Abstract
The hypothesis that the amplitude of the myogenic response is modulated by factors released from nerve endings was tested in rat tail small arteries.
A pressure myograph in conjunction with direct stimulation of nerve endings by electrical field stimulation (EFS) was used to determine rat small artery contractile reactions.
Vessel pretreatment with 10− 5 M phentolamine abolished EFS-induced reactions completely indicating that they are mediated mainly by an adrenoceptor agonist, probably noradrenaline.
In the absence and presence of 10− 5 M phentolamine, vessel diameter changes in the pressure range from 10 to 120 mmHg were not different.
Vessel stimulation by (i) EFS, (ii) noradrenaline, (iii) selective stimulation of α1- and α2-receptors, (iv) serotonin, or (v) vasopressin significantly reduced the diameter change induced by stepping pressure from 10 to 40 mmHg compared to unstimulated, control vessels.
Vessel diameter changes induced by stepping pressure from 40 to 80 and from 80 to 120 mmHg, however, were not different in vessels stimulated with EFS and noradrenaline compared to controls.
In conclusion, these data show that factors released from unstimulated adrenergic nerve endings (i.e., not stimulated by EFS) are not involved in the myogenic response. In contrast, factors released upon stimulation of nerve endings can modulate the amplitude of the myogenic response, but only at low pressures. Thus, the pressure range for myogenic blood flow autoregulation is extended to lower pressures. Myogenic autoregulation of blood flow at physiological pressures is unaltered.
Keywords: Arteries, innervation, mechanotransduction, smooth muscle
Introduction
In recent years, considerable progress has been reached in understanding the primary mechanisms of the myogenic response with increasing attention now given to the factors that modulate the myogenic response (Davis & Hill, 1999; Schubert & Mulvany, 1999; Hill et al., 2001). This is a functionally important issue, because under physiological conditions, the vessel wall is not only exposed to transmural pressure, but at the same time to substances released from the endothelium, transmitters liberated from nerve endings, conducted signals from neighboring vessel segments, and diverse metabolites. Thus, in vivo, an integration of these factors and transmural pressure exists, which may lead to a modulation of the myogenic response. This modulation can result in a change of the pressure range where pressure-induced constrictions are observed, or a change in the amplitude of pressure-induced constrictions. Hence, a precise knowledge of the modulatory characteristics of these factors on the myogenic response is important for the understanding of the role of the myogenic response in vivo.
A modulatory role of neurogenic influences on the myogenic response was suggested previously. Direct activation of neurogenic transmission, by sympathetic nerve stimulation in an in vitro cat sartorius muscle organ preparation (Ping & Johnson, 1992) or by application of phenylephrine to rat femoral arteries with a pressure range between 40 and 100 mmHg in vitro (Watanabe et al., 1993), was shown to increase the amplitude of the myogenic response. In contrast to the latter finding, adrenoceptor agonists did not alter the amplitude of the myogenic response in the above pressure range in rat cremaster small arteries (Ikeoka et al., 1992), dog renal arteries (Liu et al., 1994), or rat mesenteric arteries (Wesselman et al., 1997). The existence of these conflicting data requires a closer look at the experimental conditions of the cited studies.
In the study showing a change of the amplitude of the myogenic response by neurogenic influences, direct activation of neurogenic transmission by sympathetic nerve stimulation was used, a method most closely resembling physiological conditions. However, this study was performed on an in vitro cat sartorius muscle organ preparation (Ping & Johnson, 1992), where nerve stimulation can also affect blood flow, the metabolic state of the surrounding tissue, and the resistance distribution in the vessel network. The latter factors are able to modulate the myogenic response themselves; for example, an increased blood flow has been shown to decrease the amplitude of the myogenic response (Kuo et al., 1991; Juncos et al., 1995; Sun et al., 1995). Thus, in the cited study, the observed change of the amplitude of the myogenic response may be due to an indirect neurogenic effect mediated, for example, via a change in flow. Consequently, the investigation of the modulation of the myogenic response by neurogenic influences requires the use of experimental conditions eliminating confounding factors like changing flow.
In the studies showing no change of the amplitude of the myogenic response by neurogenic influences, these influences had been studied indirectly by topical application of transmitter substances. However, receptor subtypes reached by topically applied transmitter substances and receptor subtypes located in the synaptic cleft often differ. Importantly, different receptor subtypes have been observed to be the cause of varying effects of the transmitter noradrenaline on the amplitude of the myogenic response (Ikeoka et al., 1992). Thus, these data together with the absence of studies comparing the effect of topically applied transmitter substances and of a direct activation of neurogenic transmission on the myogenic response questions the use of topically applied transmitter substances as a substitute for a direct activation of neurogenic transmission.
In summary, due to methodological limitations of published studies, it is impossible to draw an unequivocal conclusion about the character of the modulation of the myogenic response by neurogenic influences. Therefore, the hypothesis that the amplitude of the myogenic response is modulated by neurogenic influences was tested in an in vitro model of isolated rat small arteries by directly stimulating nerve endings with use of electrical field stimulation (EFS).
Methods
The methods used in this study will be described only briefly, because they have been presented in detail previously (Fischer et al., 1996; Schubert et al., 1999).
