Abstract
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy') administration to rats produces hyperthermia if they are housed in normal or warm ambient room temperature (Ta) conditions (⩾20°C), but hypothermia when in cool conditions (Ta⩽17°C). We have now investigated some of the mechanisms involved.
MDMA (5 mg kg−1 i.p.) produced a rapid decrease in rectal temperature in rats at Ta 15°C. This response was blocked by pretreatment with the dopamine D2 receptor antagonist remoxipride (10 mg kg−1 i.p.), but unaltered by pretreatment with the D1 antagonist SCH23390 (1.1 mg kg−1 i.p.).
MDMA (5 mg kg−1) did not alter the tail temperature of rats at Ta 15°C, but decreased the tail temperature of rats at Ta 30°C.
A neurotoxic dose of MDMA (three doses of 5 mg kg−1 given 3 h apart) decreased cortical and hippocampal 5-HT content by approximately 30% 7 days later. This lesion did not influence the rise in tail temperature when rats were moved from Ta 20°C to 30°C compared to nonlesioned controls, but did result in a lower tail temperature than that of controls when they were returned to Ta 24°C.
Acute administration of MDMA (5 mg kg−1) to MDMA-lesioned rats produced a sustained decrease in tail temperature in rats housed at Ta 30°C compared to nonlesioned controls.
These data suggest that the thermoregulatory problems previously observed in MDMA-lesioned rats housed at Ta 30°C result, partially, from their inability to lose heat by vasodilation of the tail, a major heat-loss organ in this species.
Keywords: 5-Hydroxytryptamine, hypothermia, hyperthermia, tail temperature, MDMA, ecstasy, dopamine, thermoregulation, neurotoxicity
Introduction
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy') is a drug widely used by young people, particularly in dance club situations. Administration of this compound to laboratory animals when the animals are present in a room at normal (20–22°C) ambient temperature (Ta) produces acute and rapid hyperthermia (Green et al., 2003; 2004b). Human recreational users of MDMA can also suffer an acute hyperthermic response which, if severe, can result in death (Schifano, 2004). There is also evidence that the MDMA-induced hyperthermic response in rats is enhanced when the animals are present at warm Ta (30°C) (Dafters, 1995; Malberg & Seiden, 1998; Green et al., 2004a).
In contrast, when rats are housed in cool ambient room temperature conditions (Ta 17°C or lower), administration of MDMA induces a rapid hypothermic response (Gordon et al., 1991; Dafters, 1994; Dafters & Lynch, 1998). While previous work in our group indicated that the rapid increase in rectal temperature is associated with the increase in dopamine release induced by MDMA and its action on dopamine D1 receptors (Mechan et al., 2002), no investigation appears to have been made on the mechanisms involved in the hypothermic response seen in rats housed at cool Ta.
Administration of large or repeated doses of MDMA produces a long-term neurotoxic loss of 5-HT in the forebrain (Green et al., 2003). When MDMA-lesioned rats are exposed to Ta 30°C and then returned to Ta 20°C, it takes longer for their body temperature (which has increased modestly in the warm conditions compared to rats housed at an ambient room temperature of 20°C) to return to normal, compared to nonlesioned control animals. This observation was made using two different experimental approaches. Dafters & Lynch (1998) measured the duration of the hyperthermic response in lesioned rats compared to the duration of the response in the same animals prior to the neurotoxic dose of MDMA, while Mechan et al. (2001) measured the rectal temperature of parallel groups that had been pretreated 4 weeks earlier with either saline or a lesioning dose of MDMA. Both groups of investigators concluded that MDMA-lesioned rats had problems in losing heat following exposure to hot temperature and a return to normal room temperature conditions. It was suggested that this problem might be associated with the decrease in cerebral 5-HT concentration.
This problem of heat loss in lesioned rats when they are present in a warm environment was also seen in another type of study. Rats given a neurotoxic dose of MDMA 7 or more days earlier displayed a prolongation in the acute hyperthermic response, which followed a low challenge dose of MDMA when compared to saline-pretreated rats given the same challenge dose of MDMA. However, this effect was seen only in MDMA-lesioned rats housed at Ta 30°C and not when the animals were present at Ta 20°C (Green et al., 2004a).
