Abstract
The study was performed to clarify if apomorphine at the level of the rat corpus cavernosum can produce erectile responses or interfere with nerve-induced penile erection.
Apomorphine (10−9–10−4 M) exhibited a 10-fold higher potency to relax phenylephrine (Phe)- than endothelin-1 (ET-1)-induced contractions. Relaxant effects of apomorphine in Phe-activated corpus cavernosum did not change tissue levels of cyclic nucleotides, and were unaffected by inhibition of the synthesis of nitric oxide, or by inhibition of the soluble guanylate cyclase.
Relaxations by apomorphine of ET-1-contracted rat corpus cavernosum were not influenced by α2-adrenoceptor blockade (yohimbine, 10−7 M), or by the dopamine D1-like receptor antagonist SCH 23390 (10−6 M). Clozapine (10−6 M), a proposed dopamine D2-like receptor antagonist, partly reduced apomorphine-induced relaxations, and significantly altered the −log IC50 value for apomorphine.
Nerve-induced contractions of the rat corpus cavernosum were attenuated by apomorphine in a concentration-dependent and biphasic manner. Yohimbine (10−7 M) abolished the biphasic concentration–response pattern. SCH 23390 (10−6 M) attenuated the inhibitory effects of apomorphine on contractions, and significantly altered the −log IC50 value for the compound.
In anesthetized rats (50 mg kg−1 pentobarbital sodium, 10 mg kg−1 ketamine), intracavernous apomorphine (100, 300, or 1000 nmol) did not have effects on basal cavernous pressure under resting conditions, and did not affect filling or emptying rates, or peak pressures of the rat corpus cavernosum during submaximal activation of the cavernous nerve. In awake rats, apomorphine produced a maximal number of erections at 300 nmol kg−1.
In the rat isolated corpus cavernosum, pre- and postjunctional effects of apomorphine appear to involve dopamine D1- and D2-like receptors, as well as α-adrenoceptors. At relevant systemic doses of apomorphine, peripheral effects of the compound are unlikely to contribute to its proerectile effects in rats.
Keywords: Apomorphine, dopamine receptors, α-adrenoceptors, relaxation, corpus cavernosum, rat
Introduction
Dopaminergic pathways in the central nervous system (CNS) are of importance for male sexual behavior and penile erection (see Hull et al., 1999; Giuliano & Rampin, 2000; Andersson, 2001). Depending on their molecular and pharmacological properties, five subtypes of dopamine receptors, denoted D1–D5, can be distinguished and further grouped into two families, the dopamine D1-like (D1 and D5) and D2-like receptors (D2, D3, D4; see Sibley, 1999). Apomorphine is a nonselective dopamine receptor agonist that can elicit erectile responses after systemic, intrahypothalamic, intracerebroventricular, or intrathecal administration (see Andersson, 2001; Giuliano et al., 2001). It is not known which of the dopamine receptors in the CNS that mainly are responsible for the regulation of sexual functions in humans, or which dopamine receptor subtype(s) that apomorphine interact with to produce erectile responses (see Andersson, 2001; Giuliano et al., 2001).
Dopamine receptor proteins have been localized to the adventitia and media of systemic arteries (Amenta et al., 2000; Zeng et al., 2004). In mesenteric, aortic coronary, renal, lumbar, mammary, hinquarters, and forearm vascular beds or arterial preparations from various mammals, dopamine receptor agonists or apomorphine have been shown to produce vasorelaxant effects (Anwar & Mason, 1981; Ventura et al., 1984; Gyorgy & Doda, 1985; Hughes et al., 1986; 1987; Kopia & Valocik, 1989; van der Niepen et al., 1991; Han et al., 1999; Teisman et al., 2000; Zeng et al., 2004). Responses to dopamine receptor activation appear to vary depending on which arterial preparation that is studied, and some investigators have found vasoconstrictive actions of dopamine receptor agonists in postglomerular arteries, and in tail arteries from rats (Rashed & Songu-Mize, 1995; Muhlbauer et al., 2000). Dopamine receptors have also been located on nerves of the peripheral vasculature, and apomorphine has been shown to reduce the release of noradrenaline from noradrenergic nerve terminals, and to decrease nerve-mediated vasoconstriction in rats (see Soares-da-Silva, 1987; Hietala, 1988; Morgadinho et al., 1999; Murphy, 2000).
Messenger RNA for dopamine D1- and D2-like receptors has also been detected in the rat corpus cavernosum, and the respective proteins were found expressed in the nerves and vasculature of the rat erectile tissues (Hyun et al., 2002), and in human corpus cavernosum smooth muscle cells (d'Emmanuele di Villa Bianca et al., 2004). Functional effects of apomorphine in the corpus cavernosum tissue have not been thoroughly investigated. The aims of the present study was to clarify if actions by apomorphine in the rat corpus cavernosum contribute to the proerectile effect by the compound, and to try to evaluate by which mechanism(s) apomorphine may influence tonus-regulatory functions in this tissue.
Methods
Animals
In all, 67 male Sprague–Dawley rats (280–350 g) were used. The rats were kept and cared for in standard cages under clean conditions in separate quarters in a 12–12 h light to darkness cycle with free access to water and pellets. The Animal Ethics Committee of Lund University approved the experiments performed.
Functional in vitro experiments
Isometric tension measurements
In total, 41 animals were killed by carbon dioxide asphyxia followed by exsanguination. The penises were removed and placed in chilled Krebs solutions. As described previously (Hedlund et al., 1999), the tunica albuginea was carefully opened, and the erectile tissue was microsurgically dissected free. Silk ligatures were applied at both ends of the strip preparations (4 × 0.5 × 0.5 mm), which were then suspended between two metal prongs in thermostatically controlled organ baths (5 ml, 37°C) containing Krebs solution, routinely changed every 20 min, and bubbled with a mixture of 95% O2 and 5% CO2 (pH 7.4). Isometric tension was recorded by means of Grass Instruments FT03C force transducers connected to Grass 7D polygraph (Grass Instruments Co., Boston, MA, U.S.A.).
