Abstract
Acute respiratory distress syndrome (ARDS) is associated with increased superoxide (O2•−) formation in the pulmonary vasculature and negation of the bioavailability of nitric oxide (NO). Since NO inhibits NADPH oxidase expression through a cyclic GMP-mediated mechanism, sildenafil, a type V phosphodiesterase inhibitor, may be therapeutically effective in ARDS through an augmentation of NO-mediated inhibition of NADPH oxidase. Therefore, the effect of sildenafil citrate and NO-donating sildenafil (NCX 911) on O2•− formation and gp91phox (active catalytic subunit of NADPH oxidase) expression was investigated in cultured porcine pulmonary artery endothelial cells (PAECs).
PAECs were incubated with 10 nM TXA2 analogue, 9,11-dideoxy-9α,11α-methanoepoxy-prostaglandin F2α (U46619) (±sildenafil or NCX 911), for 16 h and O2•− formation measured spectrophometrically and gp91phox using Western blotting. The role of the NO-cGMP axis was studied using morpholinosydnonimine hydrochloride (SIN-1), the diethylamine/NO complex (DETA-NONOate), the guanylyl cyclase inhibitor, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one (ODQ), and the protein kinase G inhibitor, 8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-Br-cGMPS). NO release was studied using a fluorescence assay and O2•−–NO interactions by measuring nitrites.
After a 16-h incubation with 10 nM U46619, both NCX 911 and sildenafil elicited a concentration-dependent inhibition of O2•− formation and gp91phox expression, NCX 911 being more potent (IC50; 0.26 nM) than sildenafil citrate (IC50; 1.85 nM). These inhibitory effects were reversed by 1 μM ODQ and 10 μM Rp-8-Br-cGMPS. NCX 911 stimulated the formation of cGMP in PAECs and generated NO in a cell-free system to a greater degree than sildenafil citrate. The inhibitory effect of sildenafil was augmented by 1 μM SIN-1 and blocked partially by the eNOS inhibitor 10 μM N5-(1-iminoethyl)-ornithine (L-NIO). Acutely, sildenafil and NCX 911 also inhibited O2•− formation, again blocked by 1 μM ODQ. NCX 911 reacted with O2•− generated by xanthine oxidase, an effect that was inhibited by superoxide dismutase (500 U ml−1).
Since O2•− formation plays contributory role in ARDS, both sildenafil citrate and NCX 911 may be indicated for treating ARDS through suppression of NADPH oxidase expression and therefore of O2•− formation and preservation of NO bioavailability.
Keywords: Superoxide, NADPH oxidase, nitric oxide, ARDS, sildenafil
Introduction
Sildenafil (Viagra™) is widely used to treat erectile dysfunction (ED) (Rosen & Kostis, 2003). It has also become increasingly apparent that sildenafil may also be effective in treating pulmonary hypertension (Zhao et al., 2001; Sebkhi et al., 2003; Ghofrani et al., 2004; Madden et al., 2004). The therapeutic action of sildenafil is mediated through the inhibition of type V phosphodiesterase (PDE V), which increases cyclic GMP (cGMP) levels in response to nitric oxide (NO) (Jeremy et al., 1997), thereby augmenting the relaxation of vascular (and cavernosal) smooth muscle tissue (Rosen & Kostis, 2003). This effect compensates for diminished NO bioavailability (and therefore NO drive) associated with both pulmonary hypertension and ED (Jeremy et al., 1997; Rosen & Kostis, 2003). There is also an abundance of PDE V in the lung (Thomas et al., 1990), as in the corpus cavernosum (Ballard et al., 1998), which consolidates the potential use of sildenafil to treat pulmonary arterial disease.
Oxidative stress (OS), in particular superoxide (O2•−) formation, plays a central role in the aetiology of acute respiratory distress syndrome (ARDS) (Thomas et al., 1990; Chabot et al., 1998), a condition characterised by a time-dependent worsening of intrapulmonary inflammation and hypertension (Chabot et al., 1998; Weinacker & Vaszar, 2001). O2•− reacts with NO to form peroxynitrite (ONOO−) and other reactive nitrogen species, effectively reducing NO bioavailabilty (Folkerts et al., 2001; Stuart-Smith & Jeremy, 2001). NO is a vasodilator and inhibits adhesion molecule expression (Jeremy et al., 1999; 2004). A reduction of NO bioavailability may therefore not only promote vasoconstriction and pulmonary hypertension but also enhance the adhesion of leucocytes and platelets leading to augmented inflammation in ARDS (Jeremy et al., 1999; Stuart-Smith & Jeremy, 2001; Jeremy et al., 2004). In this context, endotoxin, tumour necrosis factor-alpha (TNF-α), interleukin-1α (IL-1α) and thromboxane A2 (TXA2) all promote O2•− formation via an upregulation of NADPH oxidase in isolated pulmonary artery (PA) (Muzaffar et al., 2003; 2004a, 2004b). It was suggested that this may constitute a mechanism by which pulmonary hypertension and inflammation is propagated and worsened in ARDS. It has also been demonstrated that NO is a potent inhibitor of NADPH oxidase expression, an effect mediated by a cGMP-dependent mechanism (Muzaffar et al., 2004a). It was therefore proposed that O2•− may promote ARDS, at least in part, through negation of this protective inhibitory effect of NO on NADPH oxidase expression (Muzaffar et al., 2004a). Since sildenafil prevents the hydrolysis of cGMP, through the inhibition of PDE V, it is reasonable to suggest that sildenafil may be therapeutically effective in ARDS, through an augmentation of NO-mediated suppression of O2•− formation.
The first objective of this study, therefore, was to investigate the effect of sildenafil citrate on O2•− formation, gp91phox (an active catalytic subunit of NADPH oxidase) expression and the role of cGMP in mediating effects in pig pulmonary artery endothelial cells (PAEC). A novel nitrated derivative of sildenafil (NCX 911) has been shown to release NO (Seidler et al., 2002) and as such may exert a more potent effect on the NO-cGMP axis, since the drug intrinsically drives the formation of cGMP while simultaneously blocking the hydrolysis of cGMP. This would render NCX 911 potentially more effective than sildenafil citrate in treating ARDS. Thus, the second objective was to compare the effect of NCX 911 with that of sildenafil citrate on the aforementioned parameters. Comparative effects of the two drugs on cGMP formation and on the release of NO in solution were also studied, as well as possible O2•− quenching reactions of NCX 911.
