Abstract
Nucleotides regulate various effects including vascular tone. This study was aimed to characterize P2Y receptors on endothelial cells of the aorta of C57BL6 mice. Five adjacent segments (width 2 mm) of the thoracic aorta were mounted in organ baths to measure isometric force development.
Nucleotides evoked complete (adenosine 5′ triphosphate (ATP), uridine 5′ triphosphate (UTP), uridine 5′ diphosphate (UDP); >90%) or partial (adenosine 5′ diphosphate (ADP)) relaxation of phenylephrine precontracted thoracic aortic rings of C57BL6 mice. Relaxation was abolished by removal of the endothelium and was strongly suppressed (>90%) by inhibitors of nitric oxide synthesis.
The rank order of potency was: UDP∼UTP∼ADP>adenosine 5′-[γ-thio] triphosphate (ATPγS)>ATP, with respective pD2 values of 6.31, 6.24, 6.22, 5.82 and 5.40. These results are compatible with the presence of P2Y1 (ADP>ATP), P2Y2 or P2Y4 (ATP and UTP) and P2Y6 (UDP) receptors.
P2Y4 receptors were not involved, since P2Y4-deficient mice displayed unaltered responses to ATP and UTP.
The purinergic receptor antagonist suramin exerted surmountable antagonism for all agonists. Its apparent pKb for ATP (4.53±0.07) was compatible with literature, but the pKb for UTP (5.19±0.03) was significantly higher. This discrepancy suggests that UTP activates supplementary non-P2Y2 receptor subtype(s).
Further, pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS) showed surmountable (UTP, UDP), nonsurmountable (ADP) or no antagonism (ATP).
Finally, 2′-deoxy-N6-methyladenosine3′,5′-bisphosphate (MRS2179) inhibited ADP-evoked relaxation only.
Taken together, these results point to the presence of functional P2Y1 (ADP), P2Y2 (ATP, UTP) and P2Y6 (UDP) receptors on murine aorta endothelial cells. The identity of the receptor(s) mediating the action of UTP is not fully clear and other P2Y subtypes might be involved in UTP-evoked vasodilatation.
Keywords: Nucleotides, aorta, mouse, relaxation, P2Y1, P2Y2, P2Y6, P2Y4
Introduction
Purine and pyrimidine nucleotides are released under a variety of stress circumstances. Platelet degranulation and shear stress or damage of the endothelial lining cause extracellular release of adenosine triphosphate (ATP) and uridine triphosphate (UTP) (John & Barakat, 2001; Lazarowski & Boucher, 2001). Extracellular nucleotides act through cell surface receptors which can be divided in the P2Y and the P2X receptor families (Ralevic & Burnstock, 1998). P2X receptors are membrane ion channels made by the assembly of subunits of the same (homo-oligomers) or different (hetero-oligomers) subtypes. The P2X receptor family consists of seven subtypes (P2X1–7) that are mainly activated by ATP (North, 2002). P2Y receptors consist of seven membrane spanning domains and eight subtypes have been identified: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 (Van Der Giet et al., 2002; Abbracchio et al., 2003). P2Y receptors are coupled via G-protein to phospholipase C, resulting in IP3 generation and Ca2+ release from intracellular stores, or to stimulation/inhibition of adenylate cyclase (Boarder & Hourani, 1998).
P2Y1 and P2Y2 are the major nucleotide receptors on human vascular endothelial cells (Wang et al., 2002) and mediate vasodilatation by releasing nitric oxide (NO), prostanoids or endothelium-derived hyperpolarizing factor (EDHF) (Wihlborg et al., 2003). Rat vascular endothelial cells express P2Y1 and P2Y2 receptors (Hansmann et al., 1997; Kumari et al., 2003) that mediate relaxation mainly via the NO pathway (Buvinic et al., 2002). In contrast, vascular smooth muscle cells express multiple P2Y and P2X receptors (Kunapuli & Daniel, 1998) that cause contraction. As a consequence, nucleotides are important regulators of the arterial tone.
Little is known about nucleotide receptors mediating vasodilatation of murine blood vessels. We reported that ATP induces relaxation in the murine aorta (Crauwels et al., 2003). It has already been proposed that this ATP response is mediated by P2Y1 and P2Y2 receptors (Beny, 2004). However, no rank order of potency has been determined. The present study was aimed to further characterize endothelial P2Y receptors. To this end, different nucleotides (ATP, adenosine 5′ diphosphate (ADP), uridine 5′ triphosphate (UTP), uridine 5′ diphosphate (UDP), adenosine 5′-[γ-thio] triphosphate (ATPγS)) were tested to determine their rank order of relaxation potency. Further, the functionality of the P2Y4 receptor subtype was determined in P2Y4−/0-mice. Finally, in antagonist studies with selective (2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS2179) (Kaiser & Buxton, 2002)) and nonselective (suramin and pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS) (Boarder & Hourani, 1998; Bowler et al., 2003)) P2Y antagonists, apparent pKb values were calculated. Since pKb values of competitive receptor antagonists are independent of the type and concentration of the agonist, apparent pKb values are important tools for the identification of the different P2Y subtypes.
