Abstract
The in vivo hypoglycaemic activity of a dialysed fenugreek seed extract (FSE) was studied in alloxan (AXN)-induced diabetic mice and found to be comparable to that of insulin (1.5 U kg−1). FSE also improved intraperitoneal glucose tolerance in normal mice.
The mechanism by which FSE attenuated hyperglycaemia was investigated in vitro. FSE stimulated glucose uptake in CHO-HIRc-mycGLUT4eGFP cells in a dose-dependent manner. This effect was shown to be mediated by the translocation of glucose transporter 4 (GLUT4) from the intracellular space to the plasma membrane.
These effects of FSE on GLUT4 translocation and glucose uptake were inhibited by wortmannin, a phosphatidylinositol 3-kinase (PI3-K) inhibitor, and bisindolylmaleimide 1, a protein kinase C (PKC)-specific inhibitor.
In vitro phosphorylation analysis revealed that, like insulin, FSE also induces tyrosine phosphorylation of a number of proteins including the insulin receptor, insulin receptor substrate 1 and p85 subunit of PI3-K, in both 3T3-L1 adipocytes and human hepatoma cells, HepG2. However, unlike insulin, FSE had no effect on protein kinase B (Akt) activation.
These results suggest that in vivo the hypoglycaemic effect of FSE is mediated, at least in part, by the activation of an insulin signalling pathway in adipocytes and liver cells.
Keywords: Dialysed fenugreek seed extract, hypoglycaemic activity, insulin signalling pathway, phosphorylation, in vitro cell models, glucose uptake, GLUT4 translocation
Introduction
Autophosphorylation of the insulin receptor (IR) by insulin results in the recruitment and activation of intracellular downstream signalling molecules and leads to glucose uptake and various other biological effects (White & Kahn, 1994; Saltiel & Pessin, 2002). A lack of insulin or insulin resistance, or defects in the insulin signalling pathways is the cause of diabetes mellitus, which is characterised by hyperglycaemia (Taylor, 1999).
At present, the treatment of diabetes mainly involves a sustained reduction in hyperglycaemia by the use of biguanides, thiazolidinediones, sulphonylureas, D-phenylalanine derivatives, meglitinides and α-glucosidase inhibitors in addition to insulin. However, due to unwanted side effects the efficacies of these compounds are debatable and there is a demand for new compounds for the treatment of diabetes (UKPDS Group, 1995; Moller, 2001). Hence, plants have been suggested as a rich, as yet unexplored source of potentially useful antidiabetic drugs. However, only a few have been subjected to detailed scientific investigation due to a lack of mechanism-based available in vitro assays (Oubre et al., 1997; Fabricant & Farnsworth, 2001; Habeck, 2003). Fenugreek (Trigonella foenum-graecum L., Leguminosae), one of the oldest medicinal plants, is of Mediterranean origin and cultivated worldwide. Aqueous extracts of seeds and leaves of fenugreek have been shown to possess hypoglycaemic activity and are nontoxic (Abdel-Barry et al., 1997; Zia et al., 2001; Vats et al., 2002; Basch et al., 2003), but no detailed study to elucidate the mechanism of action of these extracts at the cellular and molecular level has been performed. Hence, in the present study, a dialysed aqueous extract of fenugreek seeds was investigated in vivo for hypoglycaemic potential and its effects on insulin signalling pathways in the primary targets of insulin, adipocytes and liver cells, were examined in vitro, by the use of mechanism-based innovative contemporary strategies.
Methods
Preparation of fenugreek seed extract (FSE)
Dried, viable and fresh batches of fenugreek seeds were obtained from a commercial source (Mona spices Co. Ltd, Pune, India). Seeds were washed in distilled water, surface sterilised by soaking in 0.1% sodium hypochlorite and 0.05% nonidet P-40 (NP-40) for 30 s and rinsed thoroughly with distilled water. Seeds were ground to a fine powder in a mixer under chilled conditions, suspended in PBS (pH 7.4) containing 1 mM PMSF and protease inhibitor cocktail, filtered through three layers of cheesecloth and centrifuged at 15,000 × g at 4°C for 30 min. The clear supernatant was lyophilised, redissolved in PBS and dialysed against PBS (8000 cutoff dialysis membrane) for 24 h at 4°C; the PBS was changed every 6 h. The dialysed extract, referred to as FSE, was aliquoted, stored at −70°C in the long term and used for all further experiments.
In vivo hypoglycaemic activity of FSE in AXN-induced diabetic mice
Swiss albino mice (male, 8–10 weeks old) were housed under environmentally controlled conditions (22±2°C) with a 12 h light/dark cycle and had free access to standard rodent pellet food and water. Animals were given intraperitoneal (i.p.) injections of freshly prepared AXN (50 mg kg−1 in 0.9% sodium chloride) for 5 days. Mice with blood glucose levels of 200–300 mg dl−1 were deemed to be hyperglycaemic in this study. At 10 h before the experiments, mice were moved to new cages in which no food was available. These mice were allocated to diabetic control, insulin treated and FSE-treated groups and were injected (i.p.) with vehicle (PBS), insulin (1.5 U kg−1) and FSE (1, 5 or 15 mg kg−1), respectively. Each group contained five mice. Blood was collected, by an approved tail-cap method, before (0 min) and 90 and 240 min after the treatments for estimation of blood glucose with a rapid glucose analyser (Accu-Chek Sensor Comfort, Roche Diagnostics, Germany). Bovine pancreas insulin diluted in PBS was used as a positive test compound in all the experiments.
