Abstract
Isometric contractile responses to carbachol were studied in ileal longitudinal smooth muscle strips from wild-type mice and mice genetically lacking M2 or M3 muscarinic receptors, in order to characterize the mechanisms involved in M2 and M3 receptor-mediated contractile responses.
Single applications of carbachol (0.1–100 μM) produced concentration-dependent contractions in preparations from M2-knockout (KO) and M3-KO mice, mediated via M3 and M2 receptors, respectively, as judged by the sensitivity of contractile responses to blockade by the M2-preferring antagonist methoctramine (300 nM) or the M3-preferring antagonist 4-DAMP (30 nM).
The M2-mediated contractions were mimicked in shape by submaximal stimulation with high K+ concentrations (up to 35 mM), almost abolished by voltage-dependent Ca2+ channel (VDCC) antagonists or depolarization with 140 mM K+ medium, and greatly reduced by pertussis toxin (PTX) treatment.
The M3-mediated contractions were only partially inhibited by VDCC antagonists or 140 mM K+-depolarization medium, and remained unaffected by PTX treatment. The contractions observed during high K+ depolarization consisted of different components, either sensitive or insensitive to extracellular Ca2+.
The carbachol contractions observed with wild-type preparations consisted of PTX-sensitive and -insensitive components. The PTX-sensitive component was functionally significant only at low carbachol concentrations.
The results suggest that the M2 receptor, through PTX-sensitive mechanisms, induces ileal contractions that depend on voltage-dependent Ca2+ entry, especially associated with action potential discharge, and that the M3 receptor, through PTX-insensitive mechanisms, induces contractions that depend on voltage-dependent and -independent Ca2+ entry and intracellular Ca2+ release. In intact tissues coexpressing M2 and M3 receptors, M2 receptor activity appears functionally relevant only when fractional receptor occupation is relatively small.
Keywords: M2 receptors, M3 receptors, knockout mice, intestinal smooth muscle, carbachol, contractile response, Ca2+ mobilization, pertussis toxin
Introduction
In various gastrointestinal smooth muscles, acetylcholine and its derivatives produce contractions by activating muscarinic receptors. It is generally assumed that the M3 muscarinic receptor plays a key role in mediating this activity (Eglen et al., 1996). The M3 receptor is coupled preferentially to Gq-type G proteins, resulting in the activation of phospholipase C (PLC) and the formation of inositol trisphosphate (InsP3) and diacylglycerol (DAG) (Candell et al., 1990; Prestwich & Bolton, 1995), which are likely to participate in muscarinic receptor-mediated smooth muscle contractions. InsP3 causes Ca2+ release from intracellular stores (Komori & Bolton, 1991; Morel et al., 1997) and can also mobilize Ca2+ secondarily through Ca2+-sensitive or store-dependent mechanisms (Ito et al., 1993; Ohta et al., 1995). DAG, via activation of protein kinase C, phosphorylates various proteins (Karaki et al., 1997) and can directly activate nonselective cationic channels (Helliwell & Large, 1996; Lee et al., 2003).
Besides M3 receptors, smooth muscle tissues also express M2 receptors, which are generally more abundant than the coexpressed M3 receptors (M2 : M3=3 : 1–5 : 1). M2 receptors are coupled to pertussis toxin (PTX)-sensitive G proteins (Gi/Go), which mediate the inhibition of adenylyl cyclase (Candell et al., 1990; Griffin & Ehlert, 1992; Reddy et al., 1995). This PTX-sensitive pathway seems unlikely to induce directly smooth muscle contractions, but may reverse the relaxing effect of isoprenaline or forskolin, which activate adenylyl cyclase (Thomas et al., 1993; Thomas & Ehlert, 1994). Electrophysiological studies have identified another M2 signaling pathway involving Go-mediated activation of smooth muscle cationic channels (Inoue & Isenberg, 1990; Zholos & Bolton, 1997; Kim et al., 1998; Komori et al., 1998; Yan et al., 2003). The opening of the cationic channels results in depolarization and the activation of voltage-dependent Ca2+ channels (VDCCs), which admit Ca2+ into the cell. The M2/Go/cationic channel system may therefore participate directly in muscarinic receptor-mediated contractile responses. However, direct evidence supporting this hypothesis is still lacking.
Recently, mutant mice lacking the M2 or M3 or both the M2 and M3 receptor subtypes have been generated by the use of gene targeting techniques (Gomeza et al., 1999; Yamada et al., 2001; Matsui et al., 2000, 2002; Struckmann et al., 2003). Analysis of these knockout (KO) mice has revealed that M2 receptors, although functionally less efficacious than smooth muscle M3 receptors, can directly mediate contractions in gastric and ileal smooth muscles (Stengel et al., 2000, 2002; Matsui et al., 2002; Stengel & Cohen, 2003). However, the molecular mechanisms underlying these M2-mediated contractions remain to be elucidated. It should also be of interest to study M3 receptor-mediated contractions (in the absence of M2 receptors) in greater detail.
Therefore, in the present study, we investigated the contractile effects of carbachol on ileal longitudinal smooth muscle strips from M2-KO, M3-KO, and M2/M3-double KO mice and their corresponding wild-type (WT) strains using VDCC antagonists, PTX, 140 mM K+ medium, and other pharmacological tools or procedures. Our results clearly demonstrate that M2 and M3 receptors induce smooth muscle contractions by different molecular mechanisms.
Methods
Animals and muscle strips
The generation of mice genetically lacking M2 or M3 receptors or both M2 and M3 receptors has been described previously (Gomeza et al., 1999; Yamada et al., 2001; Struckmann et al., 2003). The genetic background of the mice used in the present study was 129J1 (50%) × CF1 (50%) for the M2-KO and their corresponding WT mice, 129SvEv (50%) × CF1 (50%) for the M3-KO and their corresponding WT mice, and 129J1 (25%) × 129SvEv (25%) × CF1 (50%) for the M2/M3-double KO mice. Animals were housed in polycarbonate-ventilated cages. The animal room was maintained at 22–25°C with a relative humidity of 40–60% and a daily light/dark cycle (07 : 00–19 : 00). Food (CRF-1 or MF; Oriental Yeust Co. Ltd, Japan) and water were supplied ad libitum.
Mice of either sex, aged more than 3 months and weighing 23–38 g, were killed by cervical dislocation. The whole intestine was then quickly excised and placed in a Petri dish filled with Tyrode solution (composition described below), from which 1.5–2 cm segments of the ileum except for the terminal 2 cm were dissected. The longitudinal muscle layer of the segment was peeled off entirely from the underlying tissues by the method of Paton & Zar (1968). The muscle layer was trimmed and cut at both ends to provide a preparation of 10±1 mm in length. One or two muscle strips were prepared from the same mouse.
