Abstract
Recently, it was reported that anesthetizing infant rats for 6 h with a combination of anesthetic drugs (midazolam, nitrous oxide, isoflurane) caused widespread apoptotic neurodegeneration in the developing brain, followed by lifelong cognitive deficits. It has also been reported that ketamine triggers neuroapoptosis in the infant rat brain if administered repeatedly over a period of 9 h. The question arises whether less extreme exposure to anesthetic drugs can also trigger neuroapoptosis in the developing brain.
To address this question we administered ketamine, midazolam or ketamine plus midazolam subcutaneously at various doses to infant mice and evaluated the rate of neuroapoptosis in various brain regions following either saline or these various drug treatments. Each drug was administered as a single one-time injection in a dose range that would be considered subanesthetic, and the brains were evaluated by unbiased stereology methods 5 h following drug treatment.
Neuroapoptosis was detected by immunohistochemical staining for activated caspase-3. It was found that either ketamine or midazolam caused a dose-dependent, statistically significant increase in the rate of neuroapoptosis, and the two drugs combined caused a greater increase than either drug alone. The apoptotic nature of the neurodegenerative reaction was confirmed by electron microscopy.
We conclude that relatively mild exposure to ketamine, midazolam or a combination of these drugs can trigger apoptotic neurodegeneration in the developing mouse brain.
Keywords: NMDA antagonist, GABAmimetic, caspase-3, substance abuse, anesthesia, brain growth spurt
Introduction
Recent studies have shown that transient exposure of infant rats or mice to several classes of drugs, including those that block NMDA glutamate receptors, those that activate GABAA receptors, and ethanol (which has both NMDA antagonist and GABAmimetic properties), triggers widespread neurodegeneration in the developing brain (Ikonomidou et al., 1999; 2000; Jevtovic-Todorovic et al., 2003). The window of vulnerability to these agents coincides with the developmental period of synaptogenesis, also known as the brain growth spurt period, which in mice and rats occurs primarily postnatally, but in humans extends from about midgestation to several years after birth (Dobbing & Sands, 1979).
The cell death process triggered by these drugs has been studied histologically and found at both light and electron microscopic levels to have classical morphological characteristics of apoptosis (Dikranian et al., 2001; Olney et al., 2002a). Studies examining gene-regulated biochemical pathways have revealed that the cell death process involves translocation of Bax protein to mitochondrial membranes where it disrupts membrane permeability, allowing extramitochondrial leakage of cytochrome c, followed by a sequence of changes culminating in activation of caspase-3. Commitment to cell death occurs prior to the caspase-3 activation (C3A) step (Young et al., 2003; 2005); therefore, immunohistochemical detection of neurons positive for C3A provides a reliable means of mapping and quantifying dying cells that have already progressed beyond the point of cell death commitment.
As ethanol acts by a combination of both NMDA and GABAA receptor-mediated mechanisms, it triggers a particularly robust neuroapoptotic response. This is important in a human context because ethanol is known to have deleterious effects on the developing human brain (fetal alcohol syndrome, FAS) (Jones & Smith, 1973; Jones et al., 1973; Clarren et al., 1978; Swayze et al., 1997). However, human relevance is not limited to ethanol and FAS. There are many agents in the human environment that have NMDA antagonist or GABAmimetic properties. Such agents include drugs that may be abused by pregnant mothers (ethanol, phencyclidine (angel dust), ketamine (Special K), nitrous oxide (laughing gas), barbiturates, benzodiazepines), and many medicinals used in obstetric and pediatric neurology (anticonvulsants) (Bittigau et al., 2002), and anesthesiology (the majority of general anesthetics are either NMDA antagonists or GABAmimetics). In pediatric anesthesia, drugs in both of these categories are often administered in combination, which signifies that the patient is being exposed to the same combination of mechanisms by which ethanol damages the human fetal brain. In a recent study (Jevtovic-Todorovic et al., 2003), infant rats were exposed for 6 h to a clinically relevant cocktail of anesthetic drugs having both NMDA antagonist and GABAmimetic properties, and it resulted in an extensive pattern of neuroapoptosis affecting many brain regions, and subsequent cognitive deficits that were particularly severe in preadolescent rats, and still detectable in adulthood.
Of the above individual drugs, ketamine is of special interest, because it is used very widely throughout the world in both emergency and operating room settings, and also is being used with increasing frequency as a recreational drug, sometimes abused by pregnant mothers. Ketamine is viewed as a safe drug, especially for children; in fact, it has been used in recent years with increased frequency in pediatric medicine, because of the observation that children are less susceptible to the psychotomimetic effects that ketamine causes in human adults (Reich & Silvay, 1989; Gilman et al., 1990). Ketamine is popular because it is easy to administer for either sedative or anesthetic purposes, and has a low incidence of cardiorespiratory depression. It is used to induce anesthesia in both obstetric and pediatric medicine, and very frequently is used to sedate pediatric patients undergoing procedures such as angiography, central line catheterization, endoscopy, intubation and fracture reduction (Bergman, 1999; Green et al., 2001; Law et al., 2003). Owing to its bronchodilator property, ketamine is also recommended for treating status asthmaticus in children (Petrillo et al., 2001), and in many countries, especially in remote areas where anesthesiologists are in short supply, ketamine is viewed as an agent of choice for general anesthesia (Pun et al., 2003).
Another anesthetic drug, midazolam, is of special interest because of its widespread use in pediatric medicine and because it is commonly used in combination with ketamine for either sedative or anesthetic purposes. Since midazolam is a GABAmimetic drug and ketamine an NMDA antagonist, the combination exposes a pediatric patient to the same dual mechanism by which ethanol damages the human fetal brain (Ikonomidou et al., 2000).