Methods
Mice
The studies were approved by the Ethical Committee of the University of Antwerp, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. C57BL6 mice (age 25±7 weeks, body weight 26.3±3.6 g) were used. P2Y4−/0 and the corresponding wild-type (WT) mice (CD1/129SV strain, age 12–18 weeks, body weight 31.3±4.5 g) were used to test the role of the P2Y4 subtype. Genotyping was carried out using two primer sets as previously described (Robaye et al., 2003).
Isolating and mounting of blood vessels
After anaesthesia (sodium pentobarbital, 75 mg kg−1, i.p.), the aorta was carefully removed, stripped of adherent tissue and dissected systematically. The root was defined as the first 1 mm of the ascending aorta outside the heart. The thoracic aorta was divided in five sequential 2 mm wide segments (TA1 → TA5) starting 3 mm from the origin of the left subclavian artery (where the azygos vein crosses the aorta) down to the diaphragm (Crauwels et al., 2003). Segments were mounted between two parallel tungsten wire hooks in 10 ml organ baths. Tension was measured isometrically with a Statham UC2 force transducer (Gould) connected to a data acquisition system (Moise 3, EMKA Technologies). Vessels were immersed in Krebs–Ringer solution (37°C, and continuously aerated with 95% O2/5% CO2, pH 7.4) with ( mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaEDTA 0.025 and glucose 11.1. Segments of the thoracic aorta and the aortic root were gradually stretched until a stable loading tension of 20 and 10 mN, respectively, was attained. Between each concentration–response curve, the Krebs–Ringer solution was replaced three times to wash out the agents.
Removal of the endothelial cells
Endothelial denudation was performed by injecting 5 ml of 0.01% Triton X 100 in Krebs–Ringer solution directly into the left ventricle, followed by washing with Krebs–Ringer solution. The effectiveness of the method for endothelial denudation was checked by the absence of responses to acetylcholine (ACh). Further immunohistochemical staining of the endothelial cells was carried out using a specific monoclonal antibody (CD31) and sections were developed with the ABC IgG method (Crauwels et al., 2003).
Vasomotor studies
Indomethacin 10 μM was present in all studies to avoid any vasomotor interference due to contractile prostanoids (Vanhoutte et al., 2005). Rings were first contracted with a depolarizing potassium solution (50 mM). After three washing steps, a cumulative concentration–response curve was made for phenylephrine (3 nM–30 μM) and the concentration (EC50) resulting in 50% of the maximal contraction (Emax) was assessed for each vessel segment. At the top phenylephrine concentration segments were exposed for 15 min to the purinergic agonist (7 × 10−5 M) followed by three washing steps. This pretreatment was used to minimize the shift between the first and second curve seen with all purinergic agonists. In relaxation studies, vessels were precontracted with their individual EC50 of phenylephrine, followed by cumulative concentration–response curves for ATP, UTP, ADP, UDP, ATPγS or adenosine. To evaluate the contribution of the endothelium, the agonist experiments were repeated in vessels without endothelium. The role of eNOS was assessed with the combination of the NOS inhibitors Nϖ-nitro-L-arginine (LNA) (300 μM) and Nϖ-nitro-L-arginine methyl ester (L-NAME) (300 μM), both incubated for 20 min.
In antagonist studies, the first cumulative concentration–response curve was performed in the absence of antagonists and was repeated three times in the presence of increasing concentrations of suramin (10–30–100 μM), PPADS (1–3–10 μM) or MRS2179 (1–3–10 μM), added to the organ bath 20 min prior to the nucleotide cumulative concentration–response curve. To minimize time effects, all results were compared to a ring that was not treated with the antagonist. In antagonist studies with UTP and UDP, only the top antagonist concentrations were used.
Calculation of pD2 and apparent Kb values
To normalize the cumulative concentration–response curves, the precontraction level was set to 100%. All results are shown as % relaxation, which is 100%−% contraction. EC50 values (pD2=−log EC50) were obtained from individual concentration–response curves by curve fitting to a four-parameter logistic function (Prism GraphPad Software; version 4.0). To classify the P2Y receptors, the apparent Kb values of each antagonist were calculated using the EC50 values of each individual ring (Furchgott, 1972) with correction for possible time effects:
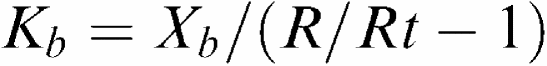 |
Xb is the antagonist concentration, R is the ratio of the EC50 with antagonist and the first EC50 without antagonist, Rt is the ratio of the EC50 of the second, third or fourth EC50 and the first EC50 in the segments not exposed to the antagonist.
