Abstract
Antiepileptic drugs (AEDs) are often utilized in the treatment of neuropathic pain. The major AED valproic acid (VPA) is of particular interest as it is thought to engage a variety of different neural mechanisms simultaneously. However, the clinical use of VPA is limited by two rare but life-threatening side effects: teratogenicity and hepatotoxicity.
We synthesized VPA's corresponding amide: valpromide (VPD), two of VPAs isomers and their corresponding amides; valnoctic acid (VCA), valnoctamide (VCD), diisopropyl acetic acid (DIA), diisopropylacetamide (DID), and VPD's congener: N-methyl-VPD (MVPD). VCD, DID and VPD are nonteratogenic, potentially nonhepatotoxic, and exhibit better anticonvuslant potency than VPA.
In this study, we assessed the antiallodynic activity of these compounds in comparison to VPA and gabapentin (GBP) using the rat spinal nerve ligation model of neuropathic pain (SNL, Chung model).
VCA and MVPD were inactive. However, VPD (20–100 mg kg− 1), VCD (20–100 mg kg− 1) and DID (20–90 mg kg− 1) produced dose-related reversal of tactile allodynia with ED50 values of 61, 52 and 58 mgkg− 1, respectively. All the amides were more potent than VPA (ED50=269 mgkg− 1). The antiallodynic effect of VPA, VPD, VCD and DID was obtained at plasma concentrations of 125, 24, 18 and 7 mg l− 1, respectively, with a good pharmacokinetic–pharmacodynamic correlation and a minimal lag response.
VCD and DID were found to have minimal motor and sedative side effects at analgesic doses, and were equipotent to GBP, currently the leading drug in neuropathic pain treatment. Consequently, VCD and DID have potential to become new drugs for the treatment of neuropathic pain.
Keywords: Neuropathic pain, spinal nerve ligation, pharmacokinetics, pharmacodynamics, valproic acid, valproic acid isomers, valpromide, valnoctamide, diisopropyl acetamide
Introduction
Neuropathic pain responds to many antiepileptic drugs (AEDs), suggesting that it shares with epilepsy underlying neurophysiological mechanisms. Specifically, most of these drugs either stabilize membrane hyperexcitability or enhance inhibitory GABA and / or glycine neurotransmission (Jensen, 2002; Rogawski & Loscher, 2004a). However, even the most effective AEDs show only modest efficacy in neuropathic pain, and some patients obtain no useful relief at all (McQuay et al., 1995). The main advantages of newer AEDs over established agents are more favorable tolerability and improved pharmacokinetics (PK) rather than improved efficacy (Nicholson, 2000; Tremont-Lukats et al., 2000). There is therefore considerable room for improvement.
Valproic acid (VPA), one of the established AEDs, is believed to act through a variety of different neural mechanism: inhibition of the voltage-dependent sodium channels, enhanced GABAergic signaling, reduced NMDA-receptor-mediated glutamate excitation and increased serotonergic inhibition (Loscher, 1999; 2002; Owens & Nemeroff, 2003). In principle, such multivalent action is highly advantageous, promising improved efficacy with reduced side effects. However, the clinical use of VPA is limited by two rare, but potentially life-threatening side effects, teratogenicity and hepatotoxicity. Partly for this reason VPA has not attracted much interest for analgesic development. We are aware of only a few randomized controlled trials in neuropathic pain, and some small open trials. These do not produce a clear picture of the drug's antinociceptive potential (Tremont-Lukats et al., 2000; Kochar et al., 2002; 2004; 2005; Otto et al., 2004).
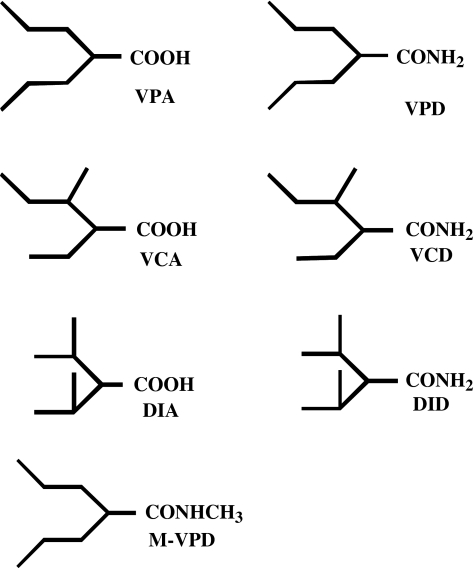
Amide analogues and derivatives of VPA have shown better anticonvulsant activity than the parent compound (Isoherranen et al., 2003a, 2003b). Valpromide (VPD, Figure 1), the primary amide of VPA, is approved as an AED and as an antipsychotic agent in several European countries (Bialer, 1991; Payen et al., 2004). VPD crosses the blood–brain barrier (BBB) more readily leading to higher CNS concentrations than VPA (Blotnik et al., 1996; Harris et al., 2003). As an AED, VPD is 3–5 times more potent than VPA and is not teratogenic in animal models. In humans, however, its better antiepileptic potency and lack of teratogenicity do not have clinical implications because, unlike in the animal models, VPD is a prodrug to VPA (Bialer, 1991).
Figure 1.
Chemical structures of valproic acid (VPA), valpromide (VPD), valnoctic acid (VCA), valnoctamide (VCD), diisopropylacetic acid (DIA), diisopropylacetamide (DID) and N-methyl-valpromide (MVPD).
Since VPD did not succeed in becoming a second-generation VPA due to its presystemic biotransformation, and in a further attempt to capitalize on the potential advantages VPA and VPD while reducing their safety liability, we have developed structural isomers of VPD that do not undergo metabolic hydrolysis to their corresponding acids (Isoherranen et al., 2003b). These agents, valnoctamide (VCD) and diisopropyl acetamide (DID), were designed to retain or enhance VPA's efficacy as an AED, and thus perhaps be superior as antinociceptive agents, while avoiding teratogenicity and hepatotoxicity (Isoherranen et al., 2003a, 2003b). Indeed, VCD and DID were more potent as anticonvulsants than VPA and their corresponding acids (and VPA structural isomers) valnoctic acid (VCA) and diisopropylacetic acid (DIA) (Haj-Yehia and Bialer, 1990; Isoherranen et al., 2003a). Moreover, unlike VPD, VCD and DID in dogs did not biotransform to their corresponding acids (Haj-Yehia & Bialer, 1988; 1990). In humans, VCD acts as a drug in its own right and not as a prodrug, and undergoes only minimal biotransformation to VCA (Barel et al., 1997).
While VPA's teratogenicity is associated with the parent compound (Nau et al., 1991), its hepatotoxicity results from biotransformation into metabolites with a terminal double bond, specifically 4-ene-VPA (Kondo et al., 1992; Baillie & Sheffels, 1995). VPA's carboxylic moiety is a structural requirement for the drug's teratotgenicity and hepatotoxicity (Grillo et al., 2001; Isoherranen et al., 2003b). Thus, both VCD and DID are nonteratogenic and are probably nonhepatotoxic due to their amide moiety, and the fact that they are not metabolized to their corresponding acids which are necessary intermediates in the formation of hepatotoxic metabolites with a terminal double bond (Figure 1, Radatz et al., 1998; Grillo et al., 2001; Okada et al., 2004).
Rather than following the old pattern of first completing the development of novel compounds as AEDs and then testing them as analgesics, we proceeded to assess the above isomers of VPA and VPD as well as its derivatives N-methyl-VPD (MVPD) for antiallodynic activity in a rat model of neuropathic pain. In addition, we assessed the PK–pharmacodynamic (PD) relationships of VPA, VPD, VCD and DID. Results are reported here.