Effects of FSE on i.p. glucose tolerance test (IPGTT) in normal mice
Swiss albino mice (male 8–10 weeks) were deprived of food for 10 h. In these animals, IPGTT was performed by administration of an i.p. injection of glucose (3 g kg−1). The blood glucose level before the injection of glucose was considered to be the basal value. FSE (15 mg kg−1) was injected 10 min after the injection of glucose. Blood samples were collected at 45, 90 and 180 min after administration of the extract and blood glucose levels were estimated. All animal experiments were performed according to guidelines approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Government of India) and with the permission of the Institute's Animal Care and Use Committee (IACUC).
Cell culture and generation of CHO-HIRc-mycGLUT4eGFP cells
A431 and HepG2 cells were maintained in DMEM supplemented with 10% foetal bovine serum (FBS). 3T3-L1 preadipocytes and 3T3-L1-mycGLUT4 cells were maintained in DMEM supplemented with 10% newborn calf serum (NBCS). 3T3-L1 and 3T3-L1-mycGLUT4 cells were differentiated as described previously (Tafuri, 1996). CHO-HIRc cells were maintained in F-12 medium containing 10% FBS. F-12 and F-12K media used in this study contained 7 mM glucose, whereas DMEM contained 25 mM glucose. Penicillin (100 U ml−1) and streptomycin (100 μg ml−1) were added routinely to cultures and all cell lines were cultivated at 37°C in a 5% CO2-enriched humidified atmosphere.
CHO-HIRc cells were cotransfected with pGreen Lantern mycGLUT4eGFP and pTk-Hyg plasmids (Clontech, Palo Alto, CA, U.S.A.), using lipofectAMINE according to the manufacturer's protocol. Clones were selected in medium containing 200 μg ml−1 hygromycin B. GFP-positive clones were isolated and verified for GLUT4 translocation using various concentrations of insulin (10–1000 nM). Verified clones (CHO-HIRc-mycGLUT4eGFP) were used for further experiments.
Effects of FSE on glucose transport in CHO-HIRc-mycGLUT4eGFP cells
[3H]-2-deoxy-D-glucose (2-DG) uptake was measured as described previously (Cheatham et al., 1994), but with the following modifications: CHO-HIRc-mycGLUT4eGFP cells (1 × 105 well−1) grown in 24-well plates were treated with insulin or FSE for 10 and 30 min, respectively, and control cells were treated with PBS. The glucose uptake was initiated by adding 0.1 mM 2-deoxy glucose containing 0.5 μCi ml−1 2-DG for 4 min at 37°C. The uptake was terminated by washing the cells with ice-cold PBS buffer containing 20 mM D-glucose and the cells solubilised with 0.1% SDS. After the amount of protein had been estimated, radioactivity incorporated into the cells was quantified with a top count microplate scintillation counter (Packard, Albertville, MN, U.S.A.). Nonspecific uptake, measured in the presence of 10 μM cytochalasin B. was subtracted from all the values. To examine the specificity of the signalling pathway, cells were pretreated with the pharmacological inhibitors wortmannin 100 nM for 20 min, or BIS-1 100 nM for 1 h, before the addition of FSE for 30 min.
GLUT4 translocation assay in CHO-HIRc-mycGLUT4eGFP and 3T3-L1-mycGLUT4 cells
CHO-HIRc-mycGLUT4eGFP cells (1 × 104 well−1) were plated onto a microscopic chamber slide (ICN, Costa Mesa, CA, U.S.A.) and serum-starved, by incubating them in DMEM containing 1 mg ml−1 BSA for 3 h, before the addition of insulin or FSE for 10 and 30 min, respectively. The treatment was terminated by washing with cold PBS and the cells fixed with 3% paraformaldehyde for 15 min at room temperature. GLUT4 translocation was investigated by using an LSM confocal microscope (Zeiss LSM 510, Heidelberg, Germany). 3T3-L1-mycGLUT4 cells were differentiated in 96-well plates. To examine the specificity of the signalling pathways, the cells were pretreated with pharmacological inhibitors. The cell surface mycGLUT4 levels were determined by an antibody-coupled optical assay, as described previously (Kamei et al., 2002) with slight modifications. Citrate buffer (pH 4.2) containing 5% ABTS and 1% H2O2 was used as the substrate and the optical absorbance of the supernatant was measured at 414 nm.
Immunoblot analysis of insulin signalling proteins in 3T3-L1 adipocytes and HepG2 cells
Differentiated 3T3-L1 adipocytes or HepG2 cells (1 × 107) were serum-starved in F-12K medium containing 0.1%. BSA for 16 h followed by a change of medium to DMEM and an additional incubation period of 1 h. Subsequently, the cells were treated with insulin or FSE for 10 and 30 min, respectively. The cells were lysed and immunoblot analysis was performed, as described previously (Chhipa et al., 2005) with appropriate antibodies.
