Abstract
Diabetes mellitus leads to a high incidence of several so-called complications, sharing similar pathophysiological features in several territories. Previous reports points at early nonenzymatic glycosylation products (Amadori adducts) as mediators of diabetic vascular complications. In the present study, we analysed a possible role for Amadori adducts as stimulators of proinflammatory pathways in human peritoneal mesothelial cells (HPMCs).
Cultured HPMCs isolated from 13 different patients (mean age 38.7±16 years) were exposed to different Amadori adducts, that is, highly glycated haemoglobin (10 nM) and glycated bovine serum albumin (0.25 mg ml−1), as well as to their respective low glycosylation controls. Amadori adducts, but not their respective controls, elicited a marked increase of NF-κB activation, as determined by electromobility shift assays and transient transfection experiments.
Additionally, Amadori adducts significantly increased the production of NF-κB-related proinflammatory molecules, including cytokines, such as TNF-α, IL-1β or IL-6, and enzymes, such as cyclooxygenase-2 and inducible nitric oxide (NO) synthase, this latter leading to the release of NO by HPMCs.
The effects of Amadori adducts were mediated by different reactive oxygen and nitrosative species (e.g. superoxide anions, hydroxyl radicals, and peroxynitrite), as they were blunted by coincubation with the appropriate scavengers. Furthermore, NO generated upon exposure to Amadori adducts further stimulated NF-κB activation, either directly or after combination with superoxide anions to form peroxynitrite.
We conclude that Amadori adducts can favour peritoneal inflammation by exacerbating changes in NO synthesis pathway and triggering NF-κB-related proinflammatory signals in human mesothelial cells.
Keywords: Diabetes, inflammation, oxidative stress, Amadori adducts, nitric oxide, nuclear factor-κB, mesothelial cells
Introduction
Diabetic patients account for approximately 40% of patients undergoing dialysis in Western Europe and United States (Stein et al., 2004). At the same time, the use of continuous ambulatory peritoneal dialysis (CAPD) is increasing as a renal replacement therapy in these patients. Although the influence of diabetes on peritoneal membrane (PM) remains debated, long-term hyperglycaemia has been suggested to be involved in several changes affecting the functionality of the PM, either in animal models or patients undergoing CAPD (Nakamoto et al., 2002; Stoenoiu et al., 2002). Indeed, such alterations of the peritoneum could be responsible, at least in part, for the higher mortality rate observed in diabetic patients compared to nondiabetic patients with peritoneal dialysis (Stein et al., 2004).
In recent years, alterations of peritoneal mesothelial cells have been proposed to be on the basis of PM dysfunction in these patients (Yañez-Mo et al., 2003; Yao et al., 2003). PM includes a monolayer of mesothelial cells, which acts as a permeability barrier. These cells share many properties with vascular endothelial cells, including the ability to synthesise nitric oxide (NO) and prostacyclin (Amore et al., 1997). Recent studies suggest that some pathophysiological mechanisms involved in diabetic microvascular complications may also be participating in PM dysfunction. Among them, an increased local release of growth factors (Mandl-Weber et al., 2002), and proinflammatory mediators (Riese et al., 1999), as well as deregulation of NO synthases (Devuyst et al., 2001) and increased accumulation of advanced glycosylation end products (AGEs) (Park et al., 2000) within the peritoneum is worth noting.
In the last years, several groups, including ours, have postulated that other products of nonenzymatic protein glycosylation, different to AGEs, can play an important role in diabetic vascular complications. Amadori adducts are the result of condensation reactions between glucose and reactive protein amino groups, which yield Schiff bases that undergo reversible rearrangement within days or weeks. Amadori adducts can, in turn, undergo irreversible changes to form AGEs after longer periods of time (Cerami et al., 1988). In the vasculature, circulating Amadori adducts, like glycated haemoglobin or glycated albumin, produce reactive oxygen species (ROS), which in turn inactivate NO, leading to endothelial dysfunction (Angulo et al., 1996; Amore et al., 1997; Peiró et al., 1998; Hattori et al., 1999; Vallejo et al., 2000a; Peiró et al., 2001; Rodríguez-Mañas et al., 2003). Amadori adducts can also activate proinflammatory redox-regulated transcription factors, including NF-κB (Hattori et al., 1999; 2002; Mandl-Weber et al., 2001; Peiró et al., 2003).
Alterations of the PM are associated with a local proinflammatory environment that may be triggered by different molecules, including the above-mentioned ROS, NO, or NF-κB. In the present work, we aimed to study the ability of Amadori adducts to induce a proinflammatory response in human peritoneal mesothelial cells (HPMCs). For this purpose, we tested the effects of both glycated haemoglobin and glycated albumin on NF-κB activity, inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) gene expression and activity, as well as on the secretion and gene expression of several proinflammatory cytokines such as IL-1β, IL-6, and TNF-α by HPMCs. In addition, a putative role for ROS and reactive nitrosative species was also analysed.
Methods
Materials
Culture plastic ware was obtained from Corning-Costar (New York, NY, U.S.A.). M199 medium, L-glutamine, and streptomycin/penicillin solutions were purchased from Biochrom KG, Berlin, Germany. Phosphate-buffered saline (PBS), foetal calf serum (FCS) and trypsin–EDTA were from Amresco (Solon, Ohio, U.S.A.), Biological Industries (Beit-Hamek, Israel), and GIBCO BRM (Paisley, U.K.), respectively. Human TNF-α was from Peprotech (London, U.K.) and IL-1β from R&D systems (Minneapolis, MN, U.S.A.). Taq DNA polymerase and dNTPs were from EGOGEN (Barcelona, Spain), while 1400W ([N-(3-aminomethyl) benzylacetamidine, 2 HCl]) was from Calbiochem (Darmstadt, Germany). Unless otherwise stated, all other reagents were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Preparation of Amadori adducts
Lyophilised human haemoglobins, nonenzymatically glycosylated at either elevated or normal levels, containing 11.1% (Catalogue no. G-1012) and 5.4% (Catalogue no. G-2012) HbA1, respectively, were purchased from Sigma Chemical Co. Before use, haemoglobins were prepared as described previously (Peiró et al., 2003). Briefly, haemoglobins were dissolved in deionised water and subsequently reduced by incubation with an excess of sodium dithionite. The haemoglobin solutions were then extensively dialysed using a 0.25 Å pore diameter (approximately 12 kDa mol wt) dialysis membrane (Visking®, Serva, Heidelberg, Germany) against deionised water containing 10 mg l−1 EDTA and continuously bubbled with N2. Oxyhaemoglobins were then aliquoted and stored at −70°C until used.
A 50 mg ml−1 solution of bovine serum albumin (BSA) (Sigma Chemical Co.) was glycosylated by incubation in PBS (pH 7.4) containing 1 M glucose under sterile and light-protected conditions for 6 days at 37°C as described previously (Peiró et al., 1998). A control solution was prepared in parallel using PBS without glucose. Glycosylation of serum albumin was verified using the thiobarbituric acid assay (Ney et al., 1981).
The absence of AGEs in the glycated oxyhaemoglobin and albumin solutions was assessed by measuring fluorescence in a Fluostar fluorometer (BMG Labtechnologies, Offenburg, Germany) at excitation maximum of 370 nm and emission maximum of 440 nm, which allows quantifying total AGEs (Sell & Monnier, 1989). A standard curve (r=0.99) was carried out using AGE-modified BSA (0.5–5 μg ml−1), prepared following a previously described method (Bucala et al., 1991). Glycated preparations did not contain significative bacterial endotoxin contamination (⩽0.5 U endotoxin ml−1), as measured with Pyrogent® plus kit (Biowhittaker Europe SPRL, Verners, Belgium).
