (1 μM) was included in the pipette solution, the peak amplitude of IBa gradually increased after establishment of a conventional whole-cell mode. As shown in Figure 4c, after approximately 10 min, when the peak amplitude IBa had become stable, application of forskolin (100 nM) had no obvious effect on IBa evoked by a depolarizing pulse to +10 mV from −70 mV (0.97±0.03, n=6; Figure 4c). Similarly, db-cAMP (0.1 and 1 mM) had little effect on IBa when PKA-IP (1 μM) had been included in the pipette solution (0.1 mM, 0.96±0.04, n=4; 1 mM, 0.92±0.04, n=5; see Figure 4c). These results suggest that the reduction of IBa is likely to occur due to PKA-dependent phosphorylation of Ca2+ channels in guinea-pig gastric antrum myocytes.
Effects of dialyzing cells with the catalytic subunit of PKA
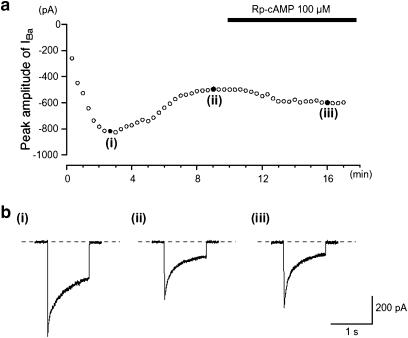
As pointed out previously, the amplitude of IBa evoked by a depolarizing pulse of +10 mV from −70 mV initially increased slightly to reach a steady-state level 4 min after establishing whole-cell recording mode (n=10). When the catalytic subunit of PKA (125 U ml−1) was included in the patch pipette, the peak amplitude of IBa, evoked by a depolarizing pulse of +10 mV from −70 mV, gradually decreased in amplitude to reach a minimum value (Figure 5a, 61% reduction (0.66±0.06, n=6) after 8 min. In the experiment shown in Figure 5, the amplitude remained stable for the next 3 min (Figure 5b(i), (ii)), and after this time, the inhibition was partially reversed by the application of Rp-cAMP (Figure 5b(iii)).
Figure 5.
Effects of dialysing cells with the catalytic subunit of PKA on IBa. The cell capacitance was 49 pF. (a) The time-dependent changes observed in IBa using patch pipette, which contained the catalytic subunit of PKA (125 U ml−1), are shown. The ordinate scale shows the peak amplitude of IBa evoked by a depolarization pulse (1 s duration) from a holding potential of −70 mV every 20 s. The abscissa scale indicates the time after formation of a conventional whole-cell recording. (b) Sample traces shows IBa recorded at the indicated points ((i)–(iii)) in (a).
cGMP-mediated inhibitory effects on IBa in guinea-pig gastric antrum
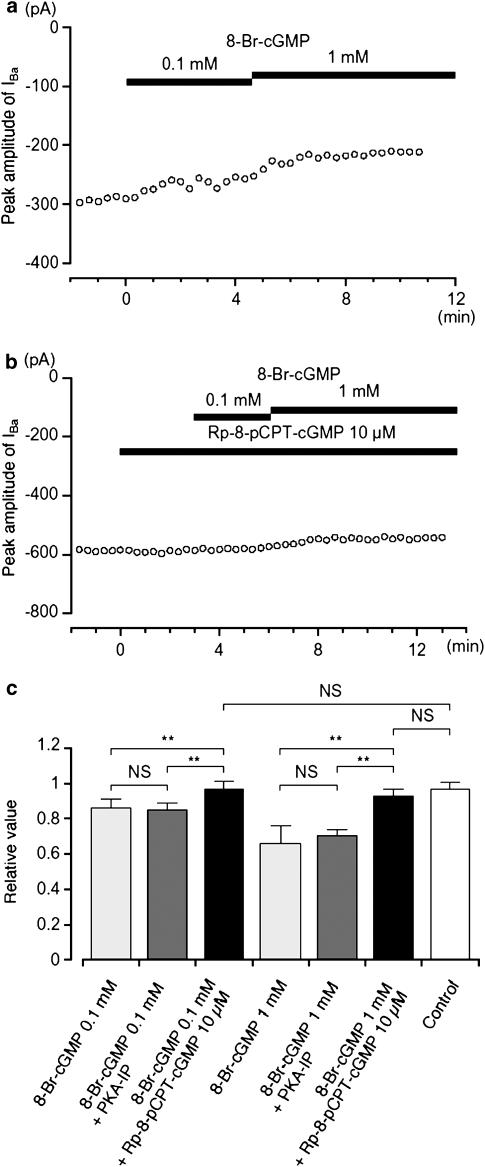