To further examine whether a loss in cerebral 5-HT concentration and therefore presumably function was involved in the abnormal thermoregulatory responses seen in MDMA-lesioned rats, we recently examined the effect on heat loss in rats housed at Ta 30°C of decreasing cerebral 5-HT function by prior injection of either the 5-HT synthesis inhibitor p-chlorophenylalanine (PCPA) or certain 5-HT receptor antagonists. Pretreatment with PCPA, methysergide or WAY100635 had the effect of prolonging the hyperthermic response to a challenge dose of MDMA when the animals were in the warm room. This again suggested strongly that it was the decrease in 5-HT function produced by a neurotoxic dose of MDMA that was responsible for the impairment in the ability of rats to lose heat in hot Ta conditions. A decrease in cerebral 5-HT function produced by either MDMA or administration of PCPA or 5-HT antagonists did not, of itself, alter the body temperature of rats housed at Ta 30°C; a challenge of either a hyperthermia-producing acute dose of MDMA or a move to cooler room conditions was required to expose the defect in thermoregulation.
In the current investigation, we have examined the effect of dopamine D1 and D2 receptor antagonists on the hypothermic response, which follows when MDMA is given to rats housed at Ta 15°C. Since heat loss in rats is primarily regulated by vasodilation of blood vessels in the tail, a major heat exchange organ in this species (Grant, 1963; Romanovsky et al., 2002), the current study has also examined the effect of an acute dose of MDMA on the tail temperature of rats exposed to warm and cool Ta, and the effect of a prior MDMA-induced neurotoxic lesion on this response.
Methods
Animals and drug administration
Male Dark Agouti rats, weighing 160–200 g, were used in all experiments (Harlan U.K. Ltd, Bicester, Oxon, U.K.). The animals were housed in groups of four, at a Ta of 20±2°C and a 12 h light/dark cycle (lights on at 07:30 h). Both food and water were freely provided. All procedures were carried out following approval by the De Montfort University Experimental Ethics Committee and in accordance with United Kingdom Home Office regulations, which ensure humane and proper care of research animals.
(±)-MDMA was obtained from Ultrafine Chemicals Ltd, Manchester, U.K. and dissolved in 0.9% w v−1 saline and injected intraperitoneally (i.p.). Remoxipride was a gift from AstraZeneca R&D Södertälje, Sweden and SCH23390 was obtained from Tocris-Cookson, Bristol. Both drugs were dissolved in 0.9% w v−1 saline and injected i.p.
Room temperature conditions
Three Ta conditions were used: ‘cool', where the room temperature was between 15 and 16°C (referred to as Ta: 15°C), ‘normal', with a room temperature of between 20 and 21°C (Ta: 20°C) and ‘warm' between 30 and 32°C (Ta: 30°C). Studies on animals at Ta 20°C and 30°C were conducted on rats grouped to simulate crowded dance club conditions. Animals were tested in a cohort group that had been housed and pretreated together. Testing was performed in polypropylene cages (50 × 25 × 15 cm3) with a light floor covering of sawdust. Appropriate control saline-injected animals were examined at the same time and in the same conditions. Animals examined at Ta 15°C were separated into individual polypropylene cages (42 × 25 × 12 cm3) when put into the cool room. This allowed each animal to have a cage area similar to that available to each grouped animal, but prevented them from huddling together, which, as we have previously observed, they do in order to minimise body heat loss.
Rats were always exposed to the room conditions for 60 min before MDMA administration, and remained in that room until completion of the acute experiment (except for the study where it is specifically stated that they were moved from Ta 30 to 24°C) and tail temperature measurements continued for a further 90 min.
MDMA-induced neurotoxic lesion
Rats housed at Ta 20°C in groups of five were injected with MDMA (5 mg kg−1 i.p.) to a total of three doses given at 3 h intervals. Control animals were injected with saline vehicle using the same time schedule. In one study, the concentration of 5-HT was examined in selected brain regions 7 days later. In another study, the effect of the lesion on tail temperature regulation was examined 7–21 days later.
Temperature measurement
Rectal temperature measurement was performed using an MC 8700 thermometer, with digital readout, and a H-RB3 rectal temperature probe (EXACON A/S, Roskilde, Denmark) lubricated with lanolin hand cream. Each rat was lightly restrained by hand for approximately 20 s, while the probe was inserted approximately 2.5 cm into its rectum and a steady reading was obtained.