Methods
Animals and surgical procedure
Experiments were performed on male Sprague–Dawley rats (Harlan Laboratories, Jerusalem) weighing 175–200 g. The procedure for inducing tactile allodynia in the spinal nerve ligation (SNL) model was as described by Kim & Chung (1992). Briefly, under ketamine–xylazine anesthesia (85 mg kg− 1, and 13 mg kg− 1, i.p., respectively), rats were placed in a prone position and paraspinal muscles on the left were separated from the L4-S2 spinous processes. Part of the L6 transverse process was removed and the underlying L4–L6 spinal nerves were identified. The L5 and L6 spinal nerves were isolated, tightly ligated with 5-0 silk, and cut just distal to the ligature. The ligature was approximately 8 mm distal to the corresponding dorsal root ganglion (DRG). Following complete homeostasis the wound was sutured in layers, the skin closed with Michel clips, topical bacteriostatic power was applied, and 20,000 units of duplo-penicillin was injected intramuscularly (i.m.). After uneventful recovery, the animals were returned to the vivarium where they were maintained in groups of 2–3 in solid-bottomed 42 × 26 cm transparent plastic shoebox cages, bedded with pine shavings. Rat dry food pellets (Kofholk, Petah Tikva, Israel, product #19510) and water were available ad libitum. The day : night cycle was 12 h : 12 h (lights on at 0600 hours). Experiments were approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Hebrew University of Jerusalem.
Sensory testing
We used a set of nine nylon von Frey monofilaments (VFF, Semmes-Weinstein monofilaments, Stoelting, Wood Dale, IL, U.S.A.) to quantify foot withdrawal in response to normally innocuous tactile stimuli. Initial bending force of the filaments (in mN) was: 5.8, 13.4, 18.7, 37.9, 57.3, 77.5, 97.4, 145.9 and 254.1 (equivalent to mass of 0.6, 1.4, 1.9, 3.9, 5.9, 7.9, 9.9, 14.9 and 25.9 g). Forces were calibrated by lowering filaments onto the pan of an electronic balance until they just bent. Standard deviation (s.d.) on repeated measurements was about ±0.2 g. Filaments were cut flush, and although they did not all have the same diameter, the same set was used for all rats.
Rats were placed on a raised wire mesh screen (gaps 4 × 4 mm), which allowed access to the plantar surface of the hindpaw from below. They were covered with a transparent plastic dome, 13 cm high, which prevented rearing on the hindlimbs. A period of 20 min was allowed for acclimation prior to sensory testing. The mid-plantar hindpaw skin just caudal to the footpads, half way between medial and lateral edges of the foot, was subjected to a series of five brief probes, spaced at about 1 s. Each such stimulus was sufficient to just bend the filament. Testing began with the filament with the weakest initial bending force (0.6 g). If the animal failed to respond with at least a momentary foot twitch / withdrawal to ⩾3 of the five probes, the next stiffest filament was tried, and so forth, using an ascending staircase protocol. Response threshold was the bending force of the first monofilament in the series that evoked a criterion ⩾3 / 5 responses. This procedure was repeated twice on each foot, alternating from side to side, with ⩾5 min rest between trials in a given animal. ‘Threshold' for each paw on a given test day was the average of the two force determinations just sufficient to evoke a criterion response. If there was no response to the stiffest filament, threshold was recorded as 26 g. Rats were included in the study only if they failed to respond to the 15 g monofilament (or weaker) on two consecutive days, two and one day before surgery.
The first postoperative tests were carried out 5 and 6 days following surgery. We set an arbitrary response criterion for demonstrating tactile allodynia on the operated side at ⩽10 g. Only animals that passed this screening test were used for drug testing. About 15% of animals operated were excluded on these grounds.
Pharmacological treatments
Anesthetic reagents: ketamine (Fort Dodge, Fort Dodge, IA, U.S.A.) and xylazine (VMD, Arendonk, Belgium). Antibiotic: duplo-penicillin (Biochimie GmbH, Kundl, Austria). Drugs: VPA, VPD, VCA, VCD, DIA, DID, MVPD and gabapentin (GBP) were tested. VPD, VCD were gifts from Sanofi Labaz (France), VPA and GBP were gifts from Teva Pharmaceuticals (Netanya, Israel). VCA, DIA, DID and MVPD were synthesized according to previously published procedures (Haj-Yehia and Bialer, 1989; 1990). The products were identified and their structure was proved by nuclear magnetic resonance spectra and by elemental microanalysis. Each compound was suspended in 0.5% methylcellulose in double distilled water (MC, vehicle) and administered intraperitoneally (i.p.) in the doses noted below, in a uniform volume of 4 ml kg− 1 body weight. The following doses were tested: VPA 200, 250, 300 and 400 mg kg− 1; VPD 20, 60, 80 and 100 mg kg− 1; VCA 100, 200 and 300 mg kg− 1; VCD 20, 40, 60, 80 and 100 mg kg− 1; DIA 200 and 300 mg kg− 1; DID 20, 40, 80 and 90 mg kg− 1; MVPD 60 and 120 mg kg− 1 and GBP 10, 30, 100 and 300 mg kg− 1. In addition, vehicle control injections were carried out using 4 ml kg− 1 MC.
Drug tests were conducted at 7, 14 and 21 days postoperative (dpo). In each rat, two different doses of a particular drug and MC were assessed in a blind randomized crossover manner. Response to VFF probing was tested before for baseline (pre-) and then 30, 60, 120, 180 and 240 min after dosing. The individual who made the behavioral assessment was unaware of the drug / dose given. The first two doses of a drug were based on its potency (ED50) as an AED in the maximal electroshock (MES) test (Table 4). I.e., one dose was just below and the second was just above the MES ED50. Based on the results of the first experiment, the next dosages were chosen in order to establish a scale of protection. VPAs first stage was 300 vs 400 mg kg− 1 vs MC (n=8 rats tested per dose) and the second stage was 200 vs 250 mg kg− 1 vs MC (n=8). VPD: 20 vs 60 mg kg− 1 vs MC (n=8); 100 vs 150 mg kg− 1 vs MC (n=8); VPD 80 mg kg− 1 vs VCA 300 mg kg− 1 vs MC (n=9). VCA: 100 vs 200 mg kg− 1 vs MC (n=8). VCD: 40 vs 80 mg kg− 1 vs MC (n=9); 20 vs 60 mg kg− 1 vs MC (n=8); 100 mg kg− 1 vs MC (n=8). DIA: 200 vs 300 mg kg− 1 vs MC (n=8). DID 40 vs 80 mg kg− 1 vs MC (n=7); 20 vs 90 mg kg− 1 vs MC (n=8). MVPD 60 vs 120 mg kg− 1 vs MC (n=9). GBP: 10 vs 30 mg kg− 1 vs MC (n=8); 100 vs 300 mg kg− 1 vs MC (n=9). A 1-week washout period was used after each drug treatment. At 5 days after each drug administration, the allodynic baseline threshold was re-evaluated, and 2 days later drugs effect was tested again.
Table 4.
Activity of AEDs as anticonvulsants (MES test) and agents that suppress tactile allodynia in the SNL model of neuropathic pain in the rat
| Drug | MES ED50 (mg kg− 1, p.o.) | SNL ED50 (mg kg− 1, i.p.) | ED50 MES / SNL | Brain-to-plasma ratio |
|---|---|---|---|---|
| VPA | 485a | 269 | 1.8 | 0.16c |
| VPD | 32b | 61 | 0.5 | 0.84c |
| VCA | NT | >300 | — | NT |
| VCD | 29c | 52 | 0.6 | 1.0c |
| DIA | NT | >300 | — | NT |
| DID | 51 | 58 | 0.9 | NT |
| MVPD | 65 | >120 | <0.54 | NT |
| GBP | 14.8a | 34d | 0.43 | 0.85e |
| FBM | 25.3a | >600d | <0.04 | 0.64f |
| LTG | 1.3a | >100d | <0.01 | 0.4g |
| CBZ | 5.4a | >30c | <0.18 | 1.0h |
Relative brain access (brain-to-plasma ratio) is also provided.
Data from White et al. (2002).
Data from Bialer et al. (1994).
Data from Blotnik et al. (1996).
Data from Hunter et al. (1997).
Data from Vajda (2002).
Data from Pellock et al. (2002).
Data from Walker et al. (2000).
Data from Spina (2002).
MES=maximal electroshock; ED50=median effective dose; SNL=spinal nerve ligation; NE=no effect; NT=not tested; CBZ=carbamazepine; FBM=felbamate; LTG=lamotrigine.