Dissection and mounting of the vessels
Male Wistar–Kyoto rats, 16–25 weeks old, were killed by stunning and subsequent decapitation according to procedures approved by the local Animal Care and Use Committee. A piece of a first order branch of the main tail artery, which is running parallel along the main tail artery (Figure 1), was isolated. These vessels have a diameter in the range from 180 to 300 μm in the fully relaxed state at 80 mmHg. The artery was fitted onto two glass cannulas, which were mounted in the experimental chamber containing experimental solution consisting of (in mM): 120 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1.0 MgSO4, 1.6 CaCl2, 0.025 EDTA, 5.5 glucose, 26 NaHCO3, 5 HEPES at pH 7.4. The experimental chamber was placed onto the stage of an inverted microscope. The vessel was transilluminated in order to allow videomicroscopic diameter determinations. The microscope image was viewed with a low-intensity camera (Hamamatsu, Japan). The signal from the camera was digitized by a frame-grabber and the internal diameter of the vessel was measured with a special program (Fischer et al., 1996).
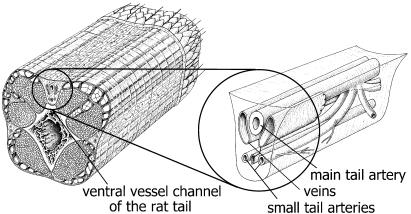
Figure 1.
The small tail artery. Left panel: An anatomic drawing of a piece of the rat tail is shown demonstrating in the cross-section the localization of the ventral vessel channel. Right panel: Magnified schematic drawing of the localization of the vessels in the ventral vessel channel. The main ventral tail artery is located just beneath the connective tissue sheet covering the vessel channel. Below this vessel, there are two thin-walled vessels, the main ventral tail veins. Deeper in the channel, two smaller vessels, the small tail arteries, are localized. These vessels are first-order branches of the main ventral tail artery running parallel along the main tail artery.
Experimental protocol
The chamber (2 ml) was continuously perfused with the experimental solution at a rate of 2 ml min− 1. One cannula was connected to a reservoir, which produced the desired intravascular pressure by gravity; the other cannula was connected to a pressure monitor. Leaking vessels were discarded at any stage of the experiment to ensure complete nonflow conditions. The pressure was increased to 80 mmHg and the vessel was lengthened up to its in vivo length and allowed to stabilize for 15 min. Thereafter, temperature was raised to 37.0±0.5°C. Probes for temperature and pH were placed in the experimental chamber. The pH was set to 7.40±0.05. The PO2 was measured to be about 150 mmHg; the PCO2 was about 40 mmHg. At the end of the warming-up period, a spontaneous (basal) myogenic tone developed, which was allowed to equilibrate for 60 min. At the end of this period, vessel diameter was about 30% smaller than the diameter at full relaxation. No additional preconstriction was employed. Thereafter, vessel viability was tested with 1 μM acetylcholine and 0.1 μM noradrenaline. Vessels showing a complete relaxation to acetylcholine and more than 20% constriction to noradrenaline were used for further experiments. All drugs were applied to the adventitial side of the vessels only. Then, the desired experiments were performed. Pressure steps were obtained by quickly changing the height of the reservoir. The experiments were always finished by a 20-min perfusion with experimental solution from which calcium was omitted and 1 mM EGTA was added to determine the fully relaxed vessel diameter at 80 mmHg. Data are given as diameter values or as % diameter change. The latter was calculated as the percent change from the diameter the vessel had just before the intervention (e.g. pressure step, agonist application).
EFS was performed with use of rectangular (2 × 5 mm) platinum plates. The vessel was placed in the center between both electrodes. All stimulation parameters are given in the results section.
In some experiments, the endothelium was removed functionally by passing air through the lumen of the vessel. During the mounting procedure, when the vessel was fixed at the first cannula only, an air bubble was introduced through this cannula. The air bubble was kept in the lumen of the vessel for 1.5 min and then removed through the open end of the vessel. After this procedure, vessel diameter did not increase upon application of 10 μM acetylcholine, which produced a complete relaxation of endothelium-intact vessels. This observation was considered to be evidence for a successful functional removal of the endothelium. In addition, noradrenaline-induced contractions and adenosine-induced relaxations were not different in endothelium-intact and endothelium-denuded vessels (see Figure 3). Thus, this procedure did not damage smooth muscle cell function.
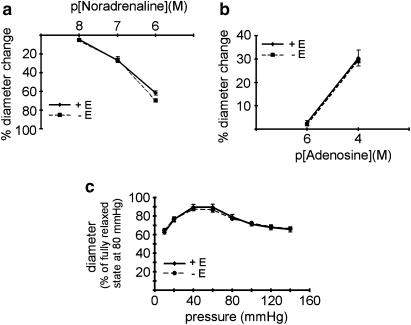
Figure 3.
Effect of endothelium removal on the myogenic response. (a) Effect of noradrenaline on vessel diameter in endothelium-intact (+ E) and endothelium-denuded (− E) vessels; no difference was observed (n=6, P=0.17). (b) Effect of adenosine on vessel diameter in endothelium-intact (+ E) and endothelium-denuded (− E) vessels; no difference was observed (n=4, P=0.89). (c) Effect of pressure on vessel diameter (myogenic response) in endothelium-intact (+ E) and endothelium-denuded (− E) vessels; no difference was observed (n=6, P=0.91).