Analysis of PKC translocation in HepG2 cells
HepG2 cells were serum-starved as described previously and treated with either insulin for 10 min or FSE for 30 min. In some wells, cells were pretreated with pharmacological inhibitors before the addition of FSE. Cells were then fixed and made permeable by the addition of 0.025% saponin for 5 min. They were then washed, incubated overnight at 4°C with PKC antibody, washed four times for 5 min each with PBS, incubated with a fluorescein isothiocyanate-conjugated rabbit antibody for 1 h, washed again and mounted using vectashield (Vector Laboratories, Burlingame, CA, U.S.A.) for visualization by confocal microscopy. Rabbit IgG was used as an isotype control.
Immunoblot detection of PKCλ and GLUT4 in the membrane fraction of 3T3-L1 adipocytes and EGFR autophosphorylation in A431 cells
Differentiated 3T3-L1 cells were serum-starved for 3 h in DMEM supplemented with 0.1% BSA before being treated with insulin or FSE for 10 and 30 min, respectively. Plasma membrane fractionation of 3T3-L1 adipocytes was performed, as described previously (Clancy & Czech, 1990) with slight modifications, in that 1 mM PMSF, 1 mM Na3VO4 and a cocktail of protease inhibitors were added to the lysis buffers. The protein obtained was transferred onto a nitrocellulose membrane and then probed with PKCλ or GLUT4 antibodies.
Similarly, A431 cells were serum-starved and treated with epidermal growth factor (EGF) (Promega, Madison, WI, U.S.A.) or insulin for 10 min, or FSE for 30 min. The cells were then processed for immunoblot analysis with phosphotyrosine or EGFR antibodies.
Statistics
The data are expressed as mean±s.e.m. Statistical comparisons were made using Student's two-tailed unpaired t-test and P-values <0.05 were considered significant.
Materials
Tritiated 2-DG (15 Ci mmol−1) and nitrocellulose membranes were obtained from Amersham Biosciences (Piscataway, NJ, U.S.A.). AXN, insulin, glucose, NP-40, wortmannin and other fine chemicals were purchased from Sigma (St Louis, MO, U.S.A.). DMEM, F-12 and F-12K media, FBS, NBCS and lipofectAMINE were purchased from Life Technologies Inc. (Rockville, MD, U.S.A.). Protease inhibitor cocktail (Tm complete) was purchased from Roche Diagnostics GmbH (Mannheim, Germany) and dialysis membrane was from Thomas Scientific (Philadelphia, PA, U.S.A.). Super signal reagent for enhanced chemiluminescence and Coomassie plus protein assay reagent were obtained from Pierce (Rockford, IL, U.S.A.). Monoclonal antibodies against phosphotyrosine (PY20), p85 and myc (9E10), polyclonal antibodies for phospho-Akt (Ser-473), IR-α, IR-β, IRS-1, Akt, PKC, PKCλ, GLUT4 and EGFR, and peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). BIS-1 was obtained from CalBiochem (San Diego, CA, U.S.A.). pGreen Lantern mycGLUT4eGFP construct (Jiang et al., 2002) was a generous gift from Dr M.P. Czech, University of Massachusetts Medical School, Worcester, MA, U.S.A. 3T3-L1 cells expressing mycGLUT4 (myc epitope in the first ectodomain of GLUT4) were kindly gifted by Dr C.R. Kahn, Joslin Diabetes Center, Boston, MA, U.S.A. CHO cells over expressing wild-type IR (CHO-HIRc) were kindly gifted by Dr M. Bernier, National Institute of Aging, Baltimore, MD, U.S.A. 3T3-L1 preadipocytes (ATCC no. CL-173), HepG2 cells (ATCC no. HB-8065) and A431 (ATCC no. CRL-1555) were obtained from ATCC, VA, U.S.A.