Methods
All reagents were obtained from the Sigma Chemical Company (Poole, Dorset, U.K.), unless stated otherwise. The water used to prepare all media and solutions was ultrapure deionised water purified to ⩾18 MΩ cm with a milli-Q ion-exchange resin system (Millipore, Watford, Herts, U.K.). Sildenafil citrate and sildenafil nitrate (NCX 911) were the kind gifts of NicOx SA (Nice, France).
Preparation of pulmonary arterial endothelial cells
Lungs were obtained from White Landrace male pigs of body weight ranging from 20 to 35 kg. All animals were given humane care in compliance with the rules and regulations of Bristol University and the U.K. Home Office. Pigs were anaesthetised with an intravenous injection of ketamine hydrochloride (10 mg kg−1; Ketaset Injection, Fort Dodge Animal Health, Southampton, U.K.) and inhaled halothane (1–2% in oxygen), exsanguinated and lungs removed. Pulmonary arteries (PAs; 3–4 mm diameter) were dissected out immediately and rinsed in Dulbecco's minimum essential medium (DMEM; GibcoBRL, Paisley, Scotland). PAECs were cultured by the explant method as described previously (Muzaffar et al., 2003; 2004a, 2004b). PAECs were grown and maintained in Endothelial Cell Growth Medium (PromoCell, Heidelberg, Germany) at 37°C in a 95% air–5% CO2 incubator. When confluent, cells were harvested by trypsinisation. All subsequent experiments were carried out using cells at passage 4, since this allows for the build up of cell numbers. We have previously demonstrated that PAECs used at passage 4 also behave in much the same way as endothelial cells in fresh, intact, PAs (Muzaffar et al., 2003).
Effect of drugs on O2•− formation and gp91phox expression
PAECs were incubated with the TXA2 analogue, 9,11-dideoxy-9α,11α-methanoepoxy-prostaglandin F2α (U46619; Calbiochem, U.K.; 10 nM) (±sildenafil citrate or NCX 911; 100 pM–10 μM) for 16 h at 37°C in a 95% air–5% CO2 incubator. Following incubation, cells were washed three times with DMEM to remove drugs. The washed cells were equilibrated in DMEM without phenol red for 10 min at 37°C in a 95% air–5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Germany). Horse heart cytochrome c (20 μM) with or without 500 U ml−1 copper–zinc superoxide dismutase (SOD) was added and the cells incubated at 37°C in a 95% air–5% CO2 incubator for 1 h. The reaction medium was removed and reduction of cytochrome c determined at 550 nm in an Anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, U.K.) and converted to μmol of O2•− using ΔE550 nm=21.1 mM−1 cm.−1 as the extinction coefficient. The reduction of cytochrome c that was inhibitable with SOD reflected actual O2•− release (Fridovich, 1985; Muzaffar et al., 2003; 2004a, 2004b). Cells were then washed with PBS, lysed with 0.1% (v v−1) Triton X-100 and total protein content measured using BCA-protein assay kit (Pierce, Rockford, IL, U.S.A.). Data are expressed as μmol of O2•− mg−1 protein h−1.
For Western analysis, following a 16-h incubation with U46619 (±sildenafil citrate or NCX 911), as described above, PAECs were washed and lysed with Tris buffer (100 mM, pH 6.8) containing 1% glycerol and 1% sodium dodecyl sulphate (SDS) (Bayraktutan et al., 1998; Muzaffar et al., 2003; 2004a, 2004b). Extracts were boiled at a 1 : 1 ratio with Tris (125 mM, pH 6.8, containing 4% (w v−1) SDS; 10% (v v−1) glycerol; 4% (v v−1) 2-mercaptoethanol; 2 mg ml−1 bromophenol blue). Total cell lysates of equal protein (40 μg) were loaded onto 10% Tris-glycine SDS gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed with a specific gp91phox monoclonal antibody (Yu et al., 1998; Muzaffar et al., 2005) (1 : 500 dilution; BD Biosciences, Oxford, U.K.). The blots were then incubated with goat anti-mouse antibody conjugated to horseradish peroxidase (1 : 2000 dilution) and developed by enhanced chemiluminescence (Amersham International). Rainbow markers (10–250 kDa; Amersham) were used for molecular weight determination.
Role of cGMP and NO in mediating effects of sildenafil and NCX 911
To determine whether the inhibitory effects of sildenafil citrate and NCX 911 on O2•− formation and gp91phox expression were mediated by cGMP, PAECs were incubated with U46619+sildenafil or U46619+NCX 911±the guanylyl cyclase inhibitor, 1H-{1,2,4}oxadiazolo{4,3-a}quinoxalin-1-one (ODQ; 10 μM), for 16 h at 37°C in a 95% air–5% CO2 incubator. Following washing of the cells, the production of O2•− was then measured by ferricytochrome c assay and gp91phox expression assessed as described above.
In order to determine whether NO enhances the potency of sildenafil, as would be expected, the effect of adding exogenous NO on the inhibitory effect of the drugs was investigated. PAECs were incubated with U46619 or sildenafil±the NO donor, 3-morpholinosydnonimine (SIN-1; 10 and 100 nM), for 16 h at 37°C in a 95% air–5% CO2 incubator. Cells were then washed in DMEM and O2•− measured by ferricytochrome c assay, as above. In order to study the role of endogenous NO, PAECs were incubated with U46619+sildenafil or U46619+NCX 911±the eNOS inhibitor, N5-(1-iminoethyl)-ornithine (L-NIO; 10 μM). Following a 16-h incubation, the formation of O2•− was then measured by ferricytochrome c assay, as above.
In order to study the more acute effects of sildenafil citrate or NCX 911 on O2•− formation, PAECs were incubated with 10 nM U46619 alone for 16 h, washed and then further incubated with sildenafil or NCX 911 (both 100 pM and 10 μM) for 1 h at 37°C in a 95% air–5% CO2 incubator and O2•− measured as above. Possible quenching effects of the drugs were studied as described below.