Experimental procedure
During an equilibration period of 40 min, tension was adjusted until a mean stable tension of 1.4±0.05 mN(n=53, N=41) was obtained. Contractile abilities of the preparations were established by adding a K+ solution (124 mM) to the organ baths, producing a mean contractile response of 6.2±0.2 mN (n=53, N=41). Concentration–response curves for L-phenylephrine (Phe, 10−9–10−4 M) or endothelin-1 (ET-1, 10−12–10−6 M) were determined by cumulative administration of the respective agonist. The agonist concentrations used (Phe, 3 × 10−6 M; ET-1, 3 × 10−8) corresponded to their approximate EC50 values, and produced stable and reproducible contractions. The cumulative effects of apomorphine (10−9–10−4 M) on Phe- or ET-1-contracted CC preparations were studied. In some experiments, effects of the compounds were investigated after pretreatment with NG-nitro-L-arginine (L-NNA) (10−4 M), or 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (10−5 M). In separate experiments, DMSO was used as an antioxidant vehicle for apomorphine. In ET-1-contracted preparations, effects of apomorphine were investigated with or without pretreatment with (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390) (10−6 M) or clozapine (10−6 M).
Electrical field stimulation was performed using two platinum electrodes, placed in parallel to strips in the organ baths. Single square-wave pulses at supramaximal stimulation (18 Hz, 20 V), and with a duration of 0.3 ms, were delivered by a Grass S48 stimulator. The train duration was 5 s and the train interval 120 s. Frequency–response relationships were determined, and when investigating the effect of drugs on electrically induced contractions, a frequency-producing activity that corresponded to 70% of maximal contraction was chosen. A preparation was regarded as stable when the amplitude of three consecutive electrically induced contractions did not differ by more than 5%. Apomorphine was then added cumulatively. The degree of inhibition was expressed as a percentage of the contraction elicited prior to the addition of the lowest concentration of drugs.
Measurements of guanosine and adenosine 3′5′-cyclic monophosphate (cGMP and cAMP)
Concentrations of cGMP and cGMP were analyzed in Phe-contracted CC preparations (controls) and in Phe-contracted preparations exposed to apomorphine (10−4 M). When the relaxant effects of apomorphine had reached steady levels, usually within 4 min, the strips were snap-frozen in liquid nitrogen and then stored in 0.5 ml of 10% trichloracetic acid at −70°C. The tissue was homogenized and then centrifuged. The pellets were dissolved and the protein content determined by the assay described by Bradford (1976). The supernatants were extracted five times and the aqueous phases were evaporated and the residues stored at −20°C. The amounts of cGMP and cAMP were quantified by using [125I]-cGMP and [125I]-cAMP RIA kits (RIANEN, Du Pont Company, Boston, MA, U.S.A.).
In vivo experiments
Intracavernous (i.c.) pressure registration in awake animals
In all, 38 male Sprague–Dawley rats (350–420 g) were anesthetized with pentobarbital sodium (Apoteket, Umeå, Sweden; 50 mg kg−1) and ketamine (Parke Davis, Barcelona, Spain; 10 mg kg−1) given intraperitoneal (i.p.) injection. Through a midline scrotal incision, entrance to the base of the penis was given. Using a 25 G needle, a small entry hole was made in the tunica albuginea of the crus cavernosum on one side and a collar-fitted heparinized (100 IE ml−1) PE50 polyethylene catheter (Becton Dickinson, Franklin Lakes, NJ, U.S.A.) was introduced into the erectile compartment. The catheter was secured by a 5/0 pouch-suture (Ethicon, Somerville, NJ, U.S.A.) in the fibrous tunica. The scrotal skin was sutured and the catheter tunnelated subcutaneously (s.c.) to the neck where it was anchored to the skin by 3/0 sutures (Ethicon, Somerville, NJ, U.S.A.). For administration of apomorphine, a PE10 polyethylene catheter (Becton Dickinson, NJ, U.S.A.) was placed s.c. and anchored to the skin of the neck.
Continuous direct measurements of i.c. pressures were performed 12 h after the implantation. Pressures were recorded with pressure transducers (OHMEDA, Model P23 XL-1, Singapore) and registered on a Grass Polygraph 7E (Grass Instrument Co., Boston, MA, U.S.A.). Apomorphine (100 μg kg−1 (300 nmol kg−1)) or vehicle (saline) was then given by s.c. injection and changes in i.c. pressure were registered during 30 min after injection of the drug or vehicle. Basal intracavernous pressure (BICP), the total number of responses, total duration of responses (the added duration of all responses), time to the first response, and peak intracavernous (PICP) were analyzed in each rat.
I.c. pressure registration and cavernous nerve stimulation
Pentobarbital sodium (50 mg kg−1; Apoteket, Umeå, Sweden) and ketamine (Ketalar®, Parke Davis, Barcelona, Spain; 10 mg kg−1), given i.p., were used for anesthesia. During the experiment, the animals (n=6) breathed spontaneously. Through a lower abdominal incision, access was given to pelvic viscera. With a midline incision in the perineum, the base of the penis was made visible. The ischiocavernous muscle covering the corpus cavernosum was bluntly divided on one side, and entrance to the underlying tunica albuginea of the crus of the corpus cavernosum was given. A 25 G needle attached to a heparinized (100 IE ml−1) polyethylene catheter (Clay Adams PE-10, Parsippany, NJ, U.S.A.) was inserted into the crus. A 27 G needle was placed into the same crus for administration of drugs. A heparinized polyethylene catheter was introduced into the carotid artery to monitor blood pressure. Continuous direct measurements of mean arterial blood pressure (MAP) and i.c. pressure were performed with pressure transducers (OHMEDA, Model P23 XL-1, Singapore) and registered by a Grass Polygraph 7E (Grass Instrument Co., Boston, MA, U.S.A.). The effect of i.c. apomorphine (100, 300, or 1000 nmol) on i.c. pressure was investigated in separate experiments.
Electrical stimulation of the cavernous nerve was performed via a slender bipolar platinum electrode, which was connected to a S48 stimulator (Grass Instrument Co., Boston, MA, U.S.A.). Each stimulation had a duration of 30 s. A resting interval of 5 min was allowed between stimulations. Voltage–response relationships (2.5, 5, 7.5, and 10 V) were determined at 20 Hz. A submaximal voltage producing approximately 50% of maximal i.c. pressure was used when investigating the effects of apomorphine (100, 300, or 1000 nmol) on nerve-induced erectile responses. The effects of apomorphine on i.c. pressure responses to cavernous nerve stimulation were evaluated 3 min after administration of the drug to the erectile compartment. Before stimulation, BICP was noted.
During tumescence, the PICP and time for the i.c. pressure to reach 80% of maximal increase (PICP-BICP) were recorded. At this point, the increase in i.c. pressure per second (ΔT80) was evaluated. After stimulation, during detumescence, the rate (ΔD20) for i.c. pressure to decrease to 20% of maximal increase was determined. A stabilizing period of 20–30 min was allowed before registration of BICP and arterial blood pressures. Drugs were given i.c. in volumes of 50 μl immediately followed by an additional 100 μl of vehicle to ensure appropriate delivery of the compound to the i.c. compartment. The latter volume was the measured dead-space of the catheter.
