Abstract
Protein tyrosine phosphatase 1B (PTP1B) acts as a physiological negative regulator of insulin signaling by dephosphorylating the activated insulin receptor (IR). Here we examine the role of PTP1B in the insulin-sensitizing action of rosiglitazone (RSG) in skeletal muscle and liver.
Fat-fed, streptozotocin-treated rats (10-week-old), an animal model of type II diabetes, and age-matched, nondiabetic controls were treated with RSG (10 μmol kg−1 day−1) for 2 weeks.
After RSG treatment, the diabetic rats showed a significant decrease in blood glucose and improved insulin sensitivity. Diabetic rats showed significantly increased levels and activities of PTP1B in the skeletal muscle (1.6- and 2-fold, respectively) and liver (1.7- and 1.8-fold, respectively), thus diminishing insulin signaling in the target tissues.
We found that the decreases in insulin-stimulated glucose uptake (55%), tyrosine phosphorylation of IRβ-subunits (48%), and IR substrate-1 (IRS-1) (39%) in muscles of diabetic rats were normalized after RSG treatment. These effects were associated with 34 and 30% decreases in increased PTP1B levels and activities, respectively, in skeletal muscles of diabetic rats. In contrast, RSG did not affect the increased PTP1B levels and activities or the already reduced insulin-stimulated glycogen synthesis and tyrosine phosphorylation of IRβ-subunits and IRS-2 in livers of diabetic rats.
RSG treatment in normal rats did not significantly change PTP1B activities and levels or protein levels of IRβ, IRS-1, and -2 in diabetic rats.
These data suggest that RSG enhances insulin activity in skeletal muscle of diabetic rats possibly by ameliorating abnormal levels and activities of PTP1B.
Keywords: Rosiglitazone, PTP1B, diabetes mellitus, type II, insulin resistance, insulin sensitivity, skeletal muscle, streptozotocin
Introduction
Resistance to the effects of insulin on glucose uptake and metabolism in skeletal muscle is a major contributor to the pathogenesis of insulin-resistant states such as obesity and type II diabetes (White & Yenush, 1998). Insulin initiates glucose uptake by binding to the insulin receptor (IR), which results in its autophosphorylation and the subsequent phosphorylation of intracellular substrates, including IR substrates (IRSs) such as IRS-1 and -2, phosphatidylinositol (PI) 3-kinase, and protein kinase B (PKB) (Quon et al., 1994; White & Kahn, 1994; Saltiel, 1996). Defective insulin signal transduction has recently been observed in skeletal muscle of type II diabetic subjects (Bjornholm et al., 1997; Kim et al., 1999).
A delicate balance is required between kinases, which transmit the signal to downstream targets, and phosphatases, which shut down signaling to prevent excessive or, in some cases, insufficient activation (Rebecca et al., 2003). Insulin resistance and diabetes are both states in which signaling pathways become deregulated, which leads to the reduction or absence of intracellular insulin activity (Steppan & Lazar, 2002). Overactivation of phosphatases is one way in which insulin signaling becomes blocked. Protein tyrosine phosphatase 1B (PTP1B), which dephosphorylates the activated IR and IRS-1, has been shown to play a major role in both insulin resistance and type II diabetes (Rondinone et al., 2002; Zinker et al., 2002; Gum et al., 2003). Recent studies showed that PTP1B knockout mice were more insulin-sensitive than normal mice and failed to gain weight despite eating a fat-rich diet (Elchebly et al., 1999; Klaman et al., 2000). In addition, a role for certain PTPs, including PTP1B, in the insulin resistance that is associated with diabetes and obesity has been suggested by clinical studies in which correlations between the levels of PTP1B expression in muscle and adipose tissue and insulin-resistant states were found (Kusari et al., 1994; Ahmad et al., 1997). Thus, PTP1B might be an attractive therapeutic target for type II diabetes and obesity.
The thiazolidinediones (TZDs) represent a class of insulin-sensitizing agents that are effective in the treatment of patients with type II diabetes (Aronoff et al., 2000; Phillips et al., 2001). The molecular targets of these compounds are thought to include the nuclear receptor peroxisome proliferator–activator receptor-γ (PPARγ), which regulates the expression of numerous genes involved in glucose and lipid metabolism (Kersten et al., 2000). Since PPARγ is most highly expressed in adipose tissue and TZDs have been shown to regulate gene expression in fat, adipocytes are speculated to be the primary site of TZD response. However, low levels of PPARγ receptor mRNA can be observed in many tissues (Kahn et al., 2000), and the concentration of PPARγ receptors in muscle, which quantitatively is the most important target tissue for insulin and plays a predominant role in TZD-induced improvement of glucose homeostasis (Baron et al., 1988; Inzucchi et al., 1998), is low (Vidal-Puig et al., 1997), thus raising a key question: Are the effects of TZDs mediated exclusively through direct action on adipocytes, with indirect effects in other tissues, or are other tissues also important targets of TZDs? Recently, Burant et al. (1997) suggested that the insulin-sensitizing action of TZDs may occur independently of adipose tissue. Some studies have even suggested that TZDs exert their effects through mechanisms that do not involve PPARγ (Olefsky, 2000; Brunmair et al., 2001). The metabolic responses to TZDs in several experimental setups are independent of PPARγ-induced gene transcription, as indicated by their rapid occurrence (Fujiwara et al., 1988; el-Kebbi et al., 1994; Furnsinn et al., 1999; 2000; Wang et al., 1999) and by a failure of the magnitude of such responses to reflect the PPARγ-activating efficacies of different TZDs (Furnsinn et al., 1999; Wang et al., 1999).
Evidence suggests that TZDs ameliorate insulin resistance and improve insulin-stimulated glucose disposal in skeletal muscle of type II diabetic subjects (Inzucchi et al., 1998), but their exact mechanism of action remains unclear. Furthermore, none of the previous studies examined the effect of rosiglitazone (RSG) on changes in the components of insulin signaling pathway in specific tissues (e.g., liver and skeletal muscle). Therefore, to determine the mechanism of the insulin-sensitizing action of TZDs, we examined the effects of RSG treatment in nondiabetic and high-fat-fed, streptozotocin (STZ)-induced diabetic rats on the activity and expression of PTP1B as well as insulin activity and signaling in skeletal muscle and liver, and combined these measurements with glucose uptake and glycogen synthesis measurements in these tissues.
