Abstract
Previous studies have shown antifibrotic effects of somatostatin. Since hepatic stellate cells (HSC) express somatostatin receptors and play a key role in hepatic fibrogenesis, we investigated the in vitro antifibrotic effect of somatostatin on rat HSC.
At day 12 after isolation, cells were exposed to different concentrations of somatostatin (10−6–10−9 mol l−1).
mRNA expression of collagen types I and III, and of smooth muscle α-actin (α-SMA) was analysed by Northern blotting. At 10−9 mol l−1, somatostatin significantly reduced mRNA expression of collagen I (72.3±10.7%; 95% confidence interval (95% CI): 45.5–99.0), collagen III (79.0±4.5%; 95% CI: 67.6–90.4) and α-SMA (65.7±5.9%; 95% CI: 51.1–80.2), as compared to control normalized at 100%. These results were confirmed by quantitative RT–PCR.
Cycloheximide experiments indicated that somatostatin has no direct transcriptional effect.
Using immunoprecipitation, we demonstrated that somatostatin also decreased de novo synthesis of collagen I (73±10%; 95% CI: 48–98%), collagen III (65±13%; 95% CI: 33–97%) and α-SMA (47±9%; 95% CI: 25–69%). Remarkably, at higher concentrations, somatostatin did not suppress collagen mRNA expression nor de novo protein synthesis. We ascribe this observation to desensitization of the cells for somatostatin.
Cell proliferation, as measured by 5-bromo-2′-deoxyuridine labelling, was not altered by somatostatin.
No significant effect on the intermediate and actin cytoskeleton were detected by immunohistochemistry and Western blotting.
Our findings imply that in vivo antifibrotic effects of somatostatin could result partially from a direct action of somatostatin on HSC, but other, in vivo effects are probably also involved.
Keywords: Fibrosis, collagen, cirrhosis, hepatic stellate cells, somatostatin, somatostatin receptors
Introduction
In the past 20 years, hepatic stellate cells (HSC) have emerged as a well-characterized cell type with a central role in the pathogenesis of hepatic fibrosis. In normal liver, these cells are liver-specific pericytes which mainly serve as vitamin A storage sites. Quiescent HSC show little proliferative activity and regulate extracellular matrix turnover in the space of Disse by secreting correct amounts of a limited number of extracellular matrix molecules, and by releasing matrix metalloproteinases and their inhibitors (Geerts, 2001). Following acute or chronic liver tissue injury, HSC undergo a process of activation which is central to the development of liver fibrosis (Friedman, 2000). Interestingly, when freshly isolated HSC are cultured on uncoated plastic, they develop all characteristics of activated cells after 3–5 days (Friedman et al., 1989). This model is very useful to study antifibrotic effects of drugs in vitro. Activated cells adapt a myofibroblast-like phenotype characterized by increased production of extracellular matrix, proliferation, contractility and motility, all of which contribute to the wound-healing process (Friedman, 2000). Activated HSC not only produce more collagens but also matrix metalloproteinases and their tissue inhibitors, although not in adequate amounts. Consequently, the delicate balance between fibrogenesis and fibrolysis is disturbed, resulting in fibrosis. Although all liver cells produce extracellular matrix, HSC are regarded as the principal matrix-producing cells, whereas Kupffer cells, endothelial cells and hepatocytes contribute only marginally to extracellular matrix formation (Brenner et al., 2000; Friedman et al., 2000; McCrudden & Iredale, 2000; Rockey, 2000). The dramatic progress in understanding the pathogenesis of liver fibrosis has directed the approach of developing antifibrotic drugs towards targeting HSC (Wu & Zern, 2000; Bataller & Brenner, 2001; Beljaars et al., 2002). Numerous drugs aiming at inhibiting the accumulation of activated HSC at the sites of liver injury and preventing the deposition of extracellular matrix are currently under investigation in in vitro and in vivo models of liver fibrosis. Many substances inhibit or reduce HSC activation (e.g. interferon-γ, trichostatin A, endothelin receptor antagonists type A, TGF-β inhibitors), proliferation (e.g. pentoxyfylline, trichostatin A, interferon-γ, angiotensin inhibitors, endothelin receptor antagonists type A) and/or extracellular matrix deposition in vitro (e.g. interferon-α and -γ, trichostatin A, nitric oxide donors, pentoxyfylline), but few of them are tolerable and effective in vivo. Although several of these agents have been studied in animals, efficacy and safety in humans are as yet largely unknown (Pinzani & Rombouts, 2004; Bataller & Brenner, 2005).
Antifibrotic effects have been attributed to somatostatin, which reduced the degree of hepatic fibrosis in different animal models. Rats treated with octreotide following bile duct ligation showed histologically significant inhibition of bile duct proliferation and peri-portal extracellular matrix deposition as compared to controls (Tracy et al., 1993; Turkcapar et al., 2003). In mice infected with Schistosoma mansoni, octreotide reduced the degree of hepatic fibrosis (Mansy et al., 1998). Finally, octreotide caused a significant decrease in fibrosis and inflammation in rats with CCl4-induced liver disease (Fort et al., 1998; Moal et al., 2002).
The mechanism by which somatostatin exerts these antifibrotic effects remains uncertain, although some possible pathways have been proposed. Somatostatin could reduce extracellular matrix by (i) modulating Kupffer cell function, (ii) through anti-inflammatory effects associated with suppression of proinflammatory cytokines or hormones and (iii) by a direct effect on stellate cells. Although HSC are key cells in the pathogenesis of liver fibrosis, a direct antifibrotic effect of somatostatin has not been investigated up to now. We have previously shown that activated rat HSC express somatostatin receptors (SSTR) and thus somatostatin could have a direct antifibrotic effect (Reynaert et al., 2001). Therefore, we investigated the in vitro influence of somatostatin on activation and proliferation of, and production of extracellular matrix proteins by cultured rat HSC.
Methods
Isolation and culture of HSC
HSC were isolated from male Wistar rats by collagenase/pronase digestion followed by density gradient centrifugation as described previously (Hellemans et al., 1999). All rats received humane care in compliance with the Institution's Guidelines for the Care and Use of Laboratory Animals in Research. Approval of the Institutes' Ethical Committee for Animal Care was obtained to perform this study. Following isolation, cells were plated at a density of 1.5 × 105 cells ml−1 in 250 ml culture flasks (Falcon, Becton Dickinson, Lincoln Park, New Jersey, U.S.A.), suspended in Dulbecco's modified Eagle's medium with 10% fetal calf serum supplemented with 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, and cultured at 37°C in a humidified atmosphere with 5% CO2 and 95% air. At day 2, cell debris and nonadherent cells were removed by washing. The medium was changed every 2 days thereafter.
Purity of cultures (at least 95%) was evaluated by examining the characteristic stellate cell shape with phase-contrast microscopy. Subcultured cells were obtained by trypsinizing primary cultures at days 8–10 when cells reached confluency. Trypsinisation was performed by incubating cell cultures for a maximum of 10 min with 0.25% trypsin containing 2 mM EDTA (GibcoBRL™, Life Technologies, Merelbeke, Belgium). Cells were then seeded in plastic dishes at a density of 3000 cells cm−2 and were subcultured. For immunophenotyping of cells, HSC were seeded at a density of 5000 cells per chamber on Falcon culture slides (Becton Dickinson Labware, Meylan, France).