All procedures described above were performed according to the guidelines approved by a local animal ethics committee of the Faculty of Applied Biological Sciences, Gifu University.
Isometric tension recording
Strips were mounted in a 5-ml organ bath filled with Tyrode solution aerated and kept at 34°C, as described previously (Unno et al., 2003a). The strips were subjected to a tension of 0.3–0.4 g and allowed to equilibrate for 30 min, after which time they were exposed briefly to hyperosmotic 70 mM KCl (70 mM K+) at 10 min intervals until reproducible contractions were obtained.
Increases in isometric tension were recorded with a force–displacement transducer (T7-30-240, Orientic, Japan) coupled with a strain DC amplifier (AS2102, San-ei, Japan), the output being displayed on an ink-writing chart recorder (U-228, Nippon Denshi Kagaku, Japan).
Carbachol concentration–response curves
To measure concentration–response curves, increasing concentrations of carbachol, differing by 3- or 3.3-fold, were applied using a ‘single-dose' protocol. Each concentration was applied for 1 min, followed by washing with fresh Tyrode solution three or four times. The time interval between successive carbachol applications varied from 8 to 20 min, since more time was required to recover spontaneous activity after administration of higher concentrations of carbachol.
The initial phase of the tension response to carbachol was used for measurement of the concentration–response curves, since this phase was greater in amplitude than any subsequent phase. Also, the initial peak, especially in strips from M3-KO mice, was immediately followed by a progressive decline so that no noticeable tonic phase could be observed thereafter. As all strips, irrespective of their source, showed spontaneous mechanical activity at rest, the carbachol-evoked contractions were measured by taking the mean peak level of the pre-existing spontaneous contractions as a base line. Carbachol-induced peak amplitudes were expressed as % of the contraction response to 70 mM K+ in the same muscle strip.
Data analysis
Carbachol concentration–response data obtained in each strip were analyzed using the computer software Delta Graph 4.0 (SPSS Inc., Chicago, IL, U.S.A.), which fits the data directly with a logistic function, providing a carbachol pD2 value (negative logarithm of EC50), a maximum response (Emax), and a slope factor for the curve (Hill slope), as described previously (Unno et al., 2003a). Averaged concentration–response curves were also constructed by direct curve fitting using the same software.
The dissociation constant (pKd) of muscarinic receptor antagonists was calculated from the following equation: pKd=log(DR−1)−log[I], where [I] denotes the concentration of the antagonist, and DR the ratio of the mean EC50 value of carbachol estimated in the absence of the antagonist divided by that estimated in the presence of the antagonist (Sawyer & Ehlert, 1999).
Values in the text are given as means±s.e.m. (n=number of muscle strips used). Student's unpaired t-test was used to determine the statistical significance of differences between two group means. For statistical comparison between multiple group means, one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test to compare between two of multiple groups were used (Unno et al., 2003a). The differences were judged to be statistically significant when P<0.05.
PTX treatment
PTX was injected into mice at the same dose (100 μg/kg body weight) and in the same way (i.p.) as described for the guinea-pig (Sawyer & Ehlert, 1999). After 70–74 h, muscle strips were prepared from the injected animals as described above. PTX treatment led to a decrease in motor activity and body weight (by ∼10%) in M3-KO mice, but no conspicuous changes in general behavior were seen in the other mouse groups injected with the toxin.
Solutions
The composition of Tyrode solution was (mM): NaCl 137, KCl 2.9, CaCl2 1.8, MgCl2 2.1, NaH2PO4 0.4, NaHCO3 11.9 and glucose 5.6. Isotonic 140 mM K+ solution was prepared by substituting KCl for NaCl. Ca2+-free 140 mM K+ solution was prepared by further omitting CaCl2 and adding 0.5 mM EGTA. To record high K+-induced contractions, a 3.5 M KCl stock solution was prepared (without any other components) and applied hyperosmotically at the desired concentration (up to 70 mM) for a period of up to 3 min.
Drugs
Carbachol chloride, N,N′-bis [6-[[(2-methoyphenyl)methyl]amino]hexyl]-1, 8-octanediamine tetrahydrochloride (methoctramine), prostaglandin F2α (PGF2α), PTX, and nicardipine were from Sigma (St Louis, MO, U.S.A.); tetrodotoxin and nifedipine were from Wako (Osaka, Japan), and 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) was from Tocris (Ballwin, MO, U.S.A.).
The individual drugs were prepared as stock solutions (100 × or higher), which were added to the organ bath (5 ml) in a volume of 10 or 30 μl to achieve the desired final concentrations. Addition of the same volume of vehicle (distilled water) produced no change in tension. The VDCC antagonists, the muscarinic receptor antagonists, and the Na+ channel blocker were added to the organ bath at least 20 min before the induction of carbachol- or K+ (70 mM)-mediated contraction responses.
Results
Carbachol-induced contractions
Isometric changes in tension were measured in longitudinal smooth muscle strips of the ileum from M2-KO, M3-KO, and M2/M3 double-KO mice and from their corresponding WT strains. The strips incubated in Tyrode solution developed basal tension and displayed spontaneous activity that continuously generated small phasic rises in tension, probably associated with the spontaneous discharge of action potentials (Bolton, 1979; Unno et al., 2003b). When exposed to 70 mM K+ in 10 min intervals, strips responded with reproducible contractions usually after the third or fourth K+ exposure. The contractile response to 70 mM K+ showed a rapid increase in tension followed by a gradual decline, during which period spontaneous contractions were arrested, probably because of depolarization-block of action potential discharge (Bolton, 1979). After returning the smooth muscle strips to normal Tyrode solution, they immediately recovered spontaneous activity. The initial peak tension generated by 70 mM K+, as measured in g, varied from ∼0.3 to 1 g among different strips, but was always clearly distinguishable from the pre-existing spontaneous contractions.