Solutions and drugs
A saline solution, containing 154 mM NaCl, and the Krebs solution was composed as follows (mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaH2PO4 1.2, and glucose 5.5. A high K+ solution (124 mM) was used, in which the NaCl in normal Krebs solution was replaced by equimolar amounts of KCl. The following drugs were used: apomorphine, l-Phe, and ET-1 (Peninsula, St Helens, U.K.), ODQ (Tocris Cookson Ltd, Bristol, U.K.), SCH 23390, yohimbine, L-NNA, and clozapine (Sigma Chemical Co., St Louis, MO, U.S.A.). Clozapine was dissolved with DMSO (Sigma Chemical Co., St Louis, MO, U.S.A.), and kept as stock solutions (10−2 M). All other drugs were dissolved in saline.
Calculations
Student's paired or unpaired two-tailed t-tests were used for statistical comparisons of two means. ANOVA with a Bonferoni post hoc test was used for the comparison of multiple means. A probability of P<0.05 was accepted as significant. When appropriate, results are given as mean±standard error of the mean (s.e.m.). Small n denotes the number of strip preparations, and capital N denotes the number of individuals. All statistical calculations were based on N.
Results
Functional in vitro experiments
Spontaneous contractile activity was not observed in any of the rat isolated corpus cavernosum preparations. Reproducible and stable contractile responses to Phe (3 × 10−6 M), or ET-1 (3 × 10−8 M), were obtained and amounted to 3.0±0.2 mN (n=21, N=15), and 3.9±0.3 mN (n=28, N=20), respectively.
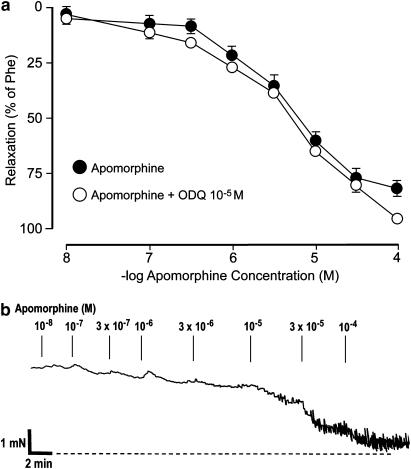
Apomorphine (10−9–10−4 M) relaxed Phe-contracted (3 × 10−6 M) corpus cavernosum preparations in a concentration-dependent manner (Figure 1). A mean relaxant effect of 60.4±4.4% (n=10, N=5) was obtained at 10−5 M, and at 10−4 M, the highest concentration investigated, the relaxant effect was 86.3±3.4% (n=10, N=5). The −log IC50 value for apomorphine in Phe-activated corpus cavernosum preparations amounted to 5.17±0.11. There was no difference between the relaxant effects of apomorphine when the compound was dissolved in DMSO vehicle (n=5, N=5) and in saline. DMSO per se did not affect Phe-induced contraction in control experiments. Pretreatment with L-NNA (10−4 M), or ODQ (10−5 M, Figure 1) had no significant effect on relaxant responses induced by apomorphine. In the presence of L-NNA or ODQ, the relaxant responses to apomorphine at 10−5 M amounted to 60.9±4.1% (n=6, N=5), and 64.9±1.3% (n=6, N=5), respectively, and the −log IC50 value for apomorphine in Phe-contracted preparations was unaffected by pretreatment with either compound.
Figure 1.
(a) Effect of apomorphine in Phe-contracted (3 × 10−6 M) preparations of rat isolated corpus cavernosum before and after pretreatment with ODQ (10−5 M). Values are given as mean and s.e.m. (b) Original tracing describing the relaxant effect by cumulative administration of apomorphine in one Phe-activated rat isolated corpus cavernosum preparation.
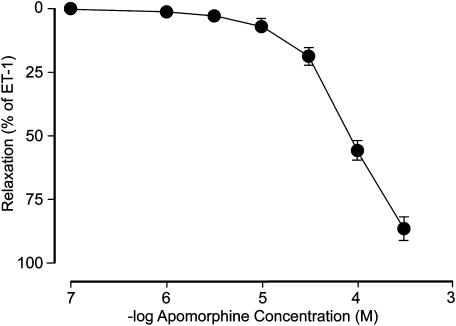
Apomorphine also produced concentration-dependent relaxant responses in ET-1 (3 × 10−8 M)-contracted corpus cavernosum preparations (Figure 2). Compared to relaxations in Phe-activated tissue, the effects on ET-1-induced contraction by apomorphine were smaller, and at 10−4 M, a mean relaxant response of 55.1±4.4% was obtained (n=10, N=8). At the highest investigated apomorphine concentration (3 × 10−4 M) in ET-1-activated corpus cavernosum preparations, a mean relaxant response of 86.6±5.5% (n=10, N=8) was obtained. The mean −log IC50 for apomorphine in preparations contracted with ET-1 was calculated to 4.10±0.07.
Figure 2.
Effect of apomorphine in ET-1-contracted (3 × 10−8 M) preparations of rat isolated corpus cavernosum. Values are given as mean and s.e.m.
In preparations pretreated with SCH 23390 (10−6 M), no effects were detected on relaxant responses at any given concentration of apomorphine in preparations contracted with ET-1. A maximal relaxant response (apomorphine 3 × 10−4 M) of 91±2.8% (n=6, N=6; NS) was obtained.
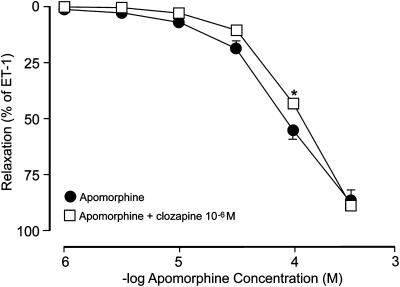
ET-1-activated preparations pretreated with 10−6 M of clozapine (Figure 3) were then exposed to cumulatively added concentrations of apomorphine. In these experiments, the concentration–response relationship to apomorphine exhibited a slight rightward shift, whereas the maximal relaxant effect (88.6±1.6%, N=8, n=8) was unaffected. In the presence of clozapine, apomorphine (10−4 M) had a relaxant effect of 42.2±2.3% (P<0.05). The −log IC50 value in the presence of clozapine in ET-1-contracted tissues amounted to 3.88±0.02 (P<0.05). The vehicle for clozapine did not have any effect on ET-induced contraction of the rat isolated corpus cavernosum preparations.