Methods
Type II diabetic rat model and treatment protocol
The type II diabetic rat model system was developed as previously described (Reed et al., 2000), with some minor modifications. Male Sprague–Dawley rats (8-week-old) from the experimental animal center of the Wuhan University weighing approximately 200 g were used for all studies. All procedures were in accordance with the guidelines of the Institute's Ethical Committee for Experimental Use of Animals and the U.K. Animals (Scientific Procedures) Act, 1986. Rats were housed five per cage in a room with a 12/12-h light/dark cycle and an ambient temperature of 22–25°C. Animals were fed either a normal chow diet consisting of (as a percentage of total kcal) 12% fat, 60% carbohydrate, and 28% protein, or a high-fat diet (HFD) consisting of 41% fat, 41% carbohydrate, and 18% protein. After 2 weeks on either diet, after an overnight fast, animals (with the exception of noninjected controls) were anesthetized with ketamine (65 mg kg−1) and xylazine (7 mg kg−1) and injected into the tail vein via a temporary indwelling 24-gauge catheter with STZ (35 mg kg−1 body weight in 0.1 M citrate-buffered saline, pH 4.5). Animals had free access to food and water after the STZ injection, and both STZ-injected and noninjected animals were maintained on their originally assigned diets (chow or fat) for the duration of the study. The development of diabetes was confirmed by determining blood glucose concentrations 72 h after STZ administration. The control rats were fasted in an identical manner and had a volume of citrate-buffered saline, equal to that of the STZ solution, injected by the same route.
The control and diabetic groups were then further subdivided into treated and untreated groups: control (n=12), control treated with RSG (Control+RSG, n=12), type II diabetes (HFD/STZ, n=12), and type II diabetes treated with RSG (10 μmol kg−1 day−1, oral gavage) (HFD/STZ+RSG, n=12). Treatment was administered daily for 2 weeks. The control group received an equal volume of vehicle (saline). At the end of each week, individual body weights were recorded, and glucose and insulin concentrations were determined under fasting and nonfasting conditions. Glycemia was assessed from tail vein blood using a One Touch II Meter (LifeScan, Milpitas, CA, U.S.A.). Insulin was determined by radio-immunoassay (RIA) using a kit from Beifang Biotech Research Center (Beijing, China). Following the completion of insulin sensitivity measurements (described below), all animals were maintained on their previous diet and treatment for 5 days. Before autopsy, after a 10-h fast, saline- and RSG-treated animals received intraperitoneal insulin 5 U kg−1 in saline with 0.1% BSA or vehicle control (saline with 0.1% BSA). Tissue samples from the liver and muscle were removed at 10 min post-treatment from both vehicle- and insulin-treated animals and flash frozen in liquid nitrogen.
Insulin sensitivity
Peripheral insulin resistance was assessed with an insulin tolerance test (ITT) (Alford et al., 1971), which measures insulin sensitivity using KITT as an index of insulin-mediated glucose metabolism. Rats fasted for 15 h before insulin challenge. A neutral insulin solution was diluted with 0.9% saline to a final concentration of 2 U ml−1, and then administered as a 2-U kg−1 body weight dose by slow intravenous injection through a tail vein. Blood samples were collected at 0 min and then at 10, 20, 30, and 60 min following administration of insulin. Blood glucose concentrations were immediately measured with a blood glucose monitor. KITT represents the percent decline in blood glucose concentration per minute and is calculated according to the formula: KITT=(0.693/t1/2) × 100, where t1/2 represents the half-life of blood glucose decay (Bonora et al., 2000), which was obtained by plotting blood glucose concentrations vs time on semilogarithmic graph paper. Lower insulin-sensitivity index (KITT) scores correspond to higher degrees of insulin resistance.
Skeletal muscle incubation procedure
Incubation media were prepared from a stock solution of Krebs–Henseleit bicarbonate buffer (KHB) supplemented with 5 mM HEPES and 0.1% bovine serum albumin (RIA grade) and continuously gassed with 95% O2/5% CO2. Rats were anaesthetized and epitrochlearis muscles were isolated and preincubated at 30°C for 30 min in KHB containing 5 mM glucose and 15 mM mannitol in the absence or presence of insulin (12 nM). Muscles were subjected to 10 min of in vitro electrical stimulation, as described previously (Ryder et al., 2000), before the final 10-min incubation.
Measurement of glucose transport activity
Following the above incubations, muscles were blotted and transferred to flasks containing 1.5 ml KHB with 1 mM, 2-deoxy-[1,2-3H]glucose (1.5 mCi mmol−1) and 39 mM [1-14C]mannitol (8 μCi mmol−1) and the same additions as in the previous incubation. The flasks were incubated at 30°C for 20 min and were continuously gassed with 95% O2/5% CO2. After incubation, the muscles were frozen between tongs cooled to the temperature of liquid nitrogen, and were stored at −80°C until 2-deoxyglucose (2-DOG) transport was measured. Frozen muscles were dissolved in 0.5 ml 1 N KOH and were then neutralized with 0.5 ml 1 N HCl. The samples were mixed and aliquots of the supernatant were counted for radioactivity in a liquid scintillation counter.
Hepatocyte culture and glycogen synthesis
Hepatocytes were isolated from RSG-treated or -untreated rats by collagenase perfusion of the liver (Agius et al., 1996). They were then suspended in minimal essential medium containing 7% newborn calf serum and seeded in multi-well plates (Agius et al., 1996). For determination of glycogen synthesis, hepatocytes were incubated in minimal essential medium containing [U-14C]glucose (2 μCi ml−1) with or without insulin (10 nM) for 3 h. Incorporation of the 14C label into glycogen was determined by ethanol precipitation (Agius et al., 1990); rates of glycogen synthesis are expressed as nmol glucose incorporated per 3 h per mg cell protein.
Lysate preparation and protein assays
Samples of frozen liver tissue (50 mg) were sonicated in 1 ml lysis buffer (buffer A) containing 20 mM Tris-HCl (pH 7.4), 1% Triton X-100, 10% glycerol, 150 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 20 mM sodium fluoride, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 10 μg ml−1 leupeptin, 1 mM benzamidine, 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, and 1 mM microcystin, and agitated for 40 min at 4°C. Detergent-insoluble material was sedimented by centrifugation at 12,000 × g for 10 min at 4°C. Muscle samples (50 mg) were homogenized and centrifuged at 100,000 × g for 1 h in ice-cold 50 mM HEPES buffer (pH 7.4) (buffer B) containing 150 mM NaCl, 10 mM sodium pyrophosphate, 2 mM Na3VO4, 10 mM NaF, 2 mM EDTA, 2 mM PMSF, 5 μg ml−1 leupeptin, 1% NP-40, and 10% glycerol.
Supernatants were collected, and the protein concentration was then measured with the Bradford protein assay reagent (Bio-Rad), using BSA as a standard.
Western blotting
Aliquots (50 μg) of muscle or liver homogenate were subjected to SDS–PAGE (7.5% gel) and electrophoretically transferred to nitrocellulose (NC) filter membranes for 5 h. NC membranes were then blocked for 2 h at room temperature with the Block Solution provided in the ECL kit. This step was followed by overnight incubation at 4°C using an α-PTP1B polyclonal antibody or antiphosphotyrosine (PY99) as the primary antibodies, as described in the figure legends. The NC membranes were then washed for 30 min using Wash Solution (ECL kit), followed by a 1-h incubation with either anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase in Block Solution. The NC membranes were washed for 30 min in Wash Solution, and the immunoreactive bands were detected by enhanced chemiluminescence. Subsequently, blot membranes were stripped for 40 min at 60°C in stripping buffer (62.5 mM Tris-HCl (pH 6.7), 2% SDS and 100 mM β-mercaptoethanol) and reprobed for β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.).