Results
The hypoglycaemic activity of FSE in AXN-induced diabetic and normal glucose-loaded mice
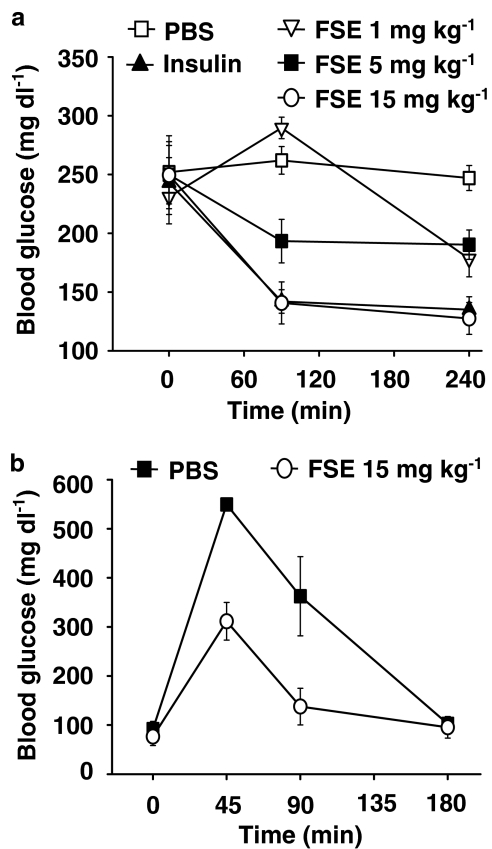
The effect of FSE on blood glucose levels in diabetic mice was investigated in vivo. FSE (i.p.) induced a dose-dependent hypoglycaemic effect in AXN-induced diabetic Swiss albino mice. FSE 15 mg kg−1 reduced the blood glucose in diabetic mice to normal levels by 4 h (Figure 1a). Overall ∼50% reduction was observed as compared to diabetic control (P<0.01). The response to FSE peaked at 90 min after its administration and the effects were comparable to that of 1.5 U kg−1 insulin. Similar results were obtained in AXN and streptozotocine-induced diabetic BALB/cJ mice (data not shown). The effects of FSE on IPGTT in glucose-treated normal Swiss albino mice were also investigated. As seen in Figure 1b, 45 min after the administration of 15 mg kg−1 FSE, the increase in serum blood glucose induced by glucose administration was significantly less (311.44±38.50 mg dl−1) than that observed in the control group injected with PBS (549.44±11.69 mg dl−1; P<0.01).
Figure 1.
In vivo hypoglycaemic activity of FSE. (a) AXN-induced diabetic Swiss albino mice were injected (i.p.) with vehicle (PBS), insulin or FSE (1, 5 or 15 mg kg−1). FSE at a dose of 15 mg kg−1 produced a significant decrease in blood glucose levels compared with PBS control (P<0.01). (b) IPGTT in glucose-loaded (3 g kg−1) normal Swiss albino mice. FSE-injected mice had significantly lower levels of blood glucose (P<0.01 vs PBS treated) at 45 and 90 min after its administration.
The effect of FSE on glucose transport in CHO-HIRc-mycGLUT4eGFP cells
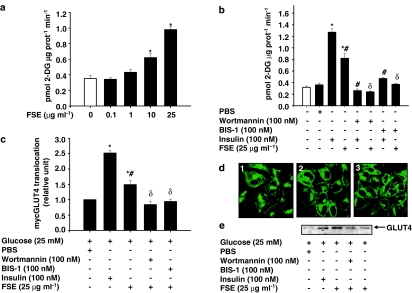
CHO-HIRc-mycGLUT4eGFP cells were incubated in the presence of various concentrations of FSE for 30 min and glucose transport was measured by determining the rates of 2-DG uptake. FSE induced a dose-dependent increase in glucose transport rates; the maximal effect was observed at a dose of 25 μg ml−1 (∼270% of basal; P<0.05; Figure 2a). However, FSE was ∼35% less potent than insulin at stimulating glucose uptake. This effect of FSE on glucose uptake was attenuated in cells pretreated with wortmannin or BIS-1 (Figure 2b). To determine whether the effect of FSE on glucose transport involves GLUT4, the translocation of GLUT4 was studied in CHO-HIRc-mycGLUT4eGFP cells, 3T3-L1-mycGLUT4 cells and 3T3-L1 adipocytes. FSE not only induced a significant increase in the GFP-associated fluorescence on the cell surface of CHO-HIRc-mycGLUT4eGFP cells but also enhanced GLUT4 content in the membrane fraction of 3T3-L1 cells (Figure 2d and e). FSE-induced mycGLUT4 translocation was increased by at least 50% (P<0.05 vs basal values) in 3T3-L1-mycGLUT4 cells (Figure 2c). Pretreatment with wortmannin and BIS-1 inhibited these effects.
Figure 2.
Effects of FSE on glucose transport and GLUT4 translocation. (a) CHO-HIRc-mycGLUT4eGFP cells were incubated with the indicated concentrations of FSE for 30 min before 2-DG uptake was measured. (b) CHO-HIRc-mycGLUT4eGFP cells were preincubated in the absence or presence of either wortmannin or BIS-1 for 20 and 60 min, respectively, followed by treatment with insulin or FSE for 10 and 30 min, respectively, before 2-DG uptake was measured. Each value shown in (a) and (b) represents the mean and s.e.m. of three independent experiments performed in triplicate (*P<0.05 vs basal; #P<0.05 vs insulin control; δP<0.05 vs FSE control). The open column represents the basal values. (c) Differentiated 3T3-L1-mycGLUT4 cells were serum-starved in DMEM and incubated in the absence or presence of wortmannin or BIS-1 for 20 and 60 min, respectively, followed by treatment with insulin or FSE for 10 and 30 min, respectively, before mycGLUT4 translocation was measured. Data represent the mean and s.e.m. of triplicate experiments and are expressed as relative translocation compared with control, which was set at 1.0 (*P<0.05 vs basal value; #P<0.05 vs insulin control; δP<0.05 vs FSE control). (d) CHO-HIRc-mycGLUT4eGFP cells were serum-starved in DMEM for 3 h followed by treatment with (1) PBS (2) insulin or (3) FSE. Cells were fixed and processed for confocal microscopy analysis. Representative pictures of at least three independent experiments are shown. (e) 3T3-L1 adipocytes were incubated in the absence or presence of wortmannin or BIS-1 for 20 and 60 min, respectively, followed by treatment with insulin or FSE for 10 and 30 min, respectively. Plasma membranes were prepared as described in the Methods section and then subjected to immunoblot analysis with antibodies against GLUT4.