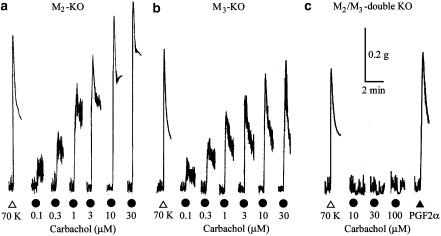
After reproducible contractions to 70 mM K+ had been observed, single, increasing concentrations of carbachol (0.01–30 or 100 μM) were applied for 1 min at intervals ranging from 8 to 20 min (see Methods). Carbachol was able to induce contractions in strips from both the M2-KO and the M3-KO groups. The initial peak amplitudes increased with increasing carbachol concentrations (Figure 1a and b), as was seen with strips from WT mice (Figure 7a). The contractile responses to carbachol (3–30 μM) were not significantly affected after application of the Na+ channel blocker tetrodotoxin (0.3 μM), suggesting that they resulted largely from a direct action of the agonist on smooth muscle.
Figure 1.
Contractions to carbachol in ileal smooth muscle strips from M2-KO (a), M3-KO (b), and M2/M3-double KO mice (c). In each strip, increasing, single concentrations of carbachol were applied for 1 min, as indicated (for details, see Methods). As a standard, a K+-induced contraction was obtained by the addition of 70 mM KCl (70 K). In (c), prostaglandin F2α (PGF2α, 0.3 μM) was applied as indicated.
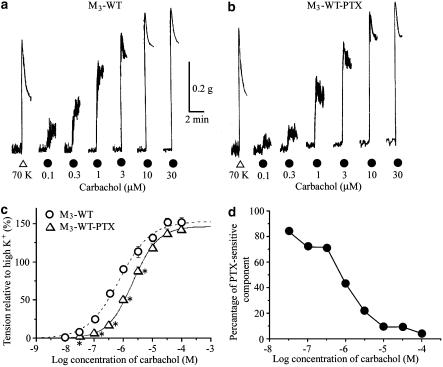
Figure 7.
Contractile effects of carbachol on ileal smooth muscle strips from WT M3 mice in the absence (a) or after treatment with PTX (b). (c) Averaged concentration–response curves for the tension increases caused by carbachol. Each point represents the mean±s.e.m. (n=8 for the control preparations; n=5 for the PTX-treated preparations). *Significantly different (P<0.05) from the corresponding control value. (d) Plot depicting the relationship between carbachol concentration and the PTX-sensitive component of the carbachol-mediated contractions. The percentage of PTX-sensitive component (P) was estimated from the data (mean relative amplitudes) shown in (c) using the following formula: P=100 (TCont−TPTX)/TCont, where TCont is the mean relative amplitude for the control preparations and TPTX is the corresponding value for samples treated with PTX. Note that the PTX-sensitive component continuously decreases with increasing carbachol concentrations.
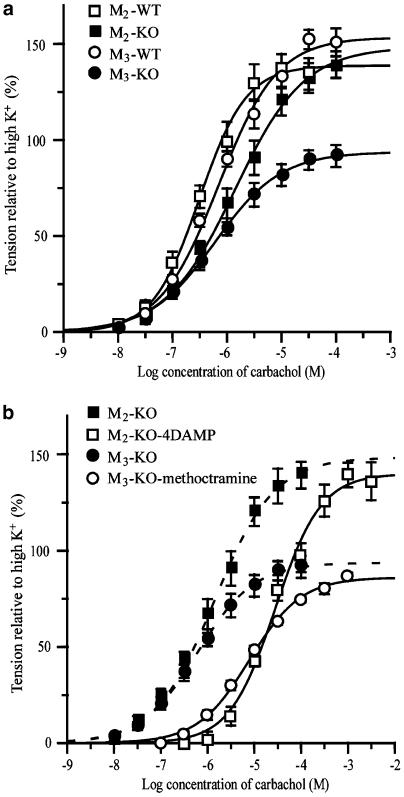
Figure 2a shows the averaged carbachol concentration–response curves obtained from the M2-KO, M3-KO, and their corresponding WT groups. Mean pD2, Emax, and Hill slope values are listed in Table 1. The carbachol Emax value found in the M3-KO group was significantly smaller than that in the M2-KO group, but there were no significant differences in pD2 values or Hill slopes between the two groups. Carbachol showed ∼2-fold reduced potency but an unchanged Emax in preparations from M2-KO mice, as compared to the corresponding WT preparations. On the other hand, the M3-KO group showed a ∼40% reduction in Emax, as compared to the corresponding WT value. There were no significant differences in any of the three parameters between the two WT groups (Table 1).
Figure 2.
Averaged concentration–response curves for the tension increases caused by carbachol in ileal smooth muscle strips from M2-KO and M3-KO mice and their respective WT controls (a). (b) Competitive antagonisms by the M2-preferring antagonist, methoctramine (300 nM), and the M3-preferring antagonist, 4-DAMP (30 nM), of carbachol-induced contractions in strips from the M2-KO and M3-KO groups. The peak tension generated by carbachol was measured from the mean peak level of pre-existing spontaneous contractions and was expressed as % of the 70 mM K+ contraction response in the same strip. In (b), the carbachol curves for the M2-KO and M3-KO groups without antagonist treatment (same as in a) are represented by the dotted curves. Each point represents the mean±s.e.m. of three to twelve measurements.
Table 1.
pD2, maximum response (Emax), and Hill coefficient for the tension increases caused by carbachol in strips from M2-KO or M3-KO type and their corresponding WT controls
| pD2 | Emax (%) | Hill | n | |
|---|---|---|---|---|
| M2-KO | 5.93±0.12a | 142.4±9.7 | 0.83±0.11 | 7 |
| +4-DAMP | 4.53±0.05d | 142.1±7.5 | 0.87±0.04 | 3 |
| +PTX | 5.82±0.05 | 136.2±4.0 | 0.92±0.06 | 6 |
| M3-KO | 6.18±0.08 | 94.8±4.9b,c | 0.76±0.06 | 12 |
| +Methoctramine | 5.14±0.06d | 83.9±9.7 | 0.88±0.05 | 5 |
| +PTX | 5.42±0.14d | 26.9±6.3d | 1.57±0.07d | 8 |
| WT for M2-KO | 6.39±0.08 | 138.3±7.1 | 1.10±0.15 | 6 |
| WT for M3-KO | 6.14±0.09 | 154.0±6.6 | 0.91±0.10 | 8 |
| +PTX | 5.68±0.06d | 147.6±4.4 | 1.02±0.07 | 5 |
Methoctramine and 4-DAMP were used at a concentration of 300 and 30 nM, respectively. Each value represents the mean±s.e.m. of the number of experiments (n). Emax values were expressed as % of 70 mM K+-induced contraction. One-way ANOVA showed that there were significant differences between the four group means (respective KO and WT groups) for pD2 and Emax.