Tyrosine phosphorylation of IRβ-subunit, IRS-1, and -2
Muscle or liver lysates (1 mg protein) were immunoprecipitated (IP) overnight at 4°C with 2 μg anti-IRβ, anti-IRS-1, or anti-IRS-2 coupled to protein A-Sepharose. The immune complex was washed three times in phosphate-buffered saline (PBS) (pH 7.4) containing 1% NP-40 and 2 mM Na3VO4, resuspended in Laemmli buffer, and boiled for 5 min. Proteins were resolved using SDS–PAGE (7.5% gel). After SDS–PAGE, electrotransfer of protein from the gel to NC membranes was performed by Western blotting. Subsequently, NC filters were incubated at 4°C overnight with PY99 at a 1 : 1000 dilution and the rest of the steps were performed as described above. Blots were stripped in stripping buffer at 50°C for 30 min and reprobed with antibodies against IR-β, IRS-1 or -2.
PTP1B protein levels and activity
The tissue homogenate was assayed in a microtiter plate at 27°C. The PTP1B assay kit was obtained from Upstate Biotechnology, Inc. The protocol outlined by the manufacturer was rigidly followed. The PTP1B protein levels were assessed by immunoblotting using polyclonal antibodies against PTP1B as described above.
Statistical analysis
All values are expressed as means±s.e. Statistical significance was determined using analysis of variance (ANOVA) followed by Tukey's test. P<0.05 was considered as significant.
Chemicals used
The reagents for SDS–PAGE and immunoblotting were obtained from Biovision (Palo Alto, CA, U.S.A.) and the apparatus was obtained from Bio-Rad (Richmond, CA, U.S.A.). Tris, NP-40, porcine insulin, and nitrocellulose (NC) membranes were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.). Protein A-Sepharose 6 MB was from Pharmacia (Upsala, Sweden). The monoclonal antiphosphotyrosine antibody (αPY, PY99) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, U.S.A.). Rabbit polyclonal anti-IR β-subunit (IRβ) antibody was purchased from Upstate Biotechnology Inc. (Lake Placid, NY, U.S.A.). The anti-rat carboxy-terminal IRS-1 antibody (Clone 8-63) was from NeoMarkers (Fremont, CA, U.S.A.). Anti-IRS-2 antibody and α-PTP1B polyclonal antibody were from Upstate Biotechnology Inc. Enhanced chemiluminescence (ECL) detection reagents were from KPL (Gaithersburg, MA, U.S.A.). All other chemicals were of the highest analytical grade.
Results
Characteristics of experimental animals
The glucose concentrations in both fasting and fed rats were significantly higher (P<0.05) in diabetic rats (HFD/STZ) than in normal control rats (Control) (Table 1). The concentrations of glucose in fasting and fed HFD/STZ rats were significantly reduced (P<0.05) after treatment with RSG at 10 μmol kg−1 day−1 for 2 weeks (HFD/STZ+RSG). It should be noted that insulin concentrations in HFD/STZ diabetic rats were similar to those in control rats. Treatment with RSG did not affect insulin concentrations in control or HFD/STZ rats. HFD/STZ diabetic rats weighed 21 g more than the normal chow-fed controls (P<0.05). There was no effect of RSG treatment on body weight in the control animals; in contrast, after 2 weeks of RSG treatment, body weight was significantly greater in treated HFD/STZ rats compared with control HFD/STZ rats (P<0.05). All data are expressed as means of values obtained from experiments performed in 12 rats.
Table 1.
Characteristics of the experimental animals
| Control | Control+RSG | HFD/STZ | HFD/STZ+RSG | |
|---|---|---|---|---|
| n | 12 | 12 | 12 | 12 |
| Blood glucose (fasting) (mM) | 6.8±0.2 | 6.2±0.2 | 18.2±1.4* | 9.3±0.4¶ |
| Blood glucose (fed) (mM) | 9.1±0.4 | 8.6±0.3 | 23.5±2.0* | 12.2±1.0¶ |
| Insulin (pM) | 164±14 | 161±13 | 177±20 | 167±15 |
| Body weight (g) | 200±2 | 206±2 | 221±3* | 239±4¶ |
| KITT (% min−1) | 9.5±1.3 | 9.6±1.2 | 5.9±0.6* | 8.2±0.9¶ |
Data are expressed as the mean±s.e.m., n indicates the number of animals studied. RSG: Rosiglitazone.
Significantly different from control group (P<0.05);
Signficantly different from HFD/STZ group (P<0.05).
Insulin sensitivity
In vivo insulin sensitivity was evaluated using the glucose disappearance constant (KITT) during an ITT. This is a simple, reasonably accurate, and rapid method for screening insulin resistance (Grulet et al., 1993), which indicates a net result of resistance to insulin action at target level, including receptor and post-receptor defects. After the HFD and STZ treatment, the KITT decreased significantly, from 9.5±1.3 to 5.9±0.6% min−1, thereby indicating a significant decrease in insulin sensitivity (P<0.05). Administration of RSG for 2 weeks significantly increased insulin sensitivity by 39% in HFD/STZ rats (P<0.05) (see Table 1).
Effects of RSG on glucose uptake in skeletal muscle from control and HFD/STZ rats
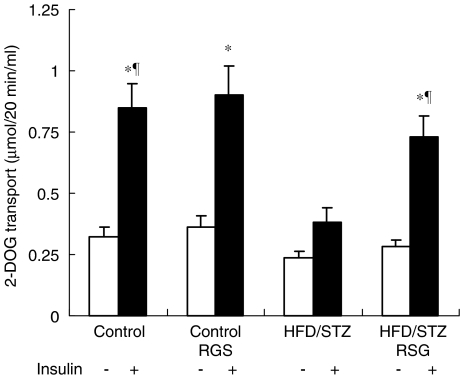
To gain insight into the mechanism of action of RSG, we measured 2-DOG uptake in skeletal muscle incubated in the presence or absence of 12 nM insulin. No significant differences in basal 2-DOG uptake in skeletal muscles were observed between the four experimental groups. However, insulin-stimulated 2-DOG uptake in skeletal muscles from HFD/STZ rats was reduced by 55% (P<0.05) compared with that of the control groups. As shown in Figure 1, RSG treatment restored insulin-stimulated 2-DOG uptake in muscles from HFD/STZ rats to near normal levels. Our data suggest that defective glucose uptake in muscles might play an important role in HFD/STZ-induced diabetes and provide evidence that RSG might profoundly improve insulin activity upon muscle glucose uptake.