The involvement of insulin signalling proteins in the cellular phosphorylation induced by FSE in 3T3-L1 adipocyte and HepG2 cells
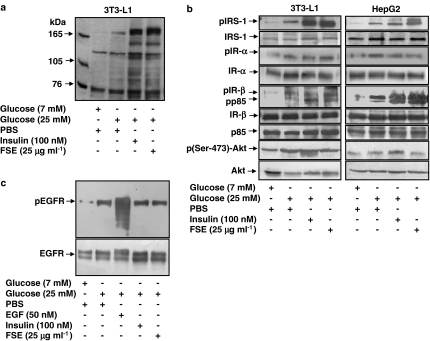
Insulin-induced stimulation of glucose transport in adipocytes requires IR-mediated tyrosine phosphorylation of IRS-1 and subsequent activation of PI3-K (Kahn & Pessin, 2002). To investigate the effects of FSE on insulin signalling, differentiated 3T3-L1 adipocytes and HepG2 cells were treated with either 25 μg ml−1 FSE or 100 nM insulin. By determining the pattern of tyrosine phosphorylation proteins involved in insulin signalling in both cell lines, the effects of FSE on the phosphorylation of IR-β and IRS-1 and a major band at 85 kDa were shown to be comparable to those seen in insulin-treated cells (Figure 3a and b), the one noticeable difference being that FSE did not promote Akt phosphorylation at Ser-473, whereas insulin did. FSE also failed to induce EGFR autophosphorylation in A431 cells (Figure 3c).
Figure 3.
Effects of FSE on insulin signalling pathways. Differentiated 3T3-L1 adipocytes or HepG2 cells were serum-starved and incubated with insulin or FSE for 10 and 30 min, respectively. The cells were washed, lysed, subjected to SDS–PAGE followed by immunoblot analysis with phospotyrosine (PY-20) antibodies or other specific antibodies and developed by enhanced chemiluminescence. (a) Tyrosine phosphorylation of cellular proteins of differentiated 3T3-L1 adipocytes. (b) Phosphorylation of IRS-1, IR-α, IR-β, p85 and Akt in 3T3-L1 adipocytes and HepG2 cells. (c) Serum-starved A431 cells were treated with EGF, insulin or FSE and washed, lysed and subjected to immunoblot analysis. The results shown are representative of three independent experiments.
The effect of FSE on PKC translocation in 3T3-L1 and HepG2 cells
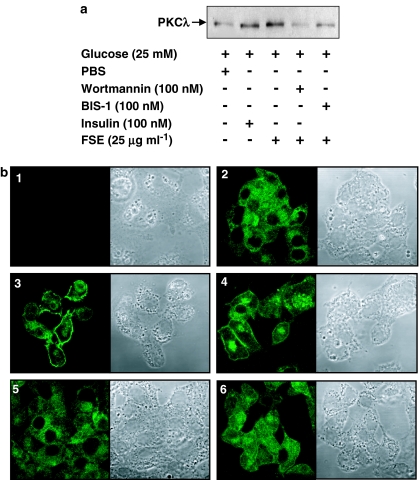
The correlation between FSE-induced IR phosphorylation and GLUT4 translocation-mediated glucose uptake was studied by following PKC activation in 3T3-L1 and HepG2 cells. A significant level of PKCλ was detected in the membrane fraction of FSE-treated as well as insulin-treated 3T3-L1 cells as compared to the cells subjected to PBS treatment (Figure 4a). Moreover, in unstimulated HepG2 cells PKC-associated immunofluorescence was broadly distributed within the cells (detected by confocal microscopy). In contrast, stimulation of cells with FSE for 30 min resulted in maximum immunofluorescence on the cell surface and this effect was indistinguishable from that observed after treating cells with 100 nM insulin for 10 min (Figure 4b). FSE-induced PKC translocation in these cells was inhibited by wortmannin and BIS-1.
Figure 4.
Effects of FSE on PKC translocation. (a) Serum-starved 3T3-L1 adipocytes were preincubated in the absence or presence of wortmannin or BIS-1 for 20 and 60 min, respectively, followed by treatment with FSE for 10 and 30 min, respectively. Plasma membranes were prepared as described in the Methods section and subjected to protein immunoblot analysis with antibodies against PKCλ. (b) HepG2 cells were serum-starved and preincubated in the absence or presence of wortmannin or BIS-1 for 20 and 60 min, respectively, followed by treatment with insulin or FSE for 10 and 30 min, respectively. Immunostaining was performed as described in the Methods section. (1) Negative control, (2) basal, (3) insulin, (4) FSE, (5) and (6) pretreated with wortmannin and BIS-1, respectively, followed by treatment with FSE. The results shown are representative of at least two independent experiments.
