Abstract
3,4-Methylenedioxymetamphetamine (MDMA) produces complex effects on body temperature, including hypo- and hyperthermic components that vary with ambient temperature and strain of rat. We have previously reported that MDMA is an α2-adrenoceptor agonist, and α2-adrenoceptor agonists such as clonidine produce hypothermia.
The purpose of this study was to investigate the effects of MDMA on core body temperature measured by radiotelemetry in conscious wild-type (WT) and α2A-knockout (α2A-KO) mice.
Clonidine (0.1 mg kg−1, subcutaneously (s.c.)) produced a hypothermic response in WT mice, but did not significantly affect temperature in α2-KO mice. MDMA (20 mg kg−1, s.c.) produced a significant hyperthermia in WT mice beginning at approximately 100 min after injection, recovering by 300 min, but produced a biphasic response, hypothermia followed by hyperthermia, in α2-KO mice.
In WT mice, following the α2A-adrenoceptor antagonist 2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole (1 mg kg−1, s.c.), MDMA (20 mg kg−1) produced an initial hypothermia.
Hence, α2-adrenoceptor agonist actions of MDMA contribute to its effects on body temperature, but in a surprising way. Although selective α2A-adrenoceptor agonism produces hypothermia, the α2A-adrenoceptor actions of MDMA alter the body temperature response to MDMA from biphasic (hypothermia followed by hyperthermia) to monophasic hyperthemia.
Keywords: MDMA, α2A-knockout mice, hypothermia, hyperthermia, clonidine
Introduction
Hyperthermia is a life-threatening acute consequence of 3,4-methylenedioxymethamphetamine (MDMA) toxicity and is often seen when the drug is used at a rave, an environment where ambient temperatures tend to be high and there is excessive physical exertion. In animal studies, it has also been shown that MDMA disrupts thermoregulation, causing either hypo- or hyperthermia depending on the ambient temperature (Malberg & Seiden, 1988). The mechanism by which MDMA disrupts thermoregulation is still unclear, but both central and peripheral mechanisms have been implicated. Since serotonergic, noradrenergic and dopaminergic neurotransmitter systems have all been implicated in the mediation of hypothermia and hyperthermia, acute increases in these neurotransmitters induced by amphetamine-like actions of MDMA (White et al., 1996), or agonist actions of MDMA at receptors for these neurotransmitters, may therefore influence the thermoregulatory system. It has also been shown that activation of the uncoupling protein-3 (UCP3), a skeletal thermogenic protein, may be involved in MDMA-induced hyperthermia (Mills et al., 2003). In addition to having an affinity for 5-hydroxytryptamine (5-HT), noradrenaline (NA) and dopamine (DA) uptake sites through which it can increase neurotransmitter accessibility to pre- and postsynaptic receptors, MDMA also has an affinity for α2-adrenoceptors in the brain (Battaglia et al., 1988). Direct agonist effects of MDMA on α2-adrenoceptors have been demonstrated both in vivo and in vitro (Lavelle et al., 1999; McDaid & Docherty, 2001).
α2-Adrenoceptors mediate pre- and postsynaptic actions of noradrenaline both in the central and peripheral nervous systems (Philipp et al., 2002). α2-Adrenoceptors have been separated into three subtypes, α2A-, α2B and α2C (Bylund et al., 1994), with α2A and α2C subtypes predominating in the central nervous system (Philipp et al., 2002). The term α2D-adrenoceptor has previously been used for the species orthologue of the human α2A-adrenoceptor, founds in rodents (see Docherty, 1998; Guimaraes & Moura, 2001). 2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole (BRL 44408) is an antagonist with selectivity for α2A-adrenoceptors (Young et al., 1989; see Docherty, 1998; Guimaraes & Moura, 2001).
Clonidine, an α2-adrenoceptor agonist, induces a hypothermia by action at central α2A-adrenoceptors (Zarrindast et al., 2003). It might be expected that actions of MDMA at α2-adrenoceptors may alter the hypo-hyperthermic actions of the agent. Hence, the aim of this study was to investigate the role of α2A-adrenoceptors in MDMA-mediated hyperthermia using both the selective α2A-adrenoceptor antagonist BRL 44408 and wild-type (WT) and α2A-adrenoceptor knockout (α2A-KO) mice.