Figure 1.
Effects of RSG on glucose uptake into skeletal muscle from control and HFD/STZ rats. Epitrochlearis muscles from saline- and RSG-treated animals were removed and incubated in the presence or absence of 12 nM insulin. 2-DOG uptake was measured as described in Methods. Values are the means±s.e.m. of six independent experiments. *P<0.05 basal vs insulin-stimulated conditions; ¶P<0.05 vs HFD/STZ-untreated rats.
Effects of RSG on glycogen synthesis in liver from control and HFD/STZ rats
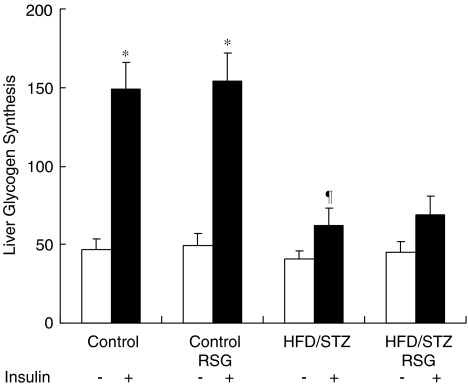
There was an ∼3-fold (P<0.05) increase in liver glycogen synthesis rate following insulin stimulation in control groups. RSG did not affect the basal or insulin-stimulated liver glycogen synthesis rate in control groups. However, insulin-stimulated glycogen synthesis in hepatocytes from HFD/STZ rats was reduced by 58% (P<0.05) compared with that of the control groups. Treatment with RSG could not improve the basal or insulin-stimulated liver glycogen synthesis rate in HFD/STZ rats (Figure 2). These results indicate that RSG had no beneficial effects on defective insulin action on liver glycogen synthesis in HFD/STZ rats.
Figure 2.
Effects of RSG on glycogen synthesis in liver from control and HFD/STZ rats. Hepatocytes were incubated for 3 h in minimal essential medium containing [U-14C]glucose (2 μCi ml−1) with or without insulin (10 nM). Incorporation of 14C label into glycogen was determined by ethanol precipitation, and rates of glycogen synthesis are expressed as nmol glucose incorporated per 3 h per mg cell protein. Values represent the means±s.e.m. for six independent experiments. *P<0.05 basal vs insulin-stimulated conditions; ¶P<0.05 vs insulin-stimulated control groups.
Effects of RSG on PTP1B expression in muscle and liver from control and HFD/STZ rats
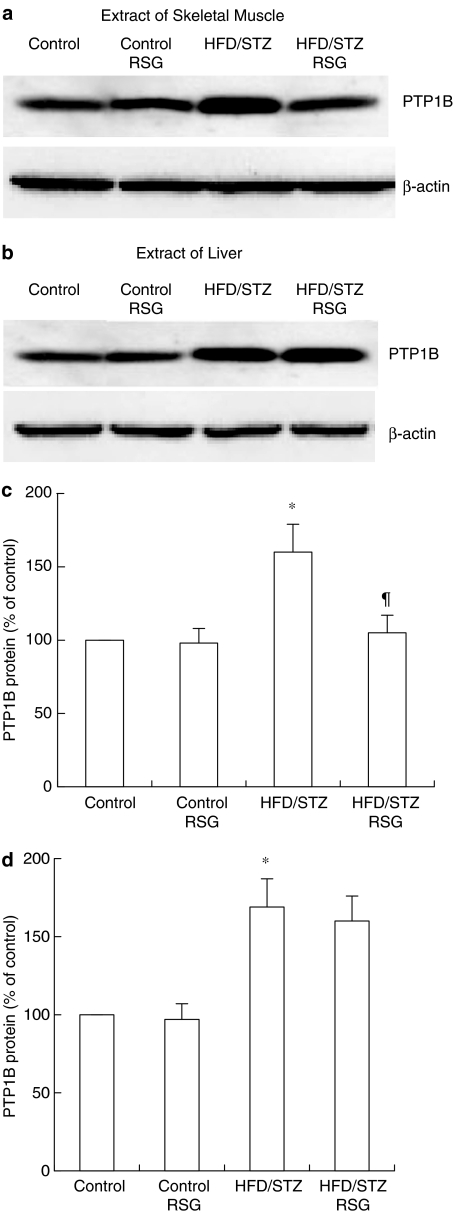
PTP1B protein levels in skeletal muscle from HFD/STZ rats were significantly increased (1.6-fold, P<0.05) compared with control rats. In HFD/STZ rats, the level of PTP1B following treatment with RSG was reduced by 34% (P<0.05) compared with control rats (Figure 3a and c). Liver PTP1B protein levels in HFD/STZ rats were also significantly increased (1.7-fold, P<0.05) compared with those in control rats. However, treatment with RSG did not affect liver PTP1B protein levels in HFD/STZ rats (Figure 3b and d). Muscle and liver PTP1B levels in normal control rats were not affected by RSG treatment. The β-actin control indicated that loaded protein amounts were equal in each lane.
Figure 3.
Effects of RSG on PTP1B protein expression in skeletal muscle and liver from control and HFD/STZ rats. Skeletal muscle and liver tissue samples were obtained from the different groups. After lysate preparation, equal amounts of muscle (a) or liver protein (b) underwent SDS–PAGE and were subjected to Western blotting with specific antibodies against PTP1B (α-PTP1B). Immunoreactive bands for PTP1B were identified using ECL, were scanned and normalized to β-actin. The control was set to 100% and the different groups are expressed as a percentage of control levels (c, d). Data are presented as the means±s.e.m. of six independent experiments. *P<0.05 vs RSG-untreated control rats. ¶P<0.05 vs RSG-untreated HFD/STZ rats.
Effects of RSG on PTP1B activity in muscle and liver from control and HFD/STZ rats
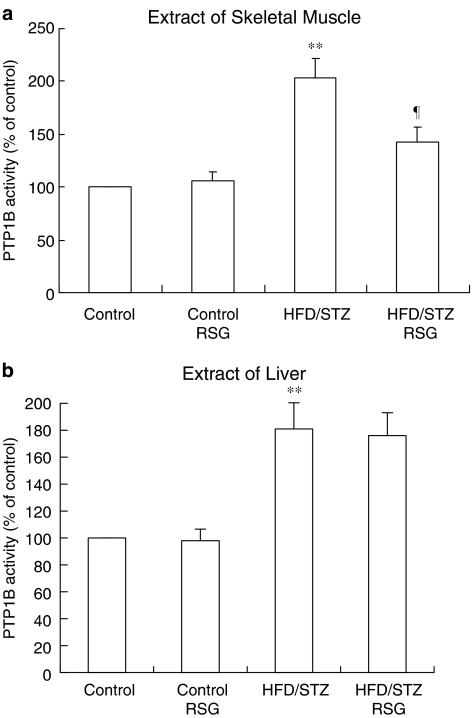
There was a 2-fold (P<0.01) increase in PTP1B activity in skeletal muscle of HFD/STZ rats. RSG reduced PTP1B activity by 30% (P<0.05) in RSG-treated HFD/STZ rats (Figure 4a). PTP1B liver activity in HFD/STZ rats was significantly increased (1.8-fold, P<0.01) compared with that in control animals. Treatment with RSG did not change liver PTP1B activity in HFD/STZ rats (Figure 4b). There were no effects of RSG on the activity of PTP1B in skeletal muscle or liver from RSG-treated control rats.