Measurement of NO release from NCX 911 and quenching of O2•− by NCX 911
NO release by NCX 911 compared to sildenafil citrate was assessed by using a fluorescent dye, 4,5-diaminofluorescein in a cell-free system (DAF-2; Sigma Chemical Co.) (Nakatsubo et al., 1998; Rathel et al., 2003). NCX 911 or sildenafil citrate solutions (0.01–100 nM) were placed in 96-well black-opaque plates (without cells). DAF-2 (1 μM final concentration) was added to each well in the dark and the plate incubated at room temperature for a further 20 min. Fluorescence was then measured at room temperature using a spectrofluorimeter (Fluorolite 1000, Dynatech Laboratories) with excitation wavelength set at 494 nm and emission wavelength at 520 nm. The bandwidth was 10 nm for both excitation and emission and the sensitivity programmed to high. DAF-2 calibration was performed using diethylamine/NO complex (DETA-NONOate).
In order to determine whether NCX 911 quenches O2•− by a direct reaction, NCX 911 was incubated in a cell-free system with xanthine (100 μM)+xanthine oxidase (0.02 U ml−1) (X/XO; generates O2•−) and nitrite formation measured using an assay based on the Griess reaction (Nims et al., 1996). The rationale for this was that NO reacts with O2•− to generate a reactive nitrogen species, which degrade rapidly to form nitrites (Nims et al., 1996). If NCX 911 is reacting with O2•− to form nitrites, then this reaction should be inhibitable with SOD. Thus, X/XO was incubated with either sildenafil citrate or NCX 911 (both at 100 nM) or DETA-NONOate (100 μM) for 1 h at room temperature, with or without SOD (500 U ml−1). Sulphanilamide and N-(1-naphthyl)ethylenediamine were then added and detected chromogenically by measuring the optical density (OD) at 540 nm using a spectrophotometer (Nims et al., 1996). Standard curves were compiled using sodium nitrite and the nitrite concentrations calculated using linear regression.
cGMP formation
In order to determine whether NCX 911 promotes cGMP formation, as one would expect if it is an NO donor, intracellular cGMP was measured using enzyme-linked immunoassay kit (R&D Systems, U.K.) (Schini et al., 1990) following incubation of PAECs with drugs. PAECs were cultured in six-well plates and upon reaching confluence were treated with 10 nM U46619+sildenafil or 10 nM U46619+NCX 911 for 16 h. All incubations were carried out in the presence of the broad-spectrum PDE inhibitor, isobuytlmethylxanthine (IBMX; 250 μM), to inhibit hydrolysis of cGMP (Jeremy et al., 1997). After incubation, media were removed and 200 μl of 0.1 N HCl containing IBMX added to the cells and incubated for 20 min to extract cGMP. cGMP concentrations were then measured according to the manufacturer's instructions. The protein content was determined by the BCA-protein assay kit and cGMP levels expressed as fmol mg−1 protein.
Effect of protein kinase G (PKG) and protein kinase A (PKA) inhibitors on O2•− formation
The role of cGMP and possibly cAMP in mediating the inhibitory effects of sildenafil citrate and NCX 911 on O2•− formation by PAECs were further investigated using the PKG inhibitor, 8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-Br-cGMPS; 10 μM; BIOLOG, Germany; Gragasin et al., 2004) or the PKA inhibitor, H89 (10 μM; Zhu et al., 2004).
Cells were incubated with U46619+sildenafil or U46619+NCX 911 (±PKG or PKA inhibitor) for 16 h at 37°C in a 95% air–5% CO2 incubator. Following washing of the cells, the production of O2•− was then measured by ferricytochrome c assay as described above. In studies on acute effects of drugs, PAECs were firstly incubated with 10 nM U46619 alone for 16 h, washed and then incubated with sildenafil citrate or NCX 911 for 1 h at 37°C. The PKA or PKG inhibitors were added 30 min prior to adding sildenafil citrate or NCX 911. O2•− was then measured by ferricytochrome c assay, as above.
Data analysis
All data represent the means of triplicate determinations; each experiment was repeated at least six times (i.e. n=six animals). Dose–response data for sildenafil and NCX 911 in Figure 1 were best fit by nonlinear regression (sigmoidal dose response, variable slope) to the following equation:
where IC50 is the concentration of sildenafil or NCX 911 resulting in 50% inhibition, X is the log of sildenafil or NCX 911 concentration, Y is the response and H is the Hill coefficient. The endothelial cells stimulated with U46619 alone were assigned for determinations of the zero value for IC50 calculations. The difference between the NCX 911 and sildenafil IC50 values with 95% confidence intervals (CI) was analysed using the F-test (P<0.05). Data analysis was carried out using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, U.S.A.).
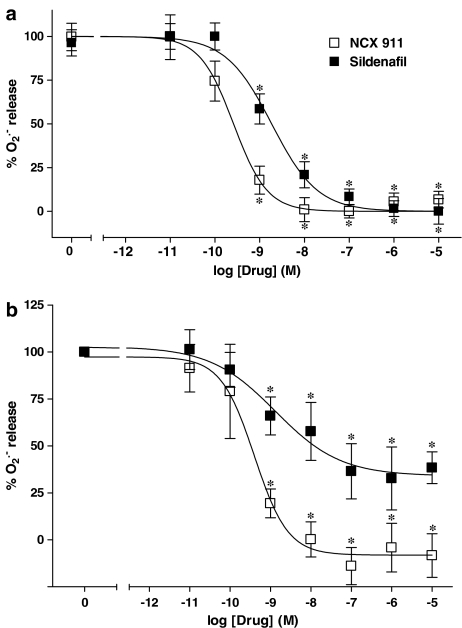
Figure 1.
Comparison of the inhibitory effects of sildenafil citrate and NCX 911 on U46619 (0; 10 nM)-induced O2•− formation from PAECs: (a) coincubated over a 16-h incubation period and (b) incubated for 1 h only following a 16 h incubation with 10 nM U46619 alone. Experimental data were averaged from six independent experiments carried out in different batches of cells. Data were normalised to U46619 responses and the curves were obtained fitting data to the sigmoidal dose–response equation described in Methods section. *P<0.01, significantly inhibited compared to U46619-treated cells (0).
The remaining data was analysed using one-way analysis of variance or post hoc unpaired, two-sided Student's t-test with a Bonferonni adjustment. Statistical significance was assumed at a value of P<0.05.
Results
As described previously (Muzaffar et al., 2004b), the TXA2 analogue, U46619, was a potent stimulator of O2•− formation by PAECs (Figure 1a). Sildenafil citrate and NCX 911 inhibited the formation of O2•− in PAECs in a concentration-dependent manner following a 16-h incubation (Figure 1a). NCX 911 (IC50: 0.26 nM with 95% CI of 0.16–0.43 nM) was approximately seven-fold (P<0.0001) more potent than sildenafil citrate (IC50: 1.85 nM with 95% CI of 1.07–3.43 nM).