8-Br-cGMP, a membrane permeant analogue of cGMP, also inhibited the amplitude of IBa, evoked by a depolarizing pulse of +10 mV from −70 mV (Figure 6a). The cumulative application of 8-Br-cGMP (0.1 and 1 mM) caused a reduction in IBa (0.1 mM, 0.86±0.05, n=5; 1 mM, 0.66±0.1, n=4) when the peak amplitude of IBa was normalized to one before application of 8-Br-cGMP. Sodium nitroprusside (SNP), a nitric oxide donor, also inhibited IBa in a concentration-dependent manner (0.1 mM, 0.84±0.07, n=4; 1 mM, 0.61±0.11, n=4). Rp-8-pCPT-cGMP (10 μM), a specific PKG inhibitor, did not affect the peak amplitude of IBa after 3 min (0.98±0.02, n=10, when the peak amplitude of IBa was normalized as one before application of Rp-8-pCPT-cGMP). However when cells were pretreated with Rp-8-pCPT-cGMP, the effects of 8-Br-cGMP (0.1 and 1 mM) were entirely abolished (0.1 mM, 0.97±0.04, n=4; 1 mM, 0.93±0.04, n=5, Figure 6b). These results, summarized in Figure 6c, suggest that the inhibition of IBa by 8-Br-cGMP is mainly due to the activation of PKG.
Figure 6.
Effects of 8-Br-cGMP on IBa using conventional whole-cell recording. (a) The time course of changes in the peak amplitude of IBa was shown before and after application of 8-Br-cGMP (0.1 and 1 mM). Time 0 indicates the time when 0.1 mM 8-Br-cGMP was applied. The cell capacitance was 42 pF. (b) Similarly, it shows that the peak amplitude of IBa was little changed by the application of 8-Br-cGMP (0.1 and 1 mM) in the presence of Rp-8-pCPT-cGMP. Time 0 indicates the time when 10 μM Rp-8-pCPT-cGMP was applied. The cell capacitance was 59 pF. (c) The effects are summarized. It can be seen that the selective inhibitor of PKG only inhibited the effects of cGMP analogues, and that the selective inhibitor of PKA only inhibited the effects of cAMP, with no cross interaction being detected. Each column shows the mean of 3–5 observations with +s.d. shown by vertical lines. Asterisks indicate a statistically significant difference, demonstrated using a paired t-test (**P<0.01).
To rule out the possibility that the inhibitory effects of cGMP on IBa was due to a ‘crossover' activation of PKA, the effects of 8-Br-cGMP were tested when PKA had been blocked by including PKA-IP (1 μM) in the pipette solution. Approximately 12 min after establishing a conventional whole-cell recording, application of 8-Br-cGMP (0.1 and 1 mM) continued to cause a concentration-dependent inhibitory effect on IBa, with the 8-Br-cGMP-induced inhibition of IBa being not significantly different in the absence or presence of PKA-IP (Figure 6c). This suggests that cGMP has little ability to activate PKA.