Figure 4.
Effects of RSG on PTP1B activity in muscle (a) and liver (b) from control and HFD/STZ rats. The muscle and liver tissue homogenate was assayed in a microtiter plate at 27°C. PTP1B activity was measured using a PTP1B assay kit. Means±s.e.m. of six independent experiments is expressed as relative to normal values, which were set as 100%. **P<0.01 vs RSG-untreated control rats. ¶P<0.05 vs RSG-untreated HFD/STZ rats.
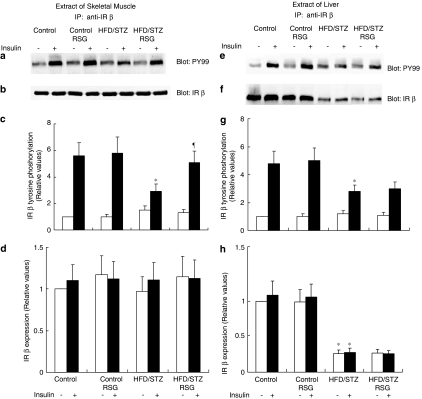
Effects of RSG on insulin-induced tyrosine phosphorylation of the IR β-subunit
In each group of animals, insulin administration resulted in an increase in IRβ-subunit tyrosine phosphorylation (Figure 5). However, in HFD/STZ rats, the skeletal muscle and liver levels of IRβ tyrosine phosphorylation that were achieved following insulin stimulation were 48 and 43% (P<0.05) lower, respectively, than those in control rats (Figure 5a, c and e, g). Treatment with RSG significantly increased the degree of IRβ tyrosine phosphorylation stimulated by insulin in skeletal muscle of HFD/STZ animals (Figure 5a and c) without having any effect on the expression of IRβ (Figure 5b and d). The results indicated that RSG did not affect insulin-stimulated IRβ tyrosine phosphorylation in HFD/STZ livers (Figure 5e and g). RSG did not alter the reduced expression of IRβ in livers from HFD/STZ rats (Figure 5f and h). No effects of RSG on IRβ tyrosine phosphorylation stimulated by insulin were observed in the skeletal muscle or liver of RSG-treated control rats.
Figure 5.
Effects of RSG on insulin-induced tyrosine phosphorylation of the IR β-subunit. A sample of muscle (left panel) or liver (right panel) tissue was obtained following injection with insulin (+) or its diluent (−). Equal amounts of solubilized protein were immunoprecipitated (IP) with an anti-IR-β antibody, followed by SDS–PAGE, and were analyzed by Western blotting with PY99 (a, e). The blots were stripped and reprobed with IR-β (b, f). Phosphorylation (c, g) and expression levels (d, h) of IR-β were quantified by densitometry and expressed relative to control (basal) samples. Error bars represent the s.e.m. of six independent experiments. *P<0.05 vs RSG-untreated control rats. ¶P<0.05 vs RSG-untreated HFD/STZ rats.
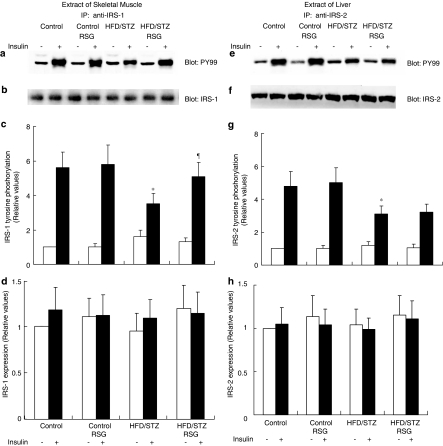
Effects of RSG on insulin-induced tyrosine phosphorylation of IRS-1 or -2
Recent studies in IRS-1 and IRS-2 gene-disrupted mice have suggested that IRS-1 is important for insulin activation of muscle glucose metabolism, whereas IRS-2 is more important for mediating insulin activation during hepatic glucose metabolism (Kahn, 1994; Previs et al., 2000). Insulin administration resulted in an increase in IRS-1 and -2 tyrosine phosphorylation in all groups of animals. Insulin-stimulated IRS-1 tyrosine phosphorylation in skeletal muscle was decreased by 39% (P<0.05) in HFD/STZ rats and returned to normal following RSG treatment (Figure 6a and c). Insulin-stimulated IRS-2 tyrosine phosphorylation in liver was decreased by 36% (P<0.05) in HFD/STZ rats, but was not improved by RSG treatment (Figure 6e and g). RSG did not alter the expression of IRS-1 in skeletal muscle (Figure 6b and d) and IRS-2 in liver (Figure 6f and h). There were no effects of RSG on IRS-1 or -2 tyrosine phosphorylation stimulated by insulin in either skeletal muscle or liver from control rats.
Figure 6.
Effects of RSG on insulin-induced tyrosine phosphorylation of IRS-1 and -2. A sample of muscle (left panel) of liver (right panel) tissue was obtained following injection with insulin (+) or its diluent (−). Equal amounts of solubilized protein were immunoprecipitated (IP) with an anti-IRS-1 or anti-IRS-2 antibody, followed by SDS–PAGE, and were analyzed by Western blotting with PY99. (a, e). The blots were stripped and reprobed with IRS-1 (b) and IRS-2 (f). Phosphorylation (c, g) and expression levels (d, h) of IRS-1 and -2 were quantified by densitometry and expressed relative to control (basal) samples. Error bars represent s.e.m. of six independent experiments. *P<0.05 vs RSG-untreated control rats. ¶P<0.05 vs RSG-untreated HFD/STZ rats.
Discussion
In the present study, we developed an animal model for type II diabetes, fat-fed, STZ-treated rats, that simulates the natural history and metabolic characteristics of patients with type II diabetes (Reed et al., 2000). In our protocol, we used a dose of 35 mg kg−1 STZ rather than the 50 mg kg−1 dose used by Reed et al. (2000). In initial experiments, we found that many animals could not tolerate the toxicity caused by 50 mg kg−1 STZ and they died from an acute infection or debility. Therefore, we used the lowest dose (35 mg kg−1) that has been shown to impair β-cells with minimal side effects in our experiments.