Somatostatin treatment of hepatic stellate cells
In in vitro experiments, somatostatin is classically used at concentrations that vary between 10−5 and 10−10 mol l−1 (Reynaert et al., 2001). Concentrations used in this study range between 10−6 and 10−9 mol l−1, which are not physiological, but which are definitely in the range of pharmacological concentrations. In pharmacological studies it has been shown that, following infusion of 250–500 μg somatostatin min−1, a plateau serum somatostatin level of ±10−8 mol l−1 is reached. Owing to the short half-life of somatostatin (in vitro: 2–3 h) (Chou et al., 1987), somatostatin (UCB, Brussels, Belgium) was added to the medium every 6 h in all experiments. Somatostatin was diluted in saline, and a stock solution of 10−4 mol l−1 was prepared for further experiments. Saline was used as control.
Northern blot hybridization analysis
HSC, 12-day old, were exposed to different concentrations (10−6–10−9 mol l−1) of somatostatin for 24 h, followed by RNA extraction using the RNeasy mini extraction Kit (QIAgen, Westburg, The Netherlands) according to the manufacturer's manual. Complementary DNA (cDNA) probes were generated by RT–PCR. Primers are summarized in Table 1. Specificity of the PCR products was confirmed by automatic sequencing (Reynaert et al., 2001). For Northern hybridization, 10 μg total RNA of each sample was fractionated on 1% agarose gels containing 3% paraformaldehyde and transferred onto a nylon membrane. Probes were labelled with 32P-deoxycytidine triphosphate using the Readyprime II labelling kit (Amersham, Little Chalfort, U.K.). Hybridization was performed using QuikHyb hybridization solution according to the manufacturer's instructions (Stratagene Europe, Amsterdam, the Netherlands) (Rombouts et al., 2002b). All experiments were performed in triplicate. Films were digitalized and the results were quantified by Kodak digital science (Kodak, Rochester, NY, U.S.A.). GAPDH, a house-keeping gene, was used for normalization of the data.
Table 1.
Primers used for RT–PCR
| Forward primer | Reverse primer | Accession no. | |
|---|---|---|---|
| α-SMA | 5′-TGTGCTGGACTCTGGAGATG-3′ | 5′-GATCACCTGCCCATCAGG-3′ | X06801 |
| Collagen I | 5′-ACGTCCTGGTGAAGTTGGTC-3′ | 5′-ACCAGGGAAGCCTCTCTCTC-3′ | BC036531 |
| Collagen III | 5′-CCTAACCAAGGCTGCAAGATG-3′ | 5′-CAGTGTGTTTAGTGCAGCCAT-3′ | XM216813 |
| GAPDH | 5′-TGATGCTGGTGCTGAGTATGTCG-′3 | 5′-AGTGAGCTTCCCGTTCAGCTCTG-3′ | BC059110 |
Inhibition of translation
Cycloheximide, a translation inhibitor, was used in combination with somatostatin to investigate whether the observed effects were due to a direct effect of somatostatin on transcription. The maximal nontoxic dose of cycloheximide (Sigma) was studied by incubating the cells with increasing concentrations of cycloheximide (0, 0.75, 1.5, 3, 6 and 12 μg ml−1) in combination with somatostatin, followed by a short incubation (5 min) with propidium iodide.
To validate the maximum protein synthesis blocking effect of cycloheximide, HSC were exposed to 0, 0.75, 1.5, 3, 6 and 12 μg ml−1 of cycloheximide for 24 h. After the initial 18 h of treatment, cells were metabolically labelled for 6 h with 50 μCi ml−1 of [35S]methionine/cysteine (Trans-35S-label, specific activity 1000 μCi mmol−1, ICN Biomedicals). Labelled media and cell layers were harvested separately and were stored at 70°C. Protein synthesis was assessed by precipitation with hot trichloroacetic acid.
After incubation of HSC with an effective, nontoxic dose of cycloheximide for 24 h and with serial concentrations of somatostatin, RNA was extracted and quantitative RT–PCR was performed, using the ABI 7700 SDS (Applied Biosystems) reaction and detection system. Primers and probes for collagen I and III were made by Assay-on-demand (collagen I: Rn01463854_g1; accession no. Z78279 and collagen III: Rn01437674_m1; accession no. X70369), whereas the primers and probe for α-SMA were made by Assays-by-Design Gene Expression Product system (Applied Biosystems) (forward: 5′-CACCGCAAATGCTTCTAAGTCA-3′; reverse: 5′-GCTGATCCACAAAACATTCACAGTT-3′; accession no. X06801). Predeveloped TaqMan Assay reagent for 18S ribosomal RNA (18SrRNA) (Applied Biosystems) was used as internal control. For analysis according to the Delta–Delta threshold (Ct) method, each Ct value was first normalized to the respective 18S rRNA Ct value of the sample and afterwards to the control. Fold induction was calculated from these Ct-values. The ratio of treated versus control was calculated for each experimental condition and was expressed as mean±standard deviation (s.d.) of three independent experiments. The statistical significance of differences between groups was determined by calculating 95% confidence intervals (95% CIs). An effect was considered statistically significant when P<0.05.
Metabolic labelling and immunoprecipitation
Metabolic labelling and immunoprecipitation were performed as described earlier by our group (Niki et al., 1996; Rombouts et al., 2001b). In short, at day 12 after isolation, fully activated cells were exposed to saline or different concentrations (10−6–10−9 mol l−1) of somatostatin for 24 h. Subsequently, cells were metabolically labelled for 24 h using 25 μCi ml−1 of Trans-35S-label (70% [35S]methionine, 15% [35S]cysteine, ICN Biomedicals, Costa Mesa, CA, U.S.A.) while exposure to test compounds continued. The labelling medium consisted of methionine/cyteine-free Dulbecco's modified Eagle's medium supplemented with 10% foetal calf serum. Media and cell layers were harvested separately. Total incorporation of Trans-35S-label into protein was determined by the hot trichloroacetic acid precipitation method (Niki et al., 1996). Total incorporation in media and cell layers was expressed per 9.6 cm2 culture dish. Labelled medium or cell layer were then subjected to immunoprecipitation, using antibodies against collagen type I (Southern Biotechnology, Birmingham, AL, U.S.A.), collagen type III (Prof. D. Schuppan) or smooth muscle α-actin (Sigma, Little Charfort, U.K.). Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Gels were immersed in Amplify (Amersham, Little Chalfort, U.K.), dried, exposed to preflashed autoradiography film (Hyperfilm MP, Amersham, Gent, Belgium) and analysed by Phosphor Imaging for quantification (BioRad, Hercules, U.S.A.).
The amount of immunoprecipitable protein was calculated per dish and per 106 cells for both medium and cell layer. Values per 106 cells were expressed relative to control culture. Total radioactivity per 106 cells was calculated by adding immunoprecipitable radioactivity of both medium and cell layer. Values were calculated from results obtained in three rats and were expressed relative to control values. To determine statistical significance between controls and treated cells, 95% CIs were calculated. Analysis was performed using Instat software (GraphPad Software Inc., San Diego, CA, U.S.A.).