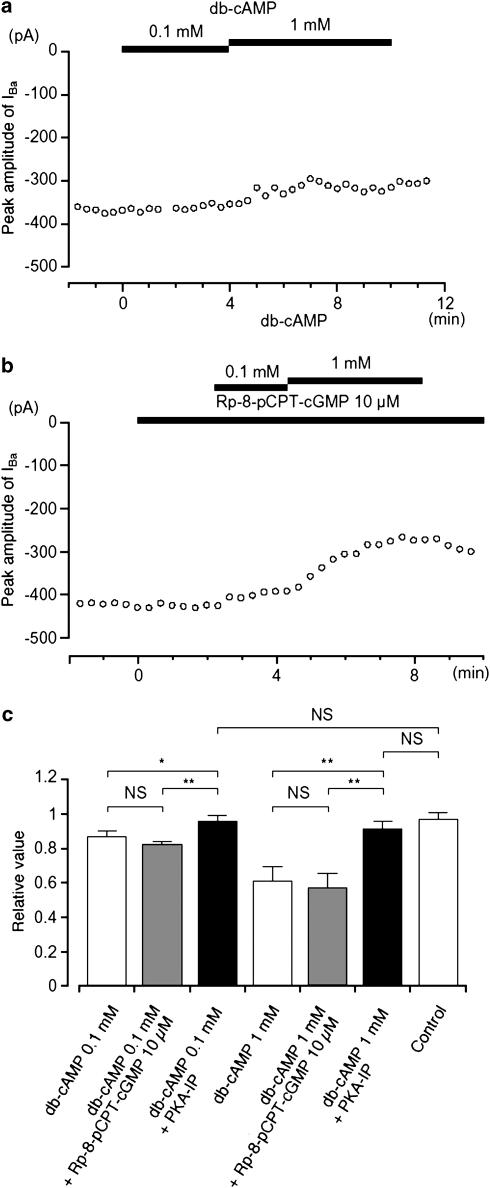
The inhibitory action of cAMP on gastric motility has also been suggested to result from its ability to crossactivate PKG, thus inhibiting L-type Ca2+ channels (Ruiz-Velasco et al., 1998). Additional experiments were therefore performed to investigate whether such a crossover action could be identified in guinea-pig gastric antrum. It was found that db-cAMP (0.1 and 1 mM) had little inhibitory effect on IBa when PKA-IP (1 μM) was included in the pipette solution (Figure 7a), suggesting that it did not activate PKG. Under control conditions, 10 μM Rp-8-pCPT-cGMP had no effect on IBa and when db-cAMP (0.1 and 1 mM) was applied cumulatively in the presence of Rp-8-pCPT-cGMP, it continued to suppress the peak amplitude of IBa in a concentration-dependent manner (Figure 7b). The relative inhibitory ratio of IBa by db-cAMP was not significantly different in the absence or presence of Rp-8-pCPT-cGMP. These results are summarized in Figure 7c.
Figure 7.
Effects of db-cAMP (0.1 and 1 mM) on IBa using conventional whole-cell recording. (a) The time course of changes in the peak amplitude of IBa before and after application of db-cAMP (0.1 and 1 mM) when PKA-IP (1 μM) was included in the pipette solution. Time 0 indicates the time when 0.1 mM db-cAMP was applied. The cell capacitance was 36 pF. (b) Similarly, it shows that the peak amplitude of IBa was little changed by the application of db-cAMP (0.1 and 1 mM) in the presence of Rp-8-pCPT-cGMP. Time 0 indicates the time when 10 μM Rp-8-pCPT-cGMP was applied. The cell capacitance was 42 pF. (c) The effects are summarized. It can be seen that the selective inhibitor of PKA only inhibited the effects of cAMP analogues and that the selective inhibitor of PKG only inhibited the effects of cGMP, with no cross interaction being detected. Each column shows the mean of 3–6 observations with +s.d. shown by vertical lines. Asterisks indicate a statistically significant difference, demonstrated using a paired t-test (*P<0.05, **P<0.01).
In order to further investigate additive inhibitory effect of cyclic nucleotides on IBa, firstly, db-cAMP was applied and subsequently, 8-Br-cGMP was applied. Similar experiments were also performed to apply cyclic nucleotides vice versa. Application of db-cAMP (1 mM) caused a significant inhibition of the peak amplitude of IBa (0.64±0.06, n=6). Approximately 5 min later, the additional application of 8-Br-cGMP (1 mM) did not cause a further significant inhibition of IBa (0.56±0.05, n=6). Similarly, the addition of 1 mM db-cAMP did not alter IBa (0.57±0.06, n=6) recorded in the presence of 1 mM 8-Br-cGMP (0.62±0.05, n=6). These results suggest that membrane-permeable cyclic nucleotides do not have additive inhibitory effects on IBa.