Tail temperature was measured by use of a MicroFlo DSP laser perfusion monitor; this was pressed lightly to the upper tail and gave a steady reading within 45 s.
Measurement of 5-HT in cerebral tissue following a neurotoxic dose of MDMA
Rats given the neurotoxic dose schedule of MDMA (see above) were killed 7 days later by cervical dislocation and decapitation, the brains rapidly removed and cortex, hippocampus and striatum dissected out on ice. Tissue was homogenised and 5-HT measured by high-performance liquid chromatography (h.p.l.c.) with electrochemical detection. The method used was based on that published in detail by Colado et al. (1997). Briefly, the mobile phase consisted of KH2PO4 (1.0 M), octanesulphonic acid (0.25 mM), EDTA (0.1 mM) and methanol (10%), and was adjusted to pH 3 with phosphoric acid, filtered and degassed. The flow rate was 1.2 ml min−1 and the working electrode potential was set at +0.8 V. The h.p.l.c. system consisted of a pump (Pharmacia LKB 2150) linked to a stainless steel reversed-phase column (C18 Phenomenex Lichrosher Select B, 5 μm, 150 × 4.6 mm2) with a pre-column and an LC-4C amperometric detector (Bioanalytical Systems Inc., Congleton, Cheshire). The current produced was monitored using integration software (SP4400 Chromjet with Nelson Chromatography Software).
Statistics
Statistical analyses of the temperature measurements were performed using the statistical computer package BMDP/386 Dynamic (BMDP Statistical Solutions, Cork, Eire). Data were analysed by analysis of variance (ANOVA) with repeated measures (program 2V). Treatment was used as the between-subjects factor and time as the repeated measure. The ANOVA values represent a main effect of treatment. Tail temperature responses were also analysed with the same program.
Analysis of tail temperature data in MDMA-lesioned rats was complex because of the effect of acute MDMA administration in both control and lesioned animals. Since the study was designed to discover whether the effect of MDMA was to alter the time course of the change, t-tests were also performed at each measurement time-point. Similarly, t-tests were performed at specific time points in the study, when rats were moved from the warm room conditions to the normal room temperature. Student's ‘t' tests were performed using GraphPad Prism v 4 for Windows (GraphPad Software, San Diego, DA, U.S.A.). In all studies, the significance level was set at P<0.05.
Results
Effect of dopamine receptor antagonists on MDMA-induced hypothermia
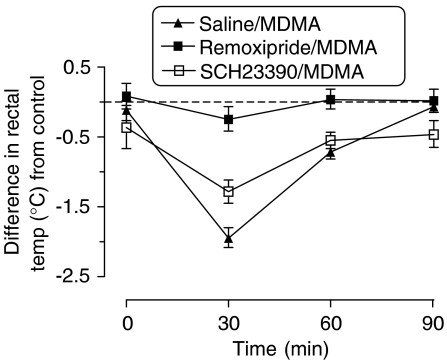
Administration of MDMA (5 mg kg−1 i.p.) to rats present at Ta 15°C produced a rapid statistically significant decrease in rectal temperature lasting more than 60 min and with a nadir of approximately 1.7°C below mean pretreatment values (Figure 1). When the dopamine D2 receptor subtype antagonist remoxipride (10 mg kg−1 i.p.) was given 20 min prior to the MDMA, it abolished the MDMA-induced hypothermic response (Figure 1). In contrast, the dopamine D1 receptor antagonist SCH23390 (1.1 mg kg−1 i.p.), when given 20 min prior to MDMA, was without effect on the hypothermic response that follows MDMA (Figure 1). Neither dopamine antagonist alone altered the basal temperature (data not shown).
Figure 1.
Effect of dopamine antagonists on the effect of MDMA on rectal temperature in rats at Ta 15°C. Graph shows the difference in the mean rectal temperature compared to the control group (saline/saline)±s.e.m. (n=5). MDMA (5 mg kg−1) produced a decrease in rectal temperature compared to saline-injected control animals (F(1,8)=190.84, P<0.0001), results being calculated from the raw data. Remoxipride (10 mg kg−1) itself had no effect on rectal temperature compared to control animals, and the response of rats pretreated with remoxipride before the MDMA was different from that of rats treated with MDMA (F(1,8)=31.2, P=0.0005). Administration of SCH23390 (1.1 mg kg−1) before MDMA did not significantly alter the response compared to rats given only MDMA (F(1,8)=1.54, P=0.2499).