5-Bromo-2′-deoxyuridine (BrdU) proliferation assay
The influence of somatostatin on cell proliferation was studied with the 5-bromo-2′-deoxyuridine (BrdU) proliferation assay (Roche, Brussels, Belgium) (Hellemans et al., 1999). Cells, 12 day-old, were plated in 24-well dishes (Falcon). Cells were allowed to attach for 24 h before exposure to different concentrations of somatostatin (10−9–10−6 mol l−1) for 48 h. After the first 24 h, media were renewed and cells were simultaneously incubated with somatostatin and 10 μM BrdU for 24 h (Boehringer, Mannheim, Germany). Following fixation and denaturation, BrdU incorporation was measured by ELISA using monoclonal peroxidase-conjugated antibodies directed against BrdU (Boehringer). Colour development was measured using a UV-VIS 3550 MICROPLATE reader (Bio-rad Laboratories). All BrdU proliferation assays were performed in triplicate in three independent cultures. To allow comparison of proliferation between different groups, results were expressed as percentage and compared to controls normalized at 100%. Means and standard deviations were calculated for all groups. To compare different groups, one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was used (Instat software). A P-value <0.05 was considered statistically significant.
Immunocytochemistry
Immunocytochemistry was performed as described previously (Geerts et al., 1998). At day 12, when cells were well attached to the glass cover slips of the culture dishes, the activated HSC were treated with saline or somatostatin for 24 h. Since in the immunoprecipitation and Northern blotting experiments, only 10−9 mol l−1 of somatostatin significantly reduced α-SMA expression, this concentration was used to examine effects of somatostatin on the cytoskeleton. Furthermore, at this concentration, somatostatin reduced HSC contractility and migration, two features for which a well-developed cytoskeleton is a prerequisite (Reynaert et al., 2001, 2004). The cells were then exposed to primary antibodies to α-SMA, desmin, vimentin, glial fibrillary acidic protein (GFAP), synemin or nestin and peroxidase-conjugated secondary antibodies. In Table 2, characteristics of the antibodies are summarized. Peroxidase was visualized by 3,3′-diaminobenzidine/H2O2 enhanced by Ni2+ and Co2+ ions, resulting in a black reaction product. After staining for nuclei with Harris Haematoxylin (Sigma, Bornem, Belgium), the cells were studied using a Zeiss Axioplan microsope (Zeiss, Jena, Germany).
Table 2.
Characteristics of antibodies used for immunohistochemistry (ICC) and Western blotting
| Antibody | Type | Source | Dilutions | |
|---|---|---|---|---|
| ICC | Western | |||
| α-SMA | Monoclonal, IgG, mouse | 1 | 1/500 | 1/10.000 |
| Vimentin | Monoclonal, IgM, mouse | 1 | 1/500 | 1/10.000 |
| Desmin | Monoclonal, IgG, mouse | 1 | 1/500 | 1/10.000 |
| GFAP | Monoclonal, IgG, mouse | 1 | 1/500 | — |
| Synemin | Polyclonal, IgG, rabbit | 2 | 1/1000 | — |
| Nestin | Polyclonal, IgG, rabbit | 3 | 1/200 | 1/7.500 |
| Secondary AB | Anti-mouse, IgG, sheepa | 4 | 1/200 | 1/20.000 |
| Secondary AB | Anti-mouse, IgM, goata | 1 | 1/250 | 1/20.000 |
| Secondary AB | Anti-rabbit, IgG, Poly-HRP, goata | 5 | 1/1 | 1/20.000 |
Peroxidase conjugated.
Sigma, Bornem, Belgium;
Courtesy Dr Z.L. Li, Biologie Moléculaire de la différenciation, Université Denis-Diderot, Paris, France;
Courtesy Dr J. Eriksson, Turku Centre for Biotechnology, University of Turku, Finland;
Amersham Biosciences, Roosendaal, The Netherlands and 5Chemicon International, Temecula, CA, U.S.A.
Western blotting
Cells, 12-day-old, were treated for 24 h with saline or somatostatin 10−9 mol l−1. Western blot analysis was performed as described (Niki et al., 1999a). Samples containing 4 μg total protein were separated using an 8% SDS–PAGE, and electroblotted onto PROTRAN BA83 nitrocellular membranes (Schleicher & Schuell, Dassel, Germany). Membranes were then reacted with primary antibodies described in Table 2. After rinsing, peroxidase-conjugated secondary antibodies were added, antigens were detected using ECL Advance Western blotting detection kit (Amersham Biosciences, Little Chalfont, U.K.) and blots were exposed to Hyperfilm (Amersham Pharmacia Biotech, U.K.). Films were digitalized and the results were quantified by Kodak digital science. The experiments were performed in duplicate.
Results
Morphological characterization of quiescent and activated stellate cells
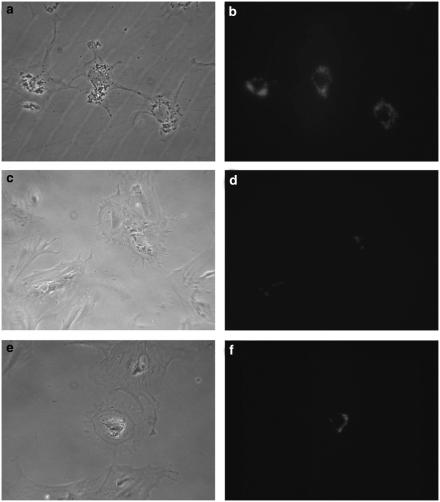
Cultured cells were characterized by phase-contrast microscopy and by autofluorescence. When freshly isolated cells were placed in culture, they initially acquired the in vivo morphological features of HSC, that is, long cytoplasmic processes and abundant cytoplasmic lipid droplets (Figure 1a and b). After 12 to 14 days in culture however, cells lost most of their lipid droplets (Figure 1d) and gained the morphology of myofibroblast-like cells (Figure 1c) (Hellemans et al., 2003). When cells were treated for 24 h with somatostatin (10−9 mol l−1), no obvious changes in morphology were observed (Figure 1e), except for a minor increase in vitamin A droplets in some cells (Figure 1f).
Figure 1.
Morphology of quiescent (a, b), activated saline-treated (c, d) and activated somatostatin-treated (e, f) stellate cells was studied with phase-contrast (left panel) and autofluoresence (right panel) microscopy. Freshly isolated cells have long, thin cytoplasmatic processes and are laden with vitamin A containing lipid droplets. In contrast, in 12-day-old activated cells, the lipid droplets have almost disappeared and are localized around the nucleus. Furthermore, the cells are larger and have no longer the typical cytoplasmatic processes of the quiescent cells. When cells were treated with somatostatin (10−9 mol l−1) for 24 h, an increase in small lipid droplets around the nucleus could be observed in some cells (original magnification × 200).
Effects of somatostatin on mRNA
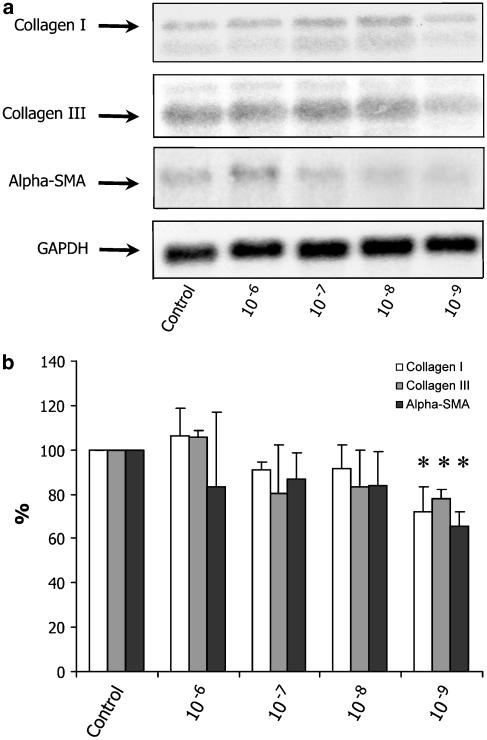
To elucidate the possible direct antifibrogenic effect of somatostatin, we exposed activated rat stellate cells to different concentrations of somatostatin. We first examined the effect of somatostatin on mRNA expression of collagen types I and III, the fibril-forming collagens that predominate in chronic liver disease, and α-SMA, a marker for HSC activation. As shown in Figure 2, collagen I and III and α-SMA mRNA levels were not significantly reduced by somatostatin 10−6 to 10−8 mol l−1. At a concentration of 10−9 mol l−1 however, somatostatin induced a significant reduction of collagen I mRNA expression (72.3±10.7%; 95% CI: 45.5–99.0), collagen III mRNA expression (79.0±4.5%; 95% CI: 67.6–90.4) and αSMA mRNA expression (65.7±5.9%; 95% CI: 51.1–80.2).