Effects of cyclic nucleotides on channel kinetics of IBa
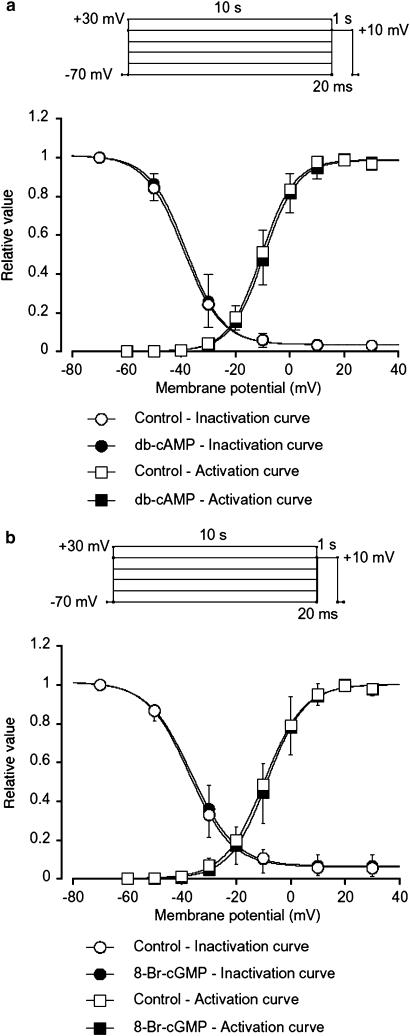
The inhibitory effects of cyclic nucleotides (db-cAMP and 8-Br-cGMP) were analysed by determining the steady-state inactivation and activation curves for IBa in guinea-pig antrum. Voltage-dependent inactivation was investigated before and after application of cyclic nucleotides using the experimental protocol shown in Figure 8 (conditioning pulse duration, 10 s; holding membrane potential, −70 mV). In the absence of cyclic nucleotide (control), inactivation of IBa occurred with depolarizing pulses positive to −50 mV. In the presence of 1 mM db-cAMP (approximately 5 min later), the voltage-dependent inactivation curve was not shifted (Figure 8a). The 50% inactivation potentials, evaluated by means of Boltzmann fitting, were −38 mV (control, n=6) and −39 mV (db-cAMP, n=6), respectively. The activation curves obtained from the current–voltage relationships, fitted to the Boltzmann equation, are shown in Figure 8a. Application of db-cAMP (1 mM) caused little shift of the activation curve (the 50% activation potentials; −10 mV (control) vs −11 mV (db-cAMP), n=6, P<0.05, Figure 8a). As shown in Figure 8b, both the inactivation and the activation curves were not significantly shifted by 8-Br-cGMP (1 mM).
Figure 8.
Effects of cyclic nucleotides (db-cAMP and 8-Br-cGMP) on the voltage-dependent activation and inactivation of IBa in guinea-pig antrum. Whole-cell recording, pipette solution Cs+-TEA+ solution containing 5 mM EGTA and the bath solution 10 mM Ba2+ containing 135 mM TEA+. Steady-state inactivation curves, obtained in the absence (control) and presence of cyclic nucleotides, were fitted to the Boltzmann equation. Peak current values were used. The steady-state inactivation curve was obtained using the double-pulse protocol (see Methods). The current measured during the test pulse is plotted against membrane potential and expressed as relative amplitude. Activation curves were obtained from the current–voltage relationships, fitting to the Boltzmann equation (see Methods). (a) The steady-state inactivation curves in the absence or presence of db-cAMP (1 mM) were drawn using the following values: (control), Imax=1.0, Vhalf=−38, k=7.0 and C=0.04; (db-cAMP, 1 mM), Imax=1.0, Vhalf=−39, k=7.0 and C=0.04. Each symbol indicates the mean of six observations with±s.d. shown by vertical lines. Some of the s.d. bars are less than the size of the symbol. (b) The steady-state inactivation curves in the absence or presence of 8-Br-cGMP (1 mM) were drawn using the following values: (control), Imax=1.0, Vhalf=−36, k=8.0 and C=0.06; (8-Br-cGMP, 1 mM), Imax=1.0, Vhalf=−37, k=7.8 and C=0.06. Each symbol indicates the mean of six observations with±s.d. shown by vertical lines. Some of the s.d. bars are less than the size of the symbol.