Figure 3.
Effect of apomorphine in ET-1-contracted (3 × 10−8 M) preparations of rat isolated corpus cavernosum before and after pretreatment clozapine (10−6 M). Values are given as mean and s.e.m. *P<0.05.
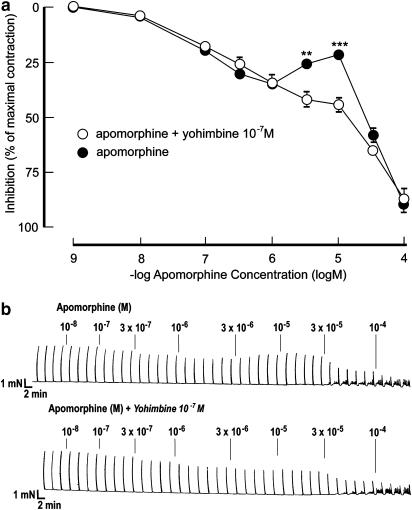
Electrical field stimulation produced reproducible, frequency-dependent, and tetrodotoxin-sensitive contractions. The concentration-dependent (10−9–10−4 M) inhibitory effects by apomorphine (n=15, N=12) on electrically induced contractions (20 V, 18 Hz) exhibited biphasic response patterns (Figure 4). A mean inhibitory effect of 34.4±2.4% was obtained at 10−6 M. At 3 × 10−5 to 10−5 M, contractile responses recovered, and amounted to 78.2±2.6% of the initial responses (i.e. ∼21% inhibition by apomorphine), whereafter inhibitory effects of 89.6±2.9% were recorded at 10−4 M. The −log IC50 value for apomorphine on contractions produced by EFS was 4.73±0.03. At baseline, no contractile effects of apomorphine were observed during these experiments.
Figure 4.
(a) Effect of apomorphine on preparations of rat isolated corpus cavernosum activated by electrical field stimulation (25 V, 20 Hz) before and after pretreatment with yohimbine (10−7 M). Values are given as mean and s.e.m. **P<0.01, ***P<0.001. (b) Original tracing describing the effect of apomorphine with or without the presence of yohimbine (10−7 M) on electrically induced contractions in one rat isolated corpus cavernosum preparation.
Pretreatment with 10−7 M of yohimbine (n=6, N=6) did not affect the initial inhibitory effects of apomorphine (10−9–10−6 M) on EFS-induced contractions. However, the ‘recovery phase' of the contractions seen at 3 × 10−6–10−5 M of apomorphine was abolished (Figure 4). At these concentrations of apomorphine, the inhibitory effects were increased from 25.2±2.1% (3 × 10−6 M) and 21.4±2.6% (10−5 M) to 39.6±3.5% (3 × 10−6 M, P<0.01) and 43.6±3.4% (10−5 M, P<0.001) in the presence of yohimbine (10−7 M). No effect of yohimbine was recorded on the inhibitory action of apomorphine at the highest concentration used. In the presence of yohimbine (10−7 M), the −log IC50 value for apomorphine on contractions produced by EFS amounted to 5.32±0.19 (P<0.01).
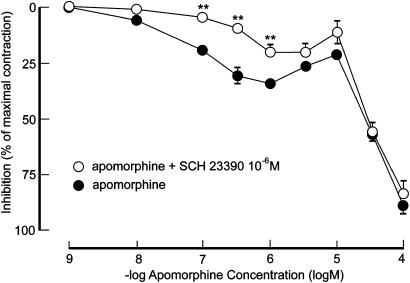
At low concentrations of apomorphine, 10−6 M of SCH 23390 attenuated inhibitory responses to apomorphine in EFS-activated preparations (Figure 5). Inhibitory effects of 19.5±2.2% (10−7 M), 30.5±3.7% (3 × 10−7 M) and 34.4±2.4% (10−6 M) were diminished to 4.8±1.5% (10−7 M, P<0.01), 10.1±2.5% (3 × 10−7 M, P<0.01) and 20.4±3.3% (10−6 M, P<0.01) in the presence of SCH 23390. The D1 receptor antagonist did not affect the biphasic inhibitory pattern or the maximal inhibitory effect of apomorphine in EFS-activated preparations. The −log IC50 value for apomorphine on contractions produced by EFS in the presence of SCH 23390 amounted to 4.73±0.02 (NS).
Figure 5.
Effect of apomorphine on preparations of rat isolated corpus cavernosum activated by electrical field stimulation (25 V, 20 Hz) before and after pretreatment with SCH 23390 (10−6 M). Values are given as mean and s.e.m. **P<0.01.
Measurements of cGMP and cAMP
Cyclic nucleotide levels were determined in Phe-contracted corpus cavernosum preparations exposed to vehicle (saline) or to apomorphine (10−4 M). In control preparations, the mean values of the content of cGMP or cAMP amounted to 35.6±4.5 or 33.0±4.9 pmol protein−1, respectively. No significant difference of the tissue content of the cyclic nucleotides occurred after administration of apomorphine, and the mean amount of cGMP and cAMP was determined to 27.7±3.0 and 34.4±4.1 pmol protein−1, respectively.
In vivo experiments
I.c. pressure registration in anesthetized animals
A mean BICP of 12±2.8 mmHg (N=8) was recorded at the beginning of the experiments. I.c. injection of apomorphine (100, 300, or 1000 nmol) did not per se exhibit any effects upon the i.c. pressure in separate experiments (N=5).