When different concentrations of antagonist were used, the Schild plot analysis was performed to determine the pA2 and the slope of the curve.
Statistical analysis
All results are expressed as mean±s.e.m.; n represents the number of mice. For statistical analysis, the SPSS for Windows package was used. A 5% level of significance was selected. Results were compared to the corresponding time control and evaluation was done using one-way analysis of variance (ANOVA) or Student's t-test.
Materials
Sodium pentobarbital (Nembutal®) was obtained from Sanofi (Brussels, Belgium), indomethacin from Federa (Belgium), antibody against CD31 from BD (Erembodegem, Belgium), ABC kit from Vektor Laboratories (Burlingame, CA, U.S.A.) and OCT from Klinipath (The Netherlands). Phenylephrine hydrochloride, LNA, L-NAME, all nucleotides and all antagonists were obtained from Sigma (Bornem, Belgium).
Results
Agonist studies
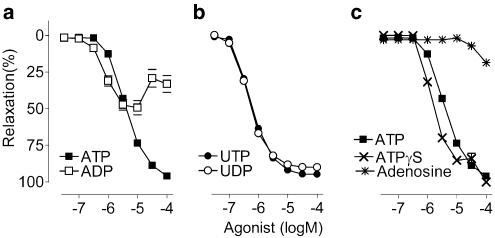
After precontraction of the thoracic aorta segments (to 12.24±3.28 mN, n=15) with the EC50 of phenylephrine (256±97 nM, n=15), nucleotides evoked concentration-dependent complete (ATP, ATPγS, UTP and UDP) or partial (ADP) relaxation (Figures 1 and 2). In the thoracic aorta, EC50 values displayed the following rank order of potency: UDP∼UTP∼ADP>ATPγS>ATP (Table 1). Adenosine, a potential degradation product of adenosine nucleotides, induced small relaxations but only at high concentrations (30 μM or more, Figure 2c).
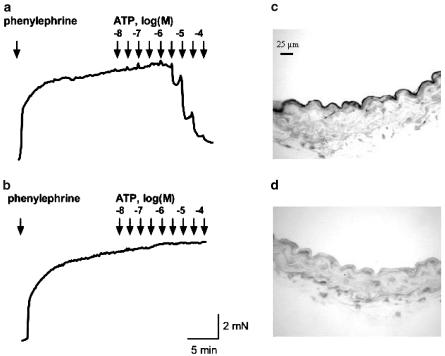
Figure 1.
Representative tracings of concentration–response curves of purinergic agonists. ATP induced relaxation in phenylephrine precontracted blood vessels with endothelium (a), but not in endothelium-denuded rings (b). Staining with an antibody against CD31 confirmed the effectiveness of the endothelial denudation. Micrographs of a vessel with endothelium (c) and an endothelial-denuded vessel (d).
Figure 2.
Cumulative concentration–response curves of phenylephrine precontracted thoracic aorta segments. (a) ATP evoked complete relaxation, whereas ADP evoked partial relaxation of the thoracic aorta. (b) UTP and UDP both evoked potent and complete relaxation. (c) ATPγS was more potent than ATP, while adenosine caused a small relaxation. Mean±s.e.m.; n=5.
Table 1.
pD2 values of nucleotides inducing endothelium-dependent relaxations in phenylephrine precontracted segments of the thoracic aorta and the aortic root
| Thoracic aorta | Aortic root | ||
|---|---|---|---|
| −log (M) | −log (M) | P-valuea | |
| ATP | 5.40±0.01 | 5.52±0.07 | 0.1281 |
| ATPγS | 5.82±0.02 | 6.12±0.08 | 0.0066 |
| ADP | 6.22±0.07 | 6.56±0.12 | 0.0401 |
| UTP | 6.24±0.02 | 6.91±0.09 | <0.0001 |
| UDP | 6.31±0.02 | 6.69±0.05 | 0.0001 |
Values represent mean±s.e.m. of five mice (ATPγS, n=3).
Aortic root versus thoracic aorta (Student's t-test).
The nucleotide-evoked relaxations disappeared after endothelial cell removal, while the phenylephrine contraction remained. In contrast, adenosine-evoked relaxation remained after removal of the endothelial cells (not shown). The efficacy of endothelial denudation was confirmed by the disappearance of CD31 staining (Figure 1) and the absence of relaxation after ACh administration (not shown).