For data analysis, the amplitude of the myogenic response was defined as the % diameter change in response to a pressure step, and vessel tone, that is, its state of activation, was calculated as published previously (Wesselman et al., 1996; VanBavel et al., 1998). Vessel tone was calculated as a dimensionless variable: tone=(Ptone–Ppass(d)) / (Pmact(d) − Ppass(d)), where Ptone is the pressure applied to the vessel for which tone is calculated and d the observed diameter at Ptone; here d is normalized to the diameter at 80 mmHg and full relaxation. Ppass(d) and Pmact(d) are the relationships between diameter and pressure of a passive vessel and a maximal active vessel, respectively, which have been determined experimentally. Thus, tone of a passive vessel corresponds to zero and that of a maximal active vessel to one.
Determination of conditions for the selective stimulation of α1- and α2-receptors
Noradrenaline in the concentration range from 1 × 10− 7 to 3 × 10− 7 M constricted the vessels at 10 mmHg. The concentration of noradrenaline was matched to produce a similar constriction compared to EFS. These constrictions were inhibited by 42.3±13.4% (n=5) by 10− 7 M idazoxan, a selective α2-receptor blocker. This idazoxan concentration is 10 times higher than its pA2 (see (Brock et al., 1997) and references therein), that is, the blocker concentration requiring a doubling of the agonist concentration to overcome the blocking effect of the antagonist. However, a 1.7-fold increase of the noradrenaline concentration was sufficient to restore the initial noradrenaline-induced contraction in the absence of idazoxan. This observation together with the finding that the selective α2-receptor agonist xylazine at 10− 5 M did not affect vessel diameter indicates that α2-receptors are not able to evoke considerable contractions on their own but play a permissive role in noradrenaline-induced contractions. Further, constrictions induced by noradrenaline were inhibited by 90.9±3.8% (n=5) by 10− 8 M prazosin, a selective α1-receptor blocker. In contrast to the findings with idazoxan, a 15-fold increase of the noradrenaline concentration in the presence of prazosin was required to restore the initial noradrenaline-induced contraction in the absence of prazosin. Taken together, these findings show that the noradrenaline-induced contraction is predominantly mediated by α1-receptors. Furthermore, constrictions induced by EFS (0.1 ms, 10 Hz, 24 mA mm− 2) were inhibited by 40.1±8.1% (n=6) by 10− 6 M idazoxan and by 47.6±6.4% (n=6) by 10− 7 M prazosin. This shows that EFS-induced constrictions are mediated by both α1- and α2-receptors. The conclusion is supported by the direct observation of neurogenic α2-receptor-mediated smooth muscle cell depolarizations in rat tail artery (Itoh et al., 1983). The latter finding also indicates that the α2-receptors are likely to have a postjunctional localization. In addition, the β-adrenoceptor agonists isoproterenol and salbutamol did not affect vessel diameter up to a concentration of 10− 6 M, and the β-adrenoceptor-antagonist propranolol at 10− 6 M did not alter contractions induced by EFS (0.1 ms, 10 Hz, 24 mA mm− 2). In summary, a selective stimulation of α1-receptors was performed by application of noradrenaline in the presence of 10− 7 M idazoxan and a selective stimulation of α2-receptors was performed by employing EFS in the presence of 10− 7 M prazosin.
Drugs and chemicals
Noradrenaline, acetylcholine, adenosine, prazosin, idazoxan, xylazine as well as the salts for the solutions were obtained from Sigma (Deisenhofen, Germany). Phentolamine was purchased from Research Biochemical International (Biotrend, Cologne, Germany), and tetrodotoxin from Molecular Probes (Leiden, The Netherlands).
Statistics
All data are means±s.e. Only one vessel was taken from each rat; thus n refers both to the number of vessels and the number of rats. Statistical analysis was performed using: independent t-test, repeated measures ANOVA, and one-way ANOVA (followed by Dunnett or Bonferoni post hoc test) as appropriate (SPSS 9.0 for Windows).
Results
Determination of conditions for a selective stimulation of nerve endings
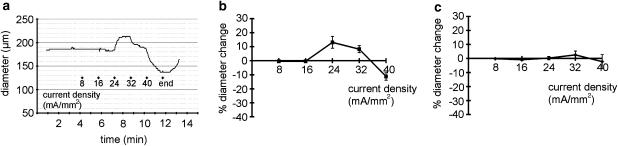
The effect of neurogenic influences on the myogenic response was studied by stimulating nerve endings with the use of EFS. Preliminary experiments showed that, depending on the stimulation parameters used, EFS either dilated or constricted the vessel. Thus in the example shown in Figure 2a, EFS (pulse duration 0.1 ms, 20 Hz) had no effect at stimulation pulse amplitudes of 8 and 16 mA mm− 2, dilated the vessel at 24 mA mm− 2 and constricted the vessel at 32 and 40 mA mm− 2. Sympathetic innervation predominates in the bed of the rat tail artery (Bao, 1993). Therefore, the observed dilation is most likely explained by an EFS-induced direct activation of endothelial and / or smooth muscle cells. Thus, the aim of the first series of experiments was to find experimental conditions for a selective stimulation of nerve endings by EFS. In a first step, nerve endings were blocked by tetrodotoxin (TTX, 10− 6 M). Vessel diameter changes were still observed in response to EFS (0.1 ms, 20 Hz, 8–40 mA mm− 2) (Figure 2b; n=7), which must result from a direct action of EFS on endothelial and / or smooth muscle cells. Next, the endothelium was removed. Vessel diameter changes were no longer observed in response to EFS (0.1 ms, 20 Hz, 8–40 mA mm− 2) in the presence of 10− 6 M TTX (Figure 2c; n=8, P=0.62), indicating that EFS does not affect smooth muscle cells directly.