Some of the results have been presented in abstract form (Bexis & Docherty, 2005).
Methods
Male WT and α2A-KO mice (22–35 g) were obtained from Jackson Laboratories (Bar Harbor, ME, U.S.A.). All studies conform to the Declaration of Helsinki and have been approved by the Department of Health and by the RCSI Research Ethics Committee.
Radiotelemetry
Under ether anaesthesia, animals were implanted with a radiotelemetric device enabling measurement of core body temperature (TAC50-PXT; Data Sciences International, St Paul, MN, U.S.A.). The implant was placed in the abdominal cavity and the abdomen was then closed. Animals were given temgesic (buprenorphine hydrochloride 0.05 mg kg−1, Schering-Plough, Welwyn, U.K., subcutaneously (s.c.)) postoperatively and allowed to recover for 14 days before experiments were performed.
On experimental days, a PhysiolTel-Receiver (model RPC-1) was placed under each animal cage, enabling recording of core body temperature. Data signals were acquired from 90 min prior to and for 300 min after drug administration. All recordings were obtained at room temperature (23±0.18°C).
Drug treatments
Since treatment of the animals and recordings were performed in the laboratory and not in the animal facility, animals were allowed to acclimatize, in their home cages, to the surroundings in the laboratory for 2 days (5–6 h per day) before administration of any drugs. Animals were injected s.c. either with vehicle (1 ml kg−1), MDMA (20 mg kg−1) or clonidine (0.1 mg kg−1). In interaction studies, the α2A-adrenoceptor antagonist BRL 44408 (1 mg kg−1) was given 30 min prior to the injection of vehicle or MDMA. Some animals were injected with test drug at least 2 days after injection of vehicle (1 ml kg−1).
Drugs
MDMA (Research Biochemicals, Natick, U.S.A. and NIDA, Bethesda, U.S.A.); clonidine HCl (Tocris, Bristol, U.K.); BRL 44408 (Sigma, Dublin, Ireland).
Drugs were dissolved in distilled water.
Statistics
Values are mean±s.e.m. from n experiments. Responses were compared between groups by repeated-measures analysis of variance (ANOVA) followed by the Bonferroni test. Statistical and graphical analysis was carried out using GraphPad Prism for Macintosh computers.
Results
Core body temperature
The resting body temperature was 35.8±0.2°C in WT animals prior to vehicle; 36.6±0.4°C in WT animals prior to BRL 44408 and 35.2±0.3°C in α2A-KO mice prior to vehicle, respectively (n=5–6). The resting body temperature values of animals from the three groups were not significantly different. However, body temperature transiently increased after the administration of vehicle or drug (see Figure 1).
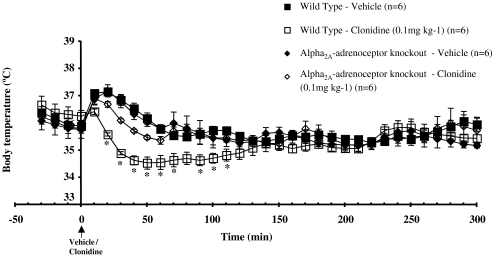
Figure 1.
Core body temperature recordings in conscious WT or α2A-adrenoceptor KO mice before and after vehicle or clonidine (0.1 mg kg−1, s.c.) administration. Vertical bars indicate the s.e.m. from six mice. *P<0.05 compared to the corresponding vehicle.
Administration of clonidine (0.1 mg kg−1, s.c.) to WT mice resulted in hypothermia with a minimum core temperature being reached by 40 min, after which the temperature rose, gradually reaching baseline levels by 300 min (Figure 1). In contrast, clonidine had no significant effect on core body temperature in α2A-KO mice (Figure 1).