PTPase is considered to be an important regulator of insulin action. Furthermore, its activation may produce insulin resistance (Goldstein, 1992), and abnormal regulation of PTPase has been reported in both animals and patients resistant to insulin (McGuire et al., 1991; Kusari et al., 1994). In this study, we found that a HFD appeared to induce skeletal muscle and liver insulin resistance by increasing the expression and activity of PTP1B, a PTPase involved in insulin action (Goldstein, 1992) (Figures 3 and 4). This result is in agreement with those of previous studies, in which increased availability of free fatty acids (FFAs) was shown to promote the expression of PTP1B in rat skeletal muscle and liver cells, and that the elevated PTP1B might mediate the insulin resistance induced by FFAs (Shao et al., 1998). In our study, the increased amount and activity of PTP1B in HFD/STZ diabetic rats resulted in decreased tyrosine phosphorylation of IRβ in muscle and liver, IRS-1 tyrosine phosphorylation in muscle, and IRS-2 tyrosine phosphorylation in liver, which might be responsible for impaired insulin-stimulated glucose uptake in muscle, glycogen synthesis in liver, and whole-body insulin sensitivity. This is the first study to demonstrate the in vivo effects of changes in PTP1B activity and expression on insulin signaling molecules in skeletal muscle and liver in HFD/STZ rats.
The present study demonstrated that administration of RSG significantly increased the body weight of HFD/STZ diabetic rats (Table 1). The mechanism of weight gain is unclear. It is currently believed that PPARγ increases adipogenesis by stimulating adipocyte differentiation (Lowell, 1999) and this may explain the effects of RSG on body weight. Most strikingly, we found that 2 weeks of RSG administration to HFD/STZ-induced type II diabetic rats improved insulin sensitivity and glycemic control by improving insulin-mediated glucose uptake into muscle but not insulin-stimulated liver glycogen synthesis. This observation is consistent with previous reports that in insulin-resistant rats given HFDs and insulin-deficient rats with STZ-induced diabetes, TZD treatment increases insulin-stimulated glucose uptake into muscle (Hofmann et al., 1991; Hulin et al., 1996; Saltiel & Olefsky, 1996). Our observation that plasma insulin concentrations were not influenced by RSG treatment in HFD/STZ rats suggests that this agent is effective in increasing peripheral glucose utilization in this type II diabetic animal model, without influencing insulin secretion. However, Pickavance et al. (1999) found that in dietary obese rats, plasma insulin levels were reduced by RSG treatment for 21 days at dosages of 3 and 10 mg kg−1 day−1, respectively, and Wang et al. (1997) indicated that 1 mg kg−1 day−1 RSG, which is approximately 3 μmol kg−1 day−1, reduced insulin levels by 42% in fatty Zucker rats after 7 and 20 days. The discrepancy of the effects of RSG on insulin secretion could be explained by the different animal models used in these studies. In dietary obese and fatty Zucker rats, plasma insulin levels are high and pancreatic β-cell function is not exhausted. RSG decreases insulin resistance by enhancing insulin activity in peripheral tissue. The pancreatic β-cells may automatically decrease insulin secretion through a negative-feedback mechanism because of the increased insulin sensitivity. One could consider that the effect of RSG on insulin secretion is indirect. In our experiment, pancreatic β-cells in HFD/STZ rats were impaired by STZ injection, resulting in relative hypoinsulinemia. The approximately normal insulin level was not altered further because of the normal insulin sensitivity restored by RSG.
The biochemical and molecular mechanisms through which RSG improves muscle sensitivity to insulin have yet to be defined, but the results of the present study shed some light on these processes. Much of the work that has demonstrated that TZDs are ligands for PPARγ has been performed in adipocytes or adipocyte models. PPARγ expression in skeletal muscle is low, only ∼10% of the expression observed in adipose tissue (Fajas et al., 1997; Vidal-Puig et al., 1997). Therefore, it has been hypothesized that adipose tissue is the major site of action of these agents, and that any improvements in skeletal muscle insulin action and glucose metabolism could be indirect due to lowering of lipid levels and reduction in activity through the glucose–fatty acid cycle (Spiegelman & Flier, 1996). However, the improvement in insulin sensitivity occurs predominantly in skeletal muscle (Petersen et al., 2000). Furthermore, TZDs have been found to enhance glucose transport even in cultured L6 muscle cells, arguing against a necessary role for adipocytes (Ciaraldi et al., 1990). It is possible that some TZD activity is independent of the PPARγ pathway. In the present study, we found that, consistent with the improvement in peripheral tissue (muscle) sensitivity to insulin, 2 weeks of RSG treatment significantly inhibited expression and activity of PTP1B and subsequently enhanced insulin-stimulated IRβ and IRS-1 tyrosine phosphorylation in skeletal muscle. Similar effects were not observed in the liver. The present results suggest that TZDs may also have direct effects in concert with the insulin signaling transduction cascade in skeletal muscle through the PTP1B regulatory pathway.
PTP1B is a well-established drug target for the treatment of type II diabetes and obesity (van Huijsduijnen et al., 2002). However, the PTP family of enzymes is large, and all are highly specific for the charged phosphotyrosine residue. Finding a selective small-molecule inhibitor of PTP1B has thus far been difficult. Numerous PTP1B inhibitor candidates are being investigated, but a breakthrough has yet to emerge (Brown, 2003). Our study has suggested that some existing antidiabetic principles per se could inhibit PTP1B. In the present study, our data showed that protein levels of PTP1B changed in parallel with changes in its activities in skeletal muscle and liver. The alterations in PTP1B activities may be partly due to changes in its protein levels. Therefore, we postulate that the inhibitory effect of RSG on PTP1B activity is more likely mediated by reducing PTP1B protein expression. However, other possible mechanisms cannot be excluded. Detailed studies to understand the mechanism of the inhibitory action of RSG on PTP1B are currently in progress in our laboratory.
In conclusion, our results indicate that HFD/STZ can impair insulin signaling via the activation of PTP1B, as well as the insulin stimulation of tyrosine phosphorylation of IRβ and IRSs in muscle and liver. It is possible that regulation of PTP1B activity is a crucial step in HFD/STZ-induced diabetes, and that RSG specifically improves the HFD/STZ-induced desensitization of IR signaling, at least partly, via the normalization of PTP1B activity in skeletal muscle. These data provide useful information for understanding the significance of PTP1B in insulin-resistant rats and the molecular mechanism of the action of RSG. Further in vitro studies are needed to determine the effects of RSG on PTP1B.
Acknowledgments
We thank Herman Rhee, Ph.D., for valuable discussions and critical review of the manuscript. We also acknowledge the Young Investigator Award to Wu Yong presented by The Division for Drug Discovery, Drug Development and Regulatory Affairs of the American Society for Pharmacology and Experimental Therapeutics, which will encourage us to work harder. This work was supported by a research grant (No. 30370673) from the National Natural Science Foundation of China and a grant (No. 30113065) from the Educational Bureau of Hubei.