An i.c. pressure of 42±1.6 mmHg (N=5) was obtained during activation of the cavernous nerve (10 V, 20 Hz). During stimulation of the cavernous nerve (Table 1), MAP was stable in each rat and amounted to 109±2.5 mmHg. At submaximal stimulation (2.5 V, 20 Hz), a mean PICP of 22±2.8 mmHg was obtained (N=5). Mean values for ΔT80 reached 9±2.1 mmHg s−1 and ΔD20 amounted to 2±0.3 mmHg s−1, respectively. Submaximal activation of the cavernous nerve did not affect MAP. At 100 or 300 nmol (i.c.), apomorphine had no effect on i.c. pressure responses to submaximal activation of the cavernous nerve (2.5 V, 20 Hz, Figure 6). The mean i.c. pressure responses amounted to 22±1.3 mmHg (N=5) and 20±3.8 mmHg (N=5). At these amounts of apomorphine, no significant effects were recorded for the mean ΔT80, which amounted to 7±1.5 and 8±2.1 mmHg s−1, respectively. The ΔD20 reached 1±0.2 and 1±0.4 mmHg s−1 for 100 and 300 nmol of apomorphine (i.c.). After i.c. administration of 1000 nmol, a mean i.c. pressure response to cavernous nerve stimulation of 19±3.7 mmHg (N=5) was obtained, with a ΔT80 of 5±1.0 mmHg s−1, and the ΔD20 amounted to 1±0.5 mmHg s−1. Irrespective of the amount given, i.c. administration of apomorphine caused a brief reduction in MAP in three out of five animals. The reduction in MAP ranged from 30 to 52 mmHg and lasted no longer than 2 min, whereafter the systemic blood pressure recovered or increased (Table 1). In two animals, 100 or 300 nmol of apomorphine (i.c.) caused increases in ICP that were similar in appearance to responses obtained after s.c. administration of the drug. These responses were seen separate from nerve-induced increases in i.c. pressure, and occurred at 6 or 8 min after administration of apomorphine. PICP for these responses amounted to 62±11 mmHg.
Table 1.
Suboptimal activation (2.5 V, 20 Hz) of the cavernous nerve (n=5)
| Apomorphine | ||||
|---|---|---|---|---|
| Control | 100 nmol | 300 nmol | 1000 nmol | |
| PICP (mmHg) | 22±2.8 | 22±1.3 | 20±3.8 | 19±3.7 |
| MAP (mmHg) | 109±2.5 | 109±3.9 | 118±8.8 | 122±8.6 |
| PICP/MAP | 0.21±0.03 | 0.20±0.02 | 0.17±0.03 | 0.15±0.02 |
| ΔT80 (mmHg s−1) | 9±2.1 | 7±1.5 | 8±2.1 | 5±1.0 |
| ΔD20 (mmHg s−1) | 2±0.3 | 1±0.2 | 1±0.4 | 1±0.5 |
Figure 6.
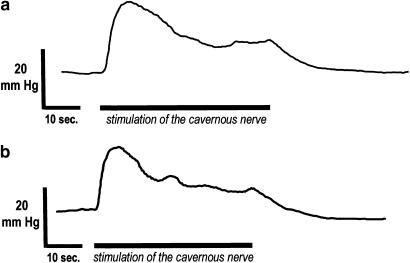
Original tracing of the i.c. pressure (ICP) response to cavernous nerve stimulation (2.5 V, 20 Hz) before (a) and after (b) i.c. administration of 300 nmol of apomorphine.
I.c. pressure registration in awake animals
A mean BICP of 17±1.8 mmHg (N=38) was recorded at the beginning of the experiments. In control experiments, rats receiving saline (s.c.) exhibited 0.1±0.1 (N=6) increases in ICP. After s.c administration of apomorphine (300 nmol kg−1 (100 μg kg−1)), a maximal number of erectile responses were recorded. At this dose, seven out of eight rats exhibited increases in i.c. pressure, and a mean number of 3.0±0.8 (N=8, P<0.05 vs vehicle) increases in i.c. pressure was recorded 8.0±3.2 min after s.c. administration of apomorphine. Peak i.c. pressure amounted to 100±11.9 mmHg, and had a mean cumulative duration of 3.6±0.8 min. Grooming behavior and yawns were observed in all animals, and usually, but not always, occurred just prior to the erectile responses. A mean of 6.5±0.6 yawns were noted during the observation period of 30 min. At doses of 10, 30, 100, or a 1000 nmol kg−1 (s.c.), apomorphine produced lower number of erections, amounting to 0.6±1.2 (N=5), 0.7±1.0 (N=7), 1.3±1.3 (N=7), and 0.2±0.4 (N=5), respectively.
Discussion
Dopamine receptors have been located to the dorsal nerves and vasculature of the penis, as well as the corpus cavernosum of the rat, and in human erectile tissue, immunoreactivities for D1- and D2-like dopamine receptors have been found in cavernous smooth muscle (Hyun et al., 2002; d'Emmanuele di Villa Bianca et al., 2004). Based on Western blot analysis, it has been suggested that dopamine D1 receptors are more abundant than D2-like receptors in the human corpus cavernosum (d'Emmanuele di Villa Bianca et al., 2004). It may be argued if an appropriate quantification of the relative distribution of the D1- and D2-like dopamine receptors can be achieved by this approach, and a careful interpretation of this finding may be in place. Still, evidence indicates the presence of dopamine receptors in the erectile tissue of rats and humans, but a functional role for of dopamine receptor-mediated signals in the penis has not been established, and it is not known if peripheral effects of apomorphine contribute to its facilitatory effect on erection.
Corresponding to findings in the human corpus cavernosum (d'Emmanuele di Villa Bianca et al., 2004), the present study demonstrates that apomorphine relaxes agonist-contracted rat isolated corpus cavernosum in a dose-dependent manner. A 10-fold higher potency was observed for apomorphine in Phe-contracted as compared to ET-1-activated tissue. It has been reported that apomorphine has affinity for α-adrenoceptors (Millan et al., 2002; Newman-Tancredi et al., 2002; Hsieh et al., 2004), and the relaxant effect of the compound in human erectile tissue contracted with Phe has been proposed to be partly mediated by an α1-adrenoceptor-antagonistic action (d'Emmanuele di Villa Bianca et al., 2004). This also appears to be valid for the rat corpus cavernosum.
The agonist concentrations used to activate the rat isolated corpus cavernosum corresponded to their approximate EC50 value. Vascular α1-adrenoceptors and ET-1 receptors mediate contractions by G-protein-coupled stimulation of phospholipase C, and by activation of the Rho/Rho kinase pathway (Guimaraes & Moura, 2001; Davenport, 2002; Wingard et al., 2003). For ETA receptors, the transduction mechanism includes increase of transmembrane calcium flux, a mechanism also suggested to mediate effects by ET-1 in the erectile tissue (Andersson, 2001). It cannot be excluded that the individual mechanisms of the receptor-mediated signal for Phe or ET-1 to produce a contraction have contributed to the different −log IC50 values obtained for apomorphine in preparations activated by either agonist.
Apomorphine may undergo auto-oxidation, and oxidized derivatives of apomorphine have been reported to exhibit differences in vascular reactivities (Abarca et al., 2003). Oxidized derivatives of apomorphine have also been suggested to disrupt endothelial function (Abarca et al., 2003). In the rat corpus cavernosum, no change in the relaxant action of apomorphine was observed when the compound was dissolved in the antioxidant DMSO.