Figure 2.
Expression of collagen I and III, and α-SMA mRNA after treatment with different concentrations of somatostatin (10−6–10−9 mol l−1) or saline (control). Representative Northern blot hybridization experiment (a). As GAPDH signal was more intense in the 10−7 and 10−8 mol l−1 experiments as compared to the control, the results of treatment in these concentrations are underestimated in this figure. Quantitative analysis, with GAPDH used for normalization of the data, is shown in panel (b). Results are expressed as mean±standard deviation, relative to the control normalized at 100% (n=3). Only a concentration of 10−9 mol l−1 resulted in a significant (*) reduction of collagen I and III, and α-SMA mRNA.
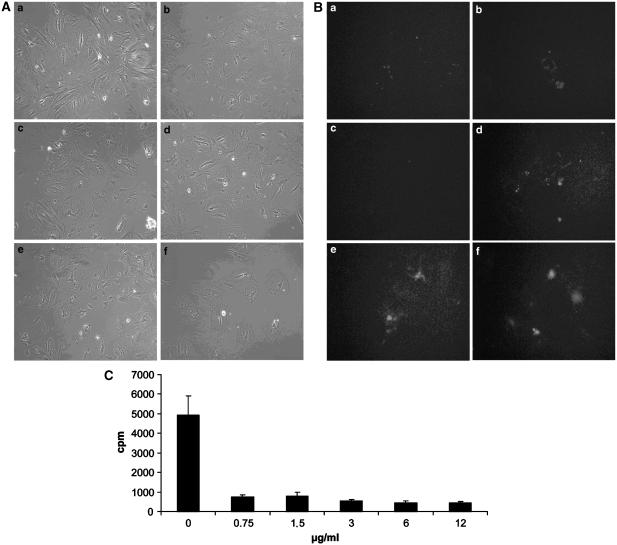
To distinguish between a direct and an indirect effect of somatostatin on transcription, we performed cyclohexamide experiments. First, we identified a nontoxic dose of cycloheximide which successfully inhibited transcription. Incubation of cultured cells with high concentrations of cycloheximide (>1.5 μg ml−1) resulted in cell detachment (Figure 3A) and apoptosis, as shown by propidium iodide fluorescence microscopy, which demonstrated the nuclei of apoptotic cells (Figure 3B). Thus, at a concentration of 3 μg ml−1, cycloheximide proved to be toxic for stellate cells. On the other hand, protein synthesis was blocked by cycloheximide in a dose equal or larger than 0.75 μg ml−1. Hence, the effective and noncytotoxic dose of 1.5 μg ml−1 was used in further experiments. The addition of somatostatin during treatment with cycloheximide did not influence the results.
Figure 3.
Effect of increasing concentrations of cycloheximide on cultured HSC. Pictures a–f were taken 24 h after, respectively, 0, 0.75, 1.5, 3, 6 and 12 μg ml−1 of cycloheximide was added to the cultures. (A) At low concentration, cycloheximide had no visible effect on cell attachment (a–c). However, a concentration of 3 μg ml−1 cycloheximide or higher (d–f) resulted in detachment of cells. (B) Fluorescence microscopy of cells treated with propidium iodide after 24 h of treatment of cycloheximide confirmed these results. Definitely, at a concentration of 3 μg ml−1 or higher, cycloheximide induced apoptosis as demonstrated by fluorescent nuclei of apoptotic cells (d–f). (C) Determination of the dose of cycloheximide which effectively blocked protein synthesis. Cells were metabolically labelled with [35S]methionine/cysteine. Protein synthesis was assessed by trichloroacetic acid precipitation. From our data, it is evident that cycloheximide blocked protein synthesis by stellate cells, even at concentrations as low as 0.75 μg ml−1. Bars represent means±s.d. of three independent experiments.
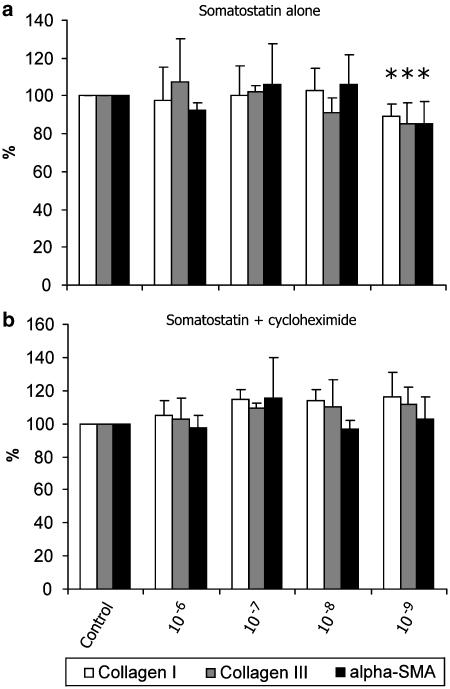
Quantitative RT–PCR confirmed the results obtained by Northern blot. The higher concentrations of somatostatin did not alter mRNA of collagen I, collagen III and α-SMA, but at a concentration of 10−9 mol l−1, somatostatin induced a small although significant decrease in collagen I (89.2±6.6%; 95% CI: 82.6–95.8), collagen III (85.4±11.0 %; 95% CI: 74.3–96.4) and α-SMA mRNA (85.3±11.8%; 95% CI: 73.4–97.2) (Figure 4a). When a combination of somatostatin (10−6–10−9 mol l−1) and cycloheximide in a concentration of 1.5 μg ml−1 was added to the cultures, somatostatin lost its effect on mRNA reduction. On the contrary, there was a trend towards increased mRNA levels (Figure 4b). The data indicate that the influence of somatostatin on collagen and α-SMA expression is indirect.
Figure 4.
Effect of somatostatin (10−6–10−9 mol l−1) in the absence (a) and presence (b) of cycloheximide (1.5 μg ml−1) on collagen I, collagen III and α-SMA mRNA as measured by quantitative RT–PCR. Controls (saline) were normalized at 100%. In accordance with the Northern blot experiments, somatostatin at a concentration of 10−9 mol l−1 induces a small, but significant reduction of collagen I and III, and α-SMA mRNA. Addition of cycloheximide to the different concentrations of somatostatin has no significant effect on mRNA, although there was a trend towards increase of mRNA. Bars represent means±s.d. of three independent experiments. *P<0.05.