Indicate values that are significantly different from the corresponding value for the M2-WT (a), M2-KO (b) and M3-WT (c) group, respectively, judged by a post hoc Bonferroni test.
Indicates that the value is significantly different from the corresponding control value, judged by Student's t-test.
In strips from M2/M3 double-KO mice, carbachol (up to 100 μM) produced no noticeable contraction, but rather inhibited the existing spontaneous activity with or without decreasing basal tension (Figure 1c). One possibility is that the inhibitory effect of carbachol is due to M1 receptor-mediated neural release of nitric oxide (Olgart & Iversen, 1999; Stengel & Cohen, 2003). PGF2α (0.3 or 1 μM), which also acts on specific G-protein-coupled receptors, induced a contraction comparable to that observed after the addition of 70 mM K+ (Figure 1c), suggesting that the strips from M2/M3-double KO mice retain an intact G-protein-linked signaling pathway leading to smooth muscle contraction. It is therefore likely that M2 and M3 receptors are entirely responsible for the carbachol-mediated contractions in mouse ileal longitudinal muscle, as described by Matsui et al. (2002).
Muscarinic receptor antagonists
We next investigated the effect of two subtype-preferring muscarinic receptor antagonists on the carbachol-mediated contractions in the M3-KO and M2-KO groups. Smooth muscle strips from M2-KO mice were exposed to the M3-preferring antagonist 4-DAMP (30 nM), which had no effect on spontaneous contractions and responsiveness to 70 mM K+. In the presence of 4-DAMP, carbachol concentration–response curves were shifted significantly to the right without a significant change in Emax or Hill slope (Figure 2b and Table 1). From the resulting 20.4-fold increase in carbachol EC50 values, the pKd of 4-DAMP was calculated to be 8.8, consistent with published pKd values for the M3 receptor (9.1 or 9.3; Eglen et al., 1996).
Similar experiments were carried out with strips from M3-KO mice using the M2-preferring antagonist methoctramine (300 nM). Methoctramine had no significant effect on spontaneous contractions and contractions induced by 70 mM K+. However, in the presence of methoctramine, carbachol concentration–response curves were shifted significantly to the right without a significant change in Emax or Hill slope (Figure 2b and Table 1). Based on the resulting 9.0-fold increase in carbachol EC50 values, the pKd of methoctramine was calculated to be 7.4, consistent with published pKd data for the M2 receptor (7.6 or 7.9; Eglen et al., 1996).
These results confirmed that the carbachol contractions in the M2-KO and M3-KO groups were mediated by M3 and M2 receptors, respectively.
Shape of M2- and M3-mediated contractions
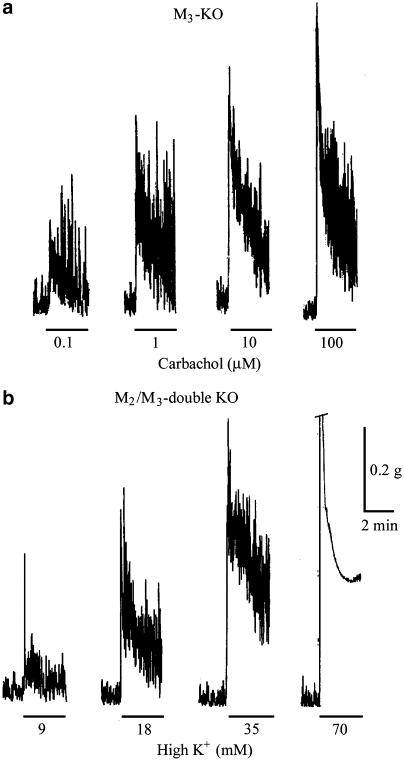
To further study the response pattern of the M2- and M3-mediated contractions, carbachol was applied for 3 min at various concentrations. Figure 3a shows the responses to 0.1, 1, 10, and 100 μM carbachol in a strip from the M3-KO group. The responses mediated by M2 receptors showed a phasic increase in tension on which spontaneous contractions were superimposed. The phasic tension response approached a level close to the pre-existing basal tension at the end of the 3 min carbachol application period.
Figure 3.
Shape of tension responses to carbachol in the M3-KO group (a) and to application of high K+ in the M2/M3-double KO group (b). Agents were applied for 3 min at different concentrations as indicated. In (b), the 70 mM K+ contraction response is cut off at its top by one calibration size (0.2 g).
M3-mediated responses to a low carbachol concentration such as 0.1 μM were similar in shape to the M2-mediated responses. At carbachol concentrations ⩾1 μM, the responses showed a clear biphasic shape characterized by an initial phasic response followed by a lower, more sustained increase in tension (Figures 4b and 5b). No spontaneous contractions were superimposed on the biphasic tension response to carbachol concentrations ⩾10 μM.
Figure 4.
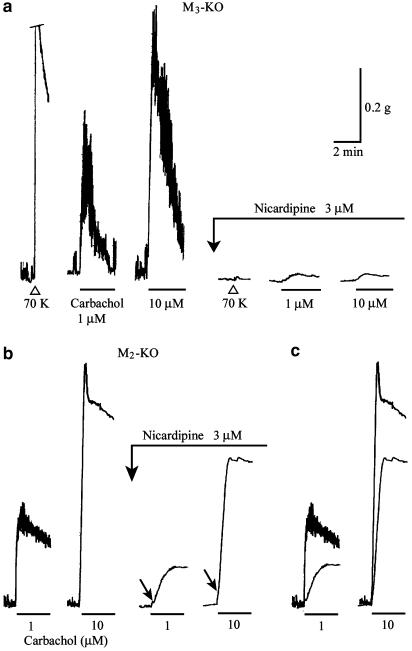
Effects of nicardipine on carbachol contractions in ileal smooth muscle strips from the M3-KO (a) and M2-KO groups (b). Carbachol was applied for 3 min at the indicated concentrations, in the absence or presence of nicardipine (3 μM). In (a), the contraction response to 70 mM K+ is cut off at its top by one calibration size (0.2 g). The arrows in (b) indicate an initial rapid rising phase (see text for details). (c) Superimposed traces of responses in the absence or presence of nicardipine (data from panel b). Note that the carbachol contractions in (a) are much more sensitive to nicardipine than those in (b).
Figure 5.