In the acute studies, that is, over 1 h following a 16-h incubation with U46619, sildenafil citrate and NCX 911 also inhibited O2•− formation in a concentration-dependent manner (Figure 1b). Again, NCX 911 (IC50: 0.39 nM with 95% CI of 0.13–1.2 nM) was more potent than sildenafil citrate (IC50: 1.32 nM with 95% CI of 0.17–1.63 nM).
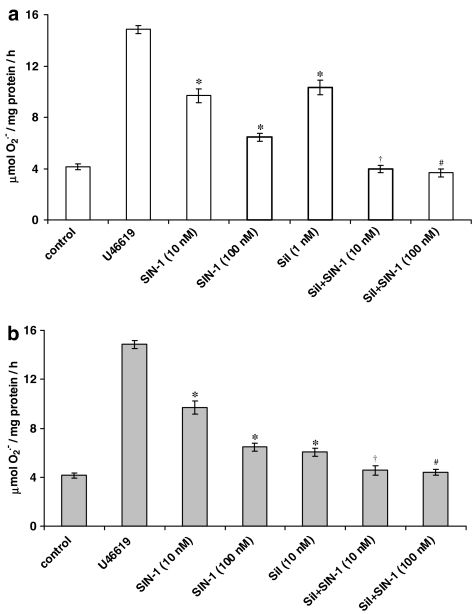
As reported previously (Muzaffar et al., 2004a), the NO donor, SIN-1, inhibited U46619-stimulated O2•− release in a concentration-dependent manner (Figure 2). The combination of both SIN-1 and sildenafil citrate enhanced the inhibition of O2•− formation compared to sildenafil citrate alone. By contrast, SIN-1 did not augment the inhibitory capacity of NCX 911 (data not shown).
Figure 2.
Effect of NO donor, SIN-1 (10 and 100 nM) on the inhibition of U46619 (10 nM)-induced O2•− formation from PAEC by coincubation with sildenafil (sil) at the following concentrations: (a) 1 nM and (b) 10 nM following 16-h incubation. Each point=mean±s.e.m., n=6. *P<0.01, significantly inhibited compared to U46619-stimulated value. †P<0.01, significantly inhibited compared to sildenafil alone or SIN-1 (10 nM). #P<0.01, significantly inhibited compared to sildenafil alone or SIN-1 (100 nM).
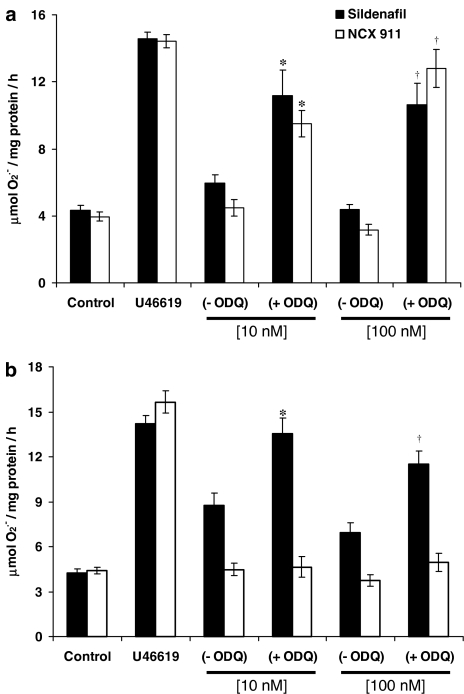
Following a 16-h incubation, the inhibitory effect of sildenafil citrate and NCX 911 on O2•− formation was reversed by coincubation with 1 μM ODQ, a guanylate cyclase inhibitor (Figure 3a). In the acute studies, ODQ only reversed the inhibitory effect of sildenafil citrate on O2•− formation, but had no significant effect on the inhibitory effect of NCX 911 (Figure 3b).
Figure 3.
Effect of ODQ (1 μM) on the (a) longer-term (16 h) and (b) acute (1 h) inhibition of U46619 (10 nM)-induced O2•− formation from PAEC by sildenafil citrate or sildenafil nitrate (NCX 911). Each point=mean±s.e.m., n=6. *P<0.01, significantly increased compared to sildenafil/sildenafil nitrate alone (10 nM). †P<0.01, significantly increased compared to sildenafil/sildenafil nitrate alone (100 nM).
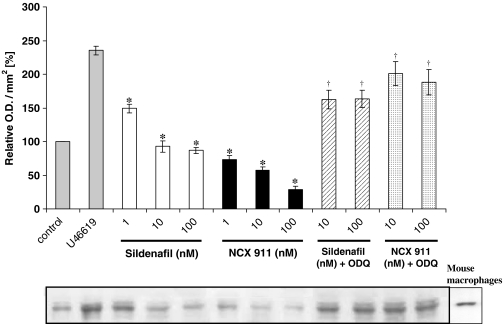
Sildenafil citrate and NCX 911 both inhibited U46619-induced protein expression of gp91phox in a dose-dependent manner, NCX 911 being significantly more potent then sildenafil alone (Figure 4). The guanylate cyclase inhibitor, ODQ, reversed the inhibitory effects of both sildenafil citrate and NCX 911 on gp91phox expression (Figure 4).
Figure 4.
Western analysis of NADPH oxidase in PAEC lysates using a monoclonal antibody directed against the extracellular epitope of gp91phox subunit of mouse NADPH oxidase. Cells were either not treated or treated with U46619 (10 nM) for 16 h with one of the following: sildenafil; sildenafil nitrate (NCX 911); or combination of sildenafil/sildenafil nitrate with ODQ (1 μM). The lower panel shows the representative blot and the upper panel the results of the densitometric analyses of six blots (expressed as relative OD/mm2). Mouse macrophages stimulated with LPS (1 μg ml−1) and INF-γ (10 ng ml−1) for 12 h were used as positive control. *P<0.05, significantly inhibited compared to U46619-treated value. †P<0.01, significantly increased compared to corresponding sildenafil/sildenafil nitrate only values.