In a prior study, we demonstrated that ketamine, when administered subcutaneously to infant rats in a series of injections spaced evenly apart over a 9-h period, caused a robust pattern of apoptotic neurodegeneration (Ikonomidou et al., 1999). We did not study the effects of a single ketamine injection. Hayashi et al. (2002) recently confirmed our findings with respect to multiple ketamine injections, but reported that a single injection of ketamine to 7-day-old rats at doses from 25 to 75 mg kg−1 did not trigger neuroapoptosis. Midazolam has not been studied for its ability, either alone or in combination with ketamine, to induce neuroapoptosis in the developing brain. To further clarify the brain damaging potential of ketamine and midazolam, we exposed infant mice to clinically relevant doses of these drugs, either individually or in combination, and evaluated the brains for evidence of acute neuroapoptosis 5 h following drug exposure.
Methods
Animal treatment and handling
All animal care procedures were in accordance with standards approved by the Animal Studies Committees of Washington University and University of Virginia. C57BL6 mice (7 days old) were used for all experimental procedures, in that mice at this age are at peak sensitivity to the neuroapoptogenic action of NMDA antagonist or GABAmimetic drugs. The study was conducted in three parts. Initially we performed a dose–response study to determine the lowest dose of ketamine effective in causing an increased number of neurons showing C3A compared to saline-treated controls. This study revealed that a single subanesthetic dose of ketamine was sufficient to trigger a significantly increased C3A response. We then administered midazolam in a single clinically relevant subanesthetic dose and determined that this also resulted in a significantly increased C3A response. Finally, we combined this dose of midazolam with a subanesthetic dose of ketamine to assess the potential of these two anesthetic drugs to trigger an exaggerated response when used in combination.
In previous studies we have observed that ethanol triggers neuroapoptosis in some brain regions earlier than in others. For example, in the caudate nucleus and certain regions of the cerebral cortex, neurons begin showing a robust display of C3A as early as 3–4 h following subcutaneous treatment, and the reaction reaches a peak in these brain regions at 5–6 h post-treatment. Therefore, we elected to expose animals subcutaneously to drugs (ketamine, midazolam or ketamine+midazolam) at time zero and kill them 5 h later for quantitative evaluation of the neuroapoptosis response in the caudate nucleus and cerebral cortex.
In early studies, we observed that male and female mouse pups were equally sensitive to injury induced by ketamine, but there was substantial litter variability in the number of neurons showing C3A, either on a spontaneous basis or in response to drug treatment. To control for litter variability we divided the litters as evenly as possible into groups of equal size, one group receiving saline and the other groups an experimental drug treatment. In most cases, we used only one or two pups for each treatment condition from each litter, and the rest of the pups were used for other experiments. We used animals of both sexes and, to the extent possible, assigned an equal number of male and female animals to each treatment condition. This resulted in mean body weights that differed very little across treatment groups (ranging from 3.31±0.11 to 3.44±0.10 g in ketamine dose–response study and from 3.16±0.28 to 3.17±0.23 g in the ketamine plus midazolam study). At the beginning of each experiment, the pups were determined to be well nourished, as was evidenced by their stomachs being full of milk (detectable through the transparent abdominal wall). Both control and experimental pups after treatment were maintained in a warm chamber (30°C) away from their mother for a 5-h observation period, then were deeply anesthetized and perfused transcardially with 4% paraformaldehyde in Tris buffer (for C3A immunohistochemistry and silver stain) or 1% paraformaldehyde and 1.5% glutaraldehyde in phosphate buffer (for electron microscopy). For C3A immunohistochemistry or silver staining, the brains were cut into 50-μm-thick sagittal sections on a Vibratome and further processed by methods described below. For electron microscopy, the brains were cut into 1 mm thick serial slabs, postfixed in 1% osmium tetroxide, dehydrated in graded concentrations of ethanol, cleared in toluene and embedded flat in araldite.
Caspase-3 immunohistochemistry
C3A immunohistochemistry was performed by methods recently described (Olney et al., 2002b). Briefly, vibratome sections (50 μm thick) were washed in 0.01 M PBS, quenched for 10 min in a solution of methanol containing 3% hydrogen peroxide, then incubated for 1 h in blocking solution (2% BSA/0.2% milk/0.1% Triton X-100 in PBS), followed by incubation overnight in rabbit antiactive caspase-3 antiserum (D175, Cell Signaling Technology, Beverly, MA, U.S.A.) diluted 1 : 1500 in blocking solution. Following incubation with D175 primary antibody, the sections were incubated for 1 h in secondary antibody (goat antirabbit 1 : 200 in blocking solution), and then reacted in the dark with ABC reagents (standard Vectastain ABC Elite Kit, Vector Labs, Burlingame, CA, U.S.A.) for 1 h. The sections were then washed three times with PBS, and incubated with VIP reagent (Vector VIP substrate kit for peroxidase, Vector Labs, Burlingame, CA, U.S.A.) to develop a purple color.
De Olmos cupric silver staining
We have found that the De Olmos cupric silver staining method, while not useful for distinguishing between apoptotic and nonapoptotic cell death processes, is an excellent method for marking and mapping dead or dying neurons. For this procedure we used a modified version (Corso et al., 1997) of the method originally described by De Olmos & Ingram (1972).
Combined light and electronmicroscopy
Tissue slabs flat embedded in araldite, as above described, were cut 1 μm thick from the caudate nucleus and frontal cortex, using glass knives (1/2 inch wide) and an MT-2B Sorval ultratome. These sections were heat dried on glass slides and stained with azure II and methylene blue for evaluation by light microscopy. For electron microscopy, areas of special interest from a given block were trimmed to a smaller size; ultrathin sections were cut and suspended over a formvar coated slot grid, stained with uranyl acetate and lead citrate and viewed in a JEOL 100C transmission electron microscope. Slot grids were used because they permit a continuous viewing field (1 × 2 mm2) uninterrupted by grid mesh bars.