Discussion
Fenugreek and other traditional plants are currently being investigated for their potential as a source of new hypoglycaemic compounds for the treatment of diabetes (Raskin et al., 2002; Habeck, 2003; Yeh et al., 2003). However, with the exception of guanidine, many of the hypoglycaemic compounds isolated from plants are small molecules such as alkaloids, flavanoids, glycosides, steroids, amino acids or minerals that are not suitable for pharmaceutical drug development (Oubre et al., 1997; Day, 1998). Therefore, to eliminate any unsuitable small molecules, an aqueous extract of fenugreek seeds was dialysed for 24 h and the hypoglycaemic potential of this dialysed extract (FSE) was investigated in vivo in AXN-induced diabetic mice. FSE significantly improved glucose homeostasis in these diabetic mice and in normal glucose-loaded mice by effectively lowering blood glucose levels. This effect of FSE on glucose levels was found to be comparable to that of insulin.
Glucose uptake by the target tissues of insulin is the rate-limiting step in hyperglycaemic conditions and it is facilitated mostly by translocation of glucose transporters from an intracellular site to the plasma membrane (Cushman & Wardzala, 1980). Aqueous extracts of elder (Sambucus nigra) and banaba (Langerstroemia speciosa) have been demonstrated to have an insulin-like effect on glucose uptake in vitro (Gray et al., 2000; Liu et al., 2001) and in the present study the mechanisms by which FSE attenuates hyperglycaemia were further investigated in vitro. Using CHO-HIRc-mycGLUT4eGFP and 3T3-L1-mycGLUT4 cells as in vitro models (Kanai et al., 1993; Dobson et al., 1996; Inoue et al., 1999), we demonstrated that FSE induces a rapid, dose-dependent stimulatory effect on cellular glucose uptake by activating cellular responses that lead to GLUT4 translocation to the cell surface. Our results also indicated that FSE contains factor(s) that might act independently of insulin to enhance glucose transporter-mediated glucose uptake.
The action of insulin is initiated by its binding to the IR. This leads to autophosphorylation of the receptor and results in increased tyrosine phosphorylation of a number of proteins including IRS-1 and p85 of PI3-K. Therefore, in our study the effects of FSE on tyrosine phosphorylation of the IR and the downstream signalling molecules in the primary cellular targets of insulin, adipocytes and liver cells were investigated using 3T3-L1 adipocytes and HepG2 cells, respectively. The results revealed that FSE activated the tyrosine phosphorylation of IR-β, subsequently enhancing tyrosine phosphorylation of IRS-1 and the p85 subunit of PI3-kinase, but had no effect on basal IR-α phosphorylation. This suggests that adipocytes and liver cells could be target sites for FSE and that it exerts its effects by activating insulin signalling pathways.
The IR belongs to a family of receptors that share a high degree of homology in the tyrosine kinase domain. The activation of receptor tyrosine kinases leads to a wide variety of biological effects ranging from metabolic regulation to deleterious neoplastic transformation (White & Kahn, 1994). To confirm the specificity of FSE and to rule out any nonspecific activation of receptor kinases, the effect of FSE on the phosphorylation of EGFR was investigated. Our findings revealed that FSE did not induce EGFR autophosphorylation in A431 cells. Taken together, these results indicate that FSE is capable of specifically activating the IR and its downstream signalling molecules in adipocytes and liver cells and is not a general sensitiser of receptor tyrosine kinase domains.
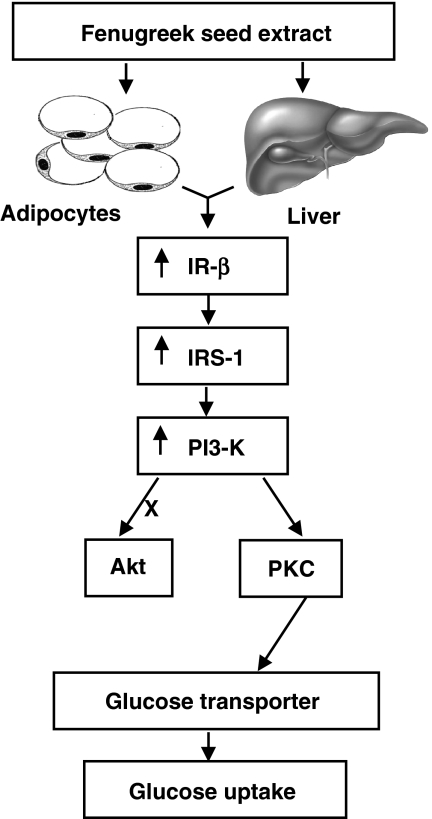
The activation of PI3-K is necessary for the metabolic action of insulin, as demonstrated in studies with the PI3-K inhibitor wortmannin (Hausdorff et al., 1999). Pretreatment of CHO-HIRc-mycGLUT4eGFP and 3T3-L1-mycGLUT4 cells with wortmannin inhibited FSE-induced GLUT4 translocation as well as glucose uptake. These results indicate that the effects of FSE are dependent on PI-3K. Insulin-elicited signals resulting in the activation of PI3-K are transmitted by two independent pathways, an Akt pathway and a PKC pathway (Saltiel & Pessin, 2002). Unlike insulin, FSE had no effect on Ser-473 phosphorylation of Akt in either 3T3-L1 or HepG2 cells. However, similar to insulin, FSE treatment resulted in translocation of PKC to the cell surface in both 3T3-L1 adipocytes and HepG2 cells. These results suggest that Akt activation is not essential for FSE-induced GLUT4 translocation and glucose uptake (Kitamura et al., 1998). To verify the involvement of PKC, cells were pretreated with BIS-1, a PKC-specific inhibitor (Toullec et al., 1991). BIS-1 inhibited translocation of PKC induced by FSE in these cells and inhibited FSE-induced GLUT4 translocation as well as glucose uptake, which suggests that PKC is involved in these two processes. Although the involvement of different PKC isoforms in the effects of FSE cannot be ruled out, the results obtained from the experiments with 3T3-L1 adipocytes indicate that, similar to insulin, FSE-induced GLUT4 translocation also requires PKCλ activation (Kotani et al., 1998). The proposed signal transduction pathways induced by FSE are schematically depicted in Figure 5.