Figure 2.
Determination of conditions for a selective stimulation of nerve endings. (a) Example of the effect of electrical field stimulation (EFS) (pulse duration 0.1 ms, 20 Hz) on vessel diameter. (b) Effect of EFS on vessel diameter in the presence of 10− 6 M tetrodotoxin (TTX) in endothelium-intact vessels showing the existence of vessel dilations and constrictions (n=7). (c) Effect of EFS on vessel diameter in the presence of 10− 6 M TTX in endothelium-denuded vessels; no effect was observed (n=8, P=0.62). Compare also to Figure 4a showing the effect of EFS on vessel diameter in the absence of TTX in endothelium-denuded vessels.
Free radicals have been shown to mediate EFS-induced vessel dilations under some circumstances (Lamb & Webb, 1984). However, 10− 3 M ascorbic acid, a scavenger of free radicals, did not affect EFS-induced responses. During continuous EFS action (0.1 ms, 20 Hz, 24 mA mm− 2), ascorbic acid application induced a vessel diameter change of 0.57±0.35% (n=3). This effect was not different from the ascorbic acid-induced diameter change in the absence of EFS (0.32±0.17%, n=3; P=0.89). These results indicate that free radicals do not mediate the EFS-induced vessel responses observed in the present study.
Effect of endothelium removal on the myogenic response
The findings presented above show that a selective action of EFS on nerve endings can only be obtained in the absence of the endothelium. Thus, we investigated the effect of endothelium removal on contractile and dilatory responses. Neither contraction induced by noradrenaline (Figure 3a; n=6, P=0.17) nor dilation induced by adenosine (Figure 3b; n=4, P=0.89) was affected by endothelium removal. Further, the myogenic response was not different in endothelium-intact and endothelium-denuded vessels in the pressure range from 10, 20, 40,…, 140 mmHg (Figure 3c; n=6, P=0.91). For the myogenic response, similar findings have been reported in a considerable number of studies (for details, see reviews by Meininger & Davis, 1992; Schubert & Mulvany, 1999). Therefore, endothelium-denuded vessels were used in all subsequent experiments.
Determination of the effect of phentolamine on EFS-induced responses
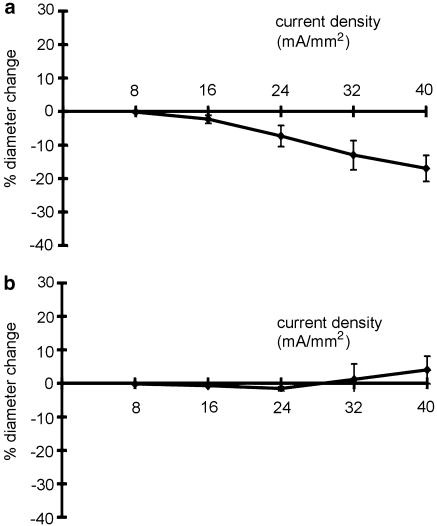
Noradrenaline is the main transmitter in the vascular bed of the main rat tail artery (Bao, 1993). The α-adrenoceptor blocker phentolamine (10− 5 M) completely blocked the contractile effect of perfusion-applied noradrenaline on the small tail artery (data not shown). Furthermore, EFS (0.1 ms, 20 Hz, 8–40 mA mm− 2) stimulated vessel constriction (Figure 4a; n=11, P<0.01); pretreatment of the vessels with 10− 5 M phentolamine abolished this effect of EFS (Figure 4b; n=6, P=0.60). This effect is similar to the action of EFS in the presence of TTX (compare to Figure 2c).
Figure 4.
Determination of the effect of phentolamine on EFS-induced response. (a) Effect of EFS on vessel diameter in endothelium-denuded vessels; a significant effect was observed (n=11, P<0.01). (b) Effect of EFS on vessel diameter in endothelium-denuded vessels in the presence of 10− 5 M phentolamine; no effect was observed (n=6, P=0.60).
Interaction of the myogenic response with neurogenic influences
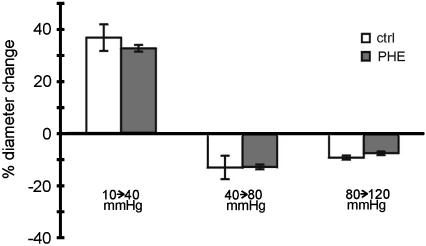
In this series of experiments, the possible involvement of adrenergic nerve endings not stimulated by EFS in the vessel response to pressure changes was tested. In the absence and the presence of 10− 5 M phentolamine, vessel diameter responses were similar for pressure steps from 10 to 40 mmHg (n=6, P=0.55), from 40 to 80 mmHg (n=7, P=0.90), and from 80 to 120 mmHg (n=6, P=0.18) (Figure 5).
Figure 5.
Lack of involvement of adrenergic nerve endings not stimulated by EFS in the myogenic response. Diameter changes to the pressure steps indicated are shown in the absence (ctrl) and in the presence of 10− 5 M phentolamine (PHE); no differences were observed (P=0.55, P=0.90 and P=0.18, respectively).