Quantitative cell counts
The brains were bisected sagittally, sectioned serially in the sagittal plane and stained immunohistochemically for C3A. For quantitative counts, every eighth section was chosen in an unbiased, systematically random sampling manner according to the principle of stereology. This permitted sampling eight to 10 sections from half of each brain. These sections were imaged and quantitatively evaluated with the help of a stereology system consisting of the following components: Stereo Investigator (MicroBrightField, Inc., Colchester, VT, U.S.A.) on a Pentium III PC, connected to a Prior Optiscan motorized stage (ES103 XYZ system, Prior Scientific Inc., Rockland, MA, U.S.A.) mounted on a Nikon Labophot-2 microscope. The boundaries of the brain regions of interest (cerebral cortex and caudate-putamen) were traced into the PC and from the tracings Stereo Investigator calculated the area in each section. In the cortex, the majority of C3A-positive profiles were in layer II, but counts were performed across the entire extent of the cerebral cortex. In the caudate-putamen, C3A-positive profiles were present in scattered or loosely clustered distribution throughout both the dorsal and ventral portions. Caspase-3 positive neurons with dendrites or axons visible were all counted. For those profiles without obvious dendritic processes, only those with a size larger than 8 μm were counted. The population estimator function of Stereo Investigator was used to mark each profile while it was counted to ensure that no profile would be missed or counted twice. To obtain an estimate of profile density (apoptotic profiles per mm2), the total number of C3A-positive profiles counted in each brain was divided by the total area within which these profiles were present. The counts were performed by an experienced histopathologist (CY), who was blind to the treatment condition.
Blood gas measurements
In a separate study, designed to rule out hypoxia/ischemia (H/I) as an explanation for the neurodegenerative response, arterial blood was collected at various time points (15, 30, 60, 120, 180 and 240 min) after ketamine at 40 mg kg−1 s. The blood sample was obtained by transcardiac aspiration from the left ventricle with a heparinized 32-gauge hypodermic needle. Bicarbonate concentration (millimoles per liter), oxygen saturation (SaO2%), pH, PaCO2 (partial pressure of carbon dioxide in mmHg) and PaO2 (partial pressure of oxygen in mmHg) were measured immediately after blood collection, using a Nova Biomedical blood gas apparatus. Seven to nine animals were used for each time point.
Drugs
Ketamine hydrochloride (Vetalar III) was obtained from Fort Dodge Animal Health (Fort Dodge, Iowa, U.S.A.). Midazolam (maleate salt) was obtained from Sigma (Saint Louis, MO, U.S.A.).
Statistical methods
All data are shown as mean±s.e.m. For statistical analysis, we used InStat 3.0 (GraphPad Software Inc., San Diego, CA, U.S.A.) running on a Macintosh PowerBook G4 with Mac OS X system. Unpaired t-test with Welch correction, one-way ANOVA or repeated measures ANOVA with Student–Newman–Keuls multiple comparisons test were used where appropriate to evaluate the effects of the various treatments.
Results
Behavior after drug treatments
Ketamine, at the highest dose administered (40 mg kg−1), caused all infant mice to lie on their back with constant paddling motions of their extremities, but did not immobilize or fully anesthetize them. There were no signs of cardiorespiratory impairment or skin discoloration. When their tails were pinched, they squeaked and pivoted, indicating preservation of at least some pain sensation. They manifested paddling movements of their paws for about 40–60 min, at which time the drug effects were beginning to wear off. At lower doses, ketamine had similar, but less prolonged and less pronounced, effects. Midazolam, at 9 mg kg−1 caused all pups to become somnolent, but without loss of righting reflex and they remained responsive to pain (tail pinch). This is consistent with the observations of Inada et al. (2004), who found that young mice do not lose the righting reflex at doses of Midazolam up to 25 mg kg−1 i.p. Paw paddling behavior was not seen following treatment with midazolam alone. Following ketamine (40 mg kg−1) together with midazolam (9 mg kg−1), the pups rapidly fell asleep and remained heavily sedated for approximately 2 h. Upon awakening, some pups exhibited paw paddling behavior prior to resuming normal ambulatory movements.
Arterial oxygen saturation after ketamine
Blood gas values measured at periodic intervals following administration of ketamine at 40 mg kg−1 s (Table 1) revealed no deviations indicative of hypoxia or ischemia. Specifically, PaCO2 values remained low (no hypoventilation), PaO2 and SaO2 remained normal (no hypoxia) and the relatively high pH/low bicarbonate values rule out hypoperfusion, hence no ischemia.
Table 1.