Tail temperature of rats present in different ambient room temperatures
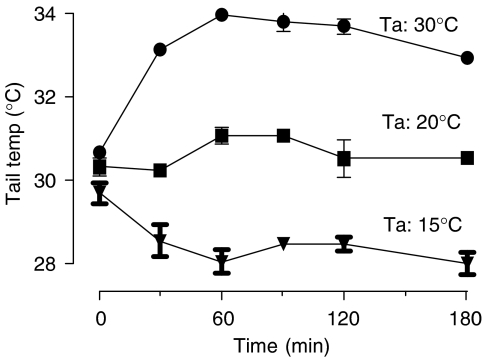
The tail temperature of rats present at Ta 20°C varied little over the 3 h observation period. Exposure of rats to Ta 30°C resulted in a rapid rise in tail temperature of approximately 3.5°C during the first 60 min, after which it remained generally stable (Figure 2). Exposure of rats to Ta 15°C produced a 1.5°C decrease in their tail temperature in the first 30 min, after which there was no further change (Figure 2).
Figure 2.
The skin temperature of the tail of rats when present in Ta 20°C or when moved at time 0 to a room at Ta 15 or 30°C. Results are shown as mean±s.e.m. (n=4). At Ta 15°C, the tail temperature dropped compared with rats at Ta 20°C (F(1,6)=122.26, P<0.0001). At Ta 30°C, the tail temperature rose above that of the rats housed at Ta 20°C (F(1,6)=159.95, P<0.0001).
Effect of low and high Ta on body and tail temperature in rats following MDMA
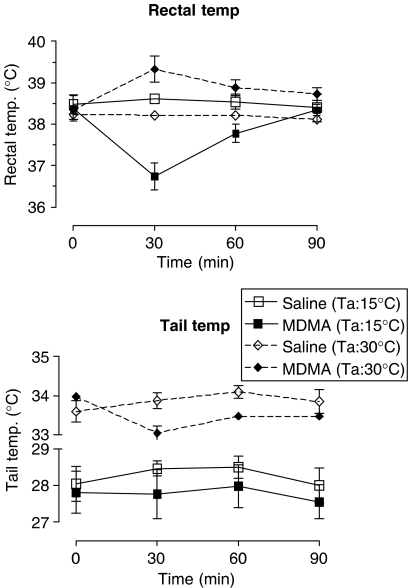
MDMA (5 mg kg−1 i.p.) administered to rats present at Ta 15°C produced a marked, rapid decrease in rectal temperature, but failed to alter the tail temperature throughout the time of the rectal hypothermic response compared to saline-treated controls (Figure 3). In contrast, this dose of MDMA administered at Ta 30°C produced a modest but sustained rectal hyperthermic response and a statistically significant decrease in tail temperature over the same period (Figure 3).
Figure 3.
The rectal temperature and tail skin temperature of rats housed at Ta 15 or 30°C and injected with MDMA (5 mg kg−1) at time 0. Graph shows the mean rectal (n=5) or tail (n=4) temperature±s.e.m. Tail temperature was unaltered by MDMA injection compared to the saline-injected group when rats were housed at Ta 15°C (F(1,8)=4.63, P=0.0637), but altered by MDMA, compared to the saline-injected group, when rats were housed at Ta 30°C (F(1,8)=8.05, P=0.0297). MDMA administration produced a decrease in rectal temperature in rats housed at Ta 15°C (F(1,8)=190.84, P<0.001) and a rise when housed at Ta 30°C (F(1,10)=13.45, P=0.0043) compared to the appropriate saline-injected control group.
Effect of a neurotoxic dose of MDMA on the cerebral 5-HT concentration
The neurotoxic dose regime of MDMA used in this study (see Methods) produced a statistically significant decrease in the concentration of 5-HT in both the cortex and hippocampus of approximately 30% compared to saline-injected controls. The decrease in the striatum was smaller (15%) and of marginal statistical significance (Table 1).
Table 1.