There were no regional differences in sensitivity or maximal amplitude among the five adjacent segments within the thoracic aorta (e.g. pD2 of ATP: TA1 5.38±0.05, TA2 5.29±0.06, TA3 5.50±0.04, TA4 5.41±0.08, TA5 5.47±0.05, one-way ANOVA, P=0.135). Therefore, the data of the five thoracic aorta segments were used to calculate the average of each thoracic aorta. Segments of the aortic root were more sensitive to ADP, UTP, UDP and ATPγS than those of the thoracic aorta (Table 1). Furthermore, ADP evoked complete relaxation of the aortic root in contrast to partial relaxation of the thoracic aorta (not shown).
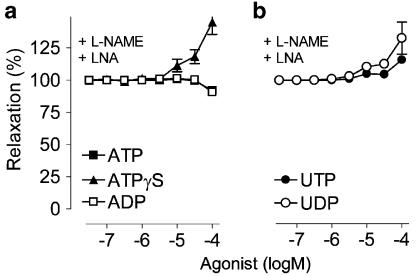
In the presence of the combination of LNA and L-NAME, the relaxation was almost completely abolished (ATP and ADP) or converted to a weak contraction (ATPγS, UTP and UDP) (Figure 3).
Figure 3.
Cumulative concentration–response curves for (a) ATP, ADP and ATPγS. (b) UTP and UDP of phenylephrine precontracted thoracic aorta segments in the presence of the combination of the NOS inhibitors LNA (300 μM) and L-NAME (300 μM). Mean±s.e.m.; n=5.
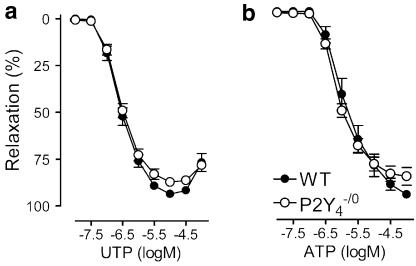
P2Y4-deficient mice
P2Y4−/0 and WT mice displayed identical relaxation curves for ATP as well as for UTP. For ATP- and UTP-evoked relaxation, P2Y4−/0 mice did not differ from WT mice, both with respect to the maximal amplitude, the slope of the curve and the sensitivity (Figure 4).
Figure 4.
Cumulative dose–response curves in the thoracic aorta segments of P2Y4−/0 and WT mice for (a) UTP and (b) ATP. Mean±s.e.m.; n=6.
Antagonist studies
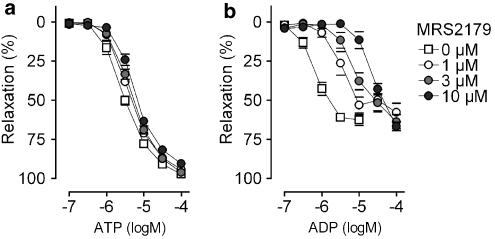
MRS2179, a selective P2Y1 receptor antagonist, caused a rightward shift of the ADP concentration–response curves (Figure 5), without affecting ATP, UTP or UDP responses (Table 2).
Figure 5.
Cumulative dose–response curves in segments of the thoracic aorta for (a) ATP and (b) ADP in the presence of MRS2179, a selective P2Y1 receptor antagonist. Mean±s.e.m.; n=5.
Table 2.
Apparent pKb values of different antagonists in isolated thoracic aorta and aortic root
| Tissue | Suramin | PPADS | MRS2179 |
|---|---|---|---|
| Agonist | −log(M) | −log(M) | −log(M) |
| Thoracic aorta | |||
| ATP | 4.53±0.07 | <5 | <5 |
| ADP | 5.55±0.11 | n.s. | 6.62±0.09 |
| UTP | 5.19±0.03 | 5.22±0.10 | <5 |
| UDP | 5.26±0.06 | 5.73±0.06 | <5 |
| Aortic root | |||
| ADP | 5.61±0.22 | 6.26±0.53 | 6.75±0.26 |
| UDP | 5.24±0.08 | 5.71±0.10 | <5 |
Values represent mean±s.e.m. of five mice, except for the aortic root: n=4. n.s.=nonsurmountable antagonism.