Blood gas profiles after a single injection of ketamine
| Time (min) | pH | PaCO2 (mmHg) | PaO2 (mmHg) | HCO3− (mEq l−1) | SaO2 (%) |
|---|---|---|---|---|---|
| 0 (n=7) | 7.64±0.01 | 18±2.0 | 145±15 | 20±2.0 | 99±0.7 |
| 15 (n=7) | 7.57±0.04 | 17±1.0 | 107±20 | 15±2.0 | 97±2.0 |
| 30 (n=9) | 7.61±0.03 | 17±2.0 | 111±14 | 17±1.3 | 98±1.0 |
| 60 (n=6) | 7.56±0.05 | 15±2.0 | 106±20 | 13±1.5 | 97±1.4 |
| 120 (n=6) | 7.58±0.04 | 22±4.0 | 104±15 | 21±1.7 | 98±1.0 |
| 180 (n=6) | 7.64±0.04 | 13±1.5 | 97±10 | 14±1.3 | 99±0.6 |
| 240 (n=6) | 7.66±0.04 | 15±1.6 | 141±11 | 17±1.9 | 99±0.2 |
Neuroapoptotic response to ketamine
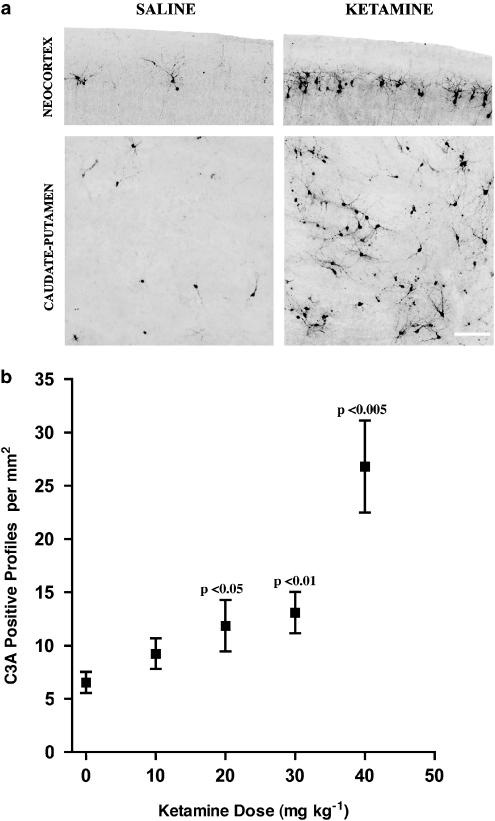
Histological evaluation of the caudate/putamen and cerebral cortex of pups treated with ketamine at 40 mg kg−1 revealed a visually impressive and statistically significant increase in neuroapoptotic (C3A-positive) profiles in both brain regions of the ketamine pups compared to the saline controls (Figure 1a and Table 2). Dose–response data were then generated from 10 litters of pups pertaining to the caudate/putamen alone and this showed that ketamine, in a dose-dependent manner, caused an increase in neuroapoptosis, which was statistically significant at 20 and 30 mg kg−1, but marginally nonsignificant at 10 mg kg−1 (Figure 1b). The dose–response curve describing these data was linear with a gradually increasing slope in the 10–30 mg kg−1 dose range, but the slope became much steeper in the transition between 30 and 40 mg kg−1, suggesting that in immature mice ketamine may become rapidly more toxic as the dose is increased from a range that produces sedation to doses approaching the anesthetic range.
Figure 1.
(a) Neuronal profiles showing caspase-3 activation (C3A) in the neocortex (layer II) and caudate-putamen of the 7-day-old mouse brain 5 h after a single subcutaneous injection of saline or ketamine, 40 mg kg−1. In the neocortex and caudate-putamen of the saline control pup, there are a few scattered C3A-positive neuronal profiles, reflecting the normal rate of physiological cell death at this developmental age. In the same brain regions of the ketamine-treated pup, the number of C3A-positive profiles is several-fold increased, consistent with the quantitative data presented in Table 2. Extreme sensitivity of layer II neocortical neurons, as shown here for ketamine, has been reported previously (Ikonomidou et al., 1999; 2000) as a feature of the apoptogenic properties of other NMDA antagonists, including MK-801, phencyclidine and ethanol. Scale bar=50 μm. (b) The apoptosis-promoting effect of ketamine is dose-dependent. At 5 h after littermates were treated with saline or ketamine, 10, 20, or 30 mg kg−1, the C3A positive profiles in the caudate-putamen region showed a linear dose-dependent increase (test for linear trend: P=0.0004, r2=0.1288). Both ketamine 20 mg kg−1 (P< 0.05) and 30 mg kg−1 (P<0.01) induced a significant increase in C3A positive profiles compared to their litter-matched controls (n=10 for each treatment group, P=0.0038, F (3, 9, 27)=5.671, repeated measures ANOVA with Student–Newman–Keuls multiple comparisons test, comparing saline,with ketamine 10, 20, and 30 mg kg−1). When the data for Ketamine 40 mg kg−1 (from Table 2) are displayed on the same graph, it is seen that, whereas the rate of apoptosis increased approximately 23% with each stepwise increase in dose in the zero to 30 mg kg−1 dose range, the rate of increase jumped by 100% as the dose was increased from 30 to 40 mg kg−1.
Table 2.
Activated caspase-3 profiles after one-time exposure to ketamine (40 mg kg−1)
| Region | Treatment | Section area examined (mm2) | Profile count | Profile density (mm−2) |
|---|---|---|---|---|
| Caudate-putamen | Saline | 20.14±1.88 | 132.6±13.9 | 7.10±0.93 |
| Ketamine | 18.56±1.36 | 466.0±69.2a | 26.80±4.33a | |
| Cerebral cortex | Saline | 100.71±6.81 | 267.0±38.6 | 2.66±0.31 |
| Ketamine | 92.41±4.71 | 787.0±68.4b | 8.63±0.74b |
P< 0.005.
P< 0.0001 compared to saline-treated controls, unpaired t-test with Welch correction, n=10 in each group.
Neuroapoptotic response to midazolam
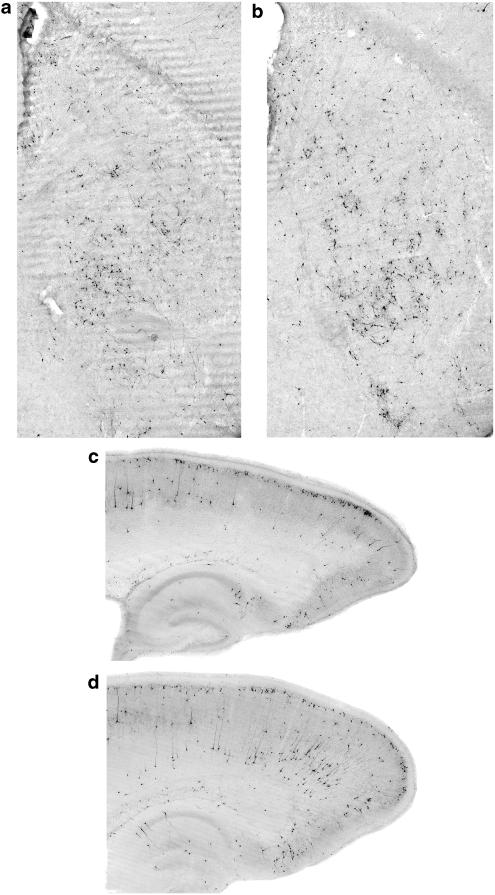
Midazolam at 9 mg kg−1 induced a neuroapoptotic response in both the cortex and caudate/putamen (Figure 2) that was not obviously different from the response to ketamine, although the pattern of C3A staining induced by midazolam tended to include a larger number of neurons distributed in the deep layers of the cortex. The apoptotic response to midazolam was statistically significant in both the caudate/putamen (24.15±5.77 profiles mm−2 vs 7.53±1.85 profiles mm−2, n=12, P< 0.01, unpaired t-test with Welch correction) and cerebral cortex (9.57±2.08 profiles mm−2 vs 2.96±0.50 profiles mm−2, n=12, P<0.01, unpaired t-test with Welch correction) and was roughly comparable in magnitude to the response to ketamine at 40 mg kg−1 (Figure 3).