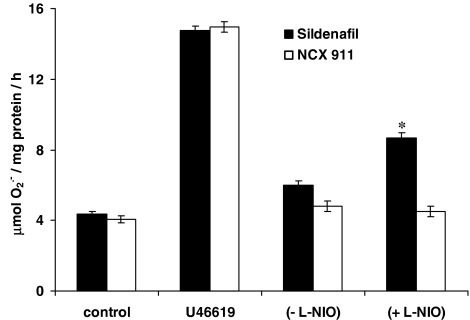
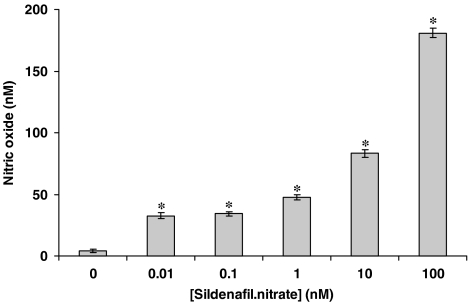
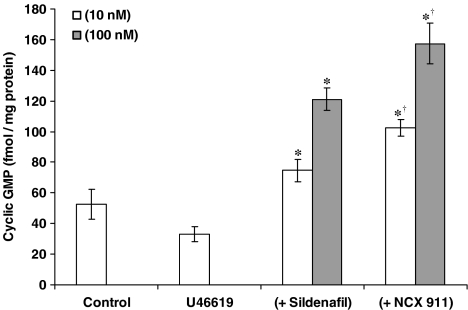
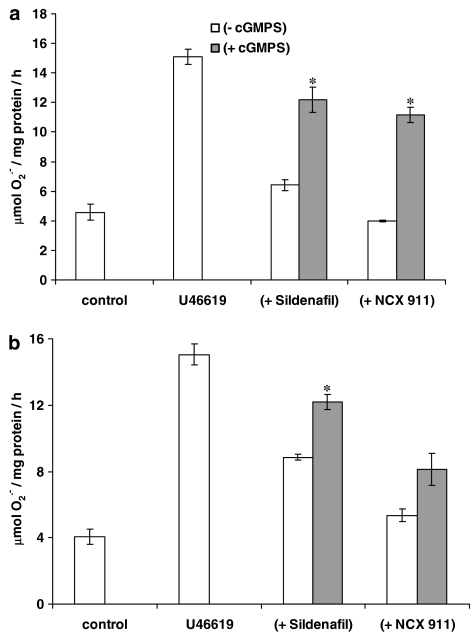
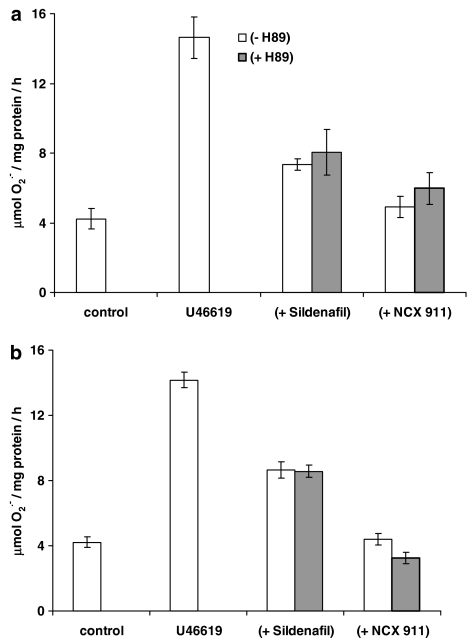
The eNOS inhibitor, L-NIO, significantly reversed the inhibitory effect of sildenafil citrate on O2•− formation and release, but had no influence on the effect of NCX 911 (Figure 5). NO was released by NCX 911 in a concentration-dependent manner as measured by the fluorescent dye, DAF-2, (Figure 6). Both sildenafil citrate and NCX 911 significantly increased cGMP levels, in a concentration-dependent manner, NCX 911 stimulating greater amounts of cGMP than sildenafil citrate (Figure 7). The PKG inhibitor, Rp-8-Br-cGMPS, reversed the inhibitory effects of both sildenafil citrate and NCX 911 following coincubation with sildenafil citrate and NCX 911 for 16 h (Figure 8a) as well as over a 1-h coincubation with drugs (Figure 8b). The effects caused by Rp-8-Br-cGMPS were, however, partial and much less effective on the short-term actions of NCX 911 (Figure 8b). The PKA inhibitor, H89, had no significant effect on the inhibitory effects of sildenafil citrate or NCX 911 over a 16-h incubation (Figure 9a) nor in the acute 1-h incubation (Figure 9b).
Figure 5.
Effect of the eNOS inhibitor, L-NIO (10 μM) on the inhibition of U46619-induced O2•− formation from PAEC by 10 nM sildenafil citrate or 10 nM sildenafil nitrate (NCX 911) following 16-h incubation. Each point=mean±s.e.m., n=6. *P<0.01, significantly increased compared to sildenafil alone.
Figure 6.
Detection of nitric oxide released by sildenafil nitrate (NCX 911) using DAF-2 in a cell-free system. DAF-2 (1 μM) was added to NCX 911 solutions of various concentrations in the dark and the reaction mixtures were incubated at room temperature for 20 min. The fluorescence was then measured as described in the Methods section. DAF-2 calibration was performed using DETA-NONOate. All data are mean±s.e.m. (n=6). *P<0.05, significantly increased compared to controls.
Figure 7.
Effect of sildenafil citrate or sildenafil nitrate (NCX 911) on cGMP formation in PAECs coincubated with U46619 (10 nM) for 16 h. Each point=mean±s.e.m., n=6. *P<0.01, significantly increased compared to controls. †P<0.01, significantly increased compared to sildenafil citrate.
Figure 8.
Effect of PKG inhibitor (cGMPS; 10 μM) on the (a) long-term (16 h) and (b) acute (1 h) inhibition of U46619 (10 nM)-induced O2•− formation from PAEC by sildenafil or sildenafil nitrate (NCX 911). Each point=mean±s.e.m., n=6. *P<0.01, significantly increased compared to sildenafil/sildenafil nitrate alone.
Figure 9.
Effect of PKA inhibitor (H89; 10 μM) on the (a) longer-term (16 h) and (b) acute (1 h) inhibition of U46619 (10 nM)-induced O2− formation from PAEC by sildenafil or sildenafil nitrate (NCX 911). Each point=mean±s.e.m., n=6.