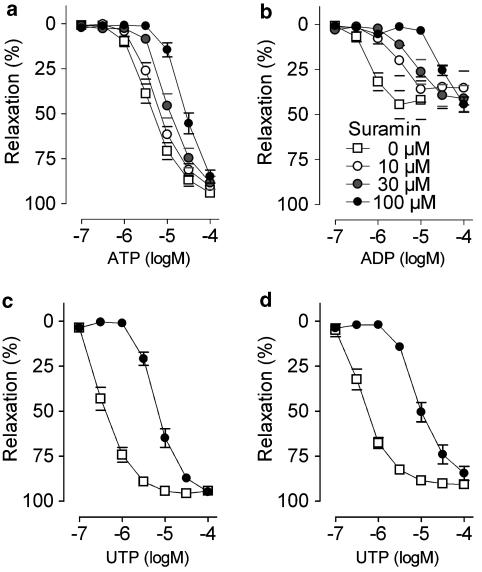
Suramin caused concentration-dependent shifts of all agonist concentration–response curves, without suppressing the maximum (Figure 6). Apparent pKb values were highest for ADP, intermediate for UTP and UDP and lowest for ATP.
Figure 6.
Cumulative dose–response curves in segments of the thoracic aorta for (a) ATP, (b) ADP, (c) UTP and (d) UDP in the presence of suramin, a nonselective purinergic receptor antagonist. Curves displayed concentration-dependent rightward shifts in the presence of suramin. Mean±s.e.m.; n=5.
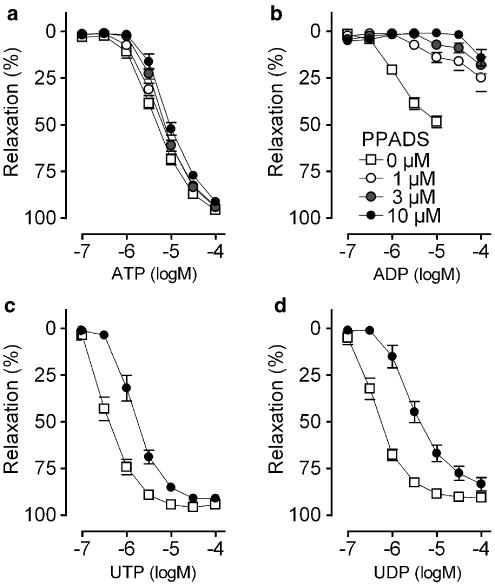
In contrast, PPADS displayed surmountable (UTP and UDP) and nonsurmountable (ADP) antagonism. Relaxation evoked by ATP was not affected by PPADS (Figure 7). The apparent pKb for UDP was higher (P=0.0039) than that for UTP (Table 2).
Figure 7.
Cumulative dose–response curves in segments of the thoracic aorta for (a) ATP, (b) ADP, (c) UTP and (d) UDP in the presence of PPADS, a nonselective purinergic receptor antagonist. PPADS showed surmountable (UTP and UDP) or nonsurmountable (ADP) antagonism, while ATP-evoked vasodilatation was not affected. Mean±s.e.m.; n=5.
Schild plot analysis of antagonists studies resulted in a pA2 of suramin of 4.48 (ATP) (confidence limits: 4.34–4.81) and of 5.64 (ADP) (confidence limits: 5.10–8.40). The pA2 of MRS2179 for ADP was 7.29 (confidence limits: 6.59–9.77). All slopes did not differ from unity. The pA2's of PPADS could not be determined since the antagonism was not surmountable.
Furthermore, apparent pKb values for ADP and UDP antagonism were not different between root and thoracic aorta (Table 2).
Discussion
Agonist studies
Nucleotides evoked relaxation of murine aorta segments. These relaxations were endothelium-dependent as removal of the endothelial cells abolished all relaxation. Three mediators are known to evoke endothelium-dependent relaxations in humans: NO, prostanoids and endothelium-derived hyperpolarizing factor (EDHF) (Wihlborg et al., 2003). The involvement of prostanoids can be excluded since indomethacin (10 μM) was always present in the Krebs–Ringer solution. The contribution of the NO pathway to the relaxation evoked by nucleotides was studied with NOS inhibitors. In the presence of the NOS inhibitors LNA and L-NAME, endothelium-dependent relaxation evoked by ATPγS, UTP and UDP was converted to a contraction while administration of ATP and ADP resulted in a small relaxation, most likely due to their degradation product adenosine. Therefore, the relaxation caused by nucleotides as seen in our experiments is solely mediated by NO derived from eNOS and neither prostanoids (indomethacin) nor endothelium-derived hyperpolarizing factor are involved. This conclusion is consistent with earlier observations of vasodilatation in mouse aortas (Crauwels et al., 2000; 2003) and is fully compatible with results of P2Y1/2-mediated vasodilatation of rat mesenteric arteries (Buvinic et al., 2002).