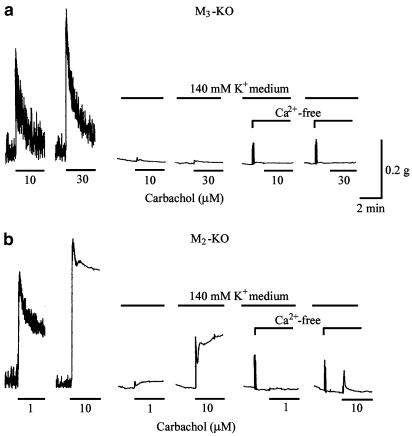
Effects of isotonic 140 mM K+ medium and removal of extracellular calcium (‘Ca2+-free') on carbachol contractions in strips from the M3-KO (a) and M2-KO groups (b). Carbachol was applied for 3 min at the indicated concentrations. The upward deflections seen at the beginning of ‘Ca2+-free' are artifacts by changing the bath solution (140 mM K+ medium) to Ca2+-free medium (140 mM K+ medium plus 0.5 mM EGTA). See text for details.
As a comparison, strips irrespective of their source were exposed to 9, 18, 35, and 70 mM K+ for 3 min each. The high K+ responses of a strip from M2/M3-double KO group are shown in Figure 3b. It can be seen that the responses to high K+ of up to 35 mM were very similar in shape to the M2-mediated responses (cf. Figure 3a and b). The response to 70 mM K+ consisted of a rapid phasic response followed by a lower sustained rise in tension, on which no spontaneous contractions were superimposed.
Calcium channel antagonist
The VDCC antagonist nicardipine was used to investigate the contribution of voltage-dependent Ca2+ entry to the M2- and M3-mediated contractions. The presence of nicardipine (3 μM) resulted in the abolition of spontaneous activity and in a marked reduction of the 70 mM K+-induced contraction to 3.0±0.9% (n=9) of the control response (Figure 4a).
Nicardipine (3 μM) also markedly inhibited the M2-mediated responses to a 3-min application of carbachol (1–100 μM), but frequently a small, slowly developing contraction remained (Figure 4a). The remaining contractions exhibited no clear concentration dependence; their size was ∼5% of the initial peak amplitude of the control response. Similar results were obtained with nifedipine (3 μM), another VDCC antagonist (n=3).
The M3-mediated response to 0.1 μM carbachol was almost completely blocked by 3 μM nicardipine (n=4; data not shown). As for the biphasic response to 1 or 10 μM carbachol, the initial phasic component was almost abolished, but the later tonic response still remained with a reduced amplitude, as shown in Figure 4b and c. Consequently, a slow-developing, sustained contraction occurred upon carbachol application in the presence of nicardipine, whose amplitude at the 3-min point corresponded to 55.0±3.8% at 1 μM and 82.7±3.4% at 10 μM carbachol (n=6 for each) of the respective control tonic components. The nicardipine-insensitive contractions often had a rapid, very small component in their initial rising phase (see the arrows in Figure 4b).
Depolarization with 140 mM K+ solution
Strips were depolarized with isotonic 140 mM K+ medium in order to investigate the components of M2- or M3-mediated contractions that are independent of electromechanical coupling. Changing the bathing solution to the high K+ medium produced a phasic increase in tension, and 5–7 min later, the tension reached a steady level near the pre-existing basal tension, where neither spontaneous activity nor any response to 70 mM K+ was seen.
In strips from the M3-KO group, when a steady resting level of tension in 140 mM K+ medium was achieved, carbachol (10–100 μM) was without effect (n=4) or produced a barely detectable increase in tension (n=3), as shown in Figure 5a. After removal of Ca2+ in the 140 mM K+ medium, no change in tension occurred upon carbachol application (Figure 5a).
In M2-KO tissues depolarized with 140 mM K+ medium, 1 or 10 μM carbachol produced a biphasic tension increase consisting of a rapid phasic response followed by a slower sustained component (Figure 5b). These components at 1 μM carbachol corresponded to 6.1±4.1, and 9.5±3.5% (n=6) of the respective corresponding components of the control response. In Ca2+-free, 140 mM K+ medium, neither component was elicited in four out of six strips, but the remaining two strips responded with a decreased contraction. The amplitudes of the initial phasic and the later tonic components at 10 μM carbachol in the 140 mM K+ medium were 23.5±4.3 and 39.6±5.2% (n=6) relative to the respective original components. After removal of the extracellular Ca2+, the tonic component was abolished, while the initial phasic still persisted, although its size was decreased to 8.8±4.1% (n=6) (Figure 5b).
The results indicated that M2-mediated contractions, in contrast to M3-mediated contractions, were largely dependent on electromechanical coupling.
PTX
The contractile effect of carbachol was examined in strips from M2-KO or M3-KO mice pretreated with PTX, which selectively uncouples Gi/Go proteins from the associated receptors (see Methods). These strips exhibited normal spontaneous activity and responsiveness to 70 mM K+.
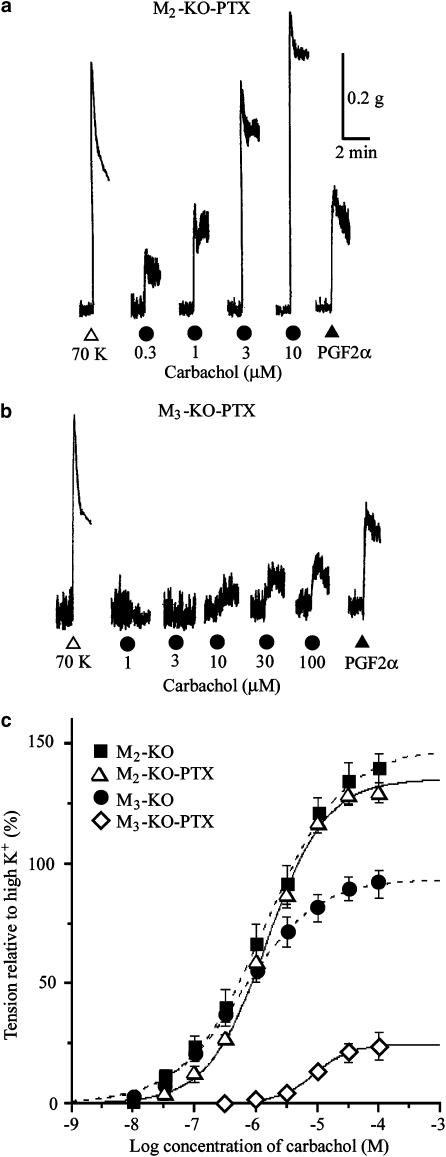
In strips from PTX-treated M2-KO mice, applications of carbachol produced concentration-dependent contractions, as shown in Figure 6a. The averaged concentration–response curve almost overlapped with the control curve (Figure 6c), and the mean values for pD2, Emax, and Hill slope were very similar to the corresponding values obtained without PTX treatment (control; Table 1). However, the contraction evoked by 3 μM PGF2α in these strips (56.7±10.2%, n=3) was significantly smaller (P<0.05) than the control response (116.0±4.2%, n=3), indicating the effectiveness of PTX treatment.