Nitrite concentrations measured following coincubation of 100 nM NCX 911 with X/XO were 10-fold greater (16±1.9 μM) than that generated by 100 nM sildenafil citrate+X/XO (1.6±0.2 μM). SOD partially reversed the formation of nitrites by NCX 911 (9.9±0.8 μM; P<0.001). As a comparative control, DETA-NONOate+X/XO also generated nitrites (33.5±1.1 μM), which was also significantly inhibited by SOD (17.2±1.4 μM). These data also demonstrate that NCX 911 reacts with O2− to generate nitrites that are inhibitable with SOD, indicating that NCX 911 possesses the capacity react with O2•−.
Discussion
The present study demonstrates for the first time that sildenafil citrate is a potent inhibitor of O2•− formation by PAECs in response to the TXA2 analogue, U46619, both acutely (over 1 h) and in the longer term (over 16 h). Following a prolonged incubation with U46619, sildenafil citrate was also a potent inhibitor of gp91phox (an active catalytic subunit of NADPH oxidase; Griendling et al., 2000) expression. It has previously been demonstrated that U46619 simulates the formation of O2•− through an upregulation of NADPH oxidase (Muzaffar et al., 2004b). Sildenafil nitrate (NCX 911) was even more potent in inhibiting O2•− formation and gp91phox expression (approximately eight-fold) by PAECs over a 16-h time course when coincubated with U46619.
These long-term effects of sildenafil citrate and NCX 911 on O2•− formation and gp91phox expression were blocked by the guanylyl cyclase inhibitor, ODQ. Since ODQ is an inhibitor of guanylyl cyclase activity, these data demonstrate that the inhibitory effect of sildenafil citrate and NCX 911 are mediated by cGMP formation. This is as one would expect since sildenafil is an inhibitor of PDE V, which hydrolyses cGMP to inactive GMP (Jeremy et al., 1997; Ballard et al., 1998). In support of this conclusion, the inhibition of PKG with Rp-8-Br-cGMPs also reversed the inhibitory effect of sildenafil citrate and NCX 911 on O2•− formation and gp91phox expression. By contrast, the PKA inhibitor had no effect indicating a lack of involvement for cyclic AMP in mediating these drug actions.
The inhibitory effect of sildenafil citrate on O2•− formation was also augmented by exogenous NO donor, SIN-1. This is consistent with an inhibition of PDE V playing a role since NO would be expected to augment the effect of sildenafil by providing drive for guanylyl cyclase (Ballard et al., 1998). It has been demonstrated that providing exogenous NO markedly augments the cGMP elevating capacity of sildenafil citrate (Jeremy et al., 1997; Ballard et al., 1998). Furthermore, the inhibition of eNOS partially reversed the effect of sildenafil citrate on O2•− formation, indicating the NO derived from eNOS mediates the effect of sildenafil, at least in part, by providing endogenous drive. In this context, it has been demonstrated that eNOS plays a key role in reducing O2•− formation by suppressing NADPH oxidase expression (Muzaffar et al., 2003). Furthermore, in the present study, sildenafil citrate did enhance intracellular cGMP levels, albeit to a lesser degree than NCX 911, which is consistent with this proposal.
By contrast, SIN-1 did not further augment the inhibitory effect of NCX 911. In the present study, NCX 911 not only released NO to a greater degree than sildenafil citrate but also augments the formation of cGMP to a greater degree than sildenafil citrate in PAECs. Thus, it is possible that NCX 911 is eliciting a maximal effect (i.e. exogenous NO cannot further augment the response). In this context, a recent study demonstrated that NO-donating aspirin and SIN-1 are potent inhibitors of NADPH oxidase expression and activity (Muzaffar et al., 2004a).
One interesting facet of the present study was the relative inhibitory potencies of sildenafil citrate and NCX 911. The IC50 of sildenafil citrate on O2•− formation and gp91phox expression after 16 h incubation in the present study was 1.85 nM. Since sildenafil citrate has been shown to inhibit PDE V activity at an IC50 of 3.5 nM (Ballard et al., 1998), these data indicate that the effects of sildenafil on O2•− formation and gp91phox expression are indeed mediated by the inhibition of PDE V. By contrast, NCX 911 was far more potent with an IC50 of 0.26 nM, which is indicative of the combined effect of PDE inhibition and NO drive.
An interim conclusion, therefore, is that the inhibitory effect of sildenafil and NCX 911 on O2•− formation is mediated through an augmentation of guanylyl cyclase activity. In turn, this blocks the upregulation of NADPH oxidase protein, which diminishes O2•− output. Apart from reducing oxidative damage this reduction would also conserve NO biovailability. Therapeutically, in ARDS, this would augment vasodilatation, contributing to the antihypertensive effects of sildenafil and reduce the adhesion of platelets and leucocytes, thereby reducing inflammation and the progression of ARDS.
Another facet of the present study was that sildenafil citrate exerted an acute inhibitory effect (i.e. over 1 h) on O2•− formation, when added to cell after a 16-h incubation with U46619, albeit at higher concentrations than that for inhibition of gp91phox in the longer term (i.e. an IC50 (acutely) of 1.32 nM). However, this concentration is still above the therapeutic plasma levels, since circulating levels of sildenafil following oral administration of the drug in man are in the range of approximately 0.5–10 μM (Jetter et al., 2002; Rosen & Kostis, 2003). This acute effect was reversed by ODQ, indicating the effect to be mediated by cGMP. As mentioned, we have demonstrated that U46619 increases O2•− formation almost exclusively by upregulating NADPH oxidase expression. Thus, these data indicate that the NO-cGMP axis plays a role in blocking not only the expression of NADPH oxidase in response to inflammation but also the intrinsic acute activity of the enzyme, a facet that may have important therapeutic implications.
As with longer-term studies, NCX 911 was more potent (IC50 (acutely) of 0.39 nM) than sildenafil citrate. However, in contrast to sildenafil citrate, ODQ failed to reverse the acute inhibitory effect of NCX 911, although in the longer term, ODQ reversed the inhibitory effect of NCX 911 on gp91phox expression. Since NO reacts with O2•− to form reactive nitrogen species, we suggest that this effect of NCX 911 may be due, in part, to a direct quenching effect on O2•− radicals. Indeed, we found NCX 911 reacts with O2•− to generate nitrites, a reaction that is inhibitable with SOD. In turn, this indicates that NCX 911 possesses the capacity to react with O2•− (i.e. quenching of O2•−). The degree of inhibition by SOD of nitrite formation by both NCX 911+X/XO and NONOate+X/XO was similar, indicating that the NO donating mechanisms of NCX 911 is similar to that of an accepted NO donor, NONOate.