Figure 5.
A proposed model for cellular effects of fenugreek seed extract on glucose homeostasis.
In conclusion, we have clearly demonstrated that a dialysed aqueous extract of fenugreek seeds possesses hypoglycaemic properties and that it stimulates insulin signalling pathways in adipocytes and liver cells. Also, the in vitro models and methods described in this study could be used for screening the activity of natural compounds suitable for the development of new anti-diabetic drugs.
Acknowledgments
We thank Dr G.C. Mishra, Director, NCCS for being very supportive and giving all the encouragement to carry out this work. We also thank Department of Biotechnology, Government of India for providing financial support. RC thanks Council for Scientific and Industrial Research for (CSIR), New Delhi, for providing fellowship. We also thank Drs D. Mitra, A. Sahu, M.V. Krishnasastry and B. Saha for critical reading of the manuscript and Ms Ashwini Atre for confocal microscopy studies.
Abbreviations
- ABTS
2,2′-azino-bis 3-ethylbenzthiazoline-6-sulphonic acid
- Akt
protein kinase B
- AXN
alloxan
- BIS-1
bisindolylmaleimide 1
- BSA
bovine serum albumin
- 2-DG
[3H]-2-deoxy-D-glucose
- DMEM
Dulbecco's modified Eagle's medium
- EGFR
epidermal growth factor receptor
- FSE
fenugreek seed extract
- GLUT4
glucose transporter 4
- IR
insulin receptor
- IRS-1
insulin receptor substrate 1
- PMSF
phenylmethylsulphonyl fluoride
- PBS
phosphate-buffered saline
- PI3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- TBS
tris-buffered saline
References
- ABDEL-BARRY J.A., ABDEL-HASSAN I.A., AL-HAKIEM M.H. Hypoglycemic and antihyperglycemic effects of Trigonella foenum-graecum leaf in normal and alloxan induced diabetic rats. J. Ethnopharmacol. 1997;58:149–155. doi: 10.1016/s0378-8741(97)00101-3. [DOI] [PubMed] [Google Scholar]
- BASCH E., ULBRICHT C., KUO G., SZAPARY P., SMITH M. Therapeutic applications of fenugreek. Altern. Med. Rev. 2003;8:20–27. [PubMed] [Google Scholar]
- CHEATHAM B., VLAHOS C.J., CHEATHAM L., WANG L., BLENIS J., KAHN C.R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHHIPA R.R., SINGH S., SURVE S.V., VIJAYAKUMAR M.V., BHAT M.K. Doxycycline potentiates antitumor effect of cyclophosphamide in mice. Toxicol. Appl. Pharmacol. 2005;202:268–277. doi: 10.1016/j.taap.2004.06.025. [DOI] [PubMed] [Google Scholar]
- CLANCY B.M., CZECH M.P. Hexose transport stimulation and membrane redistribution of glucose transporter isoforms in response to cholera toxin, dibutyryl cyclic AMP, and insulin in 3T3-L1 adipocytes. J. Biol. Chem. 1990;265:12434–12443. [PubMed] [Google Scholar]
- CUSHMAN S.W., WARDZALA L.J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 1980;25:4758–4762. [PubMed] [Google Scholar]
- DAY C. Traditional plant treatments for diabetes mellitus: pharmaceutical foods. Br. J. Nutr. 1998;80:5–6. doi: 10.1017/s0007114598001718. [DOI] [PubMed] [Google Scholar]
- DOBSON S.P., LIVINGSTONE C., GOULD G.W., TAVARE J.M. Dynamics of insulin-stimulated translocation of GLUT4 in single living cells visualized using green fluorescent protein. FEBS Lett. 1996;393:179–184. doi: 10.1016/0014-5793(96)00879-4. [DOI] [PubMed] [Google Scholar]
- FABRICANT D.S, FARNSWORTH N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY A.M., ABDEL-WAHAB Y.H., FLATT P.R. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J. Nutr. 2000;130:15–20. doi: 10.1093/jn/130.1.15. [DOI] [PubMed] [Google Scholar]
- HABECK M. Diabetes treatments get sweet help from nature. Nat. Med. 2003;9:1228. doi: 10.1038/nm1003-1228a. [DOI] [PubMed] [Google Scholar]
- HAUSDORFF S.F., FINGAR D.C., MORIOKA K., GARZA L.A., WHITEMAN E.L., SUMMERS S.A., BIRNBAUM M.J. Identification of wortmannin-sensitive targets in 3T3-L1 adipocytes. Dissociation of insulin stimulated glucose uptake and GLUT4 translocation. J. Biol. Chem. 1999;274:24677–24684. doi: 10.1074/jbc.274.35.24677. [DOI] [PubMed] [Google Scholar]