We found regional differences between the thoracic aorta and the aortic root. The aortic root was more sensitive to most nucleotides. Moreover, ADP evoked only partial relaxation in the thoracic aorta, whereas the aortic root relaxed completely. This could point to a smaller number of P2Y1 receptors, increased activity of nucleotide degrading enzymes, greater effectiveness of contractile purinergic receptors on the SMC or lower efficacy of the NO–cyclic GMP vasodilator system in the thoracic aorta. Since ATPγS also displayed greater sensitivity in the aortic root, differences in the activity of ATP-degrading enzymes seem unlikely. Moreover, ACh also showed a 0.5 log unit greater sensitivity in the aortic root (Crauwels et al., 2003), indicating that the effect is not nucleotide receptor-specific. It rather points to a greater efficacy of the NO–cyclic GMP vasodilator pathway in the aortic root.
The agonist studies provided part of the information necessary for identification of functional P2Y receptors on murine aortic endothelial cells. ADP preferentially activates P2Y1 receptors (Palmer et al., 1998). Hence, the relaxation evoked by ADP suggests the presence of P2Y1 receptors in murine aorta. However, we cannot fully exclude P2Y12 and P2Y13 receptors. These are mainly present on platelets, but recent evidence suggests that P2Y12 is also expressed in human vascular smooth muscle cells, where it induces contractions (Wihlborg et al., 2004), and in rat brain capillary endothelial cells (Simon et al., 2002).
The relaxation evoked by ATP suggests the presence of functional P2Y1, P2Y2 or P2Y4 receptors (Boarder & Hourani, 1998). ATP is very susceptible to degradation by ecto-enzymes present on the endothelial cells (Harden et al., 1997). Therefore, we tested the stable analogue ATPγS and adenosine, a potential degradation product of ATP and ADP. Aorta rings were slightly more sensitive to ATPγS than to ATP, suggesting a moderate degradation of ATP during the experiment. Adenosine induced only weak, endothelium-independent relaxations at very high concentrations, confirming previous experiments in mouse aorta (Talukder et al., 2002). Hence, a major contribution of adenosine in relaxation induced by adenosine nucleotides seems unlikely.
The relaxing effect of UTP has been attributed to P2Y2 receptors (Kumari et al., 2003), but the P2Y4 receptor might also be involved. Furthermore, an UTP-selective response has been identified in guinea pig cardiac endothelial cells (Yang et al., 1996): it is unclear if this response involves the P2Y4 receptor, since in mouse and rat that subtype is responsive to both ATP and UTP, but the properties of the guinea pig are unknown. On the other hand, the only UDP-selective receptor is the P2Y6 subtype (Communi et al., 1996; Lazarowski et al., 2001). In contrast to rat aorta (Kumari et al., 2003) and human arteries (Wihlborg et al., 2003) with a small effect of UDP in vasodilatation, the murine aortic P2Y6 receptor subtype appears to play a more prominent role. Hence, the results of the agonist studies are compatible with the presence of P2Y1 (ADP>ATP), P2Y2 or P2Y4 (ATP, UTP) and P2Y6 (UDP) receptor subtypes.
P2Y4-deficient mice
At present there are no clear reports of P2Y4 receptors mediating vasodilatation of blood vessels. However, in rat arteries, the P2Y4 subtype is associated with contractile effects (Rubino et al., 1999; Horiuchi et al., 2001) and the involvement of P2Y4 subtypes in vasodilatation of blood vessels has never been excluded. The lack of distinction between P2Y4−/0 and WT mice with respect to both ATP- and UTP-induced relaxation responses indicates that this subtype is not involved in the endothelial-dependent responses to these agonists. This finding is compatible with the observation that mRNA of the P2Y4 subtype was not detectable in murine mesenteric arteries (Vial & Evans, 2002) or murine aorta (unpublished results). Furthermore, the murine P2Y4 receptor is little sensitive to suramin inhibition (Suarez-Huerta et al., 2001), whereas suramin displaced the curves of all agonists (vide infra).
Antagonist studies
Additional information was acquired by performing antagonist studies. Relaxation of murine aorta by ADP and ATP has been already described previously (Crauwels et al., 2003; Beny, 2004). However in the present study, we used MRS2179, a selective P2Y1 receptor antagonist, to investigate more thoroughly the role of P2Y1 receptors in ADP- and ATP-evoked vasodilatation. MRS2179 antagonized ADP-evoked relaxation, but not that elicited by ATP, UTP or UDP. This indicates that P2Y12 (Wihlborg et al., 2004) or P2Y13 (Marteau et al., 2003) receptors were presumably not involved. Moreover, the fact that MRS2179 did not affect ATP-evoked relaxations to any extent points out that ATP mainly activates P2Y2 receptors in contrast to the suggestion made by Beny (2004).