Abbreviations
- 2-DOG
2-deoxyglucose
- FFAs
free fatty acids
- HFD
high-fat diet
- IR
insulin receptor
- IRSs
insulin receptor substrates
- ITT
insulin tolerance test
- KHB
Krebs–Henseleit bicarbonate buffer
- PI
phosphatidylinositol
- PKB
protein kinase B
- PPAR
peroxisome proliferator–activator receptor
- PTP1B
protein tyrosine phosphatase 1B
- RSG
rosiglitazone
- STZ
streptozotocin
- TZDs
thiazolidinediones
References
- AGIUS L., PEAK M., ALBERTI K.G. Regulation of glycogen synthesis from glucose and gluconeogenic precursors by insulin in periportal and perivenous rat hepatocytes. Biochem. J. 1990;266:91–102. doi: 10.1042/bj2660091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AGIUS L., PEAK M., NEWGARD C.B., GOMEZ-FOIX A.M., GUINOVART J.J. Evidence for a role of glucose-induced translocation of glucokinase in the control of hepatic glycogen synthesis. J. Biol. Chem. 1996;271:30479–30486. doi: 10.1074/jbc.271.48.30479. [DOI] [PubMed] [Google Scholar]
- AHMAD F., AZEVEDO J.L., CORTRIGHT R., DOHM G.L., GOLDSTEIN B.J. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J. Clin. Invest. 1997;100:449–458. doi: 10.1172/JCI119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALFORD F.P., MARTIN F.I.R., PEARSON M.J. Significance and interpretation of mildly abnormal oral glucose tolerance test. Diabetalogia. 1971;7:173–180. doi: 10.1007/BF01212550. [DOI] [PubMed] [Google Scholar]
- ARONOFF S., ROSENBLATT S., BRAITHWAITE S., EGAN J.W., MATHSEN A., SCHNEIDER R. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- BARON A.D., BRECHTEL G., WALLACE P., EDELMAN S.V. Rates and tissue sites of non-insulin and insulin-mediated glucose uptake in humans. Am. J. Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- BJORNHOLM M., KAWANO Y., LEHTIHET M., ZIERATH J.R. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997;46:524–527. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- BONORA E., TARGHER G., ALBERICHE M., BONADONNA R.C., SAGGIANI F., ZENERE M.B., MONAUNI T., MUGGEO M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- BROWN M. A tale of two necessities: breakaway technology versus diabetes. Drug Discov. Today. 2003;8:561–562. doi: 10.1016/s1359-6446(03)02770-3. [DOI] [PubMed] [Google Scholar]
- BRUNMAIR B., GRAS F., NESCHEN S., RODEN M., WAGNER L., WALDHAUSL W., FURNSINN C. Direct thiazolidinedione action on isolated rat skeletal muscle fuel handling is independent of peroxisome proliferator-activated receptor-gamma-mediated changes in gene expression. Diabetes. 2001;50:2309–2315. doi: 10.2337/diabetes.50.10.2309. [DOI] [PubMed] [Google Scholar]
- BURANT C.F., SREENAN S., HIRANO K., TAI T.A., LOHMILLER J., LUKENS J., DAVIDSON N.O., ROSS S., GRAVES R.A. Troglitazone action is independent of adipose tissue. J. Clin. Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIARALDI T.P., GILMORE A., OLEFSKY J.M., GOLDBERG M., HEIDENREICH K.A. In vitro studies on the action of CS-045. A new anti-diabetic agent. Metabolism. 1990;39:1056–1062. doi: 10.1016/0026-0495(90)90166-a. [DOI] [PubMed] [Google Scholar]
- ELCHEBLY M., PAYETTE P., MICHALISZYN E., CROMLISH W., COLLINS S., LOY A.L., NORMANDIN D., CHENG A., HIMMS-HAGEN J., CHAN C.C., RAMACHANDRAN C., GRESSER M.J., TREMBLAY M.L., KENNEDY B.P. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- EL-KEBBI I.M., ROSER S., POLLET R.J. Regulation of glucose transport by pioglitazone in cultured muscle cells. Metabolism. 1994;43:953–958. doi: 10.1016/0026-0495(94)90173-2. [DOI] [PubMed] [Google Scholar]
- FAJAS L., AUBOEUF D., RASPE E., SCHOONJANS K., LEFEBVRE A.M., SALADIN R., NAJIB J., LAVILLE M., FRUCHART J.C., DEEB S., VIDAL-PUIG A., FLIER J., BRIGGS M.R., STAELS B., VIDAL H., AUWERX J. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- FUJIWARA T., YOSHIOKA S., YOSHIOKA T., USHIYAMA I., HORIKOSHI H. Characterization of new oral antidiabetic agent CS-045: studies in KK and ob/ob mice and Zucker fatty rats. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- FURNSINN C., BRUNMAIR B., MEYER M., NESCHEN S., FURTMULLER R., RODEN M., KUHNLE H.F., NOWOTNY P., SCHNEIDER B., WALDHAUSL W. Chronic and acute effects of thiazolidinediones BM13.1258 and BM15.2054 on rat skeletal muscle glucose metabolism. Br. J. Pharmacol. 1999;128:1141–1148. doi: 10.1038/sj.bjp.0702886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNSINN C., BRUNMAIR B., NESCHEN S., RODEN M., WALDHAUSL W. Troglitazone directly inhibits CO2 production from glucose and palmitate in isolated rat skeletal muscle. J. Pharmacol. Exp. Ther. 2000;293:487–493. [PubMed] [Google Scholar]
- GOLDSTEIN B.J. Protein-tyrosine phosphatases and the regulation of insulin action. J. Cell. Biochem. 1992;48:33–42. doi: 10.1002/jcb.240480107. [DOI] [PubMed] [Google Scholar]
- GRULET H., DURIACH V., HECART A.C., GROSS A., LEUTENEGGER M. Study of the rate of early glucose disappearance following insulin injection, insulin sensitivity index. Diabetes Res. Clin. Pract. 1993;20:201–207. doi: 10.1016/0168-8227(93)90079-k. [DOI] [PubMed] [Google Scholar]
- GUM R.J., GAEDE L.L., KOTERSKI S.L., HEINDEL M., CLAMPIT J.E., ZINKER B.A., TREVILLYAN J.M., ULRICH R.G., JIROUSEK M.R., RONDINONE C.M. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003;52:21–28. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- HOFMANN C., LORENZ K., COLCA J.R. Glucose transport deficiency in diabetic animals is corrected by treatment with the oral antihyperglycemic agent pioglitazone. Endocrinology. 1991;129:1915–1925. doi: 10.1210/endo-129-4-1915. [DOI] [PubMed] [Google Scholar]
- HULIN B., MCCARTHY P.A., GIBBS E.M. The glitazone family of antidiabetic agents. Curr. Pharm. Des. 1996;2:85–102. [Google Scholar]