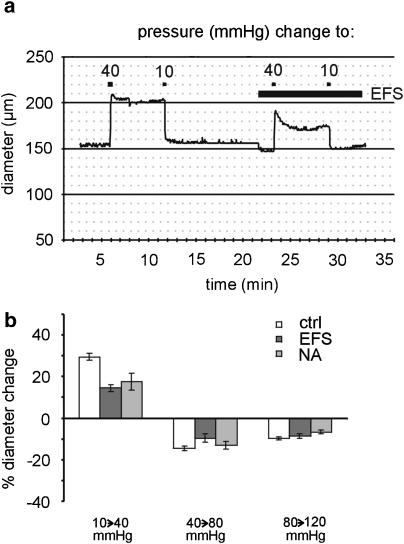
In the next series of experiments, pressure-induced vessel diameter changes were investigated during EFS (0.1 ms, 10 Hz, 24 mA mm− 2) and in the presence of noradrenaline (in the range from 1 × 10− 7 to 3 × 10− 7 M). The concentration of noradrenaline was adjusted to produce a similar constriction compared to EFS. As shown in the example in Figure 6a, under control conditions, a pressure step from 10 to 40 mmHg produced a large initial vessel dilation followed by a tiny constriction. In contrast, during EFS, the initial dilation was followed by an active constriction, that is, a myogenic response. Thus, in response to a pressure step from 10 to 40 mmHg, vessel diameter at steady state increased by 30±2% (n=11) in the control, but only by 15±2% (n=5) during EFS and by 17±4% (n=6) in the presence of noradrenaline. The effects of EFS and noradrenaline were both different from control (P<0.01) but not different from each other (Figure 6b; P=1.00). In response to pressure steps from 40 to 80 mmHg and from 80 to 120 mmHg, the steady-state vessel diameter decreased. The pressure-induced diameter changes during EFS and in the presence of noradrenaline were not significantly different from control (P=0.14 and P=0.07, respectively).
Figure 6.
Involvement of stimulated nerve endings in the myogenic response. (a) Example of the effect of a pressure change from a starting value of 10 to 40 mmHg and back to 10 mmHg on vessel diameter in the absence and presence of EFS (0.1 ms, 10 Hz, 24 mA mm− 2). (b) Diameter changes in response to the pressure steps indicated below the bars in the control (ctrl), during EFS (EFS), and in the presence of noradrenaline (NA); at the step from 10 to 40 mmHg, a significantly different effect was observed during EFS and NA compared to control (both P<0.01); at the other pressure steps, no differences were found (P=0.14 and P=0.07).
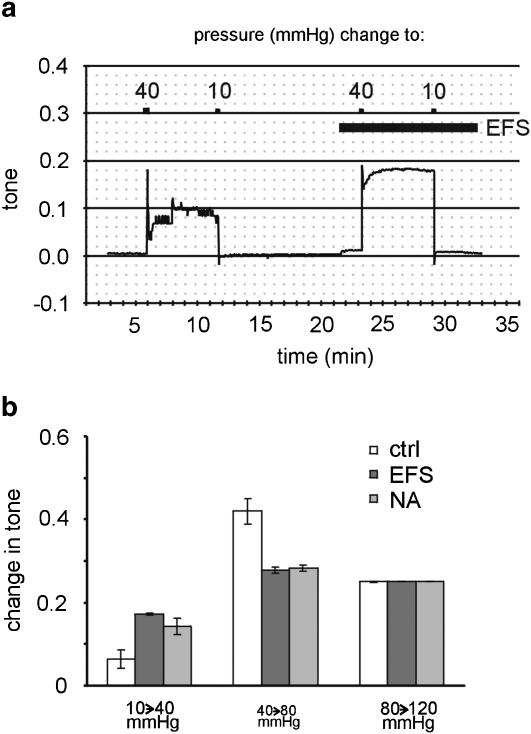
Changes in vessel tone, that is, in the activation state of the vessel, were calculated from the data presented in the previous section (see Methods). The example in Figure 7a shows that a pressure step from 10 to 40 mmHg produced an increase of tone that was augmented considerably during EFS. Thus, in response to a pressure step from 10 to 40 mmHg, tone at steady state increased by 0.065±0.022 units (n=11) in the control, but by 0.172±0.003 units (n=5) during EFS and by 0.143±0.021 units (n=6) in the presence of noradrenaline. These effects of EFS and noradrenaline were both different from control (P<0.05) but not different from each other (Figure 7b; P=1.00). In response to a pressure step from 40 to 80 mmHg, tone increased by 0.420±0.031 units (n=11) in the control, but only by 0.277±0.007 units (n=5) during EFS and by 0.282±0.008 units (n=6) in the presence of noradrenaline. The effects of EFS and noradrenaline were both different from control (P<0.01) but not different from each other (P=1.00). At a pressure step from 80 to 120 mmHg, tone increases were not significantly different in the control, during EFS and in the presence of noradrenaline (P=0.50).
Figure 7.
Changes in calculated vessel tone after different pressure steps. (a) Example of the effect of a pressure change from a starting value of 10 to 40 mmHg and back to 10 mmHg on calculated tone in the absence and presence of EFS (0.1 ms, 10 Hz, 24 mA mm− 2). (b) Changes of calculated tone in response to the pressure steps indicated below the bars in the control (ctrl), during EFS (EFS), and in the presence of noradrenaline (NA); at the steps from 10 to 40 mmHg and from 40 to 80 mmHg, a significantly different effect was observed during EFS and NA compared to control (P<0.05 and P<0.01, respectively); at the other pressure step, no difference was found (P=0.50). The data presented in this figure are based on the data shown in Figure 6.