- INOUE G., CHEATHAM B., KAHN C.R. Development of an in vitro reconstitution assay for glucose transporter 4 translocation. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14919–14924. doi: 10.1073/pnas.96.26.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Z.Y., CHAWLA A., BOSE A., WAY M., CZECH M.P. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J. Biol. Chem. 2002;277:509–515. doi: 10.1074/jbc.M108280200. [DOI] [PubMed] [Google Scholar]
- KAHN A.H., PESSIN J.E. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia. 2002;45:1475–1483. doi: 10.1007/s00125-002-0974-7. [DOI] [PubMed] [Google Scholar]
- KAMEI R., KITAGAWA Y., KADOKURA M., HATTORI F., HAZEKI O., EBINA Y., NISHIHARA T., OIKAWA S. Shikonin stimulates glucose uptake in 3T3-L1 adipocytes via an insulin-independent tyrosine kinase pathway. Biochem. Biophys. Res. Commun. 2002;292:642–651. doi: 10.1006/bbrc.2002.6714. [DOI] [PubMed] [Google Scholar]
- KANAI F., NISHIOKA Y., HAYASHI H., KAMOHARA S., TODAKA M., EBINA Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J. Biol. Chem. 1993;268:14523–14526. [PubMed] [Google Scholar]
- KITAMURA T., OGAWA W., SAKAUE H., HINO Y., KURODA S., TAKATA M., MATSUMOTO M., MAEDA T., KONISHI H., KIKKAWA U., KASUGA M. Requirement for activation of the serine–threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol. Cell. Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTANI K., OGAWA W., MATSUMOTO M., KITAMURA T., SAKAUE H., HINO Y., MIYAKE K., SANO W., AKIMOTO K., OHNO S., KASUGA M. Requirement of atypical protein kinase c lambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU F., KIM J., LI Y., LIU X., LI J., CHEN X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutr. 2001;131:2242–2247. doi: 10.1093/jn/131.9.2242. [DOI] [PubMed] [Google Scholar]
- MOLLER D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- OUBRE A.Y., CARLSON T.J., KING S.R., REAVEN G.M. From plant to patient: an ethnomedical approach to the identification of new drugs for the treatment of NIDDM. Diabetologia. 1997;40:614–617. doi: 10.1007/s001250050724. [DOI] [PubMed] [Google Scholar]
- RASKIN I., RIBNICKY D.M., KOMARNYTSKY S., ILIC N., POULEV A., BORISJUK N., BRINKER A., MORENO D.A., RIPOLL C., YAKOBY N., O'NEAL J.M., CORNWELL T., PASTOR I., FRIDLENDER B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R., PESSIN J.E. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- TAFURI S.R. Troglitazone enhances differentiation, basal glucose uptake, and GLUT1 protein levels in 3T3-L1 adipocytes. Endocrinology. 1996;137:4706–4712. doi: 10.1210/endo.137.11.8895337. [DOI] [PubMed] [Google Scholar]
- TAYLOR S.I. Deconstructing type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., COSTE H., BELLEVERGUE P., GRAND-PERRET T., AJAKANE M., BAUDET V., BOISSIN P., BOURSIER E, LORIOLLE F, LORIOLLE F., DUHAMEL L., CHARON D., KIRILOVSKY J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- U.K. Prospective Diabetes Study Group Perspectives in Diabetes. U.K. Prospective Diabetes study 16. Overview of 6 years' therapy of type 2 diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- VATS V., GROVER J.K., RATHI S.S. Evaluation of anti-hyperglycemic and hypoglycemic effect of Trigonella foenum-graecum Linn, Ocimum sanctum Linn and Pterocarpus marsupium Linn in normal and alloxanized diabetic rats. J. Ethnopharmacol. 2002;79:95–100. doi: 10.1016/s0378-8741(01)00374-9. [DOI] [PubMed] [Google Scholar]
- WHITE M.F., KAHN C.R. The insulin signaling system. J. Biol. Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- YEH G.Y., EISENBERG D.M., KAPTCHUK T.J., PHILLIPS R.S. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- ZIA T., HASNAIN S.N., HASAN S.K. Evaluation of the oral hypoglycemic effect of Trigonella foenum-graecum L (methi) in normal mice. J. Ethnopharmacol. 2001;75:191–195. doi: 10.1016/s0378-8741(01)00186-6. [DOI] [PubMed] [Google Scholar]