Cell culture
HPMCs were isolated from omental tissue from 13/15 different donors (free of any cardiovascular or peritoneal disease and nontaking anti-inflammatory drugs or antioxidants) undergoing nonurgent, nonseptic abdominal surgery, using previously described methods (Chung-Welch et al., 1997). HPMCs were routinely cultured in M199 containing 1 g l−1 of D-glucose and supplemented with 10% FCS, 100 μg ml−1 streptomycin, 100 U ml−1 penicillin, and 2.5 μg ml−1 amphotericin. At confluence, HPMC were passaged using a 0.02% EDTA–0.05% trypsin solution and split in a 1 : 2 ratio. HPMCs characterisation was based on both cell morphology (immediately prior to and at confluence, cells adopted the polygonal cobblestone-like appearance characteristic of epithelial cells and formed a monolayer) and indirect immunofluorescence staining of several human mesothelial markers (Chung-Welch et al., 1997). In brief, HPMCs showed a diffuse positive staining with an anti-von Willebrand factor antibody (Dakopatts, Glostrup, Denmark) and a marked staining with anti-cytokeratins 8 and 18, anti-E-cadherin, and anti-vimentin antibodies (all of them from Sigma Chemical Co.). HPMCs failed to express the endothelial marker PECAM-1 (CD31) (see Supplemental data, Figure 1). Cell cultures between passages two and eight were used.
Reporter plasmids
The reporter plasmid, p5 × NF-κB-Luc (Stratagene, La Jolla, CA, U.S.A.), and different luciferase-based reporter plasmids corresponding to the 5′-flanking regulatory regions of either human iNOS (7.2 hiNOS-luc; Taylor et al., 1998), human eNOS (1.33 heNOS-luc; Cieslik et al., 1999), short human COX-2 and human COX-1 (phPES2 −327/+59, phPES1 −1010/+69, respectively; Inoue et al., 1995), or human IL-6 (p1168hu.IL6P-luc; Berghe et al., 1998) genes were used.
Transient transfection and luciferase assays
Transient transfection experiments were performed as we described previously (Peiró et al., 2003). Briefly, HPMCs (105 cells) were grown in six-well plates to 80–90% confluence and the culture medium (M-199) was then replaced by vehicle medium, that is, serum-free medium supplemented with 0.1% BSA. The transfection mixture was added to cell cultures for further 18–20 h. The transfection mixture consisted of 2 μg of the above-mentioned plasmids incubated with 75 μl of DMEM and 7.5 μl of Superfect® (Quiagen Gmbh, Hilden, Germany) in vehicle medium, following the manufacturer's instructions. Following treatment with the specified agents, HPMCs were harvested and lysed with passive lysis buffer (1 ×; Promega, Madison, WI, U.S.A.), followed by one freeze/thaw cycle. The extracts were centrifuged for 30 s at 13,000 r.p.m. at 4°C, and assayed with a luciferase reporter system (Promega, Madison, WI, U.S.A.). Luciferase activity was expressed as relative luciferase units (RLUs; Peiró et al., 2003).
Western blotting and protein content
Extraction of protein homogenates and Western blotting were performed as described previously (Peiró et al., 2003). Briefly, HPMCs were extracted in lysis buffer containing 10 mmol l−1 Tris, pH 7.4, 1% SDS, 10 mmol l−1 orthovanadate, 2 mmol l−1 PMSF, and 12.5 μg ml−1 of aprotinin. Total protein extracts were diluted 3 : 1 in 4 × Laemmli's buffer and boiled for 5 min at 100°C. Proteins were equally loaded (10 μg lane−1), separated on 12% SDS–PAGE gels, and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Madrid, Spain). After blocking overnight at 4°C in 0.2% Tween-20 and 5% nonfat dry milk, the membrane was incubated for 1 h at room temperature with a monoclonal antibody against either COX-2 or iNOS (dilution 1/1000; BD Biosciences, Bedford, MA, U.S.A.), or an affinity-purified anti-nitrotyrosine polyclonal antibody (dilution 1/750; Alexis, Carlsbad, CA, U.S.A.) followed by incubation for 45 min with the respective anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (dilution 1/10,000; Chemicon, Temecula, CA, U.S.A.). Immunoreactive bands were detected using an enhanced chemiluminescence detection kit (Amersham, Arlington Hills, IL, U.S.A.) and quantified using Chemi-Imager 5.5 software from AlphaInnotec (San Leandro, CA, U.S.A.).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as previously described by others (Schreiber et al., 1989). EMSAs were performed as described previously (Peiró et al., 2003). Nuclear extracts (5 μg of protein) were incubated in the presence of 3 μg of poly-dIdC together with a commercial double-stranded 32P-labelled oligonucleotide encoding for the NF-κB consensus sequence: 5′-AGTTGAGGGGACTTTCCCAGGC-3′. DNA–protein complexes were electrophoretically separated and subjected to autoradiography. Bands were quantified by densitometry using NIH Image software.
Determination of cytokine levels
Cytokine levels in confluent cell (105 cells) culture supernatants were determined using human TNF-α, IL-1β, and IL-6 Instant ELISAs (The Bender Medsystems, Vienna, Austria), by generating a standard curve provided by the manufacturer and normalised to protein content (1 μg). Protein content of whole-cell extract and cell culture supernatants were determined using the BCA assay (Pierce, Rockford, IL, U.S.A.).
Measurement of nitrite plus nitrate
Confluent HPMCs (105 cells) were grown in six-well plates and nitrite plus nitrate production (NOx), used as an indirect quantification of NO, was measured in cell supernatants by an ozone-chemiluminescence method (Fries et al., 2003), using an NO detector (NOA® 280 analyzer, Sievers, Boulder, CO, U.S.A.). A standard curve was generated by injections of known concentrations of sodium nitrate. The levels of NOx were normalised to protein content (1 μg).
Measurement of COX activity
Confluent HPMCs (105 cells) were grown in six-well plates and COX activity was measured by the Cyclooxygenase Activity Kit (Stressgen Biotech, Madison, WI, U.S.A.) using a specific chemiluminescence substrate to detect the peroxidative activity of COX enzymes in protein extract homogenates, as specified by the manufactures. Light emission is directly proportional to COX activity in the sample. Results are expressed as RLUs normalised to protein content (1 μg).
RNA isolation and RT–Multiplex PCR (MPCR) assays
Total RNA from HPMC (105–106 cells) was obtained using RNAquous® kit (Ambion Inc., Austin, TX, U.S.A.), following the manufacturer's instructions. RT and MPCR were performed with appropriate kits (Maxim Biotech. Inc., San Francisco, CA, U.S.A.), using 1 μg of cDNA for each MPCR reaction. MPCR kit has been designed to direct the simultaneous amplification of specific ORF regions of human NOS genes and GAPDH (hNOSG-MPCR), human COX NF-κB (NF-κB1, 2) and GAPDH genes (hTNF-M052G-MPCR), or the proinflammatory cytokines IL-6, IL-1β, TNF-α, their respective receptors, and GAPDH genes (h-Inflammation-M053G-MPCR). Levels of mRNA were normalised to GAPDH transcript.