Discussion
The present experiments demonstrate that cAMP directly inhibits IBa in gastric smooth muscle cells. A similar inhibition was produced by cGMP but no interaction between either pathway could be demonstrated. Surprisingly, given the widespread inhibitory actions of β-adrenoceptor agonists on gastrointestinal motility (Kuriyama et al., 1998), cAMP has previously only been shown to facilitate the opening of L-type Ca2+ channels in gut smooth muscle cells (Koh & Sanders, 1996). When inhibition of L-type Ca2+ channels has been detected previously, it has been attributed to the activation of PKG (Koh & Sanders, 1996; Ruiz-Velasco et al., 1998). We have been unable to detect this type of interaction in smooth muscle cells from the gastric antrum. Indeed inhibition of PKG had no effect on the inhibitory effect of cAMP (Figure 7). As several workers have shown previously, we found that cGMP directly inhibited L-type Ca2+ channels but this effect did not rely on a functional PKA pathway.
Regulatory mechanisms of voltage-dependent Ca2+ currents by cAMP/PKA cascade in gastrointestinal smooth muscle
The observations on gastric antral smooth muscle cells suggest that there are many regional variations in the way in which cAMP might modify gut motility. In the canine colon, an increase in cAMP, produced by stimulating adenylate cyclase with forskolin, potentiates L-type Ca2+ channel activity, in much the same way as it does in cardiac myocytes (Trautwein & Hescheler, 1990; Koh & Sanders, 1996; Davila, 1999). In other intestinal smooth muscles, cAMP (or db-cAMP) had no effect on the amplitudes of voltage-dependent Ca2+ currents generated by L-type Ca2+ channels (rabbit jejunum, Ohya et al., 1987; guinea-pig taeniacoli, Muraki et al., 1993). An inhibition of L-type Ca2+ channels was proposed in guinea-pig gastric smooth muscle (Ozaki et al., 1992). Several inhibitory agonists that activate adenylate cyclase (such as β-adrenergic stimulators, forskolin, VIP, CGRP, etc.) were found to decrease the increases in [Ca2+]i associated with each slow wave and inhibit contraction. Although it was suggested that this might result from an inhibition of Ca2+ influx during each slow wave (Ozaki et al., 1992), a direct test of this idea was not attempted.
In the present experiments, (1) Forskolin, even at a low concentration, but not dideoxy-forskolin, was found to inhibit IBa in a concentration-dependent manner. (2) db-cAMP (100 μM–1 mM) inhibited IBa in a concentration-dependent manner. (3) In the presence of specific PKA inhibitors (Rp-cAMP or PKA-IP), application of forskolin or db-cAMP showed no inhibitory effect on IBa. (4) The amplitude IBa gradually decreased when the catalytic subunit of PKA was included in the pipette after the establishment of a conventional whole-cell recording and additional application of Rp-cAMP reversibly enhanced IBa towards the control level. (5) Similarly, isoproterenol (1–100 μM) shows a concentration-dependent inhibitory effect on IBa in guinea-pig antrum. Since the effects of low concentrations of db-cAMP and forskolin are not mediated through the activation of G proteins (reviewed by Trautwein & Hescheler, 1990; Keef et al., 2001), we suggest that the increased cAMP production, in response to stimulation of adenylate cyclase, inhibits IBa via PKA-mediated phosphorylation in the guinea-pig stomach. The simplest explanation for our findings is that phosphorylation of voltage-dependent Ca2+ channels by PKA results in a reduction of Ca2+ influx via L-type Ca2+ channels. This implies that in the gastric antrum, unlike most other tissues, there must be an inhibitory phophorylation site on L-type Ca2+ channels.
Regulatory mechanisms of voltage-dependent Ca2+ currents by cGMP/PKG cascade in gastrointestinal smooth muscle
In the gastric antrum, as it has been shown in many smooth muscles, activation of PKG led to an inhibition of IBa. Thus, the L-type Ca2+ current in human coronary artery is inhibited by nitric oxide donors which lead to the intracellular production of cGMP (Quignard et al., 1997). Similar observations were made on portal vein myoctes (Ruiz-Velasco et al., 1998). In other studies, the inhibition of L-type Ca2+ channel activity by cAMP has been attributed to the crossactivation of a presumed inhibitory PKG pathway (Koh & Sanders, 1996), but these authors failed to demonstrate the existence of such a pathway in their tissues. In the present experiments, application of 8-Br-cGMP but also of SNP reduced IBa in a concentration-dependent manner. Moreover, in the presence of a specific PKG inhibitor (Rp-8-pCRT-cGMP), application of 8-Br-cGMP caused no significant effect on IBa. These results suggest that increases of cGMP also inhibited IBa via PKG-mediated phosphorylation in gastric smooth muscle, presumably by phophorylating a distinct site.