In the present study, apomorphine-induced relaxations were unaffected by L-NNA or ODQ, suggesting that apomorphine does not interact with endothelial NO-mediated signals in the rat corpus cavernosum. No effects on the tissue levels of cGMP were observed after the addition of apomorphine to Phe-contracted erectile tissue. Species differences may account for these contrasting findings, and as suggested for the rat corpus cavernosum (Hedlund et al., 1999), endothelially derived NO may be of less functional importance for penile erection.
A dopamine D1 receptor selective agonist has been found to produce endothelium- and NO-dependent relaxations of the human corpus cavernosum (d'Emmanuele di Villa Bianca et al., 2004), and dopamine D1-like receptors have also been reported to produce vasodilation in other arterial preparations (Hughes et al., 1987; Kopia & Valocik, 1989; van der Niepen et al., 1991; Villalon et al., 2003). Under the current experimental conditions, the selective dopamine D1-like receptor antagonist, SCH 23390, had no effect on apomorphine-induced relaxations in ET-1-contracted rat corpus cavernosum. The D1-like receptors are generally held to couple to G-proteins (Gs) that stimulate adenylate cyclase (Sibley, 1999). In the rat corpus cavernosum, no effect was observed on the tissue content of cAMP by 100 μM of apomorphine, a concentration that produced an almost 90% relaxation of the Phe-induced contraction. Taken together, the present findings suggest that dopamine D1-like receptors are of no major importance for postjunctional relaxant effects by apomorphine in the rat corpus cavernosum.
In ET-1-contracted tissues, yohimbine did not influence the relaxant activity of apomorphine, and other mechanisms of action than effects at α2-adrenoceptors, appear to be involved in apomorphine-induced relaxations of ET-1-activated preparations. Clozapine can interact with all dopamine receptors, but its antagonistic properties at the D2-like dopamine receptors is considered of importance for its antipsychotic effect (Millan, 2000; Strange, 2001). Compared to the affinities for the D2, and D3 subtype, clozapine is proposed to have a high affinity for the dopamine D4 subtype receptor (Van Tol et al., 1991; Simpson et al., 1999). However, data from ligand binding assays for D2-like dopamine receptors and estimations of drug affinities to these receptors varies in the literature (Levant, 1997; Strange, 2001). In ET-1-activated rat isolated corpus cavernosum, clozapine partly counteracted the relaxant effect of apomorphine. Since SCH 23390 did not influence apomorphine-induced relaxations, the shift in IC50 value for apomorphine in the presence of clozapine may be due to blockade of D2-like dopamine receptors. In addition to G-protein-mediated inhibition of adenylate cyclase, the D2-like dopamine receptors have also been reported to modify the activities of various ion channels and to affect potassium and calcium currents (Levant, 1997). No change in the tissue contents of cAMP in the rat corpus cavernosum could be detected in the presence of apomorphine, and it may be speculated if a part of the apomorphine-induced relaxation is due to D2-like dopamine receptor-mediated effects on ion channels.
Nerve-induced contractions of the rat isolated corpus cavernosum were attenuated by apomorphine in a biphasic pattern, suggesting the involvement of more than one receptor for this effect. In the presence of yohimbine, at a concentration approximately corresponding to its Ki value for human and rat α2-adrenoceptors, the accelerated second phase of apomorphine on electrically induced contractions were abolished. Apomorphine was reported to exhibit higher affinity for α2-adrenoceptors than for α1-adrenoceptors (Newman-Tancredi et al., 2002; Hsieh et al., 2004), and the present results support that apomorphine exhibits functional α2-adrenoceptors-antagonistic properties (Newman-Tancredi et al., 2002). Prejunctional α2-adrenoceptors that have been shown to modify the release of noradrenaline from nerves in the erectile tissue (Hedlund et al., 1984; Molderings et al., 1989), and a functional role for prejunctional α2-adrenoceptors has been demonstrated in the rat corpus cavernosum (Mizusawa et al., 2002).
The shape of the concentration–response curve for apomorphine on nerve-induced contractions of the rat corpus cavernosum in the presence of yohimbine, that is, a reduction in the amplitude of the contraction, was opposite to an expected additive effect of the compounds. One explanation may be that receptors that couple to Gs, for example, the α2-adrenoceptor, exist in equilibrium between inactive and active states (Wade et al., 2001). Yohimbine has been proposed to bind preferentially to the inactive state of the α2-adrenoceptor, shifting the equilibrium between active and inactive forms, and hereby reducing the availability of the former (Wade et al., 2001). Further studies are needed to evaluate if such a relationship exists between yohimbine, apomorphine and the α2-adrenoceptor in the erectile tissue. Contractions of isolated tissues that are elicited by transmural stimulation of nerves is the combined result of pre- and postjunctional effects, and it cannot be ruled out that other than the currently investigated effects by yohimbine and/or apomorphine contribute to the appearance of the concentration–response curve of the nerve-induced contractions of the rat corpus cavernosum. As already indicated for apomorphine, yohimbine may also counteract postjunctional α1-adrenoceptor-mediated signals (Mizusawa et al., 2002).
Prejunctional dopamine receptors are proposed to modify the release of amines from nerve terminals in vascular tissues (see Soares-da-Silva, 1987). Apomorphine reduced neurogenic vasoconstrictor responses (Hietala, 1988), and counteracted nerve-induced release of noradrenaline in gastric and uterine arterial preparations by interaction with dopamine D2 receptors (Morgadinho et al., 1999). In the rat corpus cavernosum, the dopamine D1-like receptor antagonist, SCH 23390, significantly reduced the inhibitory effect of apomorphine on nerve-induced contractions. Since SCH 23390 did not affect relaxant effects by apomorphine in ET-1-contracted preparations, prejunctional dopamine D1 receptors are suggested to convey modulatory effects of apomorphine on nerve-induced contractions in this tissue.
After systemic administration, apomorphine produces erections in awake rats in a dose-dependent manner (Giuliano & Rampin, 2000; Andersson, 2001). This correlates well with the present results, and an optimum of three erectile events during a 30 min observation period was recorded at a s.c. dose of 300 nmol kg−1. In rats, at an efficacious dose of 100 nmol kg−1, the plasma concentration of apomorphine is reported to be 7.5 nM (Hsieh et al., 2004). The currently investigated i.c. doses of apomorphine (100, 300, and 1000 nmol) are expected to result in higher concentrations locally in the corpus cavernosum than those obtained at therapeutic doses for erectile dysfunction. At these amounts, i.c. apomorphine did not affect BICP under resting conditions, or filling or emptying rates, or PICP, during suboptimal activation of the cavernous nerve, and a functional peripheral site of action for the compound is not likely to occur at relevant doses.