Ethic considerations
The study was approved by the Clinical Research and Ethics Committee of Hospital Universitario de Getafe, with oral informed consent obtained from all donors.
Statistical analysis
Results are expressed as mean±s.e.m. Statistical analysis was determined by ANOVA, followed by Fisher's protected least-significance test with the level of significance chosen at P⩽0.05. n denotes the number of experiments performed in triplicate, using cell obtained from at least three different donors.
Results
Activation of NF-κB by Amadori adducts in HPMCs
In a first instance, we checked the absence of AGEs in the glycated preparations used in the present study by measuring AGE-related fluorescence at excitation maximum of 370 nm and emission maximum of 440 nm. The fluorescence values obtained in the highly glycated haemoglobin solution (HHb; 10 nM) or the glycated BSA solution (gBSA; 0.25 mg ml−1 equivalent to approximately 4 μM) were below the fluorescence values obtained with the 0 mg ml−1 concentration of the AGE-BSA in our standard curve (data not shown), therefore confirming the absence of AGEs in the glycated preparations.
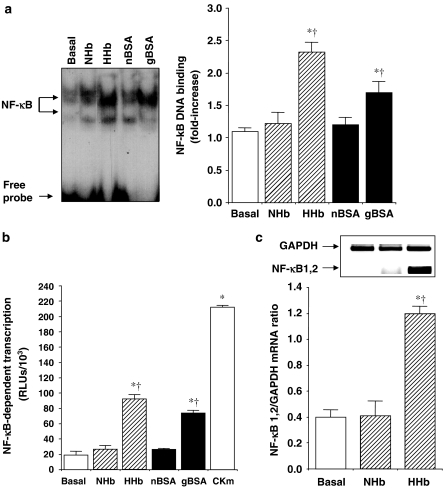
We then studied whether Amadori adducts can modify NF-κB activity in HPMCs. EMSA revealed that incubation (1 h) with both 10 nM HHb and 0.25 mg ml−1 gBSA significantly induced NF-κB-binding activity as compared with cells treated with normal-glycated haemoglobin (NHb) or nonglycated BSA (nBSA) (Figure 1a). The NF-κB-specific binding band was eliminated by the addition of a 100-fold excess of unlabelled NF-κB oligonucleotide to the reaction mixture (not shown).
Figure 1.
Amadori adducts activate NF-κB in HPMCs. (a) DNA-binding activity of NF-κB was assessed after 1 h treatment of HPMCs with either NHb or HHb (both at 10 nM) or gBSA and nBSA (both at 0.25 mg ml−1). A representative EMSA assay is shown, n=9. (b) NF-κB-dependent transcriptional activation was assessed in cells transiently transfected with p5 × NF-κB-Luc plasmid. HPMCs were exposed for 12 h to the above-described compounds, after which luciferase activity (RLUs) was measured, n=32. (c) Expression of NF-κB1 and NF-κB2 mRNAs after HPMCs exposure to HHb or NHb (both at 10 nM) was analysed by RT–MPCR assay. A representative blot and densitometry values of the ratio mRNA NF-κB1, 2/GAPDH mRNA are shown, n=9. Results are expressed as mean±s.e.m. *P⩽0.05 vs basal; †P⩽0.05 vs the respective glycosylation control.
Similarly, transiently transfected HPMCs with the p5 × NF-κB-Luc reporter plasmid showed a clear stimulation of NF-κB-dependent transcription by HHb and gBSA, but not by NHb or nBSA, after 12 h exposure (Figure 1b). Increased NF-κB-dependent transcription was observed as early as 6 h and it was sustained for at least 18 h after cell stimulation with HHb (around 1.5-, three-, 4.8-, and four-fold increase over basal at 6 h, 8 h, 12 h, and 18 h, respectively, P⩽0.05 by ANOVA) (see Supplemental data, Figure 2). A cytokine mix (CKm) consisting of IL-1β and TNF-α (both at 10 ng ml−1) was used as a positive control for the induction of NF-κB-dependent transcription. We additionally analysed the effect of HHb on mRNA levels of p50/105 (NF-κB1) and p49/100 (NF-κB2) in HPMCs (Figure 1c). The best-characterised form of NF-κB is a heterodimer formed by a 50 kDa (p50/NF-κB1) and a 65 kDa (p65/RealA) protein. RT–MPCR assays revealed an increased band corresponding to NF-κB1 and/or NF-κB2 (143 bp) in HPMCs treated with 10 nM HHb for 6 h, but not with vehicle or 10 nM NHb (Figure 1c) (see also Supplemental data, Figure 3).
Amadori adducts stimulate several NF-κB-related proinflammatory genes in HPMCs
We further studied whether Amadori adducts may affect the expression, activity, and cellular levels of several NF-κB-related proinflammatory markers. Thus, both HHb and gBSA, but not their corresponding glycosylation controls, stimulated the activity (more than three fold increase over basal) of two NF-κB-related proinflammatory enzymes such as NOS and COX (Table 1). HHb (10 nM) also induced a significant increase on the basal secretion of several NF-κB-related proinflammatory cytokines, such as IL-6 (4.9±2.6 vs 1.8±0.2 pg ml−1, P⩽0.05), TNF-α (118.0±15.1 vs 55.0±5.5 pg ml−1, P⩽0.05), and IL-1β (12.5±3.1 vs 4.3±3.2 pg ml−1, P⩽0.05), in cell supernatants. NHb failed to modify the basal production of these cytokines (see also Supplemental data, Figure 4).
Table 1.
Effect of Amadori adducts on NOS and COX enzymatic activities in HPMCs
| Effector | NOS activity (NOx, μM) | COX activity (RLUs /103) |
|---|---|---|
| None | 3.9±2.0 | 5.7±3.3 |
| NHb | 4.0±1.8 | 4.4±1.1 |
| HHb | 15.3±2.5* | 26.1±12.1* |
| HHb+L-NAME | 3.7±0.3† | ND |
| HHb+indomethacin | ND | 12.2±8.0† |
| nBSA | 4.2±0.3 | 5.3±1.2 |
| gBSA | 12.3±4.2* | 15.7±7.2* |
| CKm | 11.3±2.9* | 33.2±3.3* |
ND=not determined.
The effectors were used at the following concentrations: HHb and NHb at 10 nM, gBSA and nBSA at 0.25 mg ml−1, L-NAME at 100 μM, and indomethacin at 10 μM, n=18.
P⩽0.05 vs basal;
P⩽0.05 vs HHb-treated cells. NOx, nitrate+nitrite.