Determination of the median effective dose (ED50)
To determine ED50 as an estimate of the compounds' potency, we plotted a dose–response curve 60 min post injection except for DID and GBP, which were plotted at 120 min. These were the time points at which the largest fraction of rats tested first reached their maximal antiallodynic effect. Response was plotted as the percentage of animals tested at that time point that failed to respond to the 15 g VFF. Maximum possible response (100%) means that none of the rats tested responded to the 15 g VFF or less. ED50 was the dose that yielded 50% of the maximum possible response. This value was linearly interpolated between the dose just above and just below the ED50 value. These data were then subjected to probit analysis (Finney, 1971). 95% confidence intervals (CI) of ED50 were calculated. Efficacy of a drug was defined as the percentage of animals tested that failed to respond to the 15 g VFF at the highest dose used, at which ED50 was determined (60 or 120 min).
Analysis of plasma drug concentration
For each of the drugs VPA, VPD, VCD and DID, nine rats (320–350 g) underwent SNL surgery, and 28 days later were randomly divided into three groups of three rats per group. VPA (300 mg kg− 1), VPD (60 mg kg− 1), VCD (40 mg kg− 1) and DID (40 mg kg− 1) were administered in 4 ml kg− 1 i.p. 0.5% MC, and 400 μl of blood were collected from the amputated tail tip at each of three time points after injection. Blood was withdrawn for group 1 at 15, 60 and 180 min after dosing; for group 2 at 30, 90 and 240 min after dosing and for group 3 at 45, 120 and 360 min after dosing. Plasma was immediately separated by centrifugation at 3000 × g for 15 min and stored at −20°C until analyzed.
Plasma levels of VPA, VPD, VCD and DID were determined by gas chromatography (GC). An HP5890 series II GC equipped with FID detector, HP7673 autosampler and for VPD, VCD and DID-HP-5 capillary column (0.25 μm × 15 m × 0.25 mm) was used. The temperature program was as follows: injector temperature 250°C; initial temperature, 60°C for 3 min; gradient of 15°C min− 1 until 180°C; hold 5 min. For each drug, plasma levels obtained from three rats at each time point were averaged. In all, 25 μl of internal standard solution (50 mg l− 1 of 2,2,3,3-tetramethylcyclopropanecarboxamide (TMCD), in MeOH for the VPD and VCD assay; VPD for the DID assay) and 200 μl of 0.1 M NaOH were added to 50 μl of plasma. The mixture was vortexed, and 1 ml of chloroform was added. The phases were separated, and the chloroform was evaporated. The dry residue was redissolved in 50 μl of chloroform, and 1 μl was injected into the gas chromatograph. Calibration curves were prepared separately by spiking naïve rat plasma samples with 1.56, 2.5, 3.12, 6.25, 12.5, 25.0, 37.5, 50.0, 75.0 and 100.0 mg l− 1 of VPD, VCD and DID. The limit of quantification (LOQ) of the method was 1.56 μg ml− 1, and the coefficient of variation (CV%) was <15% at all analyzed concentrations including LOQ.
For VPA determination, a HP-FFAP capillary column (0.3 μm × 25 m × 0.2 mm) was used. The temperature program was as follows: injector temperature 280°C; oven temperature, 135°C. In total, 30 μl of internal standard solution (250 mg l− 1 of 1-methyl-1cyclohexane carboxylic acid, MCCA, in MeOH) and 125 μl of 5 N hydrochloric acid (HCl) were added to 250 μl of plasma. The mixture was vortexed, and 2 ml of chloroform was added. The phases were separated, and the 1 ml of NaOH was added. HCl 5 N (200 μl) and 2 ml of chloroform were added to the aqueous phase. The organic layer was evaporated and dry residue was redissolved in 50 μl of chloroform, and 30 μl was injected into the gas chromatograph. Calibration curves were prepared separately by spiking naïve rat plasma samples with 25.0, 50.0, 75.0, 100.0, 200.0, 300.0, 400.0, 500.0 and 600.0 mg l− 1 of VPA. LOQ of the method was 25 mg l− 1, and the coefficient of variation (CV%) was <15% at all analyzed concentrations including LOQ.
PK
PK parameters were determined by noncompartmental analysis using the pharmacokinetic software package WinNonlin, version 4.0.1 (SCI Software, Lexington, KY, U.S.A.; Yamaoka et al., 1978; Gibaldi & Perrier, 1982; Rowland & Tozer, 1995). The terminal half-life (t1 / 2) was calculated as 0.693 / β, where β is the linear terminal slope of the concentration (C)-vs-time curve. The area under the C-vs-time curve (AUC) was calculated by trapezoidal rule with extrapolation to infinity. The mean residence time (MRT) was calculated from the quotient AUMC / AUC, where AUMC is the area under the concentration–time product vs time curve from zero to infinity. Total (apparent) clearance (CL / F) was calculated from the quotient of FDose / AUCm with F being the absolute bioavailability following i.p. administration. The apparent volume of distribution (Vβ / F) was calculated from the quotient of CL and β. The peak plasma concentration (Cmax) and the time to reach Cmax (tmax) were determined empirically by visual inspection.
Neurological motor dysfunction tests
We confirmed that for VPA, VPD, VCD and DID, the highest drug dose tested was not sufficient to cause neurological motor dysfunction or sedation as this might lead to false positive results in behavioral tests of sensory response (Yanez et al., 1990; Jourdan et al., 1997). In n=6 normal rats, without nerve injury (body weight 250–350 g), changes in motor performance after VPA, VPD, VCD and DID administration were measured using an accelerating rotarod (Columbus Instruments, Columbus, OH, U.S.A.). The rotarod speed was increased from 10 to 30 r.p.m. over a 120 s period, with the maximum time spent on the rod set at 120 s. For acclimation, rats received two training trials (separated by 3–4 h) on two separate days prior to drug testing. On the day of testing, a baseline response was obtained, and rats were subsequently administered drug or vehicle. Motor performance (the time (s) to fall off the rotarod) was tested at the time (after dosing) when the ED50 was determined, that is, 60 min (VPA, VPD, VCD) or 120 min (DID). In addition, we examined our rats individually for signs of motor dysfunction and sedation as assessed by posture and gate during spontaneous and induced movement, grooming, chewing, stepping reflex and startle reflex evoked by tapping on the cage.
Statistical analysis
Results are presented as the ED50 and 95% CI. For each drug and dose, attenuation of tactile allodynia (threshold measured in g) was measured. The average response of all time points after administration (30, 60, 120, 180 and 240 min) area under the response curve, was calculated and the pretreatment value was subtracted, per rat. These areas under the response curve values for every group of rats per dose were evaluated against vehicle treatment using the nonparametric Wilcoxon one-tailed test (dose of drug tested vs vehicle on the same treatment). The results for rotarod test (mean±s.d.) were evaluated by the same statistical test (highest dose of drug tested vs vehicle). A P-value <0.05 was considered statistically significant.
Results
Baseline tactile allodynia
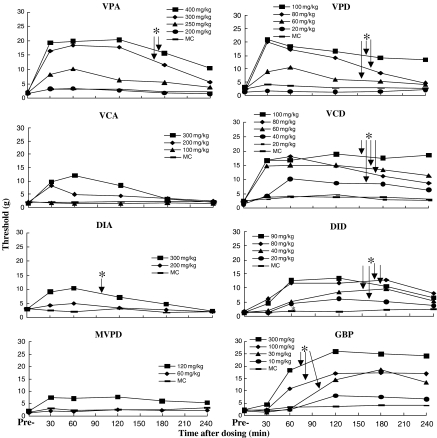
Animals were included in the experiment only if they failed to respond preoperatively to the 15 g stimulus. Following SNL surgery, animals showed brisk withdrawal responses to much weaker stimuli, reflecting tactile allodynia. Although the criterion set for inclusion in the study was response to <10 g, in fact nearly all animals responded to much weaker forces. Baseline response threshold, just before drug injection, was 1.9±1.3 g (n=114 rats). This level of response persisted in all vehicle control groups (Figure 2). In all rats, the contralateral (unoperated) hind paw failed to respond to any filament that applied <15 g at all postoperative time points. Table 1 is an example set of contralateral paw data of VPD 20 vs 60 mg kg− 1, i.p., vs MC.