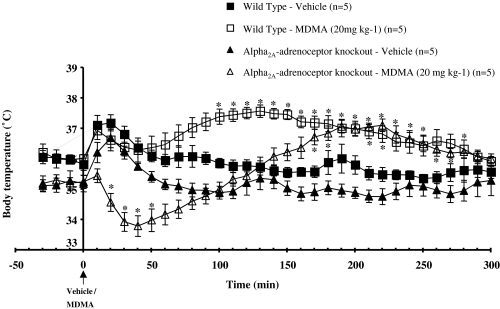
The effects of MDMA on core body temperature in WT and α2A-KO mice are shown in Figure 2. In WT mice, MDMA (20 mg kg−1, s.c.) produced hyperthermia, reaching a maximum core temperature 130 min after administration of the drug, followed by a gradual decrease in core temperature to baseline levels. In α2A-KO mice, MDMA produced a biphasic response, consisting of an initial hypothermic response followed by hyperthermia (Figure 2). The onset of hypothermia in α2A-KO mice occurred 20 min after the injection of MDMA and reached a minimum core temperature at 40 min after drug administration. The maximum core temperature was reached 220 min after the injection of MDMA, after which the temperature began to decline (Figure 2). In both WT and α2A-KO mice, vehicle produced a transient increase in body temperature, followed by a relatively stable baseline (Figure 2).
Figure 2.
Core body temperature recordings in conscious WT or α2A-adrenoceptor KO mice before and after vehicle or MDMA (20 mg kg−1, s.c.) administration. Vertical bars indicate the s.e.m. from five mice. *P<0.05 compared to the corresponding vehicle.
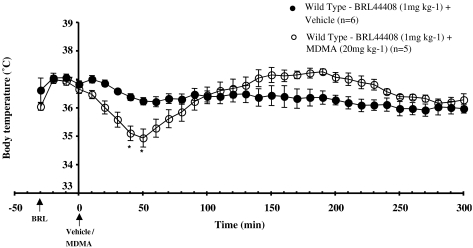
In WT mice pretreated with BRL 44408 (1 mg kg−1, s.c.), MDMA produced a significant decrease in core temperature, which was then followed by hyperthermia, although the hyperthermic response did not reach significance, as compared to the effects of vehicle (Figure 3).
Figure 3.
Core body temperature recordings in conscious WT mice administered BRL 44408 (1 mg kg−1, s.c.) 30 min prior to vehicle or MDMA (20 mg kg−1, s.c.). Vertical bars indicate the s.e.m. from five or six mice. *P<0.05 compared to the vehicle.
Discussion
In this study, a combination of pharmacology and molecular genetics was used to determine the involvement of α2A-adrenoceptors in the effects of MDMA on temperature. Before discussing the results obtained, we will first consider the drug doses chosen.
MDMA (1, 5 and 20 mg kg−1) produces dose-dependent biphasic responses on blood pressure, pressor then depressor, in the anaesthetized rat and mouse (McDaid & Docherty, 2001; Vandeputte & Docherty, 2002), but only pressor responses in conscious rats (Bexis et al., 2003). In the mouse, Carvalho et al. (2002) found that MDMA (5, 10 and 20 mg kg−1) produced dose-dependent increases in temperature, with the largest increase produced by MDMA (20 mg kg−1). Hence, we chose a dose of MDMA (20 mg kg−1) to assess the effects on temperature.
The dose of clonidine (0.1 mg kg−1) was chosen based on the results of Zarrindast et al. (2003), who found that clonidine 0.05–0.1 mg kg−1 produced dose-dependent falls in temperature. We confirmed that s.c. administration of clonidine produced hypothermia in WT mice under our experimental conditions, and that clonidine did not significantly alter body temperature when administered to α2A-KO mice, in agreement with Hunter et al. (1997). The hypothermic response elicited by clonidine is thought to be primarily mediated by activation of postsynaptic α2A-adrenoceptors in the preoptic area of the hypothalamus (Myers et al., 1987). Admittedly, clonidine and MDMA have differing effects on blood pressure, as clonidine (0.1 mg kg−1) lowers blood pressure by a central action (Zhu et al., 1999).