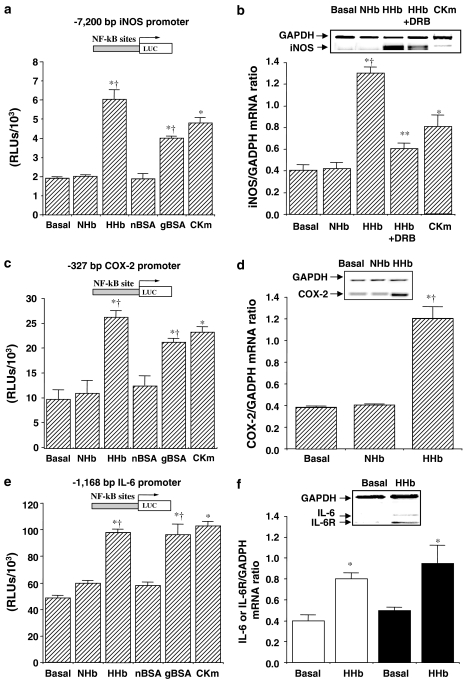
We therefore determined the influence of HHb and gBSA on the expression of NOS, COX, IL-6, TNF-α, and IL-1β genes. As assessed by transient transfection, iNOS promoter activity was significantly increased after exposure for 12 h to both Amadori adducts (Figure 2a). To confirm a possible transcriptional regulation, we tested whether inhibition of RNA synthesis was able to abrogate the increase in iNOS mRNA levels induced by HHb. To this end, we used a long half-life RNA polymerase inhibitor, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB, 10 μM). After preincubation with DRB for 1 h, HPMCs were exposed to HHb for additional 12 h. HHb-dependent iNOS mRNA upregulation was significantly reduced by DRB treatment, as shown in Figure 2b. DRB also showed a significant blockade of the iNOS promoter activity (3.19±0.3- and 1.16±0.06-fold induction over basal levels for HHb and HHb+DRB, respectively, P⩽0.05). In addition, HHb had no effect on endothelial NOS (eNOS) activity and gene expression (around 1.05±0.07-fold induction over basal, P⩽0.05) (see Supplemental data, Figure 5a).
Figure 2.
Stimulation by Amadori adducts of NF-κB-related proinflammatory gene expression. The effect of Amadori adducts on either human iNOS (a), human −327/+59 COX-2 (c), and human IL-6 (e) promoters was studied using luciferase-based reporter plasmids in transiently transfected HPMCs. Cells were treated for 12 h with HHb and NHb (both at 10 nM), gBSA, and nBSA (both at 0.25 mg ml−1) or a cytokine mixture (TNF-α+IL-1β, 10 ng ml−1 each), n=32. The effect of a 6 h treatment with the above-described compounds on iNOS (b), COX-2 (d), and IL-6 (open bars) and IL-6 receptor (solid bars) (f) mRNA levels was also determined by RT–MPCR assays. Representative blots are shown, n=9. DRB: 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (10 μM). Results are expressed as mean±s.e.m. *P⩽0.05 vs basal; †P⩽0.05 vs the respective glycosylation control; **P⩽0.05 vs HHb-treated cells.
Similarly, cell treatment with both Amadori adducts selectively stimulated the activity of a short human COX-2 promoter construct transiently transfected into HPMCs (Figure 2c), without affecting the activity of the human COX-1 gene promoter (1.04±0.01- and 0.99±0.2-fold induction over basal for HHb and gBSA, respectively, P⩽0.05). Furthermore, exposure to HHb for 6 h induced a selective upregulation of the COX-2 gene, as determined by RT–MPCR (Figure 2d). Again, no significant effect was observed on COX-1 mRNA expression upon HHb treatment (1.01±0.03-fold induction over basal, P⩽0.05) (see Supplemental data, Figure 5b).
Figure 2e shows that HHb and gBSA significantly enhanced IL-6 promoter activity. Indeed, HHb stimulated IL-6 and IL-6R mRNA expression (Figure 2f, open and solid bars, respectively). Furthermore, HHb also stimulated TNF-α and IL-1β mRNA gene expression (2.9±1- and 3.0±0.28-fold induction of basal, respectively, P⩽0.05) and their respective receptors (data not shown).
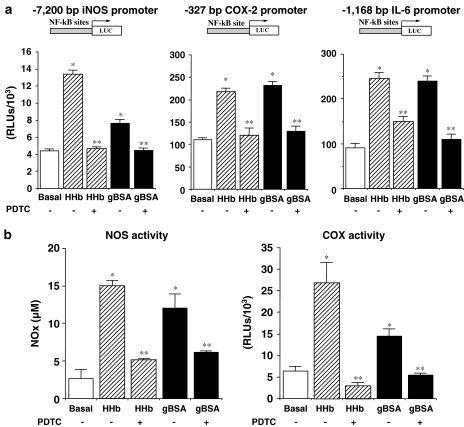
Finally, to test whether NF-κB transcriptional activation was required for these Amadori-induced effects on proinflammatory molecules, we evaluated the effect of the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC). As shown in Figure 3a, PDTC (100 μM) prevented the stimulatory effect of both HHb and gBSA on iNOS, COX-2, and IL-6 promoters (Figure 3a). In accordance with these results, PDTC abrogated HHB- and gBSA-related NOS and COX activities (Figure 3b).
Figure 3.
Blockade of Amadori-induced stimulation of NF-κB-related proinflammatory factors by PDTC. (a) HPMCs were treated for 12 h with HHb (10 nM) or gBSA (0.25 mg ml−1) either alone or in combination with PDTC (100 μM) and the luciferase-based expression of human iNOS, −327/+59 human COX-2, and human IL-6 was studied in transiently transfected HPMCs. (b) NOS and COX activities were determined after HPMCs exposure to the above-described treatments, n=32. *P⩽0.05 vs basal; **p⩽0.05 vs the correspondent Amadori (gBSA or HHb)-treated cells.
Participation of ROS on the Amadori-induced effects in HPMCs
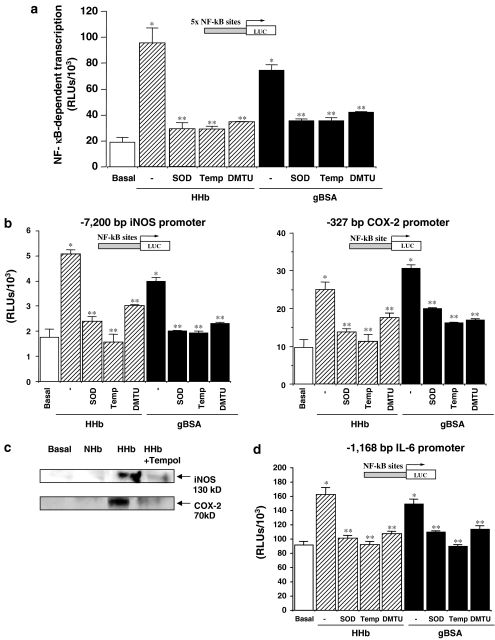
As NF-κB is a redox-regulated factor, we also analysed the effect of several ROS scavengers on the proinflammatory effects of Amadori adducts in HPMCs. As shown in Figure 4, pretreatment with superoxide dismutase (SOD, 200 U ml−1), the cell-permeable SOD-mimetic Tempol (100 μM), or dimethylthiourea (DMTU, 1 mM) prevented the stimulatory effect of both HHb and gBSA on NF-κB-dependent transcription and iNOS, COX-2 (−327/+59 bp), and IL-6 promoter activation (Figures 4a, b, and d, respectively). The ROS scavengers used did not significantly modify basal transcription levels (data not shown). Tempol also prevented HHb-induced iNOS and COX-2 protein expression, as shown by Western blotting (Figure 4c). Similarly, SOD, Tempol, and DMTU impaired the stimulatory effect of HHb on NOS and COX activities (Table 2). Tempol also prevented the increased secretion of IL6, TNF-α, or IL-1β induced by HHb in HMPCs (Table 2).
Figure 4.