Figure 2.
The effect of i.p. administration of VPA, VPD, VCA, VCD, DIA, DID, MVPD and GBP on tactile allodynia in rats with SNL neuropathy. Paw withdrawal thresholds to von Frey filaments were determined on both ipsilateral and contralateral paw prior to (Pre-) and up to 4 h after dosing, using a blinded regimen. Data show withdrawal threshold as mean values for the ipsilateral paw. None of the drugs affected contralateral withdrawal thresholds. *P<0.05 compared to vehicle (MC) by nonparametric Wilcoxon one-tailed test of area under the response curve 30–240 min after dosing (dose of drug tested vs vehicle). For clarity, s.d. error bars are not presented.
Table 1.
Contralateral paw data of VPD 20 vs 60 mg kg− 1 vs MC
| VPD dose (mg kg− 1) | |||
|---|---|---|---|
| Time | 20 | 60 | MC |
| Pre | 18.8±5.1 | 17.8±5.1 | 17.8±5.1 |
| 30 min | 21.9±5.7 | 20.5±5.9 | 19.1±5.7 |
| 60 min | 16.4±3.9 | 23.2±5.1 | 17.8±5.1 |
| 120 min | 19.1±5.7 | 17.8±5.1 | 19.1±5.7 |
| 180 min | 17.7±5.1 | 15.8±4.5 | 20.5±5.9 |
| 240 min | 16.4±3.9 | 19.1±5.7 | 17.8±5.1 |
Data presented as mean±s.d. withdrawal threshold (g).
MC=methylcellulose, vehicle.
PD: effect of drugs on tactile allodynia
All of the drugs tested except for VCA and MVPD showed significant, dose-dependent attenuation of tactile allodynia during the first few hours after i.p. injection (Figure 2). At the highest doses used, this antiallodynic drug effect was near maximal 30 min after injection. The only exceptions were DID and GBP, in which the degree of antiallodynia continued to increase after 30 min. The antiallodynic effect of the drugs began to decline 1–3 h post injection with the exception of VCD at 100 mg kg− 1 and GBP. For VPA, VPD, VCD and DID, the maximal dose used was less than that required to produce signs of neurological motor deficits or sedation.
VPA
VPA decreased tactile allodynia in a dose-dependent manner. The lowest effective (i.p.) dose was 300 mg kg− 1 (Figure 2). ED50 60 min after dosing was 269 mg kg− 1 (95% CI of 227–310 mg kg− 1). At the highest dose tested (400 mg kg− 1, i.p.), 88% (7 / 8) of rats were relieved from tactile allodynia.
VPD
VPD significantly increased the threshold at a minimal effective dose of 60 mg kg− 1, i.p. (Figure 2.) ED50 60 min after dosing was 61 mg kg− 1 (95% CI of 44–77 mg kg− 1). In all, 75% of rats failed to respond to monofilaments 15 g or less at a dose of 100 mg kg− 1, i.p.
VCA
VCA had no significant effect on tactile response threshold up to a dose of 300 mg kg− 1, i.p. (Figure 2 and Table 2), although 22% of rats failed to respond to the 15 g VFF or less at the highest dose tested.
Table 2.
Activity of VCA and DIA as antiallodynic agents at doses of 100, 200, 300 mg kg− 1
| Dose (mg kg− 1) | ||||
|---|---|---|---|---|
| Time | 100 | 200 | 300 | MC |
| VCA | ||||
| Pre | 2.1±0.9 | 1.9±0.5 | 1.5±1.1 | 1.8±1.3 |
| 30 min | 1.7±0.4 | 8.3±7.6 | 9.5±8.5 | 1.8±1.2 |
| 60 min | 1.5±0.2 | 5.0±2.9 | 12.0±9.1 | 1.8±1.6 |
| 120 min | 1.5±0.2 | 4.3±4.8 | 8.2±7.7 | 2.0±2.8 |
| 180 min | 1.7±0.5 | 3.3±3.5 | 3.2±2.6 | 2.3±0.4 |
| 240 min | 1.5±0.2 | 2.4±1.9 | 2.0±1.3 | 2.1±0.5 |
| DIA | ||||
| Pre | NT | 2.9±1.3 | 3.0±1.2 | 3.2±1.3 |
| 30 min | NT | 4.3±2.9 | 9.2±4.3 | 2.4±1.1 |
| 60 min | NT | 5.0±5.5 | 10.3±6.1 | 2.1±1.6 |
| 120 min | NT | 3.3±3.0 | 7.1±3.2 | 3.2±1.1 |
| 180 min | NT | 2.6±1.5 | 4.8±2.2 | 1.8±0.4 |
| 240 min | NT | 2.0±0.8 | 2.3±1.1 | 2.1±0.5 |
Data presented as mean±s.d. withdrawal threshold (g).
NT=not tested; MC=methylcellulose, vehicle.
VCD
The lowest dose to significantly increase the allodynic threshold, when compared to vehicle, was 40 mg kg− 1, i.p. (Figure 2). The mean effective dose (ED50) 60 min after dosing was 52 mg kg− 1 with a 95% CI of 20–84 mg kg− 1. At 100 mg kg− 1, i.p., 75% of rats were protected.
DIA
DIA was effective at a dose of 300 mg kg− 1, i.p. (Figure 2 and Table 2), 25% of rats were regarded as protected at the highest dose.
DID
The lowest dose needed to significantly increase the allodynic threshold, when compared to vehicle, was 20 mg kg− 1, i.p. (Figure 2). The response became larger in a dose-dependent manner at time points between 30 and 120 min. The mean effective dose (ED50) 120 min after dosing was 58 mg kg− 1 with a 95% CI of 18–97 mg kg− 1. There was no response to the 15 g VFF or less for 63% of rats at the highest dose tested.
MVPD
MVPD did not significantly increase the nociceptive threshold up to a dose of 120 mg kg− 1, i.p. (Figure 2), although 20% of rats failed to respond to the 15 g VFF or less at the highest dose tested. A dose of 180 mg kg− 1, i.p. caused motor dysfunction.
GBP
The lowest effective dose to produce a significant elevation of the threshold after dosing of GBP was 30 mg kg− 1, i.p. (Figure 2). ED50 120 min after dosing was 32 mg kg− 1 (95% CI of 18–50 mg kg− 1). At the highest dose used (300 mg kg− 1, i.p.) we observed minor sedation, 30 and 60 min after dosing, but not later and 100% of rats were protected. The antiallodynic effect and the neurological motor deficits of GBP were similar to those reported previously using the SNL assay (Hunter et al., 1997).
PK analysis
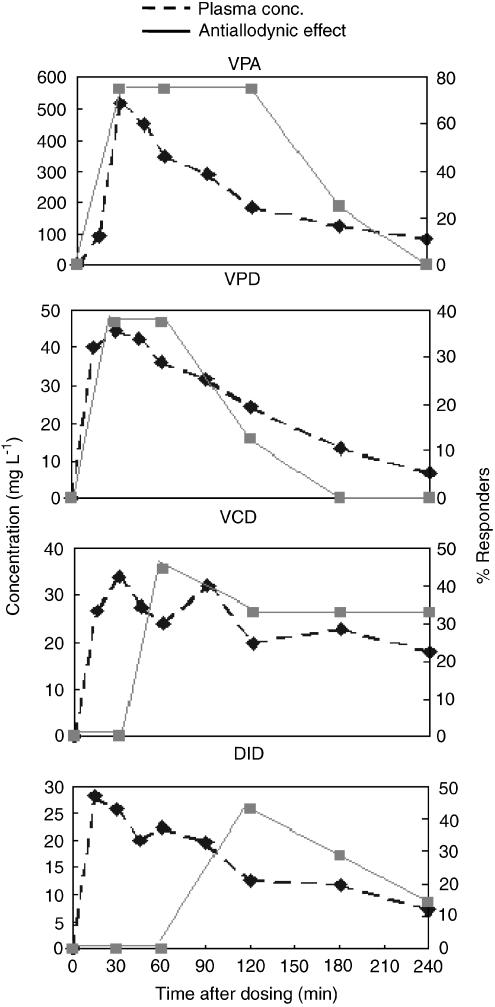
The pharmacokinetics of VPA, VPD, VCD and DID was evaluated at low effective antiallodynic doses as found in the PD study, 300, 60, 40 and 40 mg kg− 1, respectively. The PK parameters of each compound were calculated using a noncompartmental approach (Yamaoka et al., 1978; Gibaldi and Perrier, 1982) and are presented in Table 3. The antiallodynic effects of VPA, VPD, VCD and DID at these doses were associated with a minimal plasma concentrations of 125, 24, 18 and 7 mg l− 1, respectively (Figure 3).