De novo protein synthesis
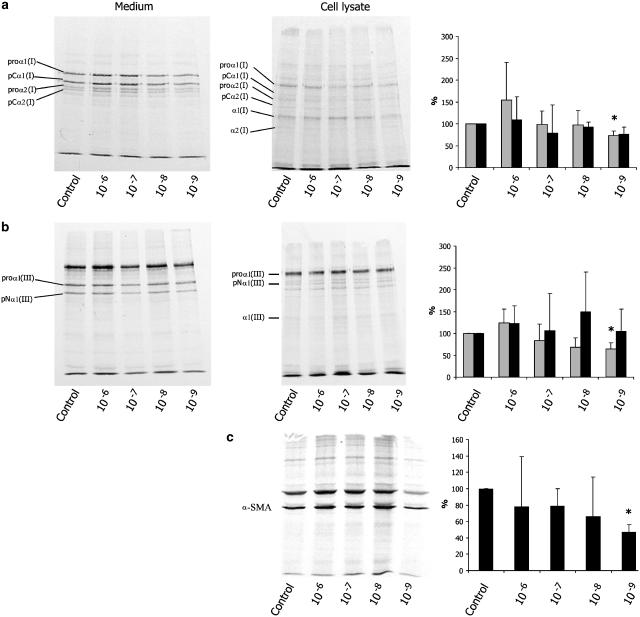
To confirm that changes of mRNA expression resulted in altered de novo protein synthesis, immunoprecipitation experiments were performed. The 12-day-old HSC were metabolically labelled with [35S]methionine/cysteine and were treated with saline (control) or somatostatin (10−6–10−9 mol l−1). With this technique, we were able to measure newly synthesized proteins in the presence of many other proteins. Furthermore, production of procollagens as well as the processed forms could be quantified. In Figure 5, representative results of immunoprecipitation experiments are shown. As reported previously (Knittel et al., 1992), collagen type I could be detected as multiple bands (Figure 5a). These bands represent different stages of collagen chain processing, that is, procollagen α1(I), procollagen α2(I), partially processed forms pCα1(I) and pCα2(I) and fully processed forms collagen α1(I) and α2(I). Furthermore, some weak bands probably represent pN collagen, a C-terminal lacking intermediate, and polypeptide fragments. In medium, intact procollagen was predominant and only little fully processed collagen was present. In contrast, in cell lysate, fully processed forms of collagen α1(I) and α2(I) were also detectable. The density of the bands of somatostatin-treated samples varied with the concentration of somatostatin. PhosphorImager quantitative analysis showed a small, statistically insignificant increase of collagen I with somatostatin 10−6 mol l−1 and a minor decrease with lower concentrations. Somatostatin at 10−9 mol l−1 induced a moderate decrease to 73±10% (95% CI: 48–98%) of collagen I synthesis as compared to control. In cell lysate, no significantly different amount of collagen I could be detected. As reported previously, 80–90% of de novo synthesized collagen is secreted into the cell culture medium (Niki et al., 1999b; Rombouts et al., 2001a). Consequently, collagen in cell lysate contributes only minimally to the total collagen synthesis.
Figure 5.
Effect of somatostatin (10−6–10−9 mol l−1) on de novo synthesis of collagen types I (a) and III (b), and of smooth muscleα-actin (c) by activated stellate cells. Connective tissue proteins from medium and cell layer were labelled with Trans-35S-label and analysed by immunoprecipitation and SDS–PAGE (left panels). Radioactivity was quantified by Phosphor Imaging (right panels). Medium is presented in grey bars, cell lysate in black bars. Since smooth muscle α-actin is not secreted in the medium, smooth muscle α-actin was only studied in the cell layer. Control values within each experiment were normalized at 100%. Results are expressed as means±standard deviation (n=3). Only treatment with somatostatin 10−9 mol l−1 resulted in a statistically significant difference as compared to control (*).
As shown in Figure 5b, antibodies to procollagen III immunoprecipitated proα1(III), the C-terminally processed pNα1(III) and the fully processed collagen α1(III). Owing to coreaction with fibronectin, the antiserum of procollagen III precipitated an extra band of fibronectin (upper band) (Knittel et al., 1992). Similar to the observations with collagen I, a small, insignificant increase in collagen III synthesis occurred in 10−6 mol l−1 somatostatin-treated cells, whereas lower concentrations resulted in a small decrease. Quantitative analysis showed that 10−9 mol l−1 somatostatin induced a statistically significant decrease to 65±13% (95% CI: 33–97%) in collagen III synthesis as compared to control. Again, no significantly different amount of collagen III could be detected in the cell lysate.
Somatostatin also reduced de novo α-SMA synthesis. This effect of somatostatin was most prominent at a concentration of 10−9 mol l−1, which suppressed α-SMA synthesis to 47±9% (95% CI: 25–69%) as compared to controls (Figure 5c).
Dynamic cellular function
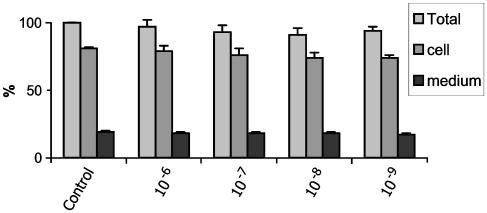
To exclude nonspecific effects on protein synthesis, we evaluated a dynamic cellular function, namely de novo total protein synthesis as measured by incorporation of trans-35S-label by trichloroacetic acid precipitation. The data, presented in Figure 6, show that administration of somatostatin in concentration range from 10−6 to 10−9 mol l−1 did not change statistically significantly the incorporation of trans-35S-label into total protein, thereby excluding unspecific toxic effects of somatostatin on protein synthesis.
Figure 6.
Evaluation of a possible cytotoxic effect of somatostatin on HSC: the observed antifibrogenic effect of somatostatin was protein specific, and not due to general cytotxicity as assumed by a sensitive dynamic cellular function, namely total protein synthesis. De novo protein synthesis was measured by incorporation of Trans-35S-label. Incorporation of Trans-35S-label into total protein was measured by trichloroacetic acid precipitation. Total incorporation into protein in control samples was normalized at 100%. Other values were recalculated accordingly. No statistically significant differences were observed, suggesting that somatostatin, in the tested concentrations, had no toxic effects on the cells severe enough to impair total protein synthesis.
Effect on cell proliferation
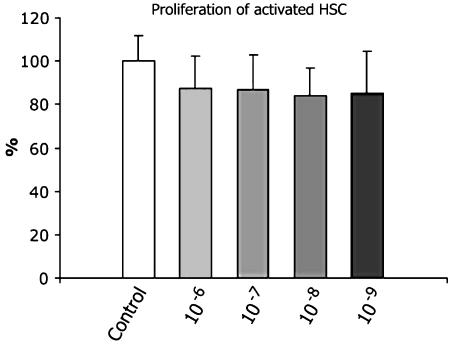
To exclude an effect of somatostatin on stellate cell proliferation, we performed BrdU proliferation experiments in 12-day-old cells. As demonstrated in Figure 7, somatostatin had no significant effect on proliferation in all of the tested concentrations. Since the possible antifibrotic effect of somatostatin was not related to changes in HSC proliferation, we further investigated a possible effect on cell activation and differentiation.
Figure 7.
Influence of somatostatin on proliferation of activated hepatic stellate cells. Cells, 12-day old, were exposed to different concentrations of somatostatin (10−6–10−9) or to vehicle alone, for 48 h. 5-Bromo-2′-deoxyuridine (BrdU) (10 μM) was added for the last 24 h followed by the quantification of BrdU incorporation. Results were normalized to control. Data are presented as mean±s.d. The histogram summarizes the quantitative data of three independent experiments. No significant differences were found between treated and untreated cells.