Figure 6.
Contractile effects of carbachol on ileal smooth muscle strips from the M2-KO (a) and M3-KO groups (b) pretreated with PTX. Increasing concentrations of carbachol and 3 μM PGF2α were applied as indicated. (c) Averaged concentration–response curves for the tension increases caused by carbachol. Each point represents the mean±s.e.m. (n=6 for the PTX-treated M2-KO group; n=8 for the PTX-treated M3-KO group). For comparison, the carbachol curves for the M2-KO and M3-KO groups without PTX treatment (taken from Figure 2) are represented by the dotted curves.
In strips from PTX-treated M3-KO mice, carbachol of up to 1 or 3 μM produced no significant contractions but inhibited existing spontaneous contractions. Higher concentrations of carbachol evoked significant contractions, although their size was considerably smaller compared with the control responses (Figure 6b and c). The mean pD2, Emax, and Hill slope values were significantly different from the control values (Table 1). The PGF2α (3 μM)-evoked contraction (49.3±2.7%, n=7) (Figure 6b) was smaller (P<0.01) than the control response (121.2±8.4%, n=5).
The results strongly suggest that M2, but not M3 receptors, utilize the PTX-sensitive G-protein-liked signaling pathway to induce contractions.
We further investigated the effect of carbachol on strips from PTX-treated WT M3 mice. As shown in Figure 7b, carbachol produced concentration-dependent contractions. The carbachol pD2 value, but not the Emax and Hill slope, differed significantly from the values obtained with strips from WT M3 mice that had not been treated with PTX (Table 1). When peak amplitudes of the carbachol contractions were compared between the PTX-treated and the control groups, there was a significant difference only at carbachol concentrations <3 μM (Figure 7c). The proportion of the PTX-sensitive component of the control contractions was estimated from the data in Figure 7c and plotted against carbachol concentrations in Figure 7d. This figure shows that the PTX-sensitive component of contractions was ∼80% at 0.3 μM carbachol, but almost undetectable at 100 μM carbachol.
Discussion
In the present work, we used M2-KO, M3-KO, or M2/M3-double KO mice as novel tools to study the functional roles of M2 and M3 muscarinic receptors in mediating contractile responses of the ileal longitudinal smooth muscle. Carbachol, a nonselective muscarinic agonist, was used to elicit contractions.
Carbachol failed to contract ileal smooth muscle strips from the M2/M3-double KO group, but was able to produce TTX-insensitive contractions in the M2-KO and M3-KO groups. These contractions were mediated by M3 and M2 receptors, respectively, as judged from studies with subtype-preferring muscarinic antagonists. These observations indicate that the M2 and M3 subtypes, but not any other muscarinic receptor subtypes, can induce smooth muscle contractions directly, as reported by Matsui et al. (2002). The carbachol Emax values obtained in the M2-KO and M3-KO groups (Table 1) indicate that the contractile efficacy of M2 receptors was significantly greater than previously reported by Matsui et al. (2002) who applied carbachol in a cumulative manner. In the present study, carbachol was applied using a single-dose protocol and the initial phasic increases in tension were evaluated. Therefore, the observed differences in the relative efficacy of M2 receptors in mediating ileal smooth muscle contractions may be due to, at least partly, the different experimental protocols used.
The relationship between receptor occupancy and contractile response has been described as response=Sh/(Sh+Kh), where S indicates the stimulus given as the product of receptor occupancy and intrinsic efficacy, K the sensitivity constant for the contractile response, and h the coefficient of cooperativity for the stimulus–response function (Sawyer & Ehlert, 1999). In the present study, carbachol pD2 values and Hill slopes were not significantly different between the M2- and the M3-mediated contractions, suggesting that M2 and M3 receptors may elicit the initial phasic contractions to carbachol with similar K and h but with different S.
Regardless of the carbachol concentration used, the M2-mediated contractions were severely reduced by VDCC antagonists or after depolarization with 140 mM K+ medium where membrane potential should be not readily altered. Moreover, the M2-mediated contractions were mimicked in shape by high K+ (up to 35 mM). Both the M2- and high K+-mediated responses showed a phasic increase in tension, which reached a peak and then progressively declined under the simultaneous occurrence of spontaneous contractions. The level of tension at 3 min after carbachol application was also similar between both responses (Figure 3). Based on these data, M2 receptor activation seems to do not more than submaximal high K+ stimulation does. In smooth muscles that can freely discharge action potentials, the spike discharge is the primary and most important mechanism by which a rise in [Ca2+]c is produced, and the tension generated by high K+ is determined by the extent of the increase in spike frequency, unless depolarization by high K+ is so extreme that spike discharge ceases (Bolton, 1979; Kohda et al., 1997). Therefore, it seems probable that the M2-mediated contractions depend largely on Ca2+ entry via VDCCs, especially associated with acceleration of spike discharge. However, voltage-independent Ca2+ entry may also make a slight contribution, since a very small contraction remained upon carbachol application in the presence of VDCC antagonists and occasionally in 140 mM K+ medium (Figures 4a and 5a).
The pronounced inhibition of M2-mediated contractions by PTX clearly demonstrates that M2 receptors utilize PTX-sensitive G-protein-linked signaling pathways to induce contractions. Studies with guinea-pig gastric and intestinal smooth muscles suggest that M2 receptor stimulation, via activation of the PTX-sensitive G protein Go, primarily opens cationic channels, which cause depolarization (Inoue & Isenberg, 1990; Komori et al., 1992, 1998; Zholos & Bolton, 1997; Kim et al., 1998; Rhee et al., 2000; Yan et al., 2003). During the course of the present study, we confirmed in WT ileal muscle cells that carbachol produces a PTX-sensitive cationic current (unpublished data). Therefore, the Go/cationic channel system is likely to mediate M2 receptor-dependent contractions by producing an inward cationic current leading to depolarization and an increased frequency of spike discharge. The M2/Go/cationic channel system in guinea-pig intestinal smooth muscles is under potent regulation by M3 receptors through Ca2+ release from intracellular stores and a permissive action on channel gating (Pacaud & Bolton, 1991; Komori et al., 1993; Bolton & Zholos, 1997; Zholos & Bolton, 1997). Thus, if M3 receptor activity is weak, muscarinic activation of the smooth muscle cationic current and consequent depolarization are poor (Okamoto et al., 2002; Unno et al., 2003a). Obviously, tissues from M3-KO mice lack such synergistic M3 effect, and hence carbachol-induced depolarization in these tissues may be small and not exceed a level sufficient for depolarization-block of spike discharge. This may agree with the observation that spontaneous contractions remained even after full activation of M2 receptors by carbachol, despite the fact that the equilibrium potential of the cationic channel-induced depolarization is around −10 mV, a level sufficient for depolarization-block of spike discharge (Bolton, 1972; Unno et al., 2003b).