Table 3.
Pharmacokinetic (PK) parameters of VPA, VPD, VCD and DID in the plasma after i.p. administration to SNL-operated rats at doses of 300, 60, 40 and 40 mg kg− 1, respectively
| PK parameters | VPA | VPD | VCD | DID |
|---|---|---|---|---|
| CL / F (l h− 1 kg− 1) | 0.2 | 0.6 | 0.3 | 0.5 |
| Vβ / F (l kg− 1) | 1.2 | 0.9 | 0.5 | 1.2 |
| T1 / 2 (h) | 4.6 | 1.0 | 1.2 | 1.5 |
| MRT (h) | 5.8 | 1.8 | 2.9 | 2.4 |
| Cmax (mg l− 1) | 515 | 44 | 37 | 28 |
| Tmax (min) | 30 | 30 | 90 | 15 |
F=absolute bioavailability; CL / F=clearance normalized by F; Vβ / F=volume of distribution normalized by F; t1 / 2=half-life; MRT=mean residence time; Cmax=peak plasma concentration; tmax=time to reach Cmax.
Figure 3.
PK–PD relationship. Time course of plasma concentration and antiallodynic effect following i.p. administration of VPA (300 mg kg− 1), VPD (60 mg kg− 1), VCD (40 mg kg− 1) and DID (40 mg kg− 1).
The PK parameters of VPA, VPD and VCD obtained in this study in operated rats (Table 3) were similar to those previously reported in nonoperated rats (Blotnik et al., 1996). VCD and DID are eliminated by metabolic clearance and they do not undergo hydrolytic biotransformation to their corresponding acids, VCA or DIA (Blotnik et al., 1996).
Rotarod test
VPA, VPD, VCD and DID had no significant effect (P>0.05, n=6) on motor performance (rotarod test) at the highest doses tested (400, 100, 100 and 90 mg kg− 1, i.p., respectively), at 60 or 120 min after dosing when compared to vehicle treatment (96.8±23.7, 98.4±19.4, 74.8±23.8 and 103.8±22.3 s, vs 118.0±2.3, respectively). MVPD at 180 mg kg− 1 but not at 120 mg kg− 1 caused convulsions.
Discussion
Antiallodynic potency and efficacy
In this study, we examined a closely related series of CNS-active isomers of VPA and its primary amide, VPD, in the SNL (Chung) model of neuropathic pain and compared their effects to those of VPA and GBP. The tested isomers were designed to retain the anticonvulsant activity, and by implication the antiallodynic activity of the parent compound VPA, but to avoid the teratogenicity and hepatotoxicity associated with VPA therapy. VPD, VCD and DID showed dose-related antiallodynic activity at doses that did not produce neurological motor deficits or sedation. Moreover, these compounds proved to have much better antiallodynic potency than VPA, with similar ED50 values to that of GBP (VCD-ED50=52 mg kg− 1, DID-ED50=58 mg kg− 1, VPD-ED50=61 mg kg− 1, GBP-ED50=34 mg kg− 1). The two structural isomers of VPA, VCA and DIA had similar antialodynic efficacy (Figure 2). However, due to high variation in the response to VCA, its effect was not statistically significant (Table 2).
The rank order of potency in suppressing allodynia, based on ED50, was GBP>VCD>DID>VPD>VPA. The rank order of efficacy at the maximal tolerated (subsedative) dose (depicted in Figure 2) was GBP>VPA>VCD=VPD>DID.
Structure–function relation: teratogenicity, hepatotoxicity and efficacy
Structure activity relationship studies indicated that in order to be teratogenic, VPA analogues and derivatives should fulfill three structural requirements: (1) posses a carboxylic acid, (2) possess a hydrogen atom at C-2 (the α-position to the carboxylic group) and (3) have branching at C-2 (Nau et al., 1991; Nau & Siemes, 1992; Bojic et al., 1996; 1998). A VPA isomer or analog lacking one of these structural requirements should not be teratogenic.
Unlike VPA's teratogenicity, that is caused by the parent compound (Nau, 1986), its hepatotoxicity is associated with P450-mediated metabolic pathways leading to the formation of hepatotoxic metabolites with a terminal double bond, 4-ene-VPA and 2,4-diene-VPA (Kondo et al., 1992; Baillie & Sheffels, 1995; Grillo et al., 2001). These metabolites are believed to form via an intermediary acyl-CoA thioester and blocking this step will lead to nonhepatotoxic VPA isomers (Grillo et al., 2001). Consequently, amides like VCD and DID, which undergo only minimal biotransformation to their corresponding acid (VCA and DIA) (Haj-Yehia & Bialer, 1990; Barel et al., 1997), cannot form the acyl-CoA thioester and therefore should not be hepatotoxic. Although VCD is a chiral molecule with two stereogenic carbons, the difference in anticonvulsant activity between racemic VCD and its individual stereoisomers was rather minor (Isoherranen et al., 2003a).
Taking into account the above structural issues, we designed and synthesized a series of CNS-active isomers and amide analogues of VPA expected to retain VPA's anticonvulsant activity but to be nonteratogenic and nonhepatotoxic (Isoherranen et al., 2003b). These are: VPD, VCA, VCD, DIA, DID and MVPD.
VCA and DIA have similar MES-ED50 in mice (269 and 238 mg kg− 1, respectively) but with no (DIA) or little (VCA) separation between doses that produce anticonvulsant activity and those that produce motor deficits and sedation (ED50=TD50). For this reason, their anticonvulsant effects were not tested in rats (Haj-Yehia & Bialer, 1989; 1990, Table 4). However, VPD and VCD were found to be broad-spectrum anticonvulsants with a wide safety margin between anticonvulsant activity and functional neurological toxicity (Table 4). DIA produced a significant decrease in allodynic response in the SNL model. Comparing VPA and DIA at a dose of 300 mg kg− 1 reveals that 75 and 25% of rats had an antiallodynic response, respectively. Owing to this relatively low percentage of responders, no further DIA doses were evaluated. VPD, VCD and DID are at least four times more potent than VPA and thus can be regarded as effective antiallodynic agents. However, MVPD does not possess any activity and is toxic at 180 mg kg− 1. In a previous study, we recently analyzed a series of cyclopropyl amide analogues of VPA in the SNL model (unpublished data). The antiallodynic activity of the leading cyclopropyl analogue was similar to that observed in this study with VPD, VCD and DID.
The peak plasma concentration (Cmax) for VPA, VPD and VCD was obtained 30 min after dosing and their maximal antiallodynic effects were observed shortly afterwards, with a reasonable parallel between plasma concentration and antiallodynic activity also at subsequent time points (Figure 3). DID was exceptional in that its Cmax was obtained 15 min after dosing, while its maximal antiallodynic effect was observed 120 min after dosing. This time of maximal PD effect was consistent with establish data on time to peak anticonvulsant effect of this compound. Since the mechanism of action of DID has not been characterized, plausible explanations for the lag time between DID maximal antiallodynic effect and its Cmax can be: (a) an effect compartment outside the plasma that is slowly equilibrated with the central compartment; (b) an antiallodynic effect that is caused by a cascade of physiological events and (c) active metabolite(s) (Rowland & Tozer, 1995).