Influence on the cytoskeleton of HSC
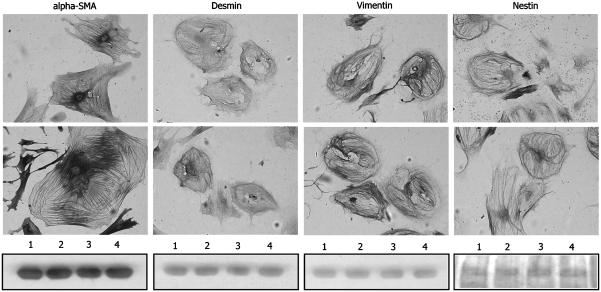
From our previous studies on the influence of somatostatin on stellate cell contraction (Reynaert et al., 2001) and migration (Reynaert et al., 2004), and from the results on α-SMA expression and synthesis in this study, we expected an effect of somatostatin on the cytoskeleton of activated HSC. Therefore, we investigated the impact of somatostatin on the expression of α-SMA, and the intermediate filament proteins desmin, vimentin, GFAP, nestin and synemin in activated HSC with immunocytochemistry. As expected, expression of α-SMA, desmin, vimentin and nestin was strong (Figure 8), whereas expression of GFAP and synemin was almost absent in activated HSC (data not shown). Treatment with somatostatin for 24 h did not induce obvious changes in the distribution of the cytoskeletal elements under study.
Figure 8.
Immunocytochemistry of 13-day-old HSC stained for α-SMA, desmin, vimentin and nestin. Cells were treated with saline (upper panel) or somatostatin 10−9 mol l−1 (middle panel) for 24 h. Somatostatin did not visibly affect the different cytoskeletal elements (original magnification × 100). Western blotting analysis (lower panel), performed to quantify the data, proved that no measurable changes occurred at the protein level following 24 h of treatment. Lanes 1 and 3 represent saline-treated cells, and lanes 2 and 4 represent SST-treated cells.
To quantify the immunocytochemical data, Western blot analysis for α-SMA, desmin, vimentin and nestin was performed. After 24 h of treatment with somatostatin 10−9 mol l−1, no changes in the total amount of these proteins could be detected (Figure 8).
Discussion and conclusions
Although other studies have attributed antifibrotic effects to octreotide, the mechanism by which this compound brings about its effects remain unknown (Tracy et al., 1993; Fort et al., 1998; Mansy et al., 1998; Moal et al., 2002; Turkcapar et al., 2003). In the present study, we demonstrate that somatostatin at 10−9 mol l−1 reduces collagen I and III mRNA and protein synthesis by activated HSC. Although there is a tendency towards decreased collagen synthesis, the higher concentrations do not have a significant effect on mRNA expression and de novo protein synthesis. How could we explain this observation? It is well known that continued and repeated stimulation of somatostatin receptors results in tolerance, tachyphylaxis or desensitization. Desensitization of the receptors by somatostatin analogues is caused by several mechanisms: receptor phosphorylation and uncoupling of the receptor both lead to rapid desensitization, whereas receptor degradation and internalization are implicated in long-term desensitization (Dohlman et al., 1991). All but SSTR 1 are to a certain extent internalized after prolonged agonist exposure (Hukovic et al., 1996). When using somatostatin analogues as antifibrotic drugs, correct dosing could thus be a problem.
Collagen I and III are fibril-forming collagens that make up the majority of scar tissue in cirrhosis. Fibrogenesis following liver injury is characterized by a four- to six-fold increase in collagen I and III. Suppression of the synthesis of these collagens is probably important in preventing cirrhosis (Rockey, 2000). Moreover, it has been demonstrated that quiescent HSC hardly synthesize any collagen types I and III, whereas the synthesis of collagen I and III increases by, respectively, 7.5 and 3.5 times in activated cells (Knittel et al., 1992). Thus, prevention of HSC activation is a principal goal in treating fibrosis (Brenner et al., 2000). Suppression of α-SMA, a well-established marker of stellate cell activation, is observed in a range of somatostatin concentrations, although it is only statistically significant at the lowest concentration (10−9 mol l−1) tested. This suggests that somatostatin partially reverts transition of quiescent into activated stellate cells.
Next, we determined whether the observed inhibitory effect of somatostatin mRNA expression was a direct effect or the result of induction or repression of other signalling pathways. Cycloheximide experiments indicated that somatostatin has no direct transcriptional effect, and that probably other pathways are involved in the reduced production of collagen and α-SMA.
To exclude the possibility that somatostatin exerts general cytotoxic effects on HSC, we also measured total protein synthesis. Total protein synthesis is not affected by somatostatin, indicating that no general toxic effects occur. Furthermore, somatostatin and somatostatin analogues have been used for prolonged periods, especially in the treatment of endocrine tumours, and appear to be nontoxic (Lamberts et al., 1996). Therefore, it is reasonable to assume that if somatostatin or analogues are to be used in the treatment of chronic liver disease, no major safety issues would need to be addressed, unless the clearance rate of the analogues is decreased in cirrhotic patients which could lead to accumulation of the drug (Ottesen et al., 1997).
Migration and contraction are also part of the wound-healing response. It has been demonstrated that contractility increases in parallel with α-SMA expression and HSC activation (Rockey et al., 1993). By analogy, migration of HSC requires a well-developed cytoskeleton (Rombouts et al., 2002a). In previous studies, we demonstrated that somatostatin inhibits endothelin-1-induced contraction of rat HSC, and reduces migration of human HSC (Reynaert et al., 2001, 2004). These effects are observed with the same concentration of somatostatin that is effective in the present study. It is therefore possible that somatostatin reduces HSC migration and contractility, by inhibiting α-SMA de novo protein synthesis. However, with Western blot, we were unable to show any effect on intermediate filament protein content following 24 h of treatment with somatostatin. This is in contradiction with the finding of reduced mRNA expression and de novo α-SMA synthesis as measured by Northern blot and immunoprecipitation. It seems that, although mRNA expression and de novo protein synthesis is reduced, total protein content is still too high to detect a difference in overall quantities of α-SMA by Western blot analysis.
Stellate cell proliferation has been studied in detail and is important in the pathogenesis of fibrosis (Friedman, 1993). Moreover, it has been shown that somatostatin has antiproliferative properties in some models (Chou et al., 1987). In our experimental set-up, no significant inhibition of proliferation could be detected, which is in agreement with our previous observations using somatostatin agonists in human HSC (Reynaert et al., 2004). It is therefore unlikely that the antifibrotic effect of somatostatin is caused by inhibition of HSC proliferation, although it remains possible that somatostatin has in vivo antiproliferative effects via inhibition of the release of insulin and reduction of the bioactivity of insulin-like growth factor, both well-recognized mitogens for HSC (Svegliati-Baroni et al., 1999). There is another possibility why proliferation is not affected in our model. Proliferation is a process that requires a cytoskeletal reorganization to increase the cell plasticity necessary to allow the cell to proliferate. Intermediate filaments are one of the cytoskeletal components that require depolymerization prior to cell proliferation. Even after 24 h of somatostatin treatment, we find no rearrangement of intermediate filaments, which is a hallmark of cells that transit from Go into the cell cycle.
Although HSC are most widely studied, other liver myofibroblasts, such as peri-portal and peri-septal fibroblasts, are probably also involved in the fibrotic process (Knittel et al., 1999; Cassiman et al., 2002). So far, SSTR expression has not been investigated in these cells and thus their contribution to the antifibrotic effect of somatostatin cannot be assessed. Furthermore, the response of these myofibroblasts to various types of liver injury has not been studied in depth.