Participation of ROS in the proinflammatory effects induced by Amadori adducts in HPMCs. (a) Transient transfection experiments were performed to measure NF-κB-dependent transcription activity after 12 h exposure to HHb (10 nM) or gBSA (0.25 mg ml−1) either alone or co-incubated with superoxide dismutase (SOD 200 U ml−1), Tempol (Temp, 100 μM) or dimethylthiourea (DMTU, 1 mM), n=18. Under the same conditions, human iNOS and −327/+59 human COX-2 (b), as well as human IL-6 (d) promoters luciferase-based reporter activities were assessed by transient transfection assays, n=18. (c) A representative immunoblot for iNOS and COX-2 protein is shown after 12 h exposure of HPMCs to HHb either alone or in combination with Tempol at the above-described concentrations, n=9. *P⩽0.05 vs basal. **P⩽0.05 vs the correspondent Amadori (gBSA or HHb)-treated cells.
Table 2.
Effects of antioxidants on NOS and COX activities, and proinflammatory cytokine levels induced by HHb in HPMCs
| Effector | NOS activity (NOx, μM) | COX activity (RLUs/103) | IL-6 (pg ml−1) | TNF-α (pg ml−1) | IL-1β (pg ml−1) |
|---|---|---|---|---|---|
| None | 3.8±2.1 | 7.5±2.4 | 1.9±0.2 | 53.9±6.2 | 4.3±2.8 |
| HHb | 14.0±1.8* | 26.9±11.1* | 5.8±2.6* | 120.0±4.0* | 12.3±3.6* |
| HHb+SOD | 4.9±0.1† | 16.7±3.8† | ND | ND | ND |
| HHb+Tempol | 5.3±0.3† | 10.8±1.1† | 2.8±0.1† | 81.0±10.1† | 6.0±1.0† |
| HHb+DMTU | 3.7±0.1† | 11.6±5.8† | ND | ND | ND |
ND=not determined.
The effectors were used at the following concentrations: HHb and NHb at 10 nM, SOD at 200 U ml−1, Tempol at 100 μM, and DMTU at 1 mM, n=9.
P⩽0.05 vs basal;
P⩽0.05 vs HHb-treated cells.
NO and peroxynitrite as mediators of HHb-induced NF-κB-dependent transcriptional activation in HPMCs
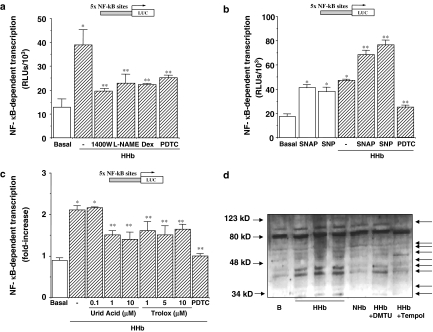
In order to investigate a potential role for NO in NF-κB activation, we performed transient transfection assays with p5 × NF-κB-Luc, in the presence of different iNOS inhibitors, either specific (1400W) or nonspecific (L-NAME). As shown in Figure 5a, both 1400W (10 μM) and L-NAME (100 μM) reduced HHb-stimulated NF-κB activity to an extent similar to that obtained in the presence of the anti-inflammatory glucocorticoid dexamethasone (Dex). In unstimulated HPMCs, NOS inhibitors and Dex failed to modify NF-κB activity by themselves (data not shown). PDTC (100 μM) was used as a control for blocking HHb-induced NF-κB activation (see Supplemental data, Figure 6).
Figure 5.
NO and peroxynitrite modulate NF-κB-dependent transcriptional activity in HPMCs. (a) HPMCs transiently transfected with pNF-κB-Luc were treated with HHb (10 nM) alone or in combination with the iNOS inhibitors 1400W (10 μM) and L-NAME (100 μM), dexamethasone (Dex, 1 μM) or PDTC (100 μM) for 12 h, after which luciferase activity was measured, n=12. (b) NF-κB-dependent transcription was further determined in HPMCs treated for the same time period with S-nitroso-N-acetylpenicillamine (SNAP, 10 μM) and sodium nitroprusside (SNP 100 μM) either alone or in the presence of HHb. HHb was also coincubated with PDTC (100 μM), n=9. (c) NF-κB-dependent transcription was determined in HPMCs treated for 8 h with HHb alone or in combination with the peroxynitrite scavengers uric acid (0.1, 1, and 10 μM) and Trolox (1, 5, and 10 μM) or PDTC (100 μM), n=9. (d) Representative immunoblot for nitrated protein expressions after exposure for 12 h to either NHb or HHb (both at 10 nM) or HHb in combination with Tempol or DMTU (100 μM and 1 mM, respectively).*P⩽0.05 vs basal; **P⩽0.05 vs HHb-treated cells.
Furthermore, incubation of HPMCs with two exogenous NO donors S-nitroso-N-acetylpenicillamine (SNAP) and sodium nitroprusside (SNP) resulted in the stimulation of this NF-κB-Luc reporter construct (Figure 5b). Both SNAP (10 μM) and SNP (100 μM) showed a cooperative effect with HHb (Figure 5b), but not with NHb (not shown).
A role for peroxynitrite in HHb-induced NF-κB activation was also analysed. Treatment of HPMCs with the peroxynitrite scavenger's uric acid (1 and 10 μM) and Trolox® (1, 5, and 10 μM) partially blocked (40–55%) the stimulation of NF-κB-dependent transcription elicited by HHb (Figure 5c). The partial nitration of different proteins (up to 45%) in HPMCs stimulated by HHb was confirmed by Western blot experiments using a 3-nitrotyrosine antibody (Figure 5d, see arrows). HHb-induced nitration was almost suppressed by coincubation with Tempol, and in a lesser extent by DMTU (Figure 5d).
Discussion
The use of CAPD is increasing as a renal replacement therapy in diabetic patients undergoing dialysis. This therapeutic option has more complications in diabetic patients than in nondiabetic patients (Stein et al., 2004). Hyperglycaemia or events linked to hyperglycaemia have risen as potentially culprit mechanisms explaining this poor outcome (Nakamoto et al., 2002; Stoenoiu et al., 2002). Hyperglycaemia is now clearly identified as a pivotal factor in other diabetes-associated complications, including the vascular ones. Among the mechanisms by which hyperglycaemia can contribute to diabetic vasculopathy, we and others have highlighted in previous works a role for early products of nonenzymatic protein glycosylation, the so-called Amadori adducts, in promoting NO-related endothelial dysfunction and inflammation in the vascular wall (Angulo et al., 1996; Amore et al., 1997; Peiró et al., 1998; Vallejo et al., 2000a; Peiró et al., 2001; Hattori et al., 2002; Peiró et al., 2003; Rodríguez-Mañas et al., 2003). We therefore aimed to analyse whether Amadori adducts may play a role in hyperglycaemia-associated PM dysfunction. We focused on the effects of Amadori adducts on mesothelial cells, which share a common embryological derivation with vascular endothelial cells (Hernando et al., 1994), and have been proposed to be on the basis of peritoneal dysfunction (Yañez-Mo et al., 2003; Yao et al., 2003) through the production of different growth factors and proinflammatory markers (Riese et al., 1999; Mandl-Weber et al., 2002).
We observed a significant stimulation of NF-κB in HPMCs treated with Amadori adducts. As a result of this, increased transcriptional activity of different proinflammatory genes, such as IL-6, TNF-α, IL-1β, iNOS, and COX-2, and increased levels of their resulting proinflammatory proteins were observed. This result is consistent with previous reports demonstrating their ability to stimulate NF-κB itself or other inflammation-related transcription factors, like activator protein 1 (AP-1), in vascular smooth muscle cells (Hattori et al., 2002; Peiró et al., 2003).