Apomorphine exhibits a complex pharmacological profile. In the rat isolated corpus cavernosum, pre- and postjunctional effects by apomorphine appear to involve dopamine D1- and D2-like receptors, as well as α1- and α2-adrenoceptors. Whereas apomorphine is effective in producing erections after systemic administration, i.c. apomorphine did not influence pressure parameters, with or without concomitant activation of the cavernous nerve. At doses of apomorphine used for treatment of erectile dysfunction, peripheral effects by the compound are unlikely to contribute to its effect on penile erection.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (Grant no. 6837).
Abbreviations
- BICP
basal intracavernous pressure
- cAMP
adenosine 3′5′-cyclic monophosphate
- cGMP
guanosine 3′5′-cyclic monophosphate
- CNS
central nervous system
- ΔD20
decrease in intracavernous pressure per second at 20% of peak intracavernous pressure
- ΔT80
increase in intracavernous pressure per second at 80% of peak intracavernous pressure
- ET-1
endothelin-1
- i.c.
intracavernous
- L-NNA
NG-nitro-L-arginine
- MAP
mean arterial blood pressure
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- PICP
peak intracavernous pressure
- SCH 23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
References
- ABARCA B., BALLESTEROS R., BIELSA P., MORAGUES J., D'OCON P., GARCIA-ZARAGOZÀ E., NOGUERA M.A. Opposite vascular activity of (R)-apomorphine and its oxidised derivatives. Endothelium-dependent vasoconstriction induced by the auto-oxidation metabolite. Eur. J. Med. Chem. 2003;38:501–511. doi: 10.1016/s0223-5234(03)00057-6. [DOI] [PubMed] [Google Scholar]
- AMENTA F., BARILI P., BRONZETTI E., FELICI L., MIGNINI F., RICCI A. Localization of dopamine receptor subtypes in systemic arteries. Clin. Exp. Hypertens. 2000;22:277–288. doi: 10.1081/ceh-100100077. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E. Pharmacology of penile erection. Pharmacol. Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- ANWAR N., MASON D.F. Actions of dopamine and apomorphine on the vasoconstrictor responses of perfused mesenteric arteries of mouse, rat and rabbit. J. Pharm. Pharmacol. 1981;33:150–154. doi: 10.1111/j.2042-7158.1981.tb13738.x. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P. International union of pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol. Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- D'EMMANUELE DI VILLA BIANCA R., SORRENTINO R., ROVIEZZO F., IMBIMBO C., PALMIERI A., DE DOMINICIS G., MONTORSI F., CIRINO G., MIRONE V. Peripheral relaxant activity of apomorphine and of a D1 selective receptor agonist on human corpus cavernosum strips. Int. J. Impot. Res. 2004;17:127–133. doi: 10.1038/sj.ijir.3901293. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., ALLARD J., RAMPIN O., DROUPY S., BENOIT G., ALEXANDRE L., BERNABE J. Spinal proerectile effect of apomorphine in the anesthetized rat. Int. J. Impot. Res. 2001;13:110–115. doi: 10.1038/sj.ijir.3900654. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., RAMPIN O. Central neural regulation of penile erection. Neurosci. Biobehav. Rev. 2000;24:517–533. doi: 10.1016/s0149-7634(00)00020-8. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- GYORGY L., DODA M. The effect of dopamine, apomorphine and pirebedil on the mesenterial blood flow of the cat. Arch. Int. Pharmacodyn. Ther. 1985;275:22–32. [PubMed] [Google Scholar]
- HAN G., KRYMAN J.P., MCMILLIN P.J., WHITE R.E., CARRIER G.O. A novel transduction mechanism mediating dopamine-induced vascular relaxation: opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J. Cardiovasc. Pharmacol. 1999;34:619–627. doi: 10.1097/00005344-199911000-00001. [DOI] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E., MATTIASSON A. Pre- and postjunctional adreno- and muscarinic receptor functions in the isolated human corpus spongiosum urethrae. J. Auton. Pharmacol. 1984;4:241–249. doi: 10.1111/j.1474-8673.1984.tb00101.x. [DOI] [PubMed] [Google Scholar]
- HEDLUND P., ALM P., ANDERSSON K.-E. NO synthase in cholinergic nerves and NO-induced relaxation in the rat isolated corpus cavernosum. Br. J. Pharmacol. 1999;127:349–360. doi: 10.1038/sj.bjp.0702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIETALA J. Effects of DA-1- and DA-2-dopamine antagonists on apomorphine-induced inhibition of peripheral sympathetic neurotransmission. J. Auton. Pharmacol. 1988;8:297–302. doi: 10.1111/j.1474-8673.1988.tb00572.x. [DOI] [PubMed] [Google Scholar]
- HSIEH G.C., HOLLINGSWORTH P.R., MARTINO B., CHANG R., TERRANOVA M.A., O'NEILL A.B., LYNCH J.J., MORELAND R.B., DONELLY-ROBERTS D.L., KOLASA T., MIKUSA J.P., MCVEY J.M., MARSH K.C., SULLIVAN J.P., BRIONI J.D. Central mechanisms regulating penile erection in conscious rats: the dopaminergic systems related to the proerectile effect of apomorphine. J. Pharmacol. Exp. Ther. 2004;308:330–338. doi: 10.1124/jpet.103.057455. [DOI] [PubMed] [Google Scholar]
- HUGHES A.D., THOM S.A., WOODALL N.M., REDMAN D., SEVER P.S. Dopamine produces forearm vasodilation following alpha-adrenoreceptor blockade by an action on vascular dopamine (DA-1) receptors in man. J. Hypertens. 1987;5:337–340. doi: 10.1097/00004872-198706000-00012. [DOI] [PubMed] [Google Scholar]
- HUGHES A., THOM S., MARTIN G., REDMAN D., HASAN S., SEVER P. The action of dopamine (DA1) receptor agonist, fenoldopam in human vasculature in vivo and in vitro. Br. J. Clin. Pharmacol. 1986;22:535–540. doi: 10.1111/j.1365-2125.1986.tb02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULL E.M., LORRAIN D.S., DU J., MATUSZEWICH L., LUMNLEY L.A., PUTNAM S.K., MOSES J. Hormone–neurotransmitter interactions in the control of sexual behaviour. Behav. Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- HYUN J.S., BIVALACQUA T.J., BAIG M.R., YANG D.Y., LEUNGWATTANAKIJ S., ABDEL-MAGEED A., KIM K.D., HELLSTRÖM W.J. Localization of peripheral dopamine D1 and D2 receptors in rat corpus cavernosum. Br. J. Pharmacol. Int. 2002;90:105–112. doi: 10.1046/j.1464-410x.2002.02789.x. [DOI] [PubMed] [Google Scholar]