Concentration of 5-HT in regions of rat brain 7 days following administration of three doses of MDMA (5 mg kg−1) each given 3 h apart
| Brain region | Tissue 5-HT conc. (ng g−1) | Statistical P-value | |
|---|---|---|---|
| Saline | MDMA | ||
| Cortex | 516±28 (5) | 370±23 (5) | 0.004 |
| Hippocampus | 311±13 (5) | 219±25 (5) | 0.011 |
| Striatum | 372±21 (5) | 314±15 (5) | 0.056 |
Results shown as mean±s.e.m. (n). The P-value was obtained following comparisons of the 5-HT concentration following MDMA compared to saline-injected control value using Student's ‘t'-test.
Effect of a prior MDMA-induced lesion on tail temperature when housed at 30°C and moved to 24°C
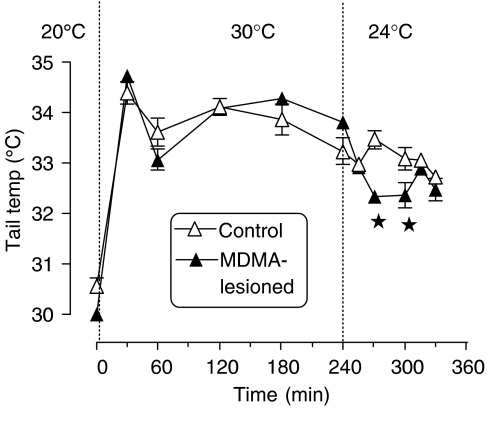
A prior neurotoxic lesion with MDMA did not influence the tail temperature of rats when housed at Ta 20°C, nor their tail response when the rats were moved and kept at Ta 30°C (Figure 4). However, when the rats were returned to Ta 24°C, the tail temperature remained elevated in the control animals, but decreased in the lesioned rats over the next 60 min (Figure 4).
Figure 4.
The tail temperature of a control group and a group subjected to a prior MDMA-induced lesion when placed in a warm environment (30°C) and when returned to a cooler room (24°C). Graph shows the mean±s.e.m. (n=4). The rectal temperature rose rapidly and similarly in both groups when exposed to Ta 30°C. When both groups were returned to Ta 24°C, the tail temperature was significantly lower (P<0.05) in the lesioned group for the 30- and 60-min time points, as indicated by the asterisks.
Effect of acute MDMA administration on the tail temperature of rats with a prior MDMA-induced lesion when housed at Ta 20 or 30°C
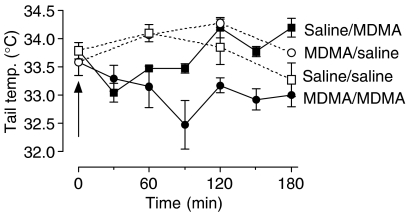
Acute administration of MDMA (5 mg kg−1) to rats at Ta 20°C did not alter the tail temperature compared to saline-injected rats in either control rats or rats subjected to a prior neurotoxic dose of MDMA (data not shown). However, MDMA (5 mg kg−1) given to rats housed at Ta 30°C produced a rapid decrease in tail temperature in control and MDMA-lesioned rats. While the tail temperature of the saline/MDMA group returned to control values within 120 min, that of the lesioned group challenged with MDMA remained lower than the lesioned control group injected with saline for more than 180 min (Figure 5).
Figure 5.
The tail temperature of a control group of rats injected with saline (saline/saline) or MDMA (saline/MDMA) (5 mg kg−1) when housed at Ta 30°C and an experimental group pretreated with an MDMA-induced neurotoxic lesion followed by either saline (MDMA/saline) or MDMA 5 mg kg−1 (MDMA/MDMA). A statistical comparison of the tail temperature following the acute MDMA dose when compared to the appropriate saline-injected control using Student's ‘t'-test indicated that the saline-pretreated group had a lower tail temperature 60 min after MDMA injection (P=0.0079), but not at 120 min (P=0.3589), while the lesioned group had a lower tail temperature at 60 (P=0.0463), 120 (P=0.0004) and 180 min (P=0.0109).
Discussion
In this study, we observed that MDMA produced a hypothermic response in rats housed in cool Ta and hyperthermia when housed in warm room conditions. These results supported other studies that have investigated the effect of MDMA (Gordon et al., 1991; Dafters, 1994; Dafters & Lynch, 1998) and its simpler congener, amphetamine (Yehuda & Wurtman, 1972a, 1972b), on the rectal temperature of rats housed at different Ta conditions.