- INZUCCHI S.E., MAGGS D.G., SPOLLETT G.R., PAGE S.L., RIFE F.S., WALTON V., SHULMAN G.I. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N. Engl. J. Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- KAHN C.R. Insulin action, diabetogenes, and the cause of type II diabetes (Banting Lecture) Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- KAHN C.R., CHEN L., COHEN S.E. Unraveling the mechanism of action of thiazolidinediones. J. Clin. Invest. 2000;106:1305–1307. doi: 10.1172/JCI11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERSTEN S., DESVERGNE B., WAHLI W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- KIM Y.B., NIKOULINA S.E., CIARALDI T.P., HENRY R.R., KAHN B.B. Normal insulin dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J. Clin. Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAMAN L.D., BOSS O., PERONI O.D., KIM J.K., MARTINO J.L., ZABOLOTNY J.M., MOGHAL N., LUBKIN M., KIM Y.B., SHARPE A.H., STRICKER-KRONGRAD A., SHULMAN G.I., NEEL B.G., KAHN B.B. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSARI J., KENNER K.A., SUH K.I., HILL D.E., HENRY R.R. Skeletal muscle protein tyrosine phosphatase activity and tyrosine phosphatase 1B protein content are associated with insulin action and resistance. J. Clin. Invest. 1994;93:1156–1162. doi: 10.1172/JCI117068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWELL B.B. PPAR: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- MCGUIRE M.C., FIELDS R.M., NYOMBA B.L., RAZ I., BOGARDUS C., TONKS N.K., SOMMERCORN J. Abnormal regulation of protein tyrosine phosphatase activities in skeletal muscle of insulin-resistant humans. Diabetes. 1991;40:939–942. doi: 10.2337/diab.40.7.939. [DOI] [PubMed] [Google Scholar]
- OLEFSKY J.M. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J. Clin. Invest. 2000;106:467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN K.F., KRSSAK M., INZUCCHI S., CLINE G.W., DUFOUR S., SHULMAN G.I. Mechanism of troglitazone action in type 2 diabetes. Diabetes. 2000;49:827–831. doi: 10.2337/diabetes.49.5.827. [DOI] [PubMed] [Google Scholar]
- PHILLIPS L., GRUNBERGER G., MILLER E., PATWARDHAN R., RAPPAPORT E.B., SALZMAN A. The Rosiglitazone Clinical Trial Study Group: Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24:308–315. doi: 10.2337/diacare.24.2.308. [DOI] [PubMed] [Google Scholar]
- PICKAVANCE L.C., TADAYYON M., WIDDOWSON P.S., BUCKINGHAM R.E., WILDING J.P. Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Br. J. Pharmacol. 1999;128:1570–1576. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREVIS S.F., WITHERS D.J., REN J.M., WHITE M.F., SHULMAN G.I. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J. Biol. Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- QUON M.J., BUTTE A.J., TAYLOR S.I. Insulin signal transduction pathways. Trends Endocrinol. Metab. 1994;5:369–376. doi: 10.1016/1043-2760(94)90104-x. [DOI] [PubMed] [Google Scholar]
- REBECCA J.G., LORI L.G., SANDRA L.K., MATTHEW H., JILL E.C., BRADLEY A.Z., JAMES M.T., ROGER G.U., MICHAEL R.J., CRISTINA M.R. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003;52:21–28. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- REED M.J., MESZAROS K., ENTES L.J., CLAYPOOL M.D., PINKETT J.G., GADBOIS T.M., REAVEN G.M. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–1396. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- RONDINONE C.M., TREVILLYAN J.M., CLAMPIT J., GUM R.J., BERG C., KROEGER P., FROST L., ZINKER B.A., REILLY R., ULRICH R., BUTLER M., MONIA B.P., JIROUSEK M.R., WARING J.F. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes. 2002;51:2405–2411. doi: 10.2337/diabetes.51.8.2405. [DOI] [PubMed] [Google Scholar]
- RYDER J.W., FAHLMAN R., WALLBERG-HENRIKSSON H., ALESSI D.R., KROOK A., ZIERATH J.R. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement of the mitogen- and stress-activated protein kinase 1. J. Biol. Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R. Diverse signaling pathways in the cellular actions of insulin. Am. J. Physiol. Endocrinol. Metab. 1996;270:E375–E385. doi: 10.1152/ajpendo.1996.270.3.E375. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R., OLEFSKY J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- SHAO J., GAO Y., YUAN Z. Free fatty acids promoting PTP1B expression in rat skeletal muscle and hepatic cells. Zhonghua Yi Xue Za Zhi. 1998;78:753–755. [PubMed] [Google Scholar]
- SPIEGELMAN B.M., FLIER J.S. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- STEPPAN C.M., LAZAR M.A. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 2002;13:18–23. [Google Scholar]
- VAN HUIJSDUIJNEN R.H., BOMBRUN A., SWINNEN D. Selecting protein tyrosine phosphatases as drug targets. Drug Discov. Today. 2002;7:1013–1019. doi: 10.1016/s1359-6446(02)02438-8. [DOI] [PubMed] [Google Scholar]
- VIDAL-PUIG A.J., CONSIDINE R.V., JIMENEZ-LINAN M., WERMAN A., PORIES W.J., CARO J.F., FLIER J.S. Peroxisome proliferator-activated receptor gene expression in human tissue: effects of obesity weight loss, and regulation by insulin and glucocorticoids. J. Clin. Invest. 1997;99:2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q., DRYDEN S., FRANKISH H.M., BING C., PICKAVANCE L., HOPKINS D., BUCKINGHAM R., WILLIAMS G. Increased feeding in fatty Zucker rats by the thiazolidinediones BRL 49653 (rosiglitazone) and the possible involvement of leptin and hypothalamic neuropeptide Y. Br. J. Pharmacol. 1997;122:1405–1410. doi: 10.1038/sj.bjp.0701535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG M., WISE S.C., LEFF T., SU T.Z. Troglitazone, an antidiabetic agent, inhibits cholesterol biosynthesis through a mechanism independent of peroxisome proliferator-activated receptor-γ. Diabetes. 1999;48:254–260. doi: 10.2337/diabetes.48.2.254. [DOI] [PubMed] [Google Scholar]
- WHITE M.F., KAHN C.R. The insulin signaling system. J. Biol. Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- WHITE M.F., YENUSH L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr. Top. Microbiol. Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- ZINKER B.A., RONDINONE C.M., TREVILLYAN J.M., GUM R.J., CLAMPIT J.E., WARING J.F., XIE N., WILCOX D., JACOBSON P., FROST L., KROEGER P.E., REILLY R.M., KOTERSKI S., OPGENORTH T.J., ULRICH R.G., CROSBY S., BUTLER M., MURRAY S.F., MCKAY R.A., BHANOT S., MONIA B.P., JIROUSEK M.R. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11357–11362. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]