To summarise, sildenafil citrate is a potent inhibitor of O2•− formation in porcine pulmonary arterial endothelial cells, both acutely and in the longer term. Acutely, this effect is mediated by the direct inhibition of U46619-induced NADPH oxidase activity and in the longer term by suppression of inducible NADPH oxidase expression. This effect, as one would predict, is mediated by the cGMP-PKG system and not by the cAMP-PKA axis. Since increased O2•− formation is associated with ARDS (Chabot et al., 1998), the administration of sildenafil may prove useful in treating ARDS. One possible drawback with using sildenafil in ARDS, at least when administered stystemically, is that of peripheral hypotension and its attendant side effects as well as a worsening mismatch of ventilation and perfusion. However, these potential problems may be overcome by the inhalational administration of sildenafil. Certainly, other drugs administered by inhalation, in particular, prostacyclin, has proven very promising in treating ARDS (Olschewski et al., 2002). Interestingly, we have demonstrated that iloprost is a potent inhibitor of NAPDH oxidase expression and O2•− formation in pulmonary arterial cells (Muzaffar et al., 2004b). In a broader context, this novel mechanism may explain, at least in part, the therapeutic effect of sildenafil citrate in reducing pulmonary hypertension (Zhao et al., 2001; Ghofrani et al., 2002; Michelakis et al., 2002; Watanabe et al., 2002), although an association between pulmonary hypertension, NAPDH oxidase and O2•− formation remains to be firmly established. The overproduction of vascular O2•− as a major aetiological component of hypertension, per se, has been firmly established, however (Li & Shah, 2004).
NCX 911 may prove even more effective than sildenafil citrate since in ARDS OS intrinsically reduces endogenous NO bioavailability (Folkerts et al., 2001). NCX 911 may compensate for this by providing NO drive. This may be particularly important where OS has become so aggressive that it completely negates all NO bioavailability. In such a scenario, sildenafil citrate would be ineffective since it requires NO to provide drive for guanylyl cyclase activity. Again, as argued above, the administration of NCX 911 by aerosol delivery may obviate systemic complications. In support of this proposal, sildenafil citrate has been shown to augment and prolong the effects of inhaled NO, clinically (Lepore et al., 2002; Michelakis et al., 2002). Furthermore, sildenafil also prevents rebound pulmonary vasoconstriction on withdrawal of inhaled NO (Lepore et al., 2002). It appears that endogenous integrity of NO formation is important in the aetiology of ARDS since inhalational NO has proved potentially beneficial in treating the condition (Klinger, 2002). However, the benefits of inhaled NO in treating ARDS are ambivalent and adverse effects have been reported (Folkerts et al., 2001; Weinberger et al., 2001; Klinger, 2002; Wang et al., 2003). These adverse effects include a life-threatening ‘rebound' increase in pulmonary vascular resistance as well as methemoglobinaemia and cellular apoptosis (Wang et al., 2003). This is probably because inhalational NO results in enormously high intrapulmonary concentrations of NO that can react with O2•− to form reactive nitrogen species such as peroxynitrite (Stuart-Smith & Jeremy, 2001). Controlling the dose of inhalational NO is difficult, if not impossible. However, inhalational dosing with drugs such as sildenafil and NCX 911 is far more controllable. Furthermore, the capacity of NCX 911 to inhibit PDE V and release NO may compensate for these negative effects of inhalational NO. Further preclinical and clinical studies are required to test these proposals.
Acknowledgments
This research was funded by the British Heart Foundation (Grant number FS/2001041).
Abbreviations
- ARDS
acute respiratory distress syndrome
- ED
erectile dysfunction
- O2•−
superoxide
- OS
oxidative stress
- PAECs
pulmonary artery endothelial cells
- PDE
phosphodiesterase
References
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BAYRAKTUTAN U., DRAPER N., LANG D., SHAH A.M. Expression of a functional neutrophil-type NADPH oxidase in cultured rat coronary microvascular endothelial cells. Cardiovasc. Res. 1998;38:256–262. doi: 10.1016/s0008-6363(98)00003-0. [DOI] [PubMed] [Google Scholar]
- CHABOT F., MITCHELL J.A., GUTTERIDGE J.M.C., EVANS T.W. Reactive oxygen species in acute lung injury. Eur. Resp. J. 1998;11:745–757. [PubMed] [Google Scholar]
- FOLKERTS G., KLOEK J., MUIJSERS R.B., NIJKAMP F.P. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- FRIDOVICH I.Cytochrome C CRC Handbook of Methods for Oxygen Radical Research 1985Boca Raton, FL: CRC Press; 121–122.ed. Greenwald, R.A. pp [Google Scholar]
- GHOFRANI H.A., PEPKE-ZABA J., BARBERA J.A., CHANNICK R., KEOGH A.M., GOMEZ-SANCHEZ M.A., KNEUSSL M., GRIMMINGER F. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43:68S–72S. doi: 10.1016/j.jacc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- GHOFRANI H.A., WIEDEMANN R., ROSE F., SCHERMULY R.T., OLSCHEWSKI H., WEISSMANN N., GUNTHER A., WALMRATH D., SEEGER W., GRIMMINGER F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- GRAGASIN F.S., MICHELAKIS E.D., HOGAN A., MOUDGIL R., HASHIMOTO K., WU X., BONNET S., HAROMY A., ARCHER S.L. The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB. J. 2004;18:1382–1391. doi: 10.1096/fj.04-1978com. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., SORESCU D., USHIO-FUKAI M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., BALLARD S.A., NAYLOR A.M., MILLER M.A., ANGELINI G.D. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br. J. Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., ROWE D., EMSLEY A.M., NEWBY A.C. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc. Res. 1999;43:580–594. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., SHUKLA N., MUZAFFAR S., HANDLEY A., ANGELINI G.D. Reactive oxygen species, vascular disease and cardiovascular surgery. Curr. Vasc. Pharmacol. 2004;2:229–236. doi: 10.2174/1570161043385691. [DOI] [PubMed] [Google Scholar]