The effect of somatostatin in the tested concentrations was moderate, and probably insufficient to explain the more pronounced effects of somatostatin and analogues observed in vivo. However, indirect antifibrotic effects through interaction with Kupffer cells (Chao et al., 1997, 1999; Maher, 2001; Valatas et al., 2004), modulation of immune response (Karalis et al., 1994; Gunal et al., 2001; Wang et al., 2001) and insulin or insulin-like growth factor (Huynh & Pollak, 1994; Svegliati-Baroni et al., 1999) could be involved. Further work in this direction is needed.
In conclusion, we demonstrate that somatostatin, at a concentration of 10−9 mol l−1, reduces collagen I and collagen III synthesis as well as α-SMA synthesis by activated rat HSC. These findings imply that part of the antifibrotic effects observed in vivo could be due to a direct antifibrotic effect of somatostatin on activated stellate cells. However, the effect of somatostatin in our in vitro study was but moderate, and insufficient to explain the more pronounced effects of somatostatin and analogues observed in vivo. Therefore, indirect effects are most likely involved. Further studies are needed to unravel the mechanisms by which somatostatin exerts its antifibrotic effects. From our results, it appears that reversal of transdifferentiation of HSC could play a role.
Acknowledgments
We are indebted to V. Andriessen, J.M. Lazou and A. Vandermonde (Laboratory for Liver Cell Biology, Vrije Universiteit Brussel, Belgium) for excellent technical assistance and to E. Quartier (Diabetes Research Center, Vrije Universiteit Brussel, Belgium) for sequencing. We are grateful to Dr D. Schuppan, Dr Z.L. Li and to Dr J. Eriksson for providing antibodies to collagen III, synemin and nestin, respectively. This study was funded by the Scientific Fund W. Gepts AZ-VUB, FWO-V (Fonds voor Wetenschappelijk Onderzoek-Vlaanderen) grants no. 1.5.618.98 and G.0068.00, OZR-VUB (Onderzoeksraad Vrije Universiteit Brussel) grants no. 1963221120, OZR234 and OZR439, and a research grant of UCB Brussels.
Abbreviations
- α-SMA
smooth muscle α-actin
- cDNA
complementary DNA
- GFAP
glial fibrillary acidic protein
- HSC
hepatic stellate cells
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SSTR
somatostatin receptor
References
- BATALLER R., BRENNER D.A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- BATALLER R., BRENNER D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELJAARS L., MEIJER D.K., POELSTRA K. Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front. Biosci. 2002;7:e214–e222. doi: 10.2741/A917. [DOI] [PubMed] [Google Scholar]
- BRENNER D.A., WATERBOER T., CHOI S.K., LINDQUIST J.N., STEFANOVIC B., BURCHARDT E., YAMAUCHI M., GILLAN A., RIPPE R.A. New aspects of hepatic fibrosis. J. Hepatol. 2000;32:32–38. doi: 10.1016/s0168-8278(00)80413-4. [DOI] [PubMed] [Google Scholar]
- CASSIMAN D., LIBBRECHT L., DESMET V., DENEF C., ROSKAMS T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- CHAO T.C., CHAO H.H., CHEN M.F., LIN J.D. Somatostatin modulates the function of Kupffer cells. Regul. Pept. 1997;69:143–149. doi: 10.1016/s0167-0115(97)00008-6. [DOI] [PubMed] [Google Scholar]
- CHAO T.C., CHAO H.H., LIN J.D., CHEN M.F. Somatostatin and octreotide modulate the function of Kupffer cells in liver cirrhosis. Regul. Pept. 1999;79:117–124. doi: 10.1016/s0167-0115(98)00150-5. [DOI] [PubMed] [Google Scholar]
- CHOU C.K., HO L.T., TING L.P., HU C.P., SU T.S., CHANG W.C., SUEN C.S., HUANG M.Y., CHANG C.M. Selective suppression of insulin-induced proliferation of cultured human hepatoma cells by somatostatin. J. Clin. Invest. 1987;79:175–178. doi: 10.1172/JCI112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHLMAN H.G., THORNER J., CARON M.G., LEFKOWITZ R.J. Model systems for the study of seven-membrane-segment receptors. Annu. Rev. Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- FORT J., OBERTI F., PILETTE C., VEAL N., GALLOIS Y., DOUAY O., ROUSSELET M.C., ROSENBAUM J., CALES P. Antifibrotic and hemodynamic effects of the early and chronic administration of octreotide in two models of liver fibrosis in rats. Hepatology. 1998;28:1525–1531. doi: 10.1002/hep.510280612. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S.L. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S.L., MAHER J.J., BISSELL D.M. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology. 2000;32:1403–1408. doi: 10.1053/jhep.2000.20243. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S.L., ROLL F.J., BOYLES J., ARENSON D.M., BISSELL D.M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J. Biol. Chem. 1989;264:10756–10762. [PubMed] [Google Scholar]
- GEERTS A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- GEERTS A., NIKI T., HELLEMANS K., DE CRAEMER D., VAN DEN BERG K., LAZOU J.M., STANGE G., VAN DE WINKEL M., DE BLESER P. Purification of rat hepatic stellate cells by side scatter-activated cell sorting. Hepatology. 1998;27:590–598. doi: 10.1002/hep.510270238. [DOI] [PubMed] [Google Scholar]
- GUNAL A.I., DUMAN S., SEN S., UNSAL A., TERZIOGLU E., AKCICEK F., BASCI A. By reducing TGF beta 1, octreotide lessens the peritoneal derangements induced by a high glucose solution. J. Nephrol. 2001;14:184–189. [PubMed] [Google Scholar]
- HELLEMANS K., GRINKO I., ROMBOUTS K., SCHUPPAN D., GEERTS A. All-trans and 9-cis retinoic acid alter rat hepatic stellate cell phenotype differentially. Gut. 1999;45:134–142. doi: 10.1136/gut.45.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLEMANS K., ROMBOUTS K., QUARTIER E., DITTIÉ A.S., KNORR A., MICHALIK L., ROGIERS V., SCHUIT F., WAHLI W., GEERTS A. PPARb regulates vitamin A metabolism-related gene expression in hepatic stellate cells undergoing activation. J. Lipid Res. 2003;44:280–295. doi: 10.1194/jlr.M200376-JLR200. [DOI] [PubMed] [Google Scholar]
- HUKOVIC N., PANETTA R., KUMAR U., PATEL Y.C. Agonist-dependent regulation of cloned human somatostatin receptor types 1–5 (hSSTR1-5): subtype selective internalization or upregulation. Endocrinology. 1996;137:4046–4049. doi: 10.1210/endo.137.9.8756582. [DOI] [PubMed] [Google Scholar]
- HUYNH H., POLLAK M. Enhancement of tamoxifen-induced suppression of insulin-like growth factor I gene expression and serum level by a somatostatin analogue. Biochem. Biophys. Res. Commun. 1994;203:253–259. doi: 10.1006/bbrc.1994.2175. [DOI] [PubMed] [Google Scholar]