The MDMA-induced hypothermic response seen in rats housed at Ta 15°C was completely blocked by the dopamine D2 receptor antagonist remoxipride, but not the D1 receptor antagonist SCH23390. This is the converse of the hyperthermic response, which we previously observed was blocked by SCH23390 but unaltered by remoxipride (Mechan et al., 2002).
The block of MDMA-induced hypothermia with remoxipride indicates the probable involvement of cerebral dopamine, since MDMA releases dopamine in the brain (see Colado et al., 2004). The fact that there was no change in tail temperature following administration of MDMA to rats at Ta 15°C suggests no change in vasotonicity also argues against a peripheral effect of dopamine.
The ‘opposite' pharmacology of the hypothermic and hyperthermic effect of MDMA may reflect either the existence of ‘cold' and ‘warm' thermosensors (Bligh, 1979) with differing pharmacology, or that the primacy of presynaptic and postsynaptic dopamine function (Hjorth & Carlsson, 1987) differs with the Ta. This appears to be a probable explanation given the observation that dopamine agonists have opposite temperature effects in normal rats and those given reserpine, where presynaptic function has presumably been compromised (Verma & Kulkarni, 1993).
The rise in rectal temperature that follows MDMA administration to rats at Ta 20 and 30°C probably involves thermogenesis, there being an increase in metabolic rate (Gordon et al., 1991) and an involvement of both the hypothalamic–pituitary–thyroid axis (Sprague et al., 2003) and also β3-adrenoreceptor activity in brown adipose tissue (Sprague et al., 2004). This induces heat generation through activation of uncoupling protein (Mills et al., 2004). Nevertheless, this increase in metabolism does not appear to activate heat-loss mechanisms and tail vasodilation, since a hyperthermic dose of MDMA to rats at Ta 20°C did not alter tail temperature of rats (Mechan et al., 2001). Furthermore, the tail temperature even decreased modestly when MDMA (5 mg kg−1) was given to rats at Ta 30°C, rather than increase, as one might expect if the animal wished to thermoregulate and lose heat. This observation contrasts with the fact that exposure of untreated rats to Ta 30°C produced a rise in rectal temperature of only 1°C (Mechan et al., 2001), but produced an increase in tail temperature of approximately 4°C (this paper). The lack of vasodilation and heat loss by the tail may be associated with the fact that MDMA can induce peripheral vasoconstriction (Gordon et al., 1991; Pedersen & Blessing, 2001).
We and others have reported that a prior MDMA-induced neurotoxic lesion, while not impairing thermoregulation at Ta 20°C, does cause problems in heat loss in rats housed at Ta 30°C. Both Dafters & Lynch (1998) and Mechan et al. (2001) found that MDMA-lesioned rats exposed to Ta 30°C had an elevated rectal temperature for a longer period than control rats when returned to a Ta 20°C room.
In order to be able to compare results obtained in the current study with these previous investigations on MDMA-induced neurotoxicity, we used a dosing schedule that would produce a loss of cerebral 5-HT similar to that previously observed (Mechan et al., 2001). The current dosing schedule produced a 5-HT loss in both the cortex and hippocampus of approximately 30%, which is similar to that produced in the earlier study of Mechan et al. (2001), and which has recently been shown to leave striatal dopamine concentration unaltered (Sanchez et al., 2004). We wanted to produce a modest loss in cerebral 5-HT content in order to make the lesion comparable to the loss in 5-HT markers that has been reported to occur in the human brain following heavy recreational use of MDMA (McCann et al., 1998). While we did not measure the 5-HT concentration in the hypothalamus (a probable key region involved in temperature regulation), our earlier study demonstrated that the loss in 5-HT content in this region is similar to that seen in the hippocampus following a neurotoxic dose of MDMA (Mechan et al., 2001).