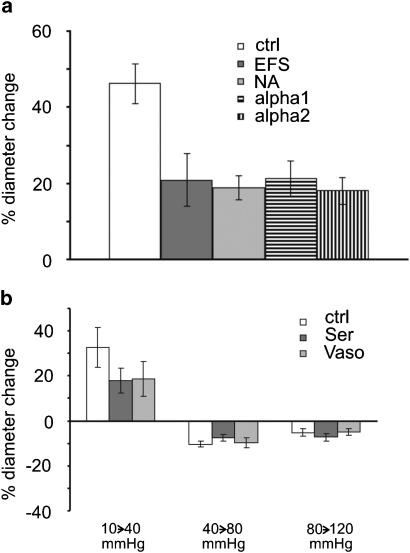
Additional experiments were performed to obtain some mechanistic information regarding the modulation of the myogenic response by neurogenic influences. Vessel diameter changes were investigated in response to a pressure step from 10 to 40 mmHg, that is, the pressure step where noradrenaline and EFS affected the myogenic response, during selective α1- and α2-receptor stimulation (see Methods). The responses to noradrenaline, EFS, selective stimulation of α1-receptors, and selective stimulation of α2-receptors were adjusted to produce a similar contraction as in the related experiments described above. Upon a pressure step from 10 to 40 mmHg, vessel diameter at steady state increased by 46±5% (n=5) in the control, but only by 21±7% (n=5) during EFS, by 19±3% (n=5) in the presence of noradrenaline, by 21±5% (n=5) during selective α1-receptor stimulation, and by 18±4% (n=5) during selective α2-receptor stimulation. The effects of EFS, noradrenaline, selective α1-receptor stimulation, and selective α2-receptor stimulation were all different from the control (P<0.01) but were not different from each other (Figure 8a; P=0.95). In addition, pressure-induced vessel diameter changes were studied in the presence of the nonadrenergic agonists serotonin and vasopressin. The responses of serotonin (in the range from 3 × 10− 9 to 1 × 10− 8 M) and vasopressin (in the range from 1 × 10− 13 to 3 × 10− 13 M) were adjusted to produce a similar constriction compared to EFS. In response to a pressure step from 10 to 40 mmHg, vessel diameter at steady state increased by 33±9% (n=5) in the control, but only by 18±5% (n=5) in the presence of serotonin and by 19±8% (n=5) in the presence of vasopressin. The effects of serotonin and vasopressin were both different from control (P<0.05) but not different from each other (Figure 8b; P=0.95). In response to pressure steps from 40 to 80 mmHg and from 80 to 120 mmHg the steady state vessel diameter decreased. However, the pressure-induced diameter changes in the presence of serotonin and vasopressin were not significantly different from control (P=0.53 and P=0.65, respectively).
Figure 8.
Effect of smooth muscle cell activation on the myogenic response. (a) Diameter changes in response to a pressure step from 10 to 40 mmHg in the control (ctrl), during EFS (EFS), in the presence of noradrenaline (NA), and during selective α1- and α2-receptor stimulation; a significantly different effect was observed during EFS, NA application, and selective α1- and α2-receptor stimulation compared to control (all P<0.01). (b) Diameter changes in response to the pressure steps indicated below the bars in the control (ctrl), in the presence of serotonin (Ser) and vasopressin (Vaso); at the step from 10 to 40 mmHg, a significantly different effect was observed during Ser and Vaso compared to control (both P<0.05); at the other pressure steps, no differences were found (P=0.53 and P=0.65).
Discussion
This study shows that the blockade of neurogenic transmission from unstimulated adrenergic nerve endings (i.e. not stimulated by EFS) does not alter pressure-induced diameter responses. Furthermore, this is the first investigation examining the modulation of the myogenic response by neurogenic influences, which employs direct activation of neurogenic transmission by EFS, that is a method that more closely resembles physiological conditions. It is shown that stimulation of nerve endings with EFS does not alter pressure-induced diameter responses in the pressure range between 40 and 120 mmHg, but increases the amplitude of the myogenic response between 10 and 40 mmHg. Similar pressure range-dependent changes of the myogenic response are observed during topical application of the transmitter substance noradrenaline and the nonadrenergic agonists serotonin and vasopressin.
This study shows that blockade of adrenoceptors, which mediate the effect of the main transmitter in this vessel, does not alter the myogenic response. Similar findings have been reported after inhibition of nerve action potentials by tetrodotoxin, after blockade of postsynaptic receptor function by receptor antagonists and after chemical vessel denervation in cat cerebral (Harder, 1984), rat cerebral (Osol & Halpern, 1985), and rat saphenous (Berczi et al., 1992) arteries. Thus, this study provides additional supporting evidence for the conclusion that the myogenic response is due to an action of transmural pressure on smooth muscle cells only, that is, indeed ‘myogenic'.