- KARALIS K., MASTORAKOS G., CHROUSOS G.P., TOLIS G. Somatostatin analogues suppress the inflammatory reaction in vivo. J. Clin. Invest. 1994;93:2000–2006. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNITTEL T., KOBOLD D., PISCAGLIA F., SAILE B., NEUBAUER K., MEHDE M., TIMPL R., RAMADORI G. Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochem. Cell. Biol. 1999;112:387–401. doi: 10.1007/s004180050421. [DOI] [PubMed] [Google Scholar]
- KNITTEL T., SCHUPPAN D., MEYER ZUM BUSCHENFELDE K.H., RAMADORI G. Differential expression of collagen types I, III, and IV by fat-storing (Ito) cells in vitro. Gastroenterology. 1992;102:1724–1735. doi: 10.1016/0016-5085(92)91736-n. [DOI] [PubMed] [Google Scholar]
- LAMBERTS S.W., VAN DER LELY A.J., DE HERDER W.W., HOFLAND L.J. Octreotide. N. Engl. J. Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- MAHER J.J. Interactions between hepatic stellate cells and the immune system. Semin. Liver Dis. 2001;21:417–426. doi: 10.1055/s-2001-17555. [DOI] [PubMed] [Google Scholar]
- MANSY S.S., YEHIA H.A., HASSAN M.M., HASSAN E.A., YOUSSEF M.M., HADI A.A., MACKENZIE C.D. Effect of octreotide on the pathology of hepatic schistosomiasis. Arzneimittelforschung. 1998;48:855–861. [PubMed] [Google Scholar]
- MCCRUDDEN R., IREDALE J.P. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol. Histopathol. 2000;15:1159–1168. doi: 10.14670/HH-15.1159. [DOI] [PubMed] [Google Scholar]
- MOAL F., CHAPPARD D., WANG J., VUILLEMIN E., MICHALAK-PROVOST S., ROUSSELET M.C., OBERTI F., CALES P. Fractal dimension can distinguish models and pharmacologic changes in liver fibrosis in rats. Hepatology. 2002;36:840–849. doi: 10.1053/jhep.2002.35533. [DOI] [PubMed] [Google Scholar]
- NIKI T., PEKNY M., HELLEMANS K., BLESER P.D., BERG K.V., VAEYENS F., QUARTIER E., SCHUIT F., GEERTS A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999a;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- NIKI T., ROMBOUTS K., DE BLESER P., DE SMET K., ROGIERS V., SCHUPPAN D., YOSHIDA M., GABBIANI G., GEERTS A. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999b;29:858–867. doi: 10.1002/hep.510290328. [DOI] [PubMed] [Google Scholar]
- NIKI T., SCHUPPAN D., DE BLESER P.J., VRIJSEN R., PIPELEERS-MARICHAL M., BEYAERT R., WISSE E., GEERTS A. Dexamethasone alters messenger RNA levels but not synthesis of collagens, fibronectin, or laminin by cultured rat fat-storing cells. Hepatology. 1996;23:1673–1681. doi: 10.1002/hep.510230651. [DOI] [PubMed] [Google Scholar]
- OTTESEN L.H., FLYVBJERG A., JAKOBSEN P., BENDTSEN F. The pharmacokinetics of octreotide in cirrhosis and in healthy man. J. Hepatol. 1997;26:1018–1025. doi: 10.1016/s0168-8278(97)80110-9. [DOI] [PubMed] [Google Scholar]
- PINZANI M., ROMBOUTS K. Liver fibrosis: from the bench to clinical targets. Dig. Liver Dis. 2004;36:231–242. doi: 10.1016/j.dld.2004.01.003. [DOI] [PubMed] [Google Scholar]
- REYNAERT H., ROMBOUTS K., VANDERMONDE A., URBAIN D., KUMAR U., BIOULAC-SAGE P., PINZANI M., ROSENBAUM J., GEERTS A. Expression of somatostatin receptors in normal and cirrhotic human liver and in hepatocellular carcinoma. Gut. 2004;53:1180–1189. doi: 10.1136/gut.2003.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNAERT H., VAEYENS F., QIN H., HELLEMANS K., CHATTERJEE N., WINAND D., QUARTIER E., SCHUIT F., URBAIN D., KUMAR U., PATEL Y.C., GEERTS A. Somatostatin suppresses endothelin-1-induced rat hepatic stellate cell contraction via somatostatin receptor subtype 1. Gastroenterology. 2001;121:915–930. doi: 10.1053/gast.2001.27971. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C. The cell and molecular biology of hepatic fibrogenesis. Clinical and therapeutic implications. Clin. Liver Dis. 2000;4:319–355. doi: 10.1016/s1089-3261(05)70113-6. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C., HOUSSET C.N., FRIEDMAN S.L. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J. Clin. Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMBOUTS K., KNITTEL T., MACHESKY L., BRAET F., WIELANT A., HELLEMANS K., DE BLESER P., GELMAN I., RAMADORI G., GEERTS A. Actin filament formation, reorganization and migration are impaired in hepatic stellate cells under influence of trichostatin A, a histone deacetylase inhibitor. J. Hepatol. 2002a;37:788–796. doi: 10.1016/s0168-8278(02)00275-1. [DOI] [PubMed] [Google Scholar]
- ROMBOUTS K., NIKI T., GREENWEL P., VANDERMONDE A., WIELANT A., HELLEMANS K., DE BLESER P., YOSHIDA M., SCHUPPAN D., ROJKIND M., GEERTS A. Trichostatin A, a histone deacetylase inhibitor, suppresses collagen synthesis and prevents TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp. Cell Res. 2002b;278:184–197. doi: 10.1006/excr.2002.5577. [DOI] [PubMed] [Google Scholar]
- ROMBOUTS K., NIKI T., WIELANT A., HELLEMANS K., SCHUPPAN D., KORMOSS N., GEERTS A. Effect of aldosterone on collagen steady state levels in primary and subcultured rat hepatic stellate cells. J. Hepatol. 2001a;34:230–238. doi: 10.1016/s0168-8278(00)00087-8. [DOI] [PubMed] [Google Scholar]
- ROMBOUTS K., WIELANT A., HELLEMANS K., SCHUPPAN D., GEERTS A. Influence of aldosterone on collagen synthesis and proliferation of rat cardiac fibroblasts. Br. J. Pharmacol. 2001b;134:224–232. doi: 10.1038/sj.bjp.0704247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEGLIATI-BARONI G., RIDOLFI F., DI SARIO A., CASINI A., MARUCCI L., GAGGIOTTI G., ORLANDONI P., MACARRI G., PEREGO L., BENEDETTI A., FOLLI F. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- TRACY T.F.J., TECTOR A.J., GOERKE M.E., KITCHEN S., LAGUNOFF D. Somatostatin analogue (octreotide) inhibits bile duct epithelial cell proliferation and fibrosis after extrahepatic biliary obstruction. Am. J. Pathol. 1993;143:1574–1578. [PMC free article] [PubMed] [Google Scholar]
- TURKCAPAR N., BAYAR S., KOYUNCU A., CEYHAN K. Octreotide inhibits hepatic fibrosis, bile duct proliferation and bacterial translocation in obstructive jaundice. Hepatogastroenterology. 2003;50:680–683. [PubMed] [Google Scholar]
- VALATAS V., KOLIOS G., MANOUSOU P., NOTAS G., XIDAKIS C., DIAMANTIS I., KOUROUMALIS E. Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells. Br. J. Pharmacol. 2004;141:477–487. doi: 10.1038/sj.bjp.0705633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J., ZHENG H., HAUER-JENSEN M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J. Pharmacol. Exp. Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- WU J., ZERN M.A. Hepatic stellate cells: a target for the treatment of liver fibrosis. J. Gastroenterol. 2000;35:665–672. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]