Figure 2.
Brains of 7-day-old mice treated with midazolam 9 mg kg−1 (a, c) or midazolam plus ketamine 40 mg kg−1 (b, d) showed robust C3A staining in cerebral cortex (c, d) and caudate-putamen region (a, b) 5 h after treatment. The combined treatment of midazolam plus ketamine was more damaging in both regions than midazolam alone.
Figure 3.
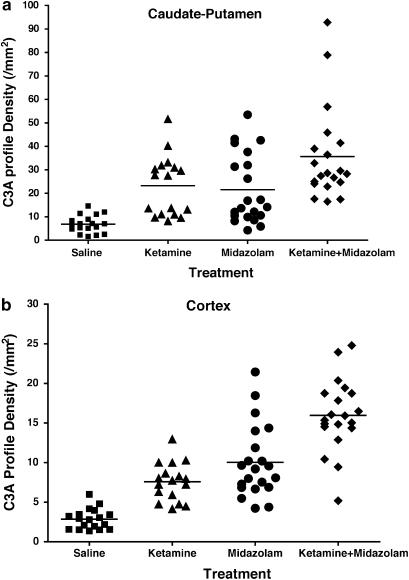
Quantitative analysis of C3A profile density revealed that combined midazolam and ketamine treatment was more effective in causing neuronal apoptosis than either drug alone. A total of 12 litters of pups were used for this analysis. (a) In caudate-putamen, ketamine plus midazolam produced a C3A profile density of 35.7±4.5 profile mm−2 (n=20), significantly higher than that of the ketamine group (23.2±3.1 profile mm−2, n=17, P<0.05) or midazolam group (21.6±3.2 profile mm−2, n=21, P<0.05). One-way ANOVA with Student–Newman–Keuls test, P=0.0162, F (2, 55)=4.447. (b) In the cerebral cortex, the C3A profile density for the ketamine plus midazolam group was 16.0±1.0 profile mm−2 (n=20), significantly higher than that of the ketamine group (7.6±0.6 profile mm−2, n=17, P<0.001) or midazolam group (10.0±4.6 profile mm−2, n=21, P<0.001). One-way ANOVA with Student–Newman–Keuls test, P<0.0001, F (2, 55)=21.209.
Neuroapoptotic response to ketamine plus midazolam
Combined administration of ketamine (40 mg kg−1) plus midazolam (9 mg kg−1) triggered a significantly greater increase in neuroapoptosis than either individual drug at these doses (Figures 2 and 3). The increase was of a magnitude suggestive of an additive mechanism.
Histological characteristics of the cell death response
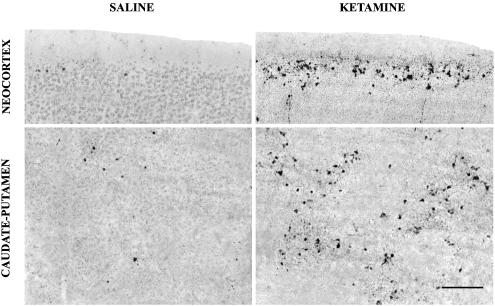
In a separate group of mouse pups, we used the De Olmos silver stain as a second method to document the pattern of neuronal cell death following ketamine administration and to compare this pattern with the pattern of C3A. The animals were killed at 7 h following ketamine administration (40 mg kg−1 s) because it requires a longer time interval for degenerating neurons to become silver positive than to show evidence of caspase 3 activation. We found at 7 h post-treatment that the silver stain revealed the same pattern of neurodegeneration that the caspase-3 stain had shown at 5 h post-treatment, indicating that a specific pattern of neuronal degeneration occurs following ketamine administration, regardless of the staining method used to detect the degenerative response (Figure 4).
Figure 4.
Sections stained by the De Olmos silver method from the neocortex (layer II) and caudate-putamen of the 7-day-old mouse brain 7 h after a single subcutaneous injection of saline or ketamine, 40 mg kg−1. The silver stain, which marks neurons that are dead or dying, reveals the same pattern of neurodegeneration that was observed in C3A-stained sections in Figure 1. At 7 h post-treatment, the affected neurons are in a more advanced stage of degeneration and, therefore, have a more condensed and fragmented appearance. Scale bar=75 μm.
The brains of an additional group of mouse pups treated with ketamine (40 mg kg−1) were evaluated by electron microscopy 5 h post-treatment. Degenerating neurons in both the cortex and caudate-putamen typically displayed ultrastructural changes (Figure 5) meeting classical criteria for neuroapoptosis (Wyllie et al., 1980; Ikonomidou et al., 1989; Dikranian et al., 2001).
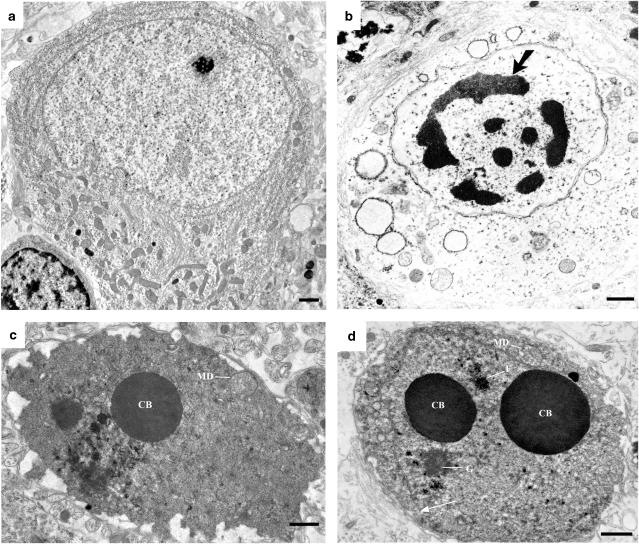
Figure 5.