- JETTER A., KINZIG-SCHIPPERS M., WALCHNER-BONJEAN M., HERING U., BULITTA J., SCHREINER P., SORGEL F., FUHR U. Effects of grapefruit juice on the pharmacokinetics of sildenafil. Clin. Pharmacol. Ther. 2002;71:21–29. doi: 10.1067/mcp.2002.121236. [DOI] [PubMed] [Google Scholar]
- KLINGER J.R. Inhaled nitric oxide in ARDS. Crit. Care Clin. 2002;18:45–68. doi: 10.1016/s0749-0704(03)00064-2. [DOI] [PubMed] [Google Scholar]
- LEPORE J.J., MAROO A., PEREIRA N.L., GINNS L.C., DEC G.W., ZAPOL W.M., BLOCH K.D., SEMIGRAN M.J. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am. J. Cardiol. 2002;90:677–680. doi: 10.1016/s0002-9149(02)02586-9. [DOI] [PubMed] [Google Scholar]
- LI J.M., SHAH A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- MADDEN B.P., SHETH A., HO T.B., PARK J.E., KANAGASABAY R.R. Potential role for sildenafil in the management of perioperative pulmonary hypertension and right ventricular dysfunction after cardiac surgery. Br. J. Anaesth. 2004;93:155–156. doi: 10.1093/bja/aeh571. [DOI] [PubMed] [Google Scholar]
- MICHELAKIS E., TYMCHAK W., LIEN D., WEBSTER L., HASHIMOTO K., ARCHER S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- MUZAFFAR S., JEREMY J.Y., ANGELINI G.D., STUART-SMITH K., SHUKLA N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., ANGELINI G.D., JEREMY J.Y. Nitroaspirins and morpholinosydnonimine, but not aspirin, inhibit the formation of superoxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation. 2004a;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., ANGELINI G.D., JEREMY J.Y.Superoxide promotes gp91phox expression in pulmonary artery endothelial cells Proc. Br. Pharmacol. Soc. 20052(abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZAFFAR S., SHUKLA N., LOBO C., ANGELINI G.D., JEREMY J.Y. Iloprost inhibits superoxide formation and gp91phox expression induced by the thromboxane A2 analogue U46619, 8-isoprostane F2a, prostaglandin F2a, cytokines and endotoxin in the pig pulmonary artery. Br. J. Pharmacol. 2004b;141:488–496. doi: 10.1038/sj.bjp.0705626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATSUBO N., KOJIMA H., KIKUCHI K., NAGOSHI H., HIRATA Y., MAEDA D., IMAI Y., IRIMURA T., NAGANO T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- NIMS R.W., COOK J.C., KRISHNA M.C., CHRISTODOULOU D., POORE C.M., MILES A.M., GRISHAM M.B., WINK D.A. Colorimetric assays for nitric oxide and nitrogen oxide species formed from nitric oxide stock solutions and donor compounds. Meth. Enzymol. 1996;268:93–105. doi: 10.1016/s0076-6879(96)68012-4. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI H., SIMONNEAU G., GALIE N., HIGENBOTTAM T., NAEIJE R., RUBIN L.J., NIKKHO S., SPEICH R., HOEPER M.M., BEHR J., WINKLER J., SITBON O., POPOV W., GHOFRANI H.A., MANES A., KIELY D.G., EWERT R., MEYER A., CORRIS P.A., DELCROIX M., GOMEZ-SANCHEZ M., SIEDENTOP H., SEEGER W. Inhaled iloprost for severe pulmonary hypertension. N. Engl. J. Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- RATHEL T.R., LEIKERT J.J., VOLLMAR A.M., DIRSCH V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proc. Online. 2003;5:136–142. doi: 10.1251/bpo55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN R.C., KOSTIS J.B. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am. J. Cardiol. 2003;92:9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- SCHINI V.B., BOULANGER C., REGOLI D., VANHOUTTE P.M. Bradykinin stimulates the production of cyclic GMP via activation of B2 kinin receptors in cultured porcine aortic endothelial cells. J. Pharmacol. Exp. Ther. 1990;252:581–585. [PubMed] [Google Scholar]
- SEBKHI A., STRANGE J.W., PHILLIPS S.C., WHARTON J., WILKINS M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- SEIDLER M., UCKERT S., WALDKIRCH E., STIEF C.G., OELKE M., TSIKAS D., SOHN M., JONAS U. In vitro effects of a novel class of nitric oxide (NO) donating compounds on isolated human erectile tissue. Eur. Urol. 2002;42:523–528. doi: 10.1016/s0302-2838(02)00397-4. [DOI] [PubMed] [Google Scholar]
- STUART-SMITH K., JEREMY J.Y. Microvessel damage in acute respiratory distress syndrome: the answer may not be NO. Br. J. Anaesth. 2001;87:272–279. doi: 10.1093/bja/87.2.272. [DOI] [PubMed] [Google Scholar]
- THOMAS M.K., FRANCIS S.H., CORBIN J.D. Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J. Biol. Chem. 1990;265:14964–14970. [PubMed] [Google Scholar]
- WANG T., EL KEBIR D., BLAISE G. Inhaled nitric oxide in 2003: a review of its mechanisms of action. Can. J. Anaesth. 2003;50:839–846. doi: 10.1007/BF03019384. [DOI] [PubMed] [Google Scholar]
- WATANABE H., OHASHI K., TAKEUCHI K., YAMASHITA K., YOKOYAMA T., TRAN Q.K., SATOH H., TERADA H., OHASHI H., HAYASHI H. Sildenafil for primary and secondary pulmonary hypertension. Clin. Pharmacol. Ther. 2002;71:398–402. doi: 10.1067/mcp.2002.123554. [DOI] [PubMed] [Google Scholar]
- WEINACKER A.B., VASZAR L.T. Acute respiratory distress syndrome: physiology and new management strategies. Annu. Rev. Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- WEINBERGER B., LASKIN D.L., HECK D.E., LASKIN J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001;59:5–16. doi: 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- YU L., QUINN M.T., CROSS A.R., DINAUER M.C. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO L., MASON N.A., MORRELL N.W., KOJONAZAROV B., SADYKOV A., MARIPOV A., MIRRAKHIMOV M.M., ALDASHEV A., WILKINS M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- ZHU B., KELLY J., VEMAVARAPU L., THOMPSON W.J., STRADA S.J. Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem. Pharmacol. 2004;68:479–491. doi: 10.1016/j.bcp.2004.03.039. [DOI] [PubMed] [Google Scholar]