Suramin, a nonselective purinergic antagonist exerted competitive and surmountable antagonism for all nucleotides and displayed three different classes of apparent pKb values, ATP, ADP and UTP/UDP, indicating that at least three different P2Y subtypes are involved. The apparent pKb values for ADP were in the same range as reported for the P2Y1 (ADPβS, pKb of 5.24) receptor of rat aorta endothelial cells (Hansmann et al., 1997).
The discrepancy between the apparent pKb values of suramin for ATP and UTP indicates that ATP and/or UTP do not solely act on P2Y2 receptor subtypes. Since ATP did not react with P2Y1 (MRS2179 data, vide supra), plausible explanations are that ATP activates (besides P2Y2 receptor subtypes) also contractile P2X4 (Yamamoto et al., 2000) or vasodilator P2Y11 receptor subtypes (Wang et al., 2002). However, to our knowledge, there is no orthologue of the P2Y11 receptor in the murine genome. Furthermore, the apparent pKb values of suramin for ATP found in this study (Table 2) are consistent with previous studies in cultured rat aortic smooth muscle cells (pA2 of 4.48) (Kumari et al., 2003) and in rat aorta endothelial cells (apparent pKb: 4.58). In contrast, the apparent pKb for UTP (Table 2) of the mouse aorta is different from those of the rat, (4.45) and (4.44) (Hansmann et al., 1997; Kumari et al., 2003). This suggests that in the murine aorta, UTP activates a supplementary P2Y subtype. The P2Y4 receptor is a potential candidate, but the experiments with P2Y4−/0 mice exclude the involvement of the P2Y4 subtype. Another candidate for UTP is the P2Y6 receptor subtype which is generally considered to be UDP-selective (Lazarowski et al., 2001). However, since UTP and UDP displayed identical apparent pKb values for suramin and had very similar concentration–response curves in the agonist studies, the presence of a UTP- and UDP-sensitive P2Y6-like receptor seems a possibility, as already proposed previously (Vial & Evans, 2002). In this hypothesis, UTP may act directly or indirectly (after breakdown to UDP) on the P2Y6 receptor. Yet, this P2Y6-like receptor hypothesis does not fully stroke with the different apparent pKb values for UTP and UDP of PPADS. Hence, the identity of the UTP receptor remains unclear and requires further investigation.
Conclusion
In murine aorta nucleotides induce an endothelium-dependent relaxation that is mediated by NO. ADP (P2Y1) induced partial relaxation that was antagonized by suramin, PPADS and MRS2179. ATP (P2Y2) caused complete relaxation that was antagonized by suramin, but not by the P2Y1-selective antagonist MRS2179. UDP (P2Y6) and UTP evoked complete relaxation that was inhibited by suramin and PPADS. Further, the results of the P2Y4−/0 mice clearly showed that the P2Y4 subtype is not involved in UTP-evoked vasodilatation. Hence, the action of UTP is mediated probably partially via P2Y2 receptors, and partially via P2Y6 receptors either directly or as a result of degradation into UDP. Taken together, these results point to the presence of functional P2Y1, P2Y2 and P2Y6 receptors on murine aorta endothelial cells.
Acknowledgments
Pieter-Jan Guns is a Research Assistant of the Fund for Scientific Research Flanders (Belgium – F.W.O.). The support by the Bilateral Scientific and Technological Collaboration Flanders – Hungary, BIL00 and by the Interuniversity Attraction Poles Programme – Belgian State – Federal Office for Scientific, Technical and Cultural Affairs, P5/02 are gratefully acknowledged. We thank Paul Fransen, Cor Van Hove and Rita Van den Bossche for technical support and helpful advice.