Antiallodynic vs anticonvulsant activity
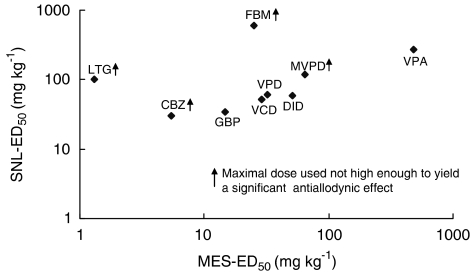
The proven analgesic activity of some AEDs in animal models of neuropathic pain, and in placebo-controlled clinical trials, naturally raises the suggestion that epilepsy and neuropathic pain share underlying neural mechanisms (Blom, 1962; McQuay et al., 1995; Zakrzewska et al., 1997; Rogawski & Loscher, 2004b). The availability of ED50 data for a series of new anticonvulsant isomers and analogues of VPD provides an opportunity for testing this hypothesis. In fact, plotting the ED50 values for these and other AEDs for which corresponding data are available (carbamazepine-CBZ, felbamate-FBM, lamotrigine-LTG) and our series of VPA analogues did not yield a significant overall positive correlation (Table 4, Figure 4). The rat-MES-ED50 data presented in Table 4 were taken from previous studies and were obtained following oral administration (Bialer et al., 1994), in collaboration with the NIH anticonvulsant screening program (White et al., 2002). Our previous studies showed that VCD's oral availability in dogs (Haj-Yehia & Bialer, 1988) and rats is complete (unpublished data). All marketed AEDs have complete oral absorption in animals and humans. GBP is only 60% absorbed in humans (Vajda, 2002) but its oral availability in rats is 79±11% (Radulovic et al., 1995). The complete oral availability of the drugs presented in Table 3 indicates the similarity between oral and i.p. administration. Some agents such as VPA, VPD, VCD and DID are nearly equipotent as anticonvulsants and analgesics (ED50 of MES / SNL 0.5–2.0). Others, however, are considerably more effective as anticonvulsants (CBZ, FBM, LTG).
Figure 4.
Plot of antiallodynic activity (SNL-ED50) and anticonvulsant activity (MES-ED50) in rats. The abbreviations for the various compounds are given in Table 4.
In a different study, several CNS-active amides of tetramethylcylcopropyl acid analogues of VPA have been assessed for their antiallodynic activity, utilizing the SNL model (unpublished data). All tested compounds showed dose-related reversal of tactile allodynia. 2,2,3,3-tetramethylcyclopropanecarboxylic acid (TMCA) and its primary amide 2,2,3,3-tetramethylcyclopropanecarboxamide (TMCD) were the only agents to exhibit antiallodynic effect without any anticonvulsant activity in the rat-MES test.
There are a number of possible explanations for this lack of overall correlation. First, although neuronal hyperexcitability may be a common denominator in epilepsy and neuropathic pain, there are diverse causes of hyperexcitability that are differentially susceptible to drug actions. For example, barbiturates and benzodiazepines suppress the hyperexcitability associated with seizure activity by enhancing inhibitory GABAergic neurotransmission (Macdonald, 2002; Olsen, 2002). These agents are ineffective as antiallodynics, probably because hyperexcitability associated with neuropathic pain is due primarily to changes in membrane hyperexcitability rather than synaptic function (Devor & Seltzer, 1999).
Drug access to active sites is another likely factor. Anticonvulsants necessarily act within the CNS. Antiallodynic agents, in contrast, may act exclusively in the PNS, or partly in the CNS and partly in the PNS. The agents included in Figure 4 vary in their ability to cross the BBB and access the brain (Table 4). PNS sites of neuronal hyperexcitability, the nerve injury site and dorsal root ganglion (DRG), are devoid of a blood–nerve-barrier (BNB) (Wadhwani & Rapoport, 1994; Devor, 1999). Different PK–PD characteristics could also contribute to differential drug potency in MES vs SNL. For these reasons, the failure of anticonvulsant and antiallodynic potency to correlate across AEDs does not seriously undermine the hypothesis that epilepsy and neuropathic pain share underlying neural mechanisms.
Mechanism of action
Pain following nerve damage is thought to result from a cascade of neurobiological events triggered in afferent conduction pathways that together result in neural hyperexcitability (Baranauskas & Nistri, 1998; Woolf & Mannion, 1999). These include, among others, upregulation of the expression of certain Na+ and Ca2 + channels in the axotomized primary sensory neurons, downregulation of certain K+ channels, increased levels of cytokines and other hyperalgesic substances in the spinal gray matter, and suppression of GABAergic neurotransmission in the spinal cord (Castro-Lopes et al., 1993; 1995; Lou et al., 2001; Moore et al., 2002; Lai et al., 2002; Watkins et al., 2003; Drew et al., 2004). Neural hyperexcitability is also the hallmark of epileptic seizure activity.
Effective AEDs and analgesics tend to act primarily on one or another of the processes that underlie neural hyperexcitability. For example, some block Na+ (CBZ, LTG) or Ca2 + channels (GBP, Lou et al., 2002), some enhance inhibitory (barbiturates, benzodiazepines) or suppress excitatory neurotransmission (NMDA-R antagonists), and some reduce levels of inflammatory mediators (e.g. NSAIDs. corticosteroids). Viable drug doses, and hence efficacy, tend to be limited by undesirable side effects such as sedation, nausea and toxicity. VPA stands out among the AEDs as a drug with multiple, simultaneous mechanisms of action: Na+ channel blockade, GABA enhancement, NMDA-R and AMPA-R antagonisms, and perhaps 5HT-R activation (Loscher, 1999; 2002). These actions bridge CNS and PNS mechanisms (Gilles et al., 2000). Simultaneous convergent actions on many target mechanisms, albeit each with relatively low affinity, implies that the dose-limiting side effect profile of the drug may be favorable. Indeed, except for the hepatotoxic and teratogenic effects of VPA observed in a small fraction of patients, the drug is well tolerated despite the high therapeutic plasma levels associated with epilepsy treatment (>0.35 mM or 50 mg l− 1, Levy et al., 2002).
Although the novel drugs tested here (VPD, VCA, VCD, DIA, DID and MVPD) were designed with the aim of avoiding teratogenicity and hepatotoxicity, all the amides had better potency as AEDs than VPA (Table 4). As antiallodynics VPD, VCD and DID were more potent than VPA. We do not know the reason for this, but presume that these valproylamides activate one or more of their presumed targets with higher affinity than VPA. VCD and DID are particularly promising agents as they showed antiallodynic activity at relatively low plasma concentrations (40 mg kg− 1 i.p., 18 and 7 mg l− 1 in plasma, respectively), and yielded the maximum possible antiallodynic effect at 100 and 90 mg kg− 1, i.p. with high tolerability as judged by the absence of noticeable motor impairment or sedation. We have not yet verified that VCD or DID has the same multiple physiological actions as VPA (Loscher, 1999). However, the structural modifications applied are not a priori expected to affect any of these actions. Overall, both VCD and DID appear to have considerable promise as lead compounds for novel analgesic drug development The fact that VCD has been utilized in Europe as an AED and antipsychotic drug and has gained clinical experience with no reported severe adverse reactions makes it an attractive choice.
Acknowledgments
The paper is abstracted from the Ph.D. thesis of Mr Ilan Winkler in partial fulfillment of requirements for the Ph.D. degree at The Hebrew University of Jerusalem. We thank Mr James Stables from the NIH-NINDS for testing the compounds in the Anticonvulsant Screening Program and Ms Pnina Raber and Anne Minert for their skillful technical assistance. This study was supported by grants from the United State-Israel Binational Science Foundation (BSF) and The Yeshaya Horowitz Fund, Jerusalem, Israel.