It is worth noting that both haemoglobin and albumin were used at concentrations that can be found in the circulation under physiological conditions (Tietz, 1990). Glycated albumin has been used in previous reports as a model for Amadori adducts, based on the close correlation found between serum glucose content and the degree of albumin glycation. However, in the experimental approach performed in our laboratory, glycated haemoglobin has been preferentially chosen as a model of circulating Amadori adduct because of the following reasons: (i) haemoglobin is very sensitive to changes in glycaemic concentrations (Jovanovic & Peterson, 1981); (ii) it circulates free in plasma at nanomolar concentrations (Tietz, 1990); and (iii) it can penetrate into the vascular tissue in nonpathological circumstances (Paredi et al., 1999). Actually, there is evidence indicating that circulating proteins, including free haemoglobin, can be incorporated into tissues either by a transcytosis mechanism, as it has been shown in mesothelial cells (Bodega et al., 2002), or by an active transmembrane transport in other cell types (Wu & Cohen, 1994). The existence of nonenzymatic protein glycosylation has been also reported during CAPD in the mesothelial layer of the human peritoneum (Posthuma et al., 2001). In addition, in vivo and in vitro kinetics data provide evidence for the formation of early-glycated proteins in the peritoneal cavity during the time course (10 h) of the routine peritoneal equilibration test (Friedlander et al., 1996). It has also been suggested that glycated proteins can move from plasma into the peritoneal cavity (Friedlander et al., 1996).
Although Amadori adducts have been described as important precursors of AGEs (Makita et al., 1992), the proinflammatory effects of the glycated solutions on HPMCs shown in this study can be attributed to Amadori adducts, as the presence of detectable AGEs in the solutions was discarded. Thus, this seems to be a separate mechanism from that induced by AGEs (Boulanger et al., 2002; Wu et al., 2002; Rashid et al., 2004), as inferred by several recent studies (Mandl-Weber et al., 2001; Hattori et al., 2002; Valencia et al., 2004). In this regard, a possible role for metal ions or endotoxin contamination was discarded mainly by the ability of Amadori proteins to release ROS even in the presence of EDTA (Vallejo et al., 2000b), or by a detection kit (see Methods), respectively.
The ability of Amadori adducts for inducing proinflammatory factors in HPMC cultures appears to be mediated by different ROS. These results are consistent with previous reports showing the ability of Amadori adducts to produce ROS, mainly superoxide anions (Vallejo et al., 2000b; Yoo et al., 2004). In this way, it is well accepted that increased oxidative stress plays a key role in the development of vascular inflammation and vasculopathy in diabetes (Spitaler & Graier, 2002). It seems therefore reasonable to propose an analogous role for ROS in the putative alterations of peritoneum membrane functionality associated to hyperglycaemia.
Concerning a role for NO in PM dysfunction, it is thought to be involved in both structural and permeability alterations (Chen et al., 2000; Mandl-Weber et al., 2002). Currently, a fact widely debated is the definitive source of local NO at the peritoneum. Our data agree with previous reports showing a barely detectable basal iNOS activity, (Chen et al., 2000; Davenport et al., 2004). However, upon treatment with Amadori adducts, a clear stimulation of iNOS activity and gene expression was observed. Thus we can conclude that, at present, several extracellular stimuli are known to induce iNOS in HPMCs, including cytokines (this work, and Chen et al., 2000), the insoluble polysaccharide zymosan, which acts as a peritonitis inducer (Yao et al., 2004), or, as shown in the present study, Amadori adducts. Although eNOS stimulation has also been involved in NO production by mesothelial cells (Reimann et al., 2004), Amadori adducts did not stimulate eNOS activity in HPMCs.
Finally, our results suggest a possible role for Amadori-induced NO or NO-derived compounds in peritoneal complications related to hyperglycaemia through the activation of NF-κB in HPMCs. This is in accordance with the fact that NO and NO-derived compounds have emerged in the last years as important modulators of gene expression through their ability to modulate several transcription factors (Liaudet et al., 2000; Cooke & Davidge, 2002). Indeed, modulation of NF-κB activity by NO appears to have an important role in the regulation of the inflammatory response (Liaudet et al., 2000). Nevertheless, in a pro-oxidant environment, NO can react with superoxide anions leading to peroxynitrite production, which may in turn further stimulate NF-κB-dependent transcription in HPMCs. Our results indicate a role for peroxynitrite as a mediator for NF-κB activation, in HPMCs, as previously proposed in vascular cells (Cooke & Davidge, 2002; Hattori et al., 2004). However, the fact that a certain degree of NF-κB-dependent transcription still occurred in the presence of peroxynitrite scavengers discards peroxynitrite as the sole mediator of NF-κB activation by glycated haemoglobin. At present, little is known about the cellular signalling pathways activated by NO and peroxynitrite in HPMCs.
In conclusion, we propose that Amadori adducts can alter mesothelial cell functionality by increasing oxidative and nitrosative stress and by activating NF-κB-related proinflammatory pathway, which may be on the basis of a low-grade proinflammatory response within the peritoneum.
External data objects
Acknowledgments
This work was supported by grants from Fondo de Investigaciones Sanitarias (01/0579 and 02/1246), Comunidad Autónoma de Madrid (GR/SAL/0899/2004), Ministerio de Educación y Ciencia (SAF-2001-1328), and Instituto de Salud Carlos III (RGDM (G03/212). Dr J. Nevado is a recipient of a Research Contract from Instituto de Salud Carlos III (FIS 99/3077). We are grateful to Drs D. Geller, G. Haegeman, K.K. Wu, and T. Tanabe for kindly providing plasmids, and Dr M. Blázquez and N. Nin for critical review of the manuscript.
Abbreviations
- AP-1
activator protein-1
- AGEs
advanced glycation end products
- CAPD
continuous ambulatory peritoneal dialysis
- DMTU
dimethylthiourea
- DRB
5,6-dichloro-1-β-D-ribofuranosylbenzimidazole
- eNOS
constitutive endothelial nitric oxide synthase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HHb
highly glycated oxyhaemoglobin
- HPMCs
human peritoneal mesothelial cells
- iNOS
inducible nitric oxide synthase
- L-NAME
Nω-nitro-L-arginine methyl ester
- NF-κB
nuclear factor-κB
- NHb
oxyhaemoglobin glycated at normal levels
- PDTC
pyrrolidine dithiocarbamate
- ROS
reactive oxygen species
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- Tempol
4-hydroxy-tempo
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp).