BRL 44408 shows selectivity for α2A (formerly α2A/D) adrenoceptors over α2B and α2C. In our own ligand-binding studies, we have found that BRL 44408 shows 10–30-fold selectivity for α2A (α2D), with pKi values (−log M) of 7.77, 6.11 and 6.28 at α2A, α2B and α2C-adrenoceptors, respectively (Cleary et al., 2002). However, BRL 44408 is also an antagonist at α1-adrenoceptors, with a pKi at α1A-adrenoceptors of 6.59 (Cleary et al., 2003). Hence, BRL 44408 shows about 10-fold selectivity for α2A- over other α2-adrenoceptors and α1A-adrenoceptors. In the pithed rat, we have shown that BRL 44408 (1 mg kg−1) produces a 0.87 log unit shift in the postjunctional potency of the α2-adrenoceptor agonist xylazine (Gavin & Docherty, 1996). Given that we could only expect 10-fold selectivity, 1 mg kg−1 was chosen as the dose to employ, producing approximately a 10-fold shift in xylazine (and presumably clonidine) potency in vivo. BRL 44408 (1 mg kg−1) is likely to have only a small effect on blood pressure, but may prolong the pressor response to MDMA, as occurred in anaesthetized mouse by knockout of the α2A-adrenoceptor (Vandeputte & Docherty, 2002).
In the present study, we have also demonstrated a role for α2A-adrenoceptors in the MDMA-induced hyperthermia. In the presence of the α2A-adrenoceptor antagonist BRL 44408, the monophasic hyperthermic response produced by MDMA in WT mice became a biphasic response with an initial hypothermia followed by a small increase in body temperature, although the hyperthermic response did not reach significance as compared to vehicle. Similarly, when α2A-KO mice were injected with MDMA, a biphasic response was seen, hypothermia followed by hyperthermia. The results are surprising since α2A-adrenoceptors are involved in producing hypothermia, as was also shown in the present study (see above). As well as being found postsynaptically where they mediate hypothermia (Myers et al., 1987), α2A-adrenoceptors are also found presynaptically as inhibitory receptors regulating release of noradrenaline (autoreceptors) and other neurotransmitters, such as dopamine and 5HT (heteroceptors), in the central and peripheral nervous systems (Philipp et al., 2002; Brede et al., 2004). It has been demonstrated that the 5HT2 receptor antagonists, ketanserin and MDL100907, and fluoxetine, a 5-HT uptake inhibitor, attenuate the hyperthermic response mediated by MDMA in mice (Fantegrossi et al., 2003). In rats, the dopamine D1-receptor antagonist SCH23390 produced a dose-dependent inhibition of hyperthermia induced by MDMA (Mechan et al., 2002). Hyperthermia elicited in mice by the 5HT-releasing amphetamine derivative, p-chloroamphetamine (PCA), is attenuated by ketanserin, SCH23390 and the dopamine depleter, α-methyl-p-tyrosine, but not by fluoxetine, or the 5HT depleter p-chlorophenylalanine (Sugimoto et al., 2000, 2001). This may indicate that dopamine plays a role in hyperthermia with 5HT2 receptors facilitating dopamine release or synthesis (Sugimoto et al., 2001). Since the monoaminergic systems are interconnected and can influence each other, it could be suggested that, under the conditions of increased extracellular levels of the three monoamines produced by MDMA, concomitant activation of the presynaptic α2A-adrenoceptor results in a component of the hyperthermic response. In the absence of α2A-adrenoceptors, this component of the hyperthermia is absent, and the resultant changes in levels of dopamine, 5HT and possibly other neurotransmitters lead to the hypothermic component seen in α2A-KO mice.