The present study shows that direct activation of neurogenic transmission by EFS increases the amplitude of the myogenic response at low transmural pressure. It should be emphasized that the smaller steady-state diameter increase during EFS in this pressure range is not the consequence of different passive vessel distensibilities. Although the initial vessel diameter of control and EFS-precontracted vessels was different, the initial passive distention of these vessels was not (33±1% in the control and 30±2% during EFS (n=5, P=0.11)). An increase of the amplitude of the myogenic response is observed also during topical application of the transmitter substance noradrenaline and the nonadrenergic agonists serotonin and vasopressin. Such a strengthening of the myogenic response at low transmural pressure has also been reported during topical application of noradrenaline in dog renal (Liu et al., 1994) and rat mesenteric (Wesselman et al., 1997) arteries. However, it should be emphasized that the similarity between the effect of EFS and of topical application of noradrenaline as found in the present study was not expected a priori. Indeed, our pilot experiments had shown that the effect of EFS and of topically applied noradrenaline is mediated by different α-receptors. Stimulation of different α-receptor subtypes has been reported to have different effects on the amplitude of the myogenic response (Ikeoka et al., 1992). Yet, the similar modulation of the myogenic response by EFS and topically applied noradrenaline, despite acting via different α-receptor subtypes, is consistent with our observations that: (i) the modulation of the myogenic response by selective stimulation of α1- and α2-receptors is the same and (ii) selective stimulation is not different from the effect of EFS or noradrenaline. Unfortunately, the number of studies addressing the modulation of the myogenic response by stimulation of different α-receptor subtypes is very small. Thus, it is currently impossible to provide an explanation for the different findings obtained in the present study and in the study cited earlier (Ikeoka et al., 1992). However, the data on the modulation of the myogenic response by selective stimulation of α1- and α2-receptors provide some insight into the mechanisms governing the modulation of the myogenic response by neurogenic influences. It is well established that α1- and α2-receptors activate different signal transduction pathways in smooth muscle cells (Guimaraes & Moura, 2001). The effects of α1-receptor stimulation are mediated predominantly by nonvoltage-operated calcium influx, calcium release, and changes in calcium sensitivity, whereas α2-receptor stimulation is mediated predominantly by voltage-operated calcium influx. Despite this difference, the modulation of the myogenic response by selective stimulation of α1- and α2-receptors was the same. Thus, the modulation of the myogenic response by neurogenic influences is independent of the particular postreceptor mechanism involved. This conclusion is supported by the observation that the nonadrenergic agonists serotonin and vasopressin produced a similar modulation of the myogenic response compared to adrenergic stimulation. In addition, the data presented in this study points to one common feature of all tested procedures for moderate vessel activation. Both EFS and topical application of agonists increase the amplitude of the myogenic response at low transmural pressure, that is, extend the myogenic range to lower pressures. This effect is achieved due to a larger change in vessel tone compared to control. The larger change in vessel tone is most likely caused by a pressure-induced increase in agonist sensitivity. Indeed, the noradrenaline concentration response curve at 40 mmHg was shifted to the left compared to 10 mmHg; the noradrenaline concentration response curves at 40 and 80 mmHg were similar (data not shown). Under the conditions of the present study, the myogenic range was extended to pressures with limited functional importance. However, during strong vasodilation, induced, for example, by tissue metabolites or substances released from the endothelium, the myogenic response occurs only at pressures considerably higher than 40 mmHg. An extension of the myogenic range to lower pressures by additional sympathetic activation can re-establish the myogenic response in the whole range of physiological pressures. In conclusion, at low transmural pressures, an active neurogenic transmission increases the amplitude of the myogenic response, thereby extending myogenic blood flow autoregulation to lower pressures. Although the activation of numerous mechanisms can apparently contribute (i.e. α1- and α2-receptor activation, serotonin, vasopressin), the effect was independent of the particular mechanism involved and was accompanied by an augmented change in vessel tone.
At medium and high transmural pressures, EFS and noradrenaline did not alter the amplitude of the myogenic response. Contrasting data have been reported in experiments using direct activation of neurogenic transmission by sympathetic nerve stimulation in an in vitro cat sartorius muscle organ preparation (Ping & Johnson, 1992). However, sympathetic nerve stimulation in an in vitro organ preparation also changes blood flow, the metabolic state of the surrounding tissue and the resistance distribution in the vessel network. Thus, a change in, for example, blood flow induced by nerve stimulation may explain the difference between the observations in the cited and the present study. Furthermore, it was reported in the literature that topical application of adrenoceptor agonists did not alter the amplitude of the myogenic response at medium and high pressures (Ikeoka et al., 1992; Liu et al., 1994; Wesselman et al., 1997). Taking into account the observed similarity between the effect of EFS and of topical application of noradrenaline on the myogenic response, the data from the literature are considered to support the findings of the present study that at medium and high transmural pressures, neurogenic influences do not alter the amplitude of the myogenic response. Thus, at physiological pressures, an active neurogenic transmission leaves myogenic blood flow autoregulation unaltered.
In summary, this study shows that a release of factors from adrenergic nerve endings not stimulated by EFS are not involved in the myogenic response. Further, by directly stimulating nerve endings with use of electrical field stimulation, that is, by using a method most closely resembling physiological conditions, a more differentiated view of the modulation of the amplitude of the myogenic response by neurogenic influences was obtained. The novel findings are that an active neurogenic transmission increases the amplitude of the myogenic response, but only at low pressures, thereby extending the pressure range with myogenic blood flow autoregulation towards lower pressures. However, a functionally more important finding was made for physiological pressures, where an active neurogenic transmission does not change the amplitude of the myogenic response, leaving myogenic blood flow autoregulation unaltered.
Acknowledgments
We thank Dr Darcy Lidington for his valuable comments on the manuscript. This work was supported by Deutsche Forschungsgemeinschaft grant SCHU 805 / 7-1.