Crossactivation between cyclic nucleotides mediated by the opposing kinases for the inhibition of voltage-dependent Ca2+ currents
Our experiments were unable to detect an interaction between the two kinase pathways. Thus, the inhibitory effects of forskolin or db-cAMP on IBa were unaffected by the presence of a specific PKG inhibitor, which was shown in the same experiments to effectively block the PKG cascade. Similarly, the inhibitory effects of 8-Br-cGMP on IBa were unaffected by a specific PKA inhibitor, which was also shown in the same experiments to effectively block the PKA cascade. However, the additional application of 8-Br-cGMP had no further additive inhibitory effect on IBa recorded from cells pretreated with db-cAMP. Taken together, we suggest that a crossaction mediated by the opposing kinases for inhibition of IBa does not occur in guinea-pig gastric antrum myocytes.
Role of endogenous PKA and PKG pathways in control of gastric smooth muscle
Recent molecular studies have revealed that a PKA phosphorylation site exists in the α1 subunit which defines the ionic pore of Ca2+ channels in cardiac and smooth muscle L-type Ca2+ channels (reviewed by Keef et al., 2001). Additional studies also reported that intracellularly located β-subunits functionally regulate Ca2+ channels by PKA (Bunemann et al., 1999). These reports suggest that PKA phosphorylation sites play an important role for regulating the functional gating of Ca2+ channels. Moreover, recently, a physical link between L-type Ca2+ channels and PKA, through a PKA-anchoring protein named AKAP-15, has been identified, suggesting that the anchoring protein may be associated with the function of L-type Ca2+ channels (Gray et al., 1998). In the present experiments, application of Rp-cAMP significantly enhanced the peak amplitude IBa. On the other hand, when a specific PKG inhibitor (Rp-8-pCRT-cGMP) was applied, there was no significant effect on the peak amplitude of IBa. These results suggest that endogenous PKA but not PKG might continuously regulate the activity of the voltage-dependent Ca2+ channels in gastric circular layer smooth muscle cells. Presumably, the PKG pathway is only activated when the smooth muscle cells are exposed to NO. However, it is controversial whether this occurs physiologically. Several reports have suggested that neurally released NO acts selectively on intramuscular interstitial cells of Cajal (ICCIM, Ward et al., 2000; Suzuki et al., 2003; Teramoto & Hirst, 2003). This might suggest that the PKG pathway is redundant, which given the complexity of the pathway seems unlikely. Similarly, several reports have suggested that neurally released catecholamines act selectively on enteric neurons (Hirst & McKirdy, 1975; Furness & Costa, 1987). This would suggest that the PKG pathway is not upregulated by neurally released transmitter; again given the complexity of the pathway this also seems unlikely.
In summary, these experiments have shown that two distinct second messenger pathways, PKA and PKG, can regulate the activity of L-type Ca2+ channels. Both pathways when activated suppress the opening of the channels and both pathways appear to have their own independent loci of action.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Research Project of International Visiting Fellowship from the Japanese Society for the Promotion of Science and the Australia Academy of Science (Noriyoshi Teramoto, Grant Number 0401003). This work was also supported by both a Grant-in-Aid for Scientific Research (B)-(2) (Noriyoshi Teramoto, Grant Number 16390067) and a Grant-in-Aid for Exploratory Research (Noriyoshi Teramoto, Grant Number 17659075) from the Japanese Society for the Promotion of Science.