Ultrastructural appearance of a normal neocortical neuron (a), a neocortical neuron undergoing hypoxia/ischemia-induced acute excitotoxic degeneration (b) and neurons in the neocortex (c) or caudate/putamen (d) undergoing acute apoptotic degeneration 5 h following exposure to ketamine. All photos are from the 7-day-old mouse brain. The earliest changes in excitotoxic neurodegeneration (b) are swelling and disintegration of cytoplasmic constituents, primarily mitochondria and endoplasmic reticulum. This is followed by formation of multiple small clumps of nuclear chromatin that distribute around the perimeter of the nucleus in a clockface configuration. These clumps then coalesce into larger irregularly shaped chromatin masses (large arrow) that migrate to the center of the nucleus, causing the nucleus to appear pyknotic. In contrast to hypoxic/ischemic excitotoxic changes, ketamine exposure causes developing neurons to display a sequence of classical changes characteristic of apoptosis. The earliest changes associated with apoptosis are relatively inconspicuous mitochondrial defects (MD) in the cytoplasm and very conspicuous formation of large spherical chromatin balls (CB) in the nucleus. These early changes are followed by fragmentation or disappearance of the nuclear membrane (thin arrow), disaggregation of the nucleolus into granular (G) and filamentous (F) components and condensation of the entire cell. The changes associated with hypoxic/ischemic neurodegeneration and apoptotic neurodegeneration are different both in nature and in sequence. Scale bar=1 μm.
Discussion
Here we demonstrate that a single subcutaneous injection of ketamine (20–40 mg kg−1) or midazolam (9 mg kg−1) triggers apoptotic neurodegeneration in the caudate nucleus and cerebral cortex of 7-day-old mice and that the two drugs together cause a more severe neuroapoptotic response than either drug alone. Our primary method for detecting the apoptotic response was C3A immunohistochemistry, a method that has proven reliable for selectively staining neurons that are undergoing apoptotic degeneration following administration of various neuroapoptosis-promoting drugs (NMDA antagonists, GABAmimetics, sodium channel blockers, ethanol) to immature rodents. In prior studies (Dikranian et al., 2001; Olney et al., 2002a), we have evaluated by both light and electron microscopy this neurodegenerative process in every stage of its evolution and demonstrated that the affected neurons, after becoming C3A-positive, progress to an advanced stage of cell death and degradation over a period of several hours. In the present study, we also determined that the degenerating neurons were selectively stained by the De Olmos silver method, which distinguishes dying or dead neurons from normal healthy neurons. Moreover, finally, we confirmed by electron microscopy that the affected neurons were dying and that the cell death process was apoptotic in nature.
In experiments with animals as small as infant rodents, it is difficult to exercise precise control over cardiorespiratory function, and this automatically raises a question whether hypoxic/ischemic mechanisms may have contributed to brain changes observed. To provide objective evidence ruling out H/I, we analyzed blood gases on arterial blood samples obtained by cardiac puncture at periodic intervals following ketamine administration. We did not detect any signs of hypoxia, hypoventilation or metabolic acidosis, and oxygen saturation remained within normal limits throughout the ketamine post-treatment period. An additional compelling reason for not attributing ketamine or midazolam-induced cell death to H/I is that this cell death response occurs very acutely (within 5 h) and meets all classical criteria for apoptosis (Wyllie et al., 1980; Dikranian et al., 2001), whereas when one exposes an infant rodent to H/I, the acute early cell death response (within 4–6 h) is excitotoxic and not apoptotic (Ikonomidou et al., 1989; Ishimaru et al., 1999; Young et al., 2004). Ultrastructural analysis is the most reliable method for distinguishing excitotoxic from apoptotic neurodegeneration, and by ultrastructural analysis the acute cell death response to anesthetic drugs is apoptotic and not excitotoxic, whereas the acute cell death response to H/I is excitotoxic and not apoptotic (Figure 5; also see Ikonomidou et al., 1989; Ishimaru et al., 1999; Dikranian et al., 2001; Young et al., 2004). In addition, anesthesia-induced cell death is heralded by a robust C3A response (Olney et al., 2002b), and the acute cell death response to H/I is not accompanied by C3A (Price et al., 2001; Young et al., 2004).
There is an apparent contradiction between our ketamine findings and those of Hayashi et al. (2002), who reported that repeated treatment of infant rats with ketamine triggered neuroapoptosis, but a single treatment at doses from 25 to 75 mg kg−1 did not. While it may be relevant that our present findings are in mice and theirs were in rats, we suspect that the discrepancy is more likely due to differences in methodology than to a species effect. To detect increases in neuroapoptosis, Hayashi et al. applied the De Olmos silver staining method 24 h following ketamine treatment. While this is an appropriate method for mapping patterns of neurodegeneration (argyrophylia) resulting from prolonged or very high drug exposure, it is not reliable for detecting or quantifying subtle increases in neuropoptosis that occur at approximately 4–5 h following low-dose treatments. Neurons that die within this early period are degraded rapidly into particulate debris. The debris particles remain visible for many hours and contribute to argyrophylia of the region, but at 24 h they are not recognizable or countable as dying neurons. In the present study, we demonstrated that C3A staining at 5 h or silver staining at 7 h documents the early neurodegenerative response, but at 24 h, the early-responding neurons would not be detectable by either method.