Abbreviations
- AED
antiepileptic drugs
- AUC
area under the concentration-vs-time curve
- AUMC
area under the concentration–time product vs time curve
- BBB
blood–brain barrier
- CBZ
carbamazepine
- CL
clearance
- Cmax
peak plasma concentration
- DIA
diisopropylacetic acid
- DID
diisopropylacetamide
- DRG
dorsal root ganglion
- FBM
felbamate
- GBP
gabapentin
- i.m.
intramuscularly
- i.p.
intraperitoneally
- LTG
lamotrigine
- MC
methylcellulose
- MES
maximal electroshock
- MRT
mean residence time
- MVPD
N-methyl-valpromide
- PD
pharmacodynamics
- PK
pharmacokinetics
- VCA
valnoctic acid
- VCD
valnoctamide
- VFF
von Frey monofilaments
- VPD
valpromide
- Vβ
volume of distribution
- VPA
valproic acid
- SNL
spinal nerve ligation
- TMCA
2,2,3,3-tetramethylcyclopropanecarboxylic acid
- TMCD
2,2,3,3-tetramethylcyclopropanecarboxamide
References
- BAILLIE T.A., SHEFFELS P.R.Valproic acid, chemistry and biotransformation Antiepileptic Drugs 1995New York: Raven Press; 589–603.4th edn. ed. Levy, R.H, Mattson, R.H. & Meldrum, B.S., pp [Google Scholar]
- BARANAUSKAS G., NISTRI A. Sensitization of pain pathways in the spinal cord: cellular mechanism. Prog. Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- BAREL S., YAGEN B., SCHURIG V., SOBACK S., PISANI F., PERUCCA E., BIALER M. Stereoselective pharmacokinetic analysis of valnocatmide in healthy subjects and epileptic patients. Clin. Pharmacol. Ther. 1997;61:442–449. doi: 10.1016/S0009-9236(97)90194-6. [DOI] [PubMed] [Google Scholar]
- BIALER M. Clinical pharmacology of valpromide. Clin. Pharmacokinet. 1991;20:114–122. doi: 10.2165/00003088-199120020-00003. [DOI] [PubMed] [Google Scholar]
- BIALER M., HAJ-YEHIA A., BADIR K., HADAD S. Can we develop improved derivatives of valproic acid. Pharm. World Sci. 1994;16:2–6. doi: 10.1007/BF01870931. [DOI] [PubMed] [Google Scholar]
- BLOM S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug. Lancet. 1962;1:839–840. doi: 10.1016/s0140-6736(62)91847-0. [DOI] [PubMed] [Google Scholar]
- BLOTNIK S., BERGMAN F., BIALER M. Disposition of valpromide, valproic acid and valnoctamide in the brain, liver, plasma and urine of rats. Drug Metab. Dispos. 1996;24:560–564. [PubMed] [Google Scholar]
- BOJIC U., EHLERS K., ELLERBECK U., BACON CL., O'DRISCOLL E., O'CONNELL C., BEREZIN V., KAWA A., LEPEKHIN E., BOCK E., REGAN C.M., NAU H. Studies on the teratogen pharmacophore of valproic acid analogues: evidence of interactions at a hydrophobic centre. Eur. J. Pharmacol. 1998;354:289–299. doi: 10.1016/s0014-2999(98)00462-2. [DOI] [PubMed] [Google Scholar]
- BOJIC U., ELMAZAR M.M.A., HAUCH R-S., NAU H. Further branching of valproate-related carboxylic acid reduces the teratogenic activity, but not the anticonvulsant activity. Chem. Res. Toxicol. 1996;9:866–870. doi: 10.1021/tx950216s. [DOI] [PubMed] [Google Scholar]
- CASTRO-LOPES J.M., MALCANGIO M., PAN B.H., BOWERY N.G. Complex change of GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995;679:289–297. doi: 10.1016/0006-8993(95)00262-o. [DOI] [PubMed] [Google Scholar]
- CASTRO-LOPES J.M., TAVARES I., COIMBRA A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- DEVOR M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;6:S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- DEVOR M., SELTZER Z.Pathophysiology of damaged nerves in relation to chronic pain Textbook of Pain 1999Edinburgh: Churchill Livingstone; 129–164.4th edn. ed. Wall, P.D. & Melzack, R, pp [Google Scholar]
- DREW G.M., SIDALL P.J., DUGGAN A.W. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- FINNEY D.J. Probit Analysis 1971Cambridge, U.K.: Cambridge University Press; 3rd edn [Google Scholar]
- GIBALDI M., PERRIER D. Pharmacokinetics 1982New York: Marcel Dekker; 199–219.2nd edn. pp [Google Scholar]
- GILLES N., CHEN H., WILSON H., LE GALL F., MONTOYA G., MOLGO J., SCHONHERR R., NICHOLSON G., HEINEMANN S.H., GORDON D. Scorpion alpha and alpha-like toxins differentially interact with sodium channels in mammalian CNS and periphery. Eur. J. Neurosci. 2000;12:2823–2832. doi: 10.1046/j.1460-9568.2000.00168.x. [DOI] [PubMed] [Google Scholar]
- GRILLO M.P., CHIELLINI G., TONELLI M., BENET L.Z. Effect of alpha-fluorination of valproic acid on valproyl-S-acyl-CoA formation in vivo in rats. Drug Metab. Dispos. 2001;29:1210–1215. [PubMed] [Google Scholar]
- HAJ-YEHIA A., BIALER M. Pharmacokinetics of a valrpomide isomer, valnoctamide in dogs. J. Pharm. Sci. 1988;77:831–834. doi: 10.1002/jps.2600771003. [DOI] [PubMed] [Google Scholar]
- HAJ-YEHIA A., BIALER M. Structure-pharmacokinetic relationship in series of valpromide isomers with entiepileptic activity. Pharm. Res. 1989;6:683–689. doi: 10.1023/a:1015934321764. [DOI] [PubMed] [Google Scholar]
- HAJ-YEHIA A., BIALER M. Structure-pharmacokinetic relationship in series of short fatty acid amides that possess anticonvulsant activity. J. Pharm. Sci. 1990;79:719–724. doi: 10.1002/jps.2600790814. [DOI] [PubMed] [Google Scholar]
- HARRIS M., CAHNDRAN S., CHAKRABORTY N., HEALTY D. Mood-satbilizers: the archeology of the concept. Bipolar Disord. 2003;5:446–452. doi: 10.1046/j.1399-5618.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- HUNTER J.C., GOGAS K.R., HEDLEY L.R., JACOBSON L.O., KASSOTAKIS L., TOMPSON J., FONTANA D.J. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur. J. Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- ISOHERRANEN N., WHITE H.S., KLEIN B.D., ROEDER M., WOODHEAD J.H., SCHURIG V., YAGEN B., BIALER M. Phramacokinetic–pharmacodynamic relationships of (2S,3S)-valnoctamide and its stereosiomer (2R,3S)-valnocatmide in rodent models of epilepsy. Pharm. Res. 2003a;20:1293–1301. doi: 10.1023/a:1025069519218. [DOI] [PubMed] [Google Scholar]
- ISOHERRANEN N., YAGEN H., BIALER M. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet. Curr. Opin. Neurol. 2003b;16:203–211. doi: 10.1097/01.wco.0000063774.81810.30. [DOI] [PubMed] [Google Scholar]
- JENSEN S.T. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur. J. Pain. 2002;6:61–68. doi: 10.1053/eujp.2001.0324. [DOI] [PubMed] [Google Scholar]
- JOURDAN D., ARDID D., BARDIN M., NEUZERET D., LANPHOUTHACOUL L., ESCHALIER A. A new automated method of pain scoring in the formalin test in rats. Pain. 1997;71:265–270. doi: 10.1016/s0304-3959(97)03366-6. [DOI] [PubMed] [Google Scholar]
- KIM S., CHUNG J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- KOCHAR D.K., JAIN N., AGARWAL R.P., SRIVASTAVA T., AGARWAL P., GUPTA S. Sodium valproate in the management of painful neuropathy in type 2 diabetes – a randomized placebo controlled study. Acta. Neurol. Scand. 2002;106:248–252. doi: 10.1034/j.1600-0404.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- KOCHAR D.K., RAWAT N., AGRAWAL R.P., VYAS A., BENIWAL R., KOCHAR S.K., GARG P. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. Q. J. Med. 2004;97:33–38. doi: 10.1093/qjmed/hch007. [DOI] [PubMed] [Google Scholar]
- KOCHAR D.K., GARG P., BUMB R.A., KOCHAR S.K., MEHTA R.D., BENIWAL R., RAWAT N. Divalproex sodium in the management of post-herpetic neuralgia: a randomized double-blind placebo-controlled study. QJ Med. 2005;98:29–34. doi: 10.1093/qjmed/hci005. [DOI] [PubMed] [Google Scholar]