Abbreviations
- db-cAMP
dibutyryl cAMP
- DMSO
dimethyl sulphoxide
- ICCIM
intramuscular interstitial cells of Cajal
- PKA
cAMP-dependent protein kinase
- PKA-IP
PKA inhibitor peptide 5-24
- PKG
cGMP-dependent protein kinase
References
- BUNEMANN M., GERHARDSTEIN B.L., GAO T., HOSEY M.M. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the β2 subunit. J. Biol. Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- BÜLBRING E., TOMITA T. Catecholamine action on smooth muscle. Pharmacol. Rev. 1987;39:49–96. [PubMed] [Google Scholar]
- DAVILA H.M. Molecular and functional diversity of voltage-gated calcium channels. Ann. NY Acad. Sci. 1999;868:102–117. doi: 10.1111/j.1749-6632.1999.tb11281.x. [DOI] [PubMed] [Google Scholar]
- FARRUGIA G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Ann. Rev. Physiol. 1999;61:45–84. doi: 10.1146/annurev.physiol.61.1.45. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., COSTA M. The Enteric Nervous System. Edinburgh: Churchill-Livingstone; 1987. [Google Scholar]
- GRAY P.C., JOHNSON B.D., WESTENBROEK R.E., HAYS L.G., YATES J.R., III, SCHEUER T., CATTERALL W.A., MURPHY B.J. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;5:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- HARTZELL H.C., MERY P.F., FISCHMEISTER R., SZABO G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature. 1991;351:573–576. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- HIRST G.D.S., MCKIRDY H.C. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J. Physiol. 1975;249:369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA T., HUME J.R., KEEF K.D. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ. Res. 1993;73:1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- KEEF K.D., HUME J.R., ZHONG J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1.2a,b) by protein kinases. Am. J. Physiol. 2001;281:C1743–C1756. [Google Scholar]
- KOH S.D., SANDERS K.M. Modulation of Ca2+ current in canine colonic myocytes by cyclic nucleotide-dependent mechanisms. Am. J. Physiol. 1996;271:C794–C803. doi: 10.1152/ajpcell.1996.271.3.C794. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., ITOH T., INOUE R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- MURAKI K., BOLTON T.B., IMAIZUMI Y., WATANABE M. Effect of isoprenaline on Ca2+ channel current in single smooth muscle cells isolated from taenia of the guinea-pig caecum. J. Physiol. 1993;471:563–582. doi: 10.1113/jphysiol.1993.sp019916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYA Y., KITAMURA K., KURIYAMA H. Modulation of ionic currents in smooth muscle balls of the rabbit intestine by intracellularly perfused ATP and cyclic AMP. Pflügers Arch. 1987;408:465–473. doi: 10.1007/BF00585070. [DOI] [PubMed] [Google Scholar]
- OZAKI H., BLONDFIELD D.P., HORI M., SANDERS K.M., PUBLICOVER N.G. Cyclic AMP-mediated regulation of excitation–contraction coupling in canine gastric smooth muscle. J. Physiol. 1992;447:351–372. doi: 10.1113/jphysiol.1992.sp019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGNARD J.F., FRAPIER J.M., HARRICANE M.C., ALBAT B., NARGEOT J., RICHARD S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J. Clin. Invest. 1997;99:185–193. doi: 10.1172/JCI119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHERMEL J.D., PARKER BOTELHO L.H. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem. J. 1988;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ-VELASCO V., ZHONG J., HUME J.R., KEEF K.D. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ. Res. 1998;82:557–565. doi: 10.1161/01.res.82.5.557. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., WARD S.M., BAYGUINOV Y.R., EDWARDS F.R., HIRST G.D.S. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J. Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGUCHI K., UEDA M., KUBO T. Effects of cAMP and cGMP on L-type calcium channel currents in rat mesenteric artery cells. Jpn. J. Pharmacol. 1997;74:179–186. doi: 10.1254/jjp.74.179. [DOI] [PubMed] [Google Scholar]
- TERAMOTO N., BRADING A.F. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br. J. Pharmacol. 1996;118:635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., HIRST G.D.S. Interaction between inhibitory and excitatory metabotropic pathways in the guinea-pig antrum. J. Physiol. 2003;550:181–189. doi: 10.1113/jphysiol.2003.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., YUNOKI T., IKAWA S., TAKANO N., TANAKA K., SEKI N., NAITO S., ITO Y. The involvement of L-type Ca2+ channels in the relaxant effects of the ATP-sensitive K+ channel opener ZD6169 on pig urethral smooth muscle. Br. J. Pharmacol. 2001;134:1505–1515. doi: 10.1038/sj.bjp.0704408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTWEIN W., HESCHELER J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu. Rev. Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- WARD S.M., BECKETT E.A., WANG X., BAKER F., KHOYI M., SANDERS K.M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]