Since ketamine has a very short half-life, only the most sensitive neurons – those that are prone to die after only a brief apoptogenic stimulus –– are likely to be affected by a single subanesthetic dose of ketamine. Although the present study was not designed to address this issue, it is possible that the combination of ketamine plus midazolam might prolong the time interval during which neuronal activity is suppressed, and thereby cause additional neurons to be killed, neurons that would not have been killed by ketamine alone. For example, we know that a high dose of ethanol causes caudate neurons to die within 3–4 h, but thalamic neurons do not begin to die until about 8 h post-treatment. A likely interpretation would be that thalamic neurons are less sensitive and do not succumb unless neuronal activity is suppressed for a longer period of time. To study this it would be necessary to examine the brain at both early and late post-treatment intervals following both brief and prolonged exposure.
Are doses of ketamine and midazolam used in the present experiments relevant to those used in human anesthesia? Doses of ketamine administered to children are in the range of 2–3 mg kg−1 i.m. for sedation, and 5–10 mg kg−1 i.m. for induction of anesthesia. However, there is an important difference between children and neonates. Ketamine is often used in neonates to prevent gross movements, and the dose required to achieve this purpose is four times greater in infants under 6 months of age than in children 6 years of age (Motoyama & Davis, 1996). Thus, it is not uncommon for neonates to be exposed to a relatively high dose of ketamine. The ED50 dose of ketamine required to induce anesthesia in mice is >80 mg kg−1 s.c. (Green et al., 1981), which is 8–16 times higher than the anesthesia-inducing/immobilizing dose for human infants. The highest dose used in our experiments (40 mg kg−1 s.c.) would be the equivalent of a 2.5–5 mg kg−1 i.m. dose for a human neonate (40 divided by 16 or 8). This is a dose range that does not immobilize human infants, just as 40 mg kg−1 did not immobilize our infant mice. The usual dose of midazolam for pediatric sedation is 0.1–0.3 mg kg−1, i.m.; however, its ED50 for causing mice to lose the righting reflex is in the range of 40 mg kg−1 i.v. (Ben-Shlomo et al., 2001; Inada et al., 2004). Thus, the doses (ketamine, 20–40 mg kg−1 s.c. and midazolam 9 mg kg−1 s.c.) shown in the present study to induce significant neuroapoptosis, are in the sedation (subanesthetic) range for an infant mouse, and would be equivalent to a sedating/subanesthetic dose for an infant human.
There is an accepted practice among anesthesiologists that when ketamine is administered for anesthetic purposes, it should be accompanied by a benzodiazepine, such as diazepam or midazolam. The basis for this practice is the clinical observation, four decades ago, that the incidence and severity of psychotic reactions induced by ketamine in adult humans could be substantially reduced by co-administration of a benzodiazepine (Reich & Silvay, 1989). Recently, there has been a growing trend to use ketamine much more frequently in pediatric than adult medicine because ketamine-induced psychosis is a phenomenon that rarely, if ever, occurs in children. The practice of administering a benzodiazepine adjunctively with ketamine has been carried over from the adult to pediatric clinical setting, despite the absence of a rationale for using this combination in pediatric patients. The finding that both ketamine and midazolam trigger neuroapoptosis in the developing mouse brain, and that exposure of the developing brain to the two drugs in combination results in additive neurotoxicity, suggests the need for a risk/benefit reevaluation.
It is generally recognized that rodent data provide an imprecise basis at best, and an irrelevant basis at worst, for evaluating human risk. For a more adequate evaluation of human risk additional research, preferably in a non-human primate species, will be needed. Steps should be taken to resolve this issue as soon as possible, in that many immature humans are being exposed throughout the world to ketamine alone or in combination with GABAmimetic anesthetics (Acworth et al., 2001).
Acknowledgments
This work was supported in part by NIA Grant AG 11355, NIDA Grant DA 05072, NICHD Grant HD 37100 (Merit award to JWO), NARSAD 2000 Toulmin Distinguished Investigator Award (JWO) and NIDA Scientist Development Award for Clinicians DA00406 (VJT). VJT is an Established Investigator of the American Heart Association (GF 10909).
Abbreviations
- C3A
caspase-3 activation
- FAS
fetal alcohol syndrome
- H/I
hypoxia/ischemia
References
- ACWORTH J.P., PURDIE D., CLARK R.C. Intravenous ketamine plus midazolam is superior to intranasal midazolam for emergency paediatric procedural sedation. Emerg. Med. J. 2001;18:39–45. doi: 10.1136/emj.18.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEN-SHLOMO I., ROSENBAUM A., HADASH O., KATZ Y. Intravenous midazolam significantly enhances the lethal effect of thiopental but not that of ketamine in mice. Pharmacol. Res. 2001;44:509–512. doi: 10.1006/phrs.2001.0900. [DOI] [PubMed] [Google Scholar]
- BERGMAN S.A. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth. Prog. 1999;46:10–20. [PMC free article] [PubMed] [Google Scholar]
- BITTIGAU P., SIFRINGER M., GENZ K., REITH E., POSPISCHIL D., GOVINDARAJALU S., DZIETKO M., PESDITSCHEK S., MAI I., DIKRANIAN K., OLNEY J.W., IKONOMIDOU C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARREN S.K., ALVORD A.C., SUMI S.M., STREISSGUTH A.P., SMITH D.W. Brain malformations related to prenatal exposure to ethanol. J. Pediatr. 1978;92:64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- CORSO T.D., SESMA M.A., TENKOVA T.I., DER T.C., WOZNIAK D.F., FARBER N.B., OLNEY J.W. Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Res. 1997;752:1–14. doi: 10.1016/s0006-8993(96)01347-9. [DOI] [PubMed] [Google Scholar]