Recently, Green et al. (2004b) observed that MDMA-lesioned rats, when administered a challenge dose of MDMA (5 mg kg−1), showed a sustained rectal hyperthermia when present in a warm (30°C) room compared to nonlesioned rats. We hypothesised that this abnormal response was also due to impaired heat loss and this proposal has been supported by the current investigation. Firstly, it was seen that when MDMA-lesioned rats were returned from Ta 30 to 24°C, their tail temperature was lower over the next 60 min than the control (nonlesioned) group. This lower tail temperature presumably resulted in the impaired heat loss seen in the lesioned rats compared to nonlesioned controls. In addition, it was found that administration of a dose of MDMA (5 mg kg−1) to control rats at Ta 30°C produced a modest decrease in tail temperature, while the same dose given to MDMA-lesioned rats resulted in a prolonged decrease in tail temperature. This is again consistent with the sustained rectal hyperthermia previously observed in lesioned rats given MDMA at Ta 30°C (Green et al., 2004b).
A recent investigation suggested that the impairment in heat dissipation following an MDMA-induced neurotoxic lesion is due to the loss in cerebral 5-HT content produced by the lesion. The fact that the effect of the MDMA-induced lesion was mimicked by administration of the 5-HT synthesis inhibitor PCPA, the nonselective 5-HT receptor antagonist methysergide and the 5-HT1A receptor antagonist WAY100635 (Saadat et al., 2005) supports this notion. The idea that normal 5-HT release is required for inducing temperature loss when rats are present at high Ta is further strengthened by the fact that PCPA pretreatment increases heat-induced mortality in rats housed at high Ta (Reid et al., 1968; Cronin, 1976).
It is interesting to note that acute administration of MDMA to rats housed at Ta 30°C induces a greater 5-HT efflux in the nucleus accumbens than that seen in rats housed at Ta 20°C (O'Shea et al., 2005), not because this region is necessarily responsible for initiating heat-loss mechanisms, but because it does suggest that region-selective changes in 5-HT, which could be involved in controlling heat-loss mechanisms, may occur.
In conclusion, these data strengthen our earlier proposal that a neurotoxic dose of MDMA produces an impairment in thermoregulation in rats exposed to high Ta. This impairment is expressed in the inability of the animal to increase heat loss through mechanisms involved in heat dissipation by the tail, a major heat-loss organ in the rat. It is possible that heavy recreational use of MDMA by humans also results in damage to 5-HT neurones in the brain, although this is by no means certain (Green et al., 2003). If this did occur, then such persons might also have impaired heat loss when further doses of the drug are taken in hot crowded dance club conditions and this requires investigation.
Abbreviations
- 5-HT
5-hydroxytryptamine
- MDMA
3,4-methylenedioxymethamphetamine
- PCPA
p-chlorophenylalanine
- SCH23390
R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro1-H-3-benzene
- Ta
ambient temperature
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane carboxamide·3HCl
References
- BLIGH J. The central neurology of mammalian thermoregulation. Neuroscience. 1979;4:1213–1236. doi: 10.1016/0306-4522(79)90153-2. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., MURRAY T.K., GREEN A.R. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (‘ecstasy') and p-chloroamphetamine but not the degeneration following fenfluramine. Br. J. Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GREEN A.R. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology. 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- CRONIN M.J. p-Chlorophenylalanine hyperthermia in a warm environment: reversal with 5-hydroxytryptophan. Brain Res. 1976;112:194–199. doi: 10.1016/0006-8993(76)90351-6. [DOI] [PubMed] [Google Scholar]
- DAFTERS R.I. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy') in rats. Psychopharmacology. 1994;114:505–508. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- DAFTERS R.I. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption and chronic dosing. Physiol. Behav. 1995;58:877–882. doi: 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- DAFTERS R.I., LYNCH E. Persistent loss of thermoregulation in the rat induced by 3,4-methylenedioxymethamphetamine (MDMA or ‘Ecstasy') but not by fenfluramine. Psychopharmacology. 1998;138:207–212. doi: 10.1007/s002130050664. [DOI] [PubMed] [Google Scholar]