The M3-mediated contractions were not significantly affected by PTX treatment, consistent with the concept that they are mediated by the Gq/PLC signaling pathway. Furthermore, the results obtained with VDCC antagonists, 140 mM K+ medium, and Ca2+-free solution suggested that the M3-mediated contractions involve multiple mechanisms, all of which lead to a rise in [Ca2+]c, including voltage-dependent Ca2+ entry associated with spike discharge or maintained activation of VDCCs under sustained depolarization, voltage-independent Ca2+ entry, and intracellular Ca2+ release. Our results also suggest that the relative contribution of these different mechanisms vary with the agonist concentrations used and/or different phases of the contraction response. Similar findings have been obtained for carbachol-induced contractions in guinea-pig stomach and Taenia caeci smooth muscle preparations (Parekh & Brading, 1991; Hishinuma et al., 1997).
The Gq/PLC signaling pathway leads to the generation of InsP3 and DAG as the key second messengers, and it is therefore likely that these agents act directly and/or indirectly to provide the Ca2+ for the M3-mediated contractions. InsP3 induces Ca2+ release from intracellular stores, but secondarily also activates the Ca2+-sensitive depolarizing Cl− current (Ito et al., 1993) and Ca2+ store-dependent Ca2+ entry via non-VDCCs (Ohta et al., 1995). DAG causes nonselective cationic channels to open allowing Ca2+ to enter the cell; Ca2+ influx can also occur secondarily via VDCCs activated by depolarization (Helliwell & Large, 1996; Lee et al., 2003). However, the precise mechanisms underlying voltage-dependent and -independent Ca2+ entry remain to be elucidated.
In ileal smooth muscle strips from M2-KO but not M3-KO mice, spontaneous contractions were decreased in the size by application of 1 or 3 μM carbachol and abolished at carbachol concentrations of 10 μM and higher. The observation may imply that M3 receptor activation exerts an inhibitory effect on action potential discharge. Consistent with this concept, we previously observed in guinea-pig ileal muscle cells that carbachol concentration-dependently suppressed Ca2+ current via VDCCs through PTX-insensitive mechanisms (Unno et al., 1995), indicative of M3 receptor-mediated VDCC inactivation. This M3 effect may cause a block of spike discharges, explaining why the spontaneous contractions were inhibited upon M3 receptor activation.
It has been suggested that only M3 receptors can mediate the contraction of various gastrointestinal smooth muscles in a direct manner (see Eglen et al., 1996). The present study indicates that the M2 subtype can also mediate this function. This notion is supported by the finding that carbachol concentration–response curves in tissues from WT mice were significantly affected by PTX treatment, which selectively inhibits M2-mediated contractions (Figure 7). It should be noted that the contribution of M2 receptors is significant only at relatively low agonist concentrations, but that M3 receptor activity is completely dominant at higher agonist concentrations (Figure 7d). This phenomenon may explain why the Emax in the WT groups (138–154%) is far different from the sum of the Emax values in the M2-KO and M3-KO groups (145 and 95%, respectively) but rather close to the M2-KO value.
Why does the contribution of M2 receptors to carbachol-induced smooth muscle contractions decrease with increasing carbachol concentrations? One possible explanation for this phenomenon may be based on the observation that M3 receptor activation leads to the inactivation of VDCCs (Unno et al., 1995). This M3 effect, which depends on fractional receptor occupation, would continuously decrease the number of VDCCs available for spike discharge, eventually preventing the M2-mediated contractile component, which relies mainly on spike discharge (see above). The loss of the M3-mediated contractile component resulting from the block of spike discharge could be readily overcome by activation of noninactivated VDCCs and other mechanisms, as mentioned above. This complex relationship between receptor occupancy and M2 and M3 receptor activity in mediating smooth muscle contractions may explain why it has been difficult in the past to detect the involvement of M2 receptors in agonist-evoked contractions in intact smooth muscle tissues.
Acknowledgments
This work was supported by a Grant-in-Aid Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Nos. 16380199 and 16780202).