In addition to the predominant α2A-adrenoceptor, it has been demonstrated that α2C-adrenoceptors also function as a presynaptic regulator of noradrenaline, but they are more prominent in sympathetic nerve endings than central noradrenergic neurons (Ho et al., 1998; Philipp et al., 2002). The α2C-adrenoceptors are also involved in inducing hypothermia, since, in the absence or overexpression of α2C-adrenoceptors, the hypothermic response to the α2-adrenoceptor agonist, dexmedetomidine, is slightly decreased (17%) or increased (12%), respectively (Sallinen et al., 1997). Indeed, α2C-adrenoceptor upregulation has been shown to occur to partly replace prejunctional α2A-adrenoceptors in rat vas deferens (Hein & Kobilka, 1998; Cleary et al., 2002). However, clonidine had no significant effect on temperature in α2A-KO mice (present results), suggesting that, either that the α2C-adrenoceptor component is small (a small but insignificant hypothermic response is present in Figure 1), or that clondine has low affinity/efficacy at α2C-adrenoceptors. MDMA has similar affinities/potencies at α2A- and α2C-adrenoceptors in ligand-binding (Lavelle et al., 1999) and functional studies (Rajamani et al., 2001), but the relative lack of importance of α2C-adrenoceptors in hypothermia and the lack of hypothermia to MDMA in WT animals still argue for a hyperthermic action of MDMA by α2A-adrenoceptor activation in WT mice. α2B-adrenoceptors mediate a centrally mediated sympathoexcitatory response (Gavras et al., 2001), but it is not clear whether this response is important in the current study.
The effects of MDMA on body temperature in mice have been examined by other authors. After a single dose of MDMA (20 mg kg−1), both a significant elevation and a significant drop in core temperature have been observed in mice (Miller & O'Callaghan, 1994; O'Shea et al., 2000; Johnson et al., 2002; Green et al., 2004). Although these discrepancies may be attributed partly to strain and sex differences, C57BL/6 mice were used in our study and all but one of the above studies (other study, Swiss Webster: O'Shea et al., 2000), and temperature changes were observed in both female and male mice (Miller & O'Callaghan, 1994; Johnson et al., 2002; Green et al., 2004).
Another mechanism by which MDMA induces hyperthermia is via cutaneous vasoconstriction, both direct and indirect due to central sympathetic activation, reducing the animal's ability to dissipate heat (Pedersen & Blessing, 2001). Although vasoconstriction is mediated predominantly by α1-adrenoceptors, α2-adrenoceptors, particularly α2A-adrenoceptors, also contribute to systemic vasoconstriction (Gavin & Docherty, 1996; Duka et al., 2000). In addition, α2c-adrenoceptors are present on veins (Gavin et al., 1997) and on cutaneous arteries, and have been shown to be involved particularly in cold induced vasoconstriction (Chotani et al., 2000). Therefore, in the absence of α2A-adrenoceptors, a loss of a component of cutaneous vasoconstriction would not explain the hypothermic response unless cutaneous vasodilating actions of MDMA become evident in the absence of α2A-adrenoceptors.
In conclusion, α2A-adrenoceptor activation prevents MDMA from inducing an initial hypothermic response. α2A-Adrenoceptor activation modulates other actions of MDMA, which in turn alter the temperature response from biphasic to monophasic.
Acknowledgments
This work was supported by the Health Research Board (Ireland). MDMA was generously supplied under the NIDA Drug Supply Program.
Abbreviations
- BRL 44408
2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole
- 5-HT
5-hydroxytryptamine
- KO
knockout
- MDMA
3,4-methylenedioxymethamphetamine
- WT
wild type
References
- BATTAGLIA G., BROOKS B.P., KULSAKDINUN C., DE SOUZA E.B. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur. J. Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- BEXIS S., DOCHERTY J.R. Role of alpha2-adrenoceptors in the effects of MDMA on body temperature in the mouse. Proceedings of the British Pharmacological Society at. 2005. [DOI] [PMC free article] [PubMed]