- GORDON C.J., WATKINSON W.P., O'CALLAGHAN J.P., MILLER D.B. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol. Biochem. Behav. 1991;14:644–653. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- GRANT R.T. Vasodilatation and body warming in the rat. J. Physiol. 1963;167:311–317. doi: 10.1113/jphysiol.1963.sp007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A.R., MECHAN A.O., ELLIOTT J.M., O'SHEA E., COLADO M.I. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA ‘Ecstasy') Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., O'SHEA E., COLADO M.I. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur. J. Pharmacol. 2004a;500:3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., SANCHEZ V., O'SHEA E., SAADAT K.S., ELLIOTT J.M., COLADO M.I. Effect of ambient temperature and a prior neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA) on the hyperthermic response of rats to a single or repeated (‘binge' ingestion) low dose of MDMA. Psychopharmacology. 2004b;173:264–269. doi: 10.1007/s00213-003-1725-2. [DOI] [PubMed] [Google Scholar]
- HJORTH S., CARLSSON A. Postsynaptic dopamine (DA) receptor stimulator properties of the putative DA autoreceptor-selective agonist B-HT 920 uncovered by co-treatment with the D-1 agonist SK&F 38393. Psychopharmacology. 1987;93:534–537. doi: 10.1007/BF00207249. [DOI] [PubMed] [Google Scholar]
- MALBERG J.E., SEIDEN L.S. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCANN U.D., SZABO A., SCHEFFEL U., DANNALS R.F., RICAURTE G.A. Neurotoxic effects in human brains after use of 3,4-methylenedioxymethamphetamine (‘Ecstasy') viewed by positron emission tomography. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- MECHAN A.O., ESTEBAN B., O'SHEA E., ELLIOTT J.M., COLADO M.I., GREEN A.R. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') to rats. Br. J. Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHAN A.O., O'SHEA E., ELLIOTT J.M., COLADO M.I., GREEN A.R. A neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) to rats results in a long term defect in thermoregulation. Psychopharmacology. 2001;155:413–418. doi: 10.1007/s002130100735. [DOI] [PubMed] [Google Scholar]
- MILLS E.M., RUSYNIAK D.E., SPRAGUE J.E. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J. Mol. Med. 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- O'SHEA E., ESCOBEDO I., ORIO L., SNACHEZ V., NAVARRO M., GREEN A.R., COLADO M.I.Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats Neuropsychopharmacology 2005(in press) [DOI] [PubMed]
- PEDERSEN N.P., BLESSING W.W. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J. Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID W.D., VOLICER L., SMOOKER H., BEAVEN M.A., BRODIE B.B. Brain amines and temperature regulation. Pharmacology. 1968;1:329–344. doi: 10.1159/000135983. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., SHIMANSKY Y.P. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- SAADAT K.S., O'SHEA E., COLADO M.I., ELLIOTT J.M., GREEN A.R. The role of 5-HT in the impairment of thermoregulation in MDMA (‘ecstasy')-pretreated rats. Psychopharmacology. 2005;179:884–890. doi: 10.1007/s00213-004-2106-1. [DOI] [PubMed] [Google Scholar]
- SANCHEZ V., O'SHEA E., SAADAT K.S., ELLIOTT J.M., COLADO M.I., GREEN A.R. Effect of repeated (‘binge') dosing of MDMA to rats housed at normal and high temperature on neurotoxic damage to cerebral 5-HT and dopamine neurones. J. Psychopharmacol. 2004;18:412–416. doi: 10.1177/026988110401800312. [DOI] [PubMed] [Google Scholar]
- SCHIFANO F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology. 2004;173:242–248. doi: 10.1007/s00213-003-1730-5. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J.E., BANKS M.L., COOK V.J., MILLS E.M. Hypothalamic–pituitary–thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) J. Pharmacol. Exp. Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J.E., BRUTCHER R.E., MILLS E.M., CADEN D., RUSYNIAK D.E. Attenuation of 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoreceptor antagonists. Br. J. Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERMA A., KULKARNI K. Differential role of dopamine receptor subtypes in thermoregulation and stereotypic behaviour in naïve and reserpinized rats. Arch. Int. Pharmacodyn. 1993;324:17–32. [PubMed] [Google Scholar]
- YEHUDA S., WURTMAN R.J. The effects of D-amphetamine and related drugs on colonic temperatures of rats kept at various ambient temperatures. Life Sci. 1972a;11:851–859. doi: 10.1016/0024-3205(72)90101-4. [DOI] [PubMed] [Google Scholar]
- YEHUDA S., WURTMAN R.J. Release of brain dopamine as the probable mechanism for the hypothermic effect of D-amphetamine. Nature. 1972b;240:477–478. doi: 10.1038/240477a0. [DOI] [PubMed] [Google Scholar]