Abbreviations
- [Ca2+]c
cytosolic concentration of Ca2+
- DAG
diacylglycerol
- 4-DAMP
1,1-dimethyl-4-diphenylacetoxypiperidinium iodide
- EGTA
ethyleneglycol-bis (2-aminoethyl ether) N,N,N′,N′,-tetraacetic acid
- InsP3
inositol-1,4,5-trisphosphate
- KO mice
knockout mice
- methoctramine
N,N′-bis [6-[[(2-methoyphenyl)methyl]amino]hexyl]-1,8-octanediamine tetrahydrochloride
- PGF2α
prostaglandin F2α
- PLC
phospholipase C
- PTX
pertussis toxin
- VDCCs
voltage-dependent Ca2+ channels
- WT mice
wild-type mice
References
- BOLTON T.B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J. Physiol. 1972;220:647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979;59:647–671. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- CANDELL L.M., YUN S.H., TRAN L.L, EHLERT F.J. Differential coupling of subtypes of the muscarinic receptor to adenylate cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol. Pharmacol. 1990;38:689–697. [PubMed] [Google Scholar]
- EGLEN R.M., HEGDE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- GOMEZA J., SHANNON H., KOSTENIS E., FELDER C., ZHANG L., BRODKIN J., GRINGERG A., SHENG H., WESS J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN M.T., EHLERT F.J. Specific inhibition of isoproterenol-stimulated cyclic AMP accumulation by M2 muscarinic receptors in rat intestinal smooth muscle. J. Pharmacol. Exp. Ther. 1992;263:221–225. [PubMed] [Google Scholar]
- HELLIWELL R.M., LARGE W.A. α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J. Physiol. 1996;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HISHINUMA S., HONGO I., MATSUMOTO Y., NARITA F., KUROKAWA M. Contracting effects of carbachol, McN-A-343 and AHR-602 on Ca2+-mobilization and Ca2+-influx pathways in Taeniacaeci. Br. J. Pharmacol. 1997;122:985–992. doi: 10.1038/sj.bjp.0701467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Acetylcholine activates nonselective cation channels in guinea-pig ileum through a G-protein. Am. J. Physiol. 1990;258:C1–C6. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- ITO S., OHTA T., NAKAZATO Y. Inward current activated by carbachol in rat intestinal smooth muscle cells. J. Physiol. 1993;470:395–409. doi: 10.1113/jphysiol.1993.sp019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., SIM J.H., CHO C.H., JUHNN Y.S., SUH S.H., SO I., KIM K.W. Suppression of the carbachol-activated nonselective cationic current by antibody against α subunit of Go protein in guinea-pig gastric myocytes. Pflugers Arch. 1998;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- KOHDA M., KOMORI S., UNNO T., OHASHI H. Characterization of action potential-triggered [Ca2+]i transients in single smooth muscle cells of guinea-pig ileum. Br. J. Pharmacol. 1997;122:477–486. doi: 10.1038/sj.bjp.0701407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J. Physiol. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., PACAUD P., OHASHI H., BOLTON T.B. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflugers Arch. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., UNNO T., NAKAYAMA T., OHASHI H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- LEE Y.M., KIM B.J., KIM H.J., YANG D.K., ZHU M.H., LEE K.P., SO I., KIM K.W. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am. J. Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., FUJIKAWA T., JIANG J., TAKAHASHI S., MANABE T., TAKETO M.M. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J. Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUI M., MOTOMURA D., KARASAWA H., FUJIKAWA T., JIANG J., KOMIYA Y., TAKAHASHI S., TAKETO M.M. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL J.L., MACREZ N., MIRONNEAU J. Specific Gq protein involvement in muscarinic M3 receptor-induced phosphatidylinositol hydrolysis and Ca2+ release in mouse duodenal myocytes. Br. J. Pharmacol. 1997;121:451–458. doi: 10.1038/sj.bjp.0701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHTA T., KAWAI K., ITO S., NAKAZATO Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth muscle of the rat. Br. J. Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO H., PRESTWICH S.A., ASAI S., UNNO T., BOLTON T.B., KOMORI S. Muscarinic agonist potencies at three different effector systems linked to the M2 or M3 receptor in longitudinal smooth muscle of guinea-pig small intestine. Br. J. Pharmacol. 2002;135:1765–1775. doi: 10.1038/sj.bjp.0704642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLGART C., IVERSEN H.H. Nitric oxide-dependent relaxation induced by M1 muscarinic receptor activation in the rat small intestine. Br. J. Pharmacol. 1999;127:309–313. doi: 10.1038/sj.bjp.0702529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACAUD P., BOLTON T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B., BRADING A.F. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of guinea-pig stomach. Br. J. Pharmacol. 1991;104:412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W.D., ZAR M.A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J. Physiol. 1968;194:13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTWICH S.A., BOLTON T.B. G-protein involvement in muscarinic receptor-stimulation of inositol phosphates in longitudinal smooth muscle from the small intestine of the guinea-pig. Br. J. Pharmacol. 1995;114:119–126. doi: 10.1111/j.1476-5381.1995.tb14915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY H., WATSON N., FORD A.P., EGLEN R.M. Characterization of the interaction between muscarinic M2 receptors and β-adrenoceptor subtypes in guinea-pig isolated ileum. Br. J. Pharmacol. 1995;114:49–56. doi: 10.1111/j.1476-5381.1995.tb14904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHEE J.C., RHEE P.L., PARK M.K., SO I., UHM D.Y., KIM K.W., KANG T.M. Muscarinic receptors controlling the carbachol-activated nonselective cationic current in guinea pig gastric smooth muscle cells. Jpn. J. Pharmacol. 2000;82:331–337. doi: 10.1254/jjp.82.331. [DOI] [PubMed] [Google Scholar]
- SAWYER G.W., EHLERT F.J. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea-pig colon: evidence for M2/M3 receptor interactions. J. Pharmacol. Exp. Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- STENGEL P.W., COHEN M.L. M1 receptor-mediated nitric oxide-dependent relaxation unmasked in stomach fundus from M3 receptor knockout mice. J. Pharmacol. Exp. Ther. 2003;304:675–682. doi: 10.1124/jpet.102.042283. [DOI] [PubMed] [Google Scholar]
- STENGEL P.W., GOMEZA J., WESS J., COHEN M.L. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J. Pharmacol. Exp. Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- STENGEL P.W., YAMADA M., WESS J., COHEN M.L. M3 receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder and trachea in vitro. Am. J. Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- STRUCKMANN N., SCHWERING S., WIEGAND S., GSCHNELL A., YAMADA M., KUMMER W., WESS J., HABERBERGER R.V. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol. Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- THOMAS E.A., BAKER S.A., EHLERT F.J. Functional role for the M2 muscarinic receptor in smooth muscle of guinea pig ileum. Mol. Pharmacol. 1993;44:102–110. [PubMed] [Google Scholar]
- THOMAS E.A., EHLERT F.J. Pertussis toxin blocks M2 muscarinic receptor-mediated effects on contraction and cyclic AMP in the guinea pig ileum, but not M3-mediated contractions and phosphoinositide hydrolysis. J. Pharmacol. Exp. Ther. 1994;271:1042–1050. [PubMed] [Google Scholar]
- UNNO T., KOMORI S., OHASHI H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J. Physiol. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNNO T., KWON S.-C., OKAMOTO H., IRIE Y., KATOH Y., MATSUYAMA H., KOMORI S. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br. J. Pharmacol. 2003a;139:337–350. doi: 10.1038/sj.bjp.0705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNNO T., MATSUYAMA H., KOMORI S. Muscarinic signal transduction in gastrointestinal smooth muscle. Recent Res. Dev. Physiol. 2003b;1:577–597. [Google Scholar]
- YAMADA M., MIYAKAWA T., DUTTAROY A., YAMANAKA A., MORIGUCHI T., MAKITA R., OGAWA M., CHOU C.J., XIA B., CRAWLEY J.N., FELDER C.C., DENG C.-X., WESS J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- YAN H.-D., OKAMOTO H., UNNO T., TSYTSYURA Y.D., PRESTWICH S.A., KOMORI S., ZHOLOS A.V., BOLTON T.B. Effects of G protein-specific antibodies and Gβγsubunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 2003;139:605–615. doi: 10.1038/sj.bjp.0705289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]