- KONDO T., KANEKO S., OTANI K., ISHID A.M., HIRANO T., FUKUSHIMA Y., MURANAKA H., KOIDE N., YOKOYAMA M. Associations between risk factors for valproate hepatotoxicity and altered valproate metabolism. Epilepsia. 1992;33:172–177. doi: 10.1111/j.1528-1157.1992.tb02302.x. [DOI] [PubMed] [Google Scholar]
- LAI J., GOLD M.S., KIM C.S., BIAN D., OSSIPOV M.H., HUNTER J.C., PORRECA F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin resistant sodium channel. NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- LEVY R.H., SHEN D.D., ABBOTT F.S., RIGGS W., HACHAD H.Valproic acid, chemistry and biotransformation Antiepileptic Drugs 2002Philadelphia: Lipincott Williams & Wilkins; 780–800.5th edn. ed. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E. pp [Google Scholar]
- LOSCHER W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- LOSCHER W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- LOU Z.D., CHAPLAN S.R., HIGUERA E.S., SORKIN L.S., STAUDERMAN K.A., WILLIAMS M.E., YAKSH T.L. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. Neuroscience. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOU Z.D., CALCUTT N.A., HIGUERA E.S., VALDER C.R., SONG Y.-H., SVENSSON C.I., MYERS R.R. Injury type-spesific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- MACDONALD R.L.Benzodiazepines – mechanisms of action Antiepileptic drugs 2002Philadelphia: Lipincott Williams & Wilkins; 489–495.5th edn. ed. Levy, R.H, Mattson, R.H., Meldrum, B.S. & Perucca, E. pp [Google Scholar]
- MCQUAY H., CARROLL D., JADAD A.R., WIFFEN P., MOORE R.A. Anticonvulsant drugs for management of pain: a systematic review. Br. Med. J. 1995;311:1047–1052. doi: 10.1136/bmj.311.7012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE K.A., KOHNO T., KARCHEWSKI L.A., SCHOLZ J., BABA H., WOOLF C.J. Partial peripheral injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAU H. Valproic acid teratogenesis in mice after various administration and phenobarbital-pretreatment regimens: the parent drug and not of the metabolites assayed in is implicated as teratogen. Fund. Appl. Toxicol. 1986;6:662–668. doi: 10.1016/0272-0590(86)90179-x. [DOI] [PubMed] [Google Scholar]
- NAU H., HAUCK R.-S., EHELRS K. Valproic acid induced neural tube defects in mouse and humans: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol. Toxicol. 1991;69:310–321. doi: 10.1111/j.1600-0773.1991.tb01303.x. [DOI] [PubMed] [Google Scholar]
- NAU H., SIEMES H. Differentiation between valproate-induced anticonvulsant effect. Tertaogencity and hepatotoxicity. Pharm. Weekblad Sci. Edit. 1992;14:101–107. doi: 10.1007/BF01962697. [DOI] [PubMed] [Google Scholar]
- NICHOLSON B. Gabapentin use in neuropathic pain syndromes. Acta. Neurol. Scand. 2000;101:359–371. doi: 10.1034/j.1600-0404.2000.0006a.x. [DOI] [PubMed] [Google Scholar]
- OKADA A., KURIHARA H., AOKI Y., BIALER M., FUJIWARA M. Amidic modification of valproic acid reduces skeletal teratogenicity in mice. Birth Defects Res. B. Dev. Reprod. Toxicol. 2004;71:47–53. doi: 10.1002/bdrb.10057. [DOI] [PubMed] [Google Scholar]
- OLSEN R.W.Phenobarbital and other barbiturates-Mechanisms of action Antiepileptic Drugs 2002489–495.5th edn. ed. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E. pp
- OTTO M., BACH F.W., JENSEN T.S., SINDRUP S.H. Valproic acid has no effect on pain in polyneuropathy: a randomized, controlled trial. Neurology. 2004;27:285–288. doi: 10.1212/wnl.62.2.285. [DOI] [PubMed] [Google Scholar]
- OWENS M.J., NEMEROFF C.B. Pharmacology of valproate. Psychopharmacol. Bull. 2003;37 Suppl 2:17–24. [PubMed] [Google Scholar]
- PAYEN C., FRANTZ P., MARTIN O., PARANT F., MOULSMA M., PLACE C., DESCOTES J. Delayed toxicity following acute ingestion of valpromide. Hum. Exp. Toxicol. 2004;23:145–148. doi: 10.1191/0960327104ht430oa. [DOI] [PubMed] [Google Scholar]
- PELLOCK JM, PERHACH JL, SOFIA RD.Felbamate Antiepileptic Drugs 2002Philadelphia: Lippincott Williams & Wilkins; 301–318.5th edn. ed. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E. pp [Google Scholar]
- RADATZ M., EHLERS K., YAGEN B., BIALER M., NAU H. Valnoctamide, valpromide and valnoctic acid are much less teratogenic in mice than valproic acid. Epilepsy Res. 1998;30:41–48. doi: 10.1016/s0920-1211(97)00095-8. [DOI] [PubMed] [Google Scholar]
- RADULOVIC L.L., TURCK D., VON HODENBERG A, VOLLMER O., NCNALLY W.P., DEHARDT P.D., HANSON B.J., BOCKBRADER H.N., CHANG T. Disposition of gabapentin (neutontin) in mice, rats, dogs and monkeys. Drug Metab. Dispos. 1995;23:441–448. [PubMed] [Google Scholar]
- ROGAWSKI M.A., LOSCHER W. The neurobiology of antiepileptic drugs. Neuroscience. 2004a;5:553–562. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- ROGAWSKI M.A., LOSCHER W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat. Med. 2004b;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- ROWLAND M., TOZER T. Clinical Pharamcokeintics. Baltimore: Williams & Wlkins; 1995. Pharmacological Response; pp. 340–365. [Google Scholar]
- SPINA E.Carbamazepine-chemistry, biotarnsoformation, pharmacokinetics and interactions Antiepileptic Drugs 2002Philadelphia: Lippincott Williams & Wilkins; 232–246.5th edn. ed. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E., pp [Google Scholar]
- TREMONT-LUKATS W.I., MEGEFF C., BACKONJA M.M. Anticonvulsants for neuropathic pain syndromes mechanisms of action and place in therapy. Drugs. 2000;60:1029–1052. doi: 10.2165/00003495-200060050-00005. [DOI] [PubMed] [Google Scholar]
- VAJDA F.J.E.Gabapentin-chemistry, biotarnsoformation, pharmacokinetics and interactions Antiepileptic Drugs 2002Philadelphia: Lippincott Williams & Wilkins; 335–339.5th edn. eds. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E., pp [Google Scholar]
- WADHWANI K.C., RAPOPORT S.I. Transport properties of vertebrate blood-nerve barrier: comparison with blood–brain barrier. Prog. Neurobiol. 1994;43:235–279. doi: 10.1016/0301-0082(94)90002-7. [DOI] [PubMed] [Google Scholar]
- WALKER M.C., TONG X., PERRY H., ALAVIJEH M.S., PATSALOS P.N. Comparison of serum, cerebrospinal fluid and brain extracellular fluid pharmacokinetics of lamotrigine. Br. J. Pharmacol. 2000;130:242–248. doi: 10.1038/sj.bjp.0703337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS L.R., MILLIGAN E.D., MAIER S.F. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- WHITE H.S., WOOHHEAD J.H., WILCOX K.S., STABLES J.P., KUPFERBERG H.J., WOLF H.H.Discovery and preclinical development of antiepileptic drugs Antiepileptic Drugs 2002Philadelphia: Lippincott Williams & Wilkins; 36–48.5th edn. ed. Levy, R.H., Mattson, R.H., Meldrum, B.S. & Perucca, E. pp [Google Scholar]
- WOOLF C.J., MANNION R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- YAMAOKA K., NAKAGAWA T., UNO T. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 1978;6:547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- YANEZ A., SABBE M.B., STEVENS C.W., YAKSH T.L. Interaction of midazolam and morphine in the spinal cord of the rat. Neuropharmacology. 1990;29:359–364. doi: 10.1016/0028-3908(90)90094-8. [DOI] [PubMed] [Google Scholar]
- ZAKRZEWSKA J.M., CHAUDHRY Z., NURMIKKO T.J., PATTON D., MULLENS E.L. Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73:223–230. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]