Abbreviations
- EFS
electrical field stimulation
- TTX
tetrodotoxin
References
- BAO J.X. Sympathetic neuromuscular transmission in rat tail artery – a study based on electrochemical, electrophysiological and mechanical recording. Acta Physiol. Scand. 1993;148:1–58. [PubMed] [Google Scholar]
- BERCZI V., STEKIEL W.J., CONTNEY S.J., RUSCH N.J. Pressure-induced activation of membrane K+ current in rat saphenous artery. Hypertension. 1992;19:725–729. doi: 10.1161/01.hyp.19.6.725. [DOI] [PubMed] [Google Scholar]
- BROCK J.A., MCLACHLAN E.M., RAYNER S.E. Contribution of alpha-adrenoceptors to depolarization and contraction evoked by continuous asynchronous sympathetic nerve activity in rat tail artery. Br. J. Pharmacol. 1997;120:1513–1521. doi: 10.1038/sj.bjp.0701055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M.J., HILL M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- FISCHER J.-G., MEWES H., HOPP H.-H., SCHUBERT R. Analysis of pressurized resistance vessel diameter changes with a low cost digital image processing device. Comp. Meth. Prog. Biomed. 1996;50:23–30. doi: 10.1016/0169-2607(96)01726-9. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HARDER D.R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ. Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- HILL M.A., ZOU H., POTOCNIK S.J., MEININGER G.A., DAVIS M.J. Invited review: arteriolar smooth muscle mechanotransduction: Ca(2 +) signaling pathways underlying myogenic reactivity. J. Appl. Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- IKEOKA K., NISHIGAKI K., OHYANAGI M., FABER J.E. In vitro analysis of alpha-adrenoceptor interactions with the myogenic response in resistance vessels. J. Vasc. Res. 1992;29:313–321. doi: 10.1159/000158946. [DOI] [PubMed] [Google Scholar]
- ITOH T., KITAMURA K., KURIYAMA H. Roles of extrajunctional receptors in the response of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J. Physiol. 1983;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNCOS L.A., GARVIN J., CARRETERO O.A., ITO S. Flow modulated myogenic responses in isolated microperfused rabbit afferent arteriols via endothelium-derived nitric oxide. J. Clin. Invest. 1995;95:2741–2748. doi: 10.1172/JCI117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUO L., CHILIAN W.M., DAVIS M.J. Interaction of pressure-induced and flow-induced responses in porcine coronary resistance vessels. Am. J. Physiol. 1991;261:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- LAMB F.S., WEBB R.C. Vascular effects of free radicals generated by electrical stimulation. Am. J. Physiol. 1984;247:H709–H714. doi: 10.1152/ajpheart.1984.247.5.H709. [DOI] [PubMed] [Google Scholar]
- LIU Y.P., HARDER D.R., LOMBARD J.H. Myogenic activation of canine small renal arteries after nonchemical removal of the endothelium. Am. J. Physiol. 1994;267:H302–H307. doi: 10.1152/ajpheart.1994.267.1.H302. [DOI] [PubMed] [Google Scholar]
- MEININGER G.A., DAVIS M.J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. 1992;263:H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- OSOL G., HALPERN W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am. J. Physiol. 1985;249:H914–H921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- PING P., JOHNSON P.C. Role of myogenic response in enancing autoregulation of flow during sympathetic nerve stimulation. Am. J. Physiol. 1992;263:H1177–H1184. doi: 10.1152/ajpheart.1992.263.4.H1177. [DOI] [PubMed] [Google Scholar]
- SCHUBERT R., LEHMANN G., SEREBRYAKOV V.N., MEWES H., HOPP H.-H. cAMP-dependent protein kinase is in an active state in rat small arteries possessing a myogenic tone. Am. J. Physiol. 1999;277:H1145–H1155. doi: 10.1152/ajpheart.1999.277.3.H1145. [DOI] [PubMed] [Google Scholar]
- SCHUBERT R., MULVANY M.J. The myogenic response: established facts and attractive hypotheses. Clin. Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- SUN D., HUANG A., KOLLER A., KALEY G. Flow-dependent dilation and myogenic constriction interact to establish the resistance of skeletal muscle arteriols. Microcirculation. 1995;2:289–295. doi: 10.3109/10739689509146775. [DOI] [PubMed] [Google Scholar]
- VANBAVEL E., WESSELMAN J.P.M., SPAAN J.A.E. Myogenic activation and calcium sensitivity of cannulated rat mesenteric small arteries. Circul. Res. 1998;82:210–220. doi: 10.1161/01.res.82.2.210. [DOI] [PubMed] [Google Scholar]
- WATANABE J., KEITOKU M., HANGAI K., KARIBE A., TAKISHIMA T. Alpha-adrenergic augmentation of myogenic response in rat arterioles – role of protein kinase-C. Am. J. Physiol. 1993;264:H547–H552. doi: 10.1152/ajpheart.1993.264.2.H547. [DOI] [PubMed] [Google Scholar]
- WESSELMAN J.P.M., SCHUBERT R., VANBAVEL E., NILSSON H., MULVANY M.J. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am. J. Physiol. 1997;272:H2241–H2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- WESSELMAN J.P.M., VANBAVEL E., PFAFFENDORF M., SPAAN J.A.E. Voltage-operated calcium channels are essential for the myogenic responsiveness of cannulated rat mesenteric small arteries. J. Vasc. Res. 1996;33:32–41. doi: 10.1159/000159129. [DOI] [PubMed] [Google Scholar]