References
- AMORE A., CIRINA P., MITOLA S., PERUZZI L., GIANOGLIO B., RABBONE I., SACCHETTI C., CERUTTI F., GRILLO C., COPPO R. Nonenzymatically glycated albumin (Amadori adducts) enhances nitric oxide synthase activity and gene expression in endothelial cells. Kidney Int. 1997;51:27–35. doi: 10.1038/ki.1997.4. [DOI] [PubMed] [Google Scholar]
- ANGULO J., SÁNCHEZ-FERRER C.F., PEIRÓ C., MARÍN J., RODRÍGUEZ-MAÑAS L. Impairment of endothelium-dependent relaxations by increasing percentages of glycated human haemoglobin. Hypertension. 1996;28:583–592. doi: 10.1161/01.hyp.28.4.583. [DOI] [PubMed] [Google Scholar]
- BERGHE W.V., PLAISANCE S., BOONE E., DE BOSSCHER K., SCHIMITZ M.L., FIERS W., HAEGEMAN G. P38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathway are regulated for nuclear-factor κB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- BODEGA F., ZACCHI L., AGOSTINI E. Albumin transcytosis in mesothelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L3–L11. doi: 10.1152/ajplung.00157.2001. [DOI] [PubMed] [Google Scholar]
- BOULANGER E., WAUTIER M.P., WAUTIER J.C., BOVAL B., PANIS Y., WERNERT N., DANZE P.M., DEQUIEDT P. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 2002;61:148–156. doi: 10.1046/j.1523-1755.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- BUCALA R., TRACEY K.J., CERAMI A. Advanced glycosylation products quench nitric oxide mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERAMI A., VLASSARA H., BROWNLEE M. Role of advanced glycosylation products in complications of diabetes. Diabetes Care. 1988;1:73–79. [PubMed] [Google Scholar]
- CHEN J.Y., CHIU J.H., CHEN T.W., YANG W.C., YANG A.H. Human peritoneal mesothelial cells produce nitric oxide: induction by cytokines. Periton. Dialysis Int. 2000;20:772–777. [PubMed] [Google Scholar]
- CHUNG-WELCH N., PATTON W.F., SHEPRO D., CAMBRIA R.P. Two-stage isolation procedure for obtaining homogenous populations of microvascular endothelial and mesothelial cells from human omentum. Microvas. Res. 1997;54:121–134. doi: 10.1006/mvre.1997.2039. [DOI] [PubMed] [Google Scholar]
- CIESLIK K., LEE L.H., TANG J.L., WU K.K. Transcriptional regulation of endothelial nitric oxide synthase by an interaction between casein kinase 2 and protein phosphatase 2A. J. Biol. Chem. 1999;274:34669–34675. doi: 10.1074/jbc.274.49.34669. [DOI] [PubMed] [Google Scholar]
- COOKE C.M., DAVIDGE S.T. Peroxynitrite increases iNOS through NF-κB and decreases prostacyclin synthase in endothelial cells. Am. J. Physiol. Cell. Physiol. 2002;282:C395–C402. doi: 10.1152/ajpcell.00295.2001. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A., FERNANDO R.L., ROBSON R., VARGHESE Z. Nitric oxide production by peritoneal mesothelial cells. Int. J. Artif. Organs. 2004;27:15–23. doi: 10.1177/039139880402700105. [DOI] [PubMed] [Google Scholar]
- DEVUYST O., COMBET S., CNOPS Y., STOENOIU M. Regulation of NO synthase isoforms in the peritoneum: implications for ultrafiltration failure in peritoneal dialysis. Nephrol. Dial. Transplant. 2001;16:675–678. doi: 10.1093/ndt/16.3.675. [DOI] [PubMed] [Google Scholar]
- FRIEDLANDER M.A., WU Y.C., ELGAWISH A., MONNIER V.M. Early and advanced glycosylation end-products. Kinetics of formation and clearance in peritoneal dialysis. J. Clin. Invest. 1996;97:728–735. doi: 10.1172/JCI118471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIES D.M., PAXINOU E., THEMISTOCLEOUS M., SWANBERG E., GRIENDLING K.K., SALVEMINI D., SLOT J.W., HEIJNEN H.F., HAZEN S.L., ISCHIROPOULUS H. Expression of inducible nitric-oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells. J. Biol. Chem. 2003;25:22901–22907. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., BANBA N., GROSS S.S., KASAI K. Glycated serum albumin-induced nitric oxide production in vascular smooth muscle cell by nuclear-factor κB-dependent transcriptional activation of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 1999;259:128–132. doi: 10.1006/bbrc.1999.0736. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., KASSAI K., GROSS S.S. NO suppresses while peroxynitrite sustains NF-κB: a paradigm to rationalize cytoprotective and cytotoxic actions attributed to NO. Cardiovasc. Res. 2004;63:31–40. doi: 10.1016/j.cardiores.2004.03.014. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., SUZIKI M., HATTORI S., KASAI K. Vascular smooth muscle cell activation by glycated albumin (Amadori adducts) Hypertension. 2002;39:22–28. doi: 10.1161/hy1201.097300. [DOI] [PubMed] [Google Scholar]
- HERNANDO A., GARCIA-HONDUVILLA N., BELLO J.M., BUJAN J., NAVLET J. Coatings for vascular prostheses: mesothelial cell express specific markers for muscle cells and have biological activity similar to that of endothelial cells. Eur. J. Endovasc. Surg. 1994;8:531–536. doi: 10.1016/s0950-821x(05)80586-9. [DOI] [PubMed] [Google Scholar]
- INOUE H., YOKOYAMA C., HARA S., TONE Y., TANABE T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J. Biol. Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- JOVANOVIC I., PETERSON C.M. The clinical utility of glycated hemoglobin. Am. J. Med. 1981;70:331–338. doi: 10.1016/0002-9343(81)90770-1. [DOI] [PubMed] [Google Scholar]
- LIAUDET L., GARCIA-SORIANO F., SZABO C. Biology of nitric oxide signalling. Crit. Care Med. 2000;28:N37–N49. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- MAKITA Z., VLASSARA H., RAYFIELD E., CARTWRIGHT K., FRIEDMAN E., RODBY R., CERAMI A., BUCALA R. Hemoglobin-AGE: a circulating marker of advanced glycosylation. Science. 1992;258:651–653. doi: 10.1126/science.1411574. [DOI] [PubMed] [Google Scholar]
- MANDL-WEBER S., COHEN C.D., HASLINGER B., KRETZLER M., SITTER T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002;61:570–578. doi: 10.1046/j.1523-1755.2002.00143.x. [DOI] [PubMed] [Google Scholar]
- MANDL-WEBER S., HASLINGER B., SCHALKWIJK C.G., SITTER T. Early glycated albumin, but not advanced glycated albumin, methylglyoxal, or 3-deoxyglucosonee increases the expression of PAI-1 in human peritoneal mesothelial cells. Periton. Dialysis Int. 2001;21:487–494. [PubMed] [Google Scholar]
- NAKAMOTO H., IMAI H., KAWANISHI H., NAKAMOTO M., MINAKUCHI J., KUMON S., WATANABE S SHIOHIRA Y., ISHII T., KAWAHARA T., TSUZAKI K., SUZUKI H. Effect of diabetes on peritoneal function assessed by personal dialysis capacity test in patients undergoing CAPD. Am. J. Kidney Dis. 2002;40:1045–1054. doi: 10.1053/ajkd.2002.36343. [DOI] [PubMed] [Google Scholar]
- NEY K.A., COLLEY K.J., PIZZO S.V. The standardization of the thiobarbituric acid assay for nonenzymatic glycosylation of human serum albumin. Anal. Biochem. 1981;118:294–300. doi: 10.1016/0003-2697(81)90585-6. [DOI] [PubMed] [Google Scholar]