- BEXIS S., VANDEPUTTE C., DOCHERTY J.R. Effects of chronic treatment with MDMA on pre and postjunctional responsiveness in the rat. Br. J. Pharmacol. 2003;138:195. [Google Scholar]
- BREDE M., PHILIPP M., KNAUS A., MUTHIG V., HEIN L. Alpha2-adrenergic receptor subtypes – novel functions uncovered in gene-targeted mouse models. Biol. Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBERG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., JR, TRENDELENBURG U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CARVALHO M., CARVALHO F., REMIAO F., PEREIRA M.L., PIRES-DAS-NEVES R., BASTOS M.L. Effect of 3,4-methylenedioxymeth-amphetamine (‘ecstasy') on body temperature and liver antioxidant status in mice: influence of ambient temperature. Arch. Toxicol. 2002;76:166–172. doi: 10.1007/s00204-002-0324-z. [DOI] [PubMed] [Google Scholar]
- CHOTANI M.A., FLAVAHAN S., MITRA S., DAUNT D., FLAVAHAN N. Silent alpha2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- CLEARY L., VANDEPUTTE C., DOCHERTY J.R. Investigation of neurotransmission in the vas deferens from alpha2A/D-adrenoceptor knockout mice. Br. J. Pharmacol. 2002;136:857–864. doi: 10.1038/sj.bjp.0704791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEARY L., VANDEPUTTE C., DOCHERTY J.R. Investigation of postjunctional alpha1- and alpha2-adrenoceptor subtypes in vas deferens from wild-type and alpha2A/D-adrenoceptor knockout mice. Br. J. Pharmacol. 2003;138:1069–1076. doi: 10.1038/sj.bjp.0705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCHERTY J.R. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur. J. Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- DUKA I., GAVRAS I., JOHNS C., HANDY D.E., GAVRAS H. Role of the postsynaptic alpha2-adrenergic receptor subtypes in catecholamine-induced vasoconstriction. Gen. Pharmacol. 2000;34:101–106. doi: 10.1016/s0306-3623(00)00051-3. [DOI] [PubMed] [Google Scholar]
- FANTEGROSSI W.E., GODLEWSKI T., KARABENICK R.L., STEPHENS J.M., ULLRICH T., RICE K.C., WOODS J.H. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (‘ecstasy') and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology. 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- GAVIN K.T., COLGAN M.-P., MOORE D., SHANIK G., DOCHERTY J.R. Alpha2c-adrenoceptor mediate contractile responses to noradrenaline in the human saphenous vein. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:406–411. doi: 10.1007/pl00004961. [DOI] [PubMed] [Google Scholar]
- GAVIN K.T., DOCHERTY J.R. Investigation of the subtype of alpha2-adrenoceptor mediating pressor responses in the pithed rat. Eur. J. Pharmacol. 1996;318:81–87. doi: 10.1016/s0014-2999(96)00780-7. [DOI] [PubMed] [Google Scholar]
- GAVRAS I., MANOLIS A.J., GAVRAS H. The alpha2-adrenergic receptors in hypertension and heart failure: experimental and clinical studies. J. Hypertens. 2001;19:2115–2124. doi: 10.1097/00004872-200112000-00001. [DOI] [PubMed] [Google Scholar]
- GREEN R.A., O'SHEA E., COLADO M.I. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur. J. Pharmacol. 2004;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HEIN I., KOBILKA B.K. Clinical and molecular pharmacology of adrenergic receptors. Naunyn-Schmeideberg's Arch. Pharmacol. 1998;358:R575. [Google Scholar]
- HO S.L., HONNER V., DOCHERTY J.R. Investigation of the subtypes of alpha2-adrenoceptor mediating prejunctional inhibition in rat atrium and cerebral cortex. Naunyn-Schmeideberg's Arch. Pharmacol. 1998;357:634–639. doi: 10.1007/pl00005218. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., FONTANA D.J., HEDLEY L.R., JASPER J.R., LEWIS R., LINK R.E., SECCHI R., SUTTON J., EGLEN R.M. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E.A., O'CALLAGHAN J.P., MILLER D.B. Chronic treatment with supraphysiological levels of corticosterone enhances D-MDMA-induced dopaminergic neurotoxicity in the C57BL/6J female mouse. Brain Res. 2002;933:130–138. doi: 10.1016/s0006-8993(02)02310-7. [DOI] [PubMed] [Google Scholar]