- KOPIA G.A., VALOCIK R.E. Demonstration of specific dopamine-1 receptor-mediated coronary vasodilation in the anesthetized dog. J. Pharmacol. Exp. Ther. 1989;248:215–221. [PubMed] [Google Scholar]
- LEVANT B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol. Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- MILLAN M.J. Improving the treatment of schizophrenia; focus on serotonin (5-HT)1A receptors. J. Pharmacol. Exp. Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- MILLAN M.J., MAIOFISS L., CUSSAC D., AUDINOT V., BOUTIN J.A., NEWMAN TANCREDI A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned receptor subtypes. J. Pharmacol. Exp. Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- MIZUSAWA H., HEDLUND P., SJUNNESSON J., BRIONI J.D., SULLIVAN J.P., ANDERSSON K.-E. Enhancement of apomorphine-induced penile erection in the rat by a selective α1D-adrenoceptor antagonist. Br. J. Pharmacol. 2002;136:701–708. doi: 10.1038/sj.bjp.0704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., GOTHERT M., VAN AHLEN H., PORST H. Noradrenaline release in human corpus cavernosum and its modulation via presynaptic alpha 2-adrenoreceptors. Fund. Clin. Pharmacol. 1989;3:497–504. doi: 10.1111/j.1472-8206.1989.tb00684.x. [DOI] [PubMed] [Google Scholar]
- MORGADINHO M.T., FONTES RIBEIRO C.A., MACEDO T.R. Presynaptic dopamine receptors involved in the inhibition of noradrenaline and dopamine release in the human gastric and uterine arteries. Fund. Clin. Pharmacol. 1999;13:662–670. doi: 10.1111/j.1472-8206.1999.tb00378.x. [DOI] [PubMed] [Google Scholar]
- MUHLBAUER B., KUSTER E., LUIPPOLD G. Dopamine D3 receptors in the rat kidney: role in physiology and pathophysiology. Acta Physiol. Scand. 2000;168:219–223. doi: 10.1046/j.1365-201x.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- MURPHY M.B. Dopamine: a role in the pathogenesis and treatment of hypertension. J. Hum. Hypertens. 2000;14 Suppl 1:S47–S50. doi: 10.1038/sj.jhh.1000987. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., CUSSAC D., AUDINOT V., NICOLAS J.-P., DE CEUNINCK F., BOUTIN J.A., MILLAN M.J. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D2-like receptor and α1/α2-adrenoceptor. J. Pharmacol. Exp. Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- RASHED S.M., SONGU-MIZE E. Regulation of Na+-pump activity by dopamine in rat tail arteries. Eur. J. Pharmacol. 1995;33:150–154. doi: 10.1016/0014-2999(95)00363-p. [DOI] [PubMed] [Google Scholar]
- SIBLEY D.R. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu. Rev. Pharmacol. Toxicol. 1999;39:313–341. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- SIMPSON M.M., BALLESTEROS J.A., CHIAPPA V., CHEN J., SUEHIRO M., HARTMAN D.S., GODEL T., SNYDER L.A., SAKMAR T.P., JAVITCH J.A. Dopamine D4/D2 receptor selectivity is determined by a divergent aromatic microdomain contained within the second, third, and seventh membrane-spanning segments. Mol. Pharmacol. 1999;56:1116–1126. doi: 10.1124/mol.56.6.1116. [DOI] [PubMed] [Google Scholar]
- SOARES-DA-SILVA P. Dopamine released from nerve terminals activates prejunctional dopamine receptors in dog mesenteric arterial vessels. Br. J. Pharmacol. 1987;91:591–599. doi: 10.1111/j.1476-5381.1987.tb11252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE P.G. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol. Rev. 2001;53:119–133. [PubMed] [Google Scholar]
- TEISMAN A.C., BUIKEMA H., VAN VELDHUISEN D.J., DE ZEEUW D., VAN GILST W.H. Direct vasodilating effects of the new dopaminergic agonist Z1046 in human arteries. J. Cardiovasc. Pharmacol. 2000;35:581–585. doi: 10.1097/00005344-200004000-00011. [DOI] [PubMed] [Google Scholar]
- VAN DER NIEPEN P., SCHOORS D.F., DUPONT A.G. Hypotensive and regional hemodynamic effects of the dopamine receptor agonist SK & F 85174 in the anesthetized rat. Arch. Int. Pharmacodyn. Ther. 1991;313:98–107. [PubMed] [Google Scholar]
- VAN TOL H.H., BUNZOW J.R., GUAN H.C., SUNAHARA R.K., SEEMAN P., NIZNIK H.B., CIVELLI O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature (London) 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- VENTURA A.L., KLEIN W.L., DE MELLO F.G. Differential ontogenesis of D1 and D2 dopaminergic receptor in the chick embryo retina. Brain Res. 1984;314:217–223. doi: 10.1016/0165-3806(84)90044-0. [DOI] [PubMed] [Google Scholar]
- VILLALON C.M., RAMIREZ-SAN JUAN E., SANCHEZ-LOPEZ A., BRAVO G., WILLEMS E.W., SAXENA P.R., CENTURION D. Pharmacological profile of the vascular responses to dopamine in the canine external carotid circulation. Pharmacol. Toxicol. 2003;92:165–172. doi: 10.1034/j.1600-0773.2003.920406.x. [DOI] [PubMed] [Google Scholar]
- WADE S.M., LAN K., MOORE D.J., NEUBIG R.R. Inverse agonist activity at the α2A-adrenergic receptor. Mol. Pharmacol. 2001;59:532–542. doi: 10.1124/mol.59.3.532. [DOI] [PubMed] [Google Scholar]
- WINGARD C.J., HUSAIN S., WILLIAMS J., JAMES S. RhoA-Rho kinase mediates synergistic ET-1 and phenylephrine contraction of rat corpus cavernosum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1145–R1152. doi: 10.1152/ajpregu.00329.2003. [DOI] [PubMed] [Google Scholar]
- ZENG C., WANG D., YANG Z., WANG Z., ASICO L.D., WILCOX C.S., EINSNER G.M., WELCH W.J., FELDER R.A., JOSE P.A. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]