- PAREDI P., BIERNACKI W., INVERNIZZI G., KHARITONOV S.A., BARNES P.J. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease. Chest. 1999;116:1007–1011. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- PARK M.S., LEE H.A., CHU W.S., YANG D.H., HWANG S.D.Peritoneal accumulation of AGE and peritoneal membrane permeability Periton. Dialysis Int. 200020452–460.12 [PubMed] [Google Scholar]
- PEIRÓ C., ANGULO J., RODRÍGUEZ-MAÑAS L., LLERGO J.L., VALLEJO S., CERCAS E., SÁNCHEZ-FERRER C.F. Vascular smooth muscle cell hypertrophy induced by glycosylated human oxyhaemoglobin. Br. J. Pharmacol. 1998;125:637–644. doi: 10.1038/sj.bjp.0702097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIRÓ C., LAFUENTE N., MATESANZ CERCAS E., LLERGO J.L., VALLEJO S., RODRÍGUEZ-MAÑAS L., SÁNCHEZ-FERRER C.F. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br. J. Pharmacol. 2001;133:967–974. doi: 10.1038/sj.bjp.0704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEIRÓ C., MATESANZ N., NEVADO J., LAFUENTE N., CERCAS E., AZCUTIA V., VALLEJO S., RODRÍGUEZ-MAÑAS L., SÁNCHEZ-FERRER C.F. Glycated human oxyhaemoglobin activates nuclear factor-κB and activator protein-1 in cultured human aortic smooth muscle. Br. J. Pharmacol. 2003;140:681–690. doi: 10.1038/sj.bjp.0705483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTHUMA N., TER WEE P.M., NIESSEN H., DONKER A.J., VERNRUGH H.A., SCHALKWIJK C.G. Amadori albumin and advanced glycation end-product formation in peritoneal dialysis using icodextrin. Periton. Dialysis Int. 2001;21:43–51. [PubMed] [Google Scholar]
- RASHID G., BENCHETRIT S., FISHMAN D., BERHEIM J. Effect of advanced glycation end-products on gene expression and synthesis of TNF-alpha and endothelial nitric oxide synthase by endothelial cell. Kidney Int. 2004;66:1099–1106. doi: 10.1111/j.1523-1755.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- REIMANN D., DACHS D., MEYE C., GROSS P. Amino-acid-based peritoneal dialysis solution stimulates mesothelial nitric oxide production. Periton. Dial. Int. 2004;24:378–384. [PubMed] [Google Scholar]
- RIESE J., DENZEL C., ZOWE M., MEHER C., HOHENBERGER W., HAUPT W. Secretion of IL-6, monocyte chemoattractant protein-1, macrophage inflammatory protein-1-alpha, and TNF-alpha by cultured intact human peritoneum. Eur. Surg. Res. 1999;31:281–288. doi: 10.1159/000008704. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ-MAÑAS L., ANGULO J., VALLEJO S., PEIRÓ C., SÁNCHEZ-FERRER A., CERCAS E., SÁNCHEZ-FERRER C.F. Early and intermediate Amadori glycated adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia. 2003;46:556–566. doi: 10.1007/s00125-003-1056-1. [DOI] [PubMed] [Google Scholar]
- SCHREIBER M.M., MATTHIAS P., MULLER M.M., SCHAFFNER W. Rapid detection of octamer binding proteins with ‘mini extracts' prepared from a small number of cell. Nucleic Acid Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELL D.R., MONNIER V.M. Structure elucidation of a senescence crosslink from human extracellular matrix. Implication of pentoses in the aging process. J. Biol. Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- SPITALER M.M., GRAIER W.F. Vascular targets of redox signalling in diabetes mellitus. Diabetologia. 2002;45:476–494. doi: 10.1007/s00125-002-0782-0. [DOI] [PubMed] [Google Scholar]
- STEIN G., FUNFSTUCK R., SCHIEL R. Diabetes mellitus and dialysis. Min. Urol. Nefrol. 2004;56:289–303. [PubMed] [Google Scholar]
- STOENOIU M.S., DE VRIESSE A.S., MOULIN P., FERON O., LAMEIRE M., DEVUYST O. Experimental diabetes induces functional and structural changes in the peritoneum. Kidney Int. 2002;62:668–678. doi: 10.1046/j.1523-1755.2002.00487.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR B.S., De VERA M.E., GANSTER R.W., Wang K., SHAPIRO R.A., MORRIS S.M., JR., BILLIAR T.R., GELLER D.A. Multiple NF-κB enhancer elements regulate cytokine induction of human inducible nitric oxide synthase gene. J. Biol. Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- TIETZ N.W. Clinical Guide to Laboratory Tests. Philadelphia, PA: WB Saunders Co.; 1990. pp. 284–285. [Google Scholar]
- VALENCIA J.V., MONE M., KOEHNE C., REDISKE J., HUGHES T.E. Binding of receptor for advanced glycation end product (RAGE) ligands is not sufficient to induce inflammatory signals: lack of activity on endotoxin-free albumin-derived advanced glycation end products. Diabetologia. 2004;47:844–852. doi: 10.1007/s00125-004-1392-9. [DOI] [PubMed] [Google Scholar]
- VALLEJO S., ANGULO J., PEIRÓ C., NEVADO J., SÁNCHEZ-FERRER A., PETIDIER R., SÁNCHEZ-FERRER C.F., RODRÍGUEZ-MAÑAS L. Highly glycated oxyhemoglobin impairs nitric oxide relaxations in human mesenteric microvessels. Diabetologia. 2000a;43:83–90. doi: 10.1007/s001250050011. [DOI] [PubMed] [Google Scholar]
- VALLEJO S., ANGULO J., PEIRÓ C., SÁNCHEZ-FERRER A., CERCAS E., NEVADO J., SÁNCHEZ-FERRER C.F., RODRÍGUEZ-MAÑAS L. Correction of glycated oxyhemoglobin-induced impairment of endothelium-dependent vasodilatation by glycacide. J. Diabetes Complicat. 2000b;14:207–214. doi: 10.1016/s1056-8727(00)00080-5. [DOI] [PubMed] [Google Scholar]
- WU C.H., HUANG C.M., LIN C.H., HO Y.S., CHEN C.M., LEE H.M. Advanced glycosylation end products induce NF-kappaB dependent iNOS expression in RAW 264.7 cells. Mol. Cell. Endocrinol. 2002;194:9–17. doi: 10.1016/s0303-7207(02)00212-5. [DOI] [PubMed] [Google Scholar]
- WU V.Y., COHEN M.P. Receptor specific for Amadori-modified glycated albumin on murine endothelial cells. Biochem. Biophys. Res. Commun. 1994;199:1088. doi: 10.1006/bbrc.1994.1106. [DOI] [PubMed] [Google Scholar]
- YAÑEZ-MO M., LARA-PEZZI E., SELGAS R., RAMIREZ-HUESCA M., DOMÍNGUEZ-JIMÉNEZ C., JIMÉNEZ-HEFFERNAN A., AGUILERA A., SÁNCHEZ-TOMERO J.A., BAJO M.A., ÁLVAREZ V., CASTRO M.A., DEL PESO G., CIRUJEDA A., GAMALLO C, SÁNCHEZ-MADRID F., LÓPEZ-CABRERA M. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003;348:403–412. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- YAO V., MCCAULEY R., COOPER D., PLATELL C., HALL J.C. Zymosan induces nitric oxide production by peritoneal mesothelial cells. ANZ J. Surg. 2004;74:266–269. doi: 10.1111/j.1445-2197.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- YAO V., PLATELL C., HALL J.C. Role of peritoneal mesothelial cells in peritonitis. Br. J. Surg. 2003;90:1187–1194. doi: 10.1002/bjs.4373. [DOI] [PubMed] [Google Scholar]
- YOO C., SONG C., KIM B., HONHG H., LEE H. Glycated albumin induces superoxide generation in mesangial cells. Cell. Physiol. Biochem. 2004;14:361–368. doi: 10.1159/000080346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.