- DE OLMOS J.S., INGRAM W.R. The projection field of the stria terminalis in the rat brain. An experimental study. J. Comp. Neurol. 1972;146:303–334. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- DIKRANIAN K., ISHIMARU M.J., TENKOVA T., LABRUYERE J., QIN Y.Q., IKONOMIDOU C., OLNEY J.W. Apoptosis in the in vivo mammalian forebrain. Neurobiol. Dis. 2001;8:359–379. doi: 10.1006/nbdi.2001.0411. [DOI] [PubMed] [Google Scholar]
- DOBBING J., SANDS J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G., RALL T., NIES A., TAYLOR P. The Pharmacological Basis of Therapeutics. New York: Pergamon Press, Inc; 1990. [Google Scholar]
- GREEN C.J., KNIGHT J., PRECIOUS S., SIMPKIN S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab. Anim. 1981;15:163–170. doi: 10.1258/002367781780959107. [DOI] [PubMed] [Google Scholar]
- GREEN S.M., DENMARK T.K., CLINE J., ROGHAIR C., ABD ALLAH S., ROTHROCK S.G. Ketamine sedation for pediatric critical care procedures. Pediatr. Emerg. Care. 2001;17:244–248. doi: 10.1097/00006565-200108000-00004. [DOI] [PubMed] [Google Scholar]
- HAYASHI H., DIKKES P., SORIANO S.G. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr. Anaesth. 2002;12:770–774. doi: 10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C., BITTIGAU P., ISHIMARU M.J., WOZNIAK D.F., KOCH C., GENZ K., PRICE M.T., STEFOVSKA V., HORSTER F., TENKOVA T., DIKRANIAN K., OLNEY J.W. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C., BOSCH F., MIKSA M., BITTIGAU P., VOCKLER J., DIKRANIAN K., TENKOVA T.I., STEFOVSKA V., TURSKI L., OLNEY J.W. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- IKONOMIDOU C., PRICE M.T., MOSINGER J.L., FRIERDICH G., LABRUYERE J., SALLES K.S., OLNEY J.W. Hypobaric–ischemic conditions produce glutamate-like cytopathology in infant rat brain. J. Neurosci. 1989;9:1693–1700. doi: 10.1523/JNEUROSCI.09-05-01693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INADA T., ASAI T., YAMADA M., SHINGU K. Propofol and midazolam inhibit gastric emptying and gastrointestinal transit in mice. Anesth. Analg. 2004;99:1102–1106. doi: 10.1213/01.ANE.0000130852.53082.D5. [DOI] [PubMed] [Google Scholar]
- ISHIMARU M.J., IKONOMIDOU C., TENKOVA T.I., DER T.C., DIKRANIAN K., SESMA M.A., OLNEY J.W. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J. Comp. Neurol. 1999;408:461–476. [PubMed] [Google Scholar]
- JEVTOVIC-TODOROVIC V., HARTMAN R.E., IZUMI Y., BENSHOFF N.D., DIKRANIAN K., ZORUMSKI C.F., OLNEY J.W., WOZNIAK D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES K.L., SMITH D.W., ULLELAND C.N., STREISSGUTH A.P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;I:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- JONES K.L., SMITH D.W. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;ii:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- LAW A.K., NG D.K., CHAN K.K. Use of intramuscular ketamine for endoscopy sedation in children. Pediatr. Int. 2003;45:180–185. doi: 10.1046/j.1442-200x.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- MOTOYAMA E.K., DAVIS P.J. Smith's Anesthesia for Infants and Children. St Louis: Mosby-Year Book, Inc; 1996. [Google Scholar]
- OLNEY J.W., TENKOVA T., DIKRANIAN K., QIN Y.Q., LABRUYERE J., IKONOMIDOU C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res. Dev. Brain Res. 2002a;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- OLNEY J.W., TENKOVA T., DIKRANIAN K., MUGLIA L.J., JERMAKOWICZ W.J., D'SA C., ROTH K.A. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol. Dis. 2002b;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- PETRILLO T.M., FORTENBERRY J.D., LINZER J.F., SIMON H.K. Emergency department use of ketamine in pediatric status asthmaticus. J. Asthma. 2001;38:657–664. doi: 10.1081/jas-100107543. [DOI] [PubMed] [Google Scholar]
- PRICE M.T., TENKOVA T., OLNEY J.W. Caspase-3 activation accompanies apoptotic but not excitotoxic neurodegeneration in the developing mouse retina and brain. Soc. Neurosci. Abs. 2001;27:450. [Google Scholar]
- PUN M.S., THAKUR J., POUDYAL G., GURUNG R., RANA S., TABIN G., GOOD W.V., RUIT S. Ketamine anaesthesia for paediatric ophthalmology surgery. Br. J. Ophthalmol. 2003;87:535–537. doi: 10.1136/bjo.87.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH D.L., SILVAY G. Ketamine: an update on the first twenty-five years of clinical experience. Can. J. Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- SWAYZE V.W., II, JOHNSON V.P., HANSON J.W., PIVEN J., SATO Y., ANDREASEN N.C. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- WYLLIE A.H., KERR J.F., CURRIE A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- YOUNG C., KLOCKE B.J., TENKOVA T., CHOI J., LABRUYERE J., QIN Y.Q., HOLTZMAN D.M., ROTH K.A., OLNEY J.W. Ethanol-induced neuronal apoptosis in vivo requires BAX in the developing mouse brain. Cell Death Differ. 2003;10:1148–1155. doi: 10.1038/sj.cdd.4401277. [DOI] [PubMed] [Google Scholar]
- YOUNG C., ROTH K.A., KLOCKE B.J., WEST T., HOLTZMAN D.M., LABRUYERE J., QIN Y., DIKRANIAN K., OLNEY J.W.Role of caspase-3 in ethanol-induced developmental neurodegeneration Neurobiol. Dis. 2005(in press) [DOI] [PubMed]
- YOUNG C., TENKOVA T., DIKRANIAN K., OLNEY J.W. Excitotoxic versus apoptotic mechanisms of neuronal cell death in perinatal hypoxia/ischemia. Curr. Mol. Med. 2004;4:77–85. doi: 10.2174/1566524043479158. [DOI] [PubMed] [Google Scholar]