- LAVELLE A., HONNER V., DOCHERTY J.R. Investigation of the prejunctional alphal-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br. J. Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALBERG J.E., SEIDEN L.S. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 1988;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDAID J., DOCHERTY J.R. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetized rat. Br. J. Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHAN A.O., ESTEBAN B., O'SHEA E., ELLIOTT J.M., COLADO I., GREEN A.R. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') to rats. Br. J. Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER D.B., O'CALLAGHAN J.P. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- MILLS E.M., BANKS M.L., SPRAGUE J.E., FINKEL T. Uncoupling the agony from ecstasy. Nature. 2003;246:403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- MYERS R.D., BELESLIN D.B., REZVANI A.H. Hypothermia: role of α1- and α2-noradrenergic receptors in the hypothalamus of the cat. Pharmacol. Biochem. Behav. 1987;26:373–379. doi: 10.1016/0091-3057(87)90132-8. [DOI] [PubMed] [Google Scholar]
- O'SHEA E., ESTEBAN B., CAMARERO J., GREEN A.R., COLADO M.I. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy') on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2000;40:65–74. doi: 10.1016/s0028-3908(00)00106-4. [DOI] [PubMed] [Google Scholar]
- PEDERSEN N.P., BLESSING W.W. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J. Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPP M., BREDE M., HEIN L. Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough. Am. J. Physiol. 2002;283:287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- RAJAMANI K., LEONG S., LAVELLE A., DOCHERTY J.R. Prejunctional actions of methylenedioxymethamphetamine in vas deferens from wild-type and alpha2A/D knockout mice. Eur. J. Pharmacol. 2001;423:223–228. doi: 10.1016/s0014-2999(01)01118-9. [DOI] [PubMed] [Google Scholar]
- SALLINEN J., LINK R.E., HAAPALINNA A., VITAMAA T., KULATUNGA M., SJOHOLM B., MACDONALD E., PELTO-HUIKKO M., LEINO T., BARSH G., KOBILKA B.K., SCHEININ M. Genetic alteration of α2c-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective α2-adrenoceptor agonist. Mol. Pharmacol. 1997;51:36–46. doi: 10.1124/mol.51.1.36. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., OHKURA M., INOUE K., YAMADA J. Involvement of the 5-HT2 receptor in hyperthermia induced by p-chloroamphetamine, a serotonin-releasing drug in mice. Eur. J. Pharmacol. 2000;403:225–228. doi: 10.1016/s0014-2999(00)00585-9. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., OHKURA M., INOUE K., YAMADA J. Involvement of serotonergic and dopaminergic mechanisms in hyperthermia induced by a serotonin-releasing drug, p-chloroamphetamine in mice. Eur. J. Pharmacol. 2001;430:265–268. doi: 10.1016/s0014-2999(01)01386-3. [DOI] [PubMed] [Google Scholar]
- VANDEPUTTE C., DOCHERTY J.R. Vascular actions of 3,4 methylenedioxy-methamphetamine in alpha(2A/D)-adrenoceptor knockout mice. Eur. J. Pharmacol. 2002;457:45–49. doi: 10.1016/s0014-2999(02)02661-4. [DOI] [PubMed] [Google Scholar]
- WHITE S.R., OBRADOVIC T., IMEL K.M., WHEATON M.J. The effects of methylenedioxymethamphetamine (MDMA, ‘ecstasy') on monoaminergic neurotransmission in the central nervous system. Prog. Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- YOUNG P., BERGE J., CHAPMAN H., CAWTHORNE M.A. Novel alpha 2-adrenoceptor antagonists show selectivity for alpha 2A- and alpha 2B-adrenoceptor subtypes. Eur. J. Pharmacol. 1989;168:381–386. doi: 10.1016/0014-2999(89)90801-7. [DOI] [PubMed] [Google Scholar]
- ZARRINDAST M.-R., SADEGHI S., SAHEBGHARANI M. Influence of α-adrenoceptor agonists and antagonists on imipramine-induced hypothermia in mice. Pharmacol. Toxicol. 2003;93:48–53. doi: 10.1034/j.1600-0773.2003.930107.x. [DOI] [PubMed] [Google Scholar]
- ZHU Q.M., LESNICK J.D., JASPER J.R., MACLENNAN S.J., DILLON M.P., EGLEN R.M., BLUE D.R., Jr Cardiovascular effects of rilmenidine, moxonidine and clonidine in conscious wild-type and D79N alpha2A-adrenoceptor transgenic mice. Br. J. Pharmacol. 1999;126:1522–1530. doi: 10.1038/sj.bjp.0702429. [DOI] [PMC free article] [PubMed] [Google Scholar]