Abbreviations
- ACh
acetylcholine
- ADP
adenosine 5′ diphosphate
- ATP
adenosine 5′ triphosphate
- ATPγS
adenosine 5′-[γ-thio] triphosphate
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- PPADS
pyridoxal-phosphate-6-azophenyl-2′-4′-disulphonic acid
- UDP
uridine 5′ diphosphate
- UTP
uridine 5′ triphosphate
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENY J.L. Characterization of purine receptors in mouse thoracic aorta. J. Cardiovasc. Pharmacol. 2004;44:171–177. doi: 10.1097/00005344-200408000-00005. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOWLER J.W., BAILEY R.J., NORTH R.A., SURPRENANT A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUVINIC S., BRIONES R., HUIDOBRO-TORO J.P. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br. J. Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., PARMENTIER M., BOEYNAEMS J.M. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- CRAUWELS H.M., VAN HOVE C.E., HERMAN A.G., BULT H. Heterogeneity in relaxation mechanisms in the carotid and the femoral artery of the mouse. Eur. J. Pharmacol. 2000;404:341–351. doi: 10.1016/s0014-2999(00)00619-1. [DOI] [PubMed] [Google Scholar]
- CRAUWELS H.M., VAN HOVE C.E., HOLVOET P., HERMAN A.G., BULT H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc. Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoreceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines. Handbook of experimental pharmacology 1972Berlin: Springer; 283–335.eds. Blaschko, H. & Muscholl, E., Vol. 33, pp [Google Scholar]
- HANSMANN G., BULTMANN R., TULUC F., STARKE K. Characterization by antagonists of P2-receptors mediating endothelium-dependent relaxation in the rat aorta. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:641–652. doi: 10.1007/pl00005101. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K., LAZAROWSKI E.R., BOUCHER R.C. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol. Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- HORIUCHI T., DIETRICH H.H., TSUGANE S., DACEY R.G., JR Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H767–H776. doi: 10.1152/ajpheart.2001.280.2.H767. [DOI] [PubMed] [Google Scholar]
- JOHN K., BARAKAT A.I. Modulation of ATP/ADP concentration at the endothelial surface by shear stress: effect of flow-induced ATP release. Ann. Biomed. Eng. 2001;29:740–751. doi: 10.1114/1.1397792. [DOI] [PubMed] [Google Scholar]
- KAISER R.A., BUXTON I.L. Nucleotide-mediated relaxation in guinea-pig aorta: selective inhibition by MRS2179. Br. J. Pharmacol. 2002;135:537–545. doi: 10.1038/sj.bjp.0704476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMARI R., GOH G., NG L.L., BOARDER M.R. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br. J. Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C. UTP as an extracellular signaling molecule. News Physiol. Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., ROCHELLE L.G., O'NEAL W.K., RIBEIRO C.M., GRUBB B.R., ZHANG V., HARDEN T.K., BOUCHER R.C. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J. Pharmacol. Exp. Ther. 2001;297:43–49. [PubMed] [Google Scholar]
- MARTEAU F., LE POUL E., COMMUNI D., LABOURET C., SAVI P., BOEYNAEMS J.M., GONZALEZ N.S. Pharmacological characterization of the human P2Y13 receptor. Mol. Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- PALMER R.K., BOYER J.L., SCHACHTER J.B., NICHOLAS R.A., HARDEN T.K. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROBAYE B., GHANEM E., WILKIN F., FOKAN D., VAN DRIESSCHE W., SCHURMANS S., BOEYNAEMS J.M., BEAUWENS R. Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol. Pharmacol. 2003;63:777–783. doi: 10.1124/mol.63.4.777. [DOI] [PubMed] [Google Scholar]
- RUBINO A., ZIABARY L., BURNSTOCK G. Regulation of vascular tone by UTP and UDP in isolated rat intrapulmonary arteries. Eur. J. Pharmacol. 1999;370:139–143. doi: 10.1016/s0014-2999(99)00150-8. [DOI] [PubMed] [Google Scholar]
- SIMON J., FILIPPOV A.K., GORANSSON S., WONG Y.H., FRELIN C., MICHEL A.D., BROWN D.A., BARNARD E.A. Characterization and channel coupling of the P2Y12 nucleotide receptor of brain capillary endothelial cells. J. Biol. Chem. 2002;277:31390–31400. doi: 10.1074/jbc.M110714200. [DOI] [PubMed] [Google Scholar]
- SUAREZ-HUERTA N., POUILLON V., BOEYNAEMS J., ROBAYE B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur. J. Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- TALUKDER M.A., MORRISON R.R., MUSTAFA S.J. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H49–H57. doi: 10.1152/ajpheart.2002.282.1.H49. [DOI] [PubMed] [Google Scholar]
- VAN DER GIET M., GIEBING G., TOLLE M., SCHMIDT S. The role of P2Y receptors in the control of blood pressure. Drug News Perspect. 2002;15:640–646. doi: 10.1358/dnp.2002.15.10.740237. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., FELETOU M., TADDEI S. Endothelium-dependent contractions in hypertension. Br. J. Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol. Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- WANG L., KARLSSON L., MOSES S., HULTGARDH-NILSSON A., ANDERSSON M., BORNA C., GUDBJARTSSON T., JERN S., ERLINGE D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- WIHLBORG A.K., MALMSJO M., EYJOLFSSON A., GUSTAFSSON R., JACOBSON K., ERLINGE D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIHLBORG A.K., WANG L., BRAUN O.O., EYJOLFSSON A., GUSTAFSSON R., GUDBJARTSSON T., ERLINGE D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler. Thromb. Vasc. Biol. 2004;24:1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., ANDO J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 2000;87:385–391. doi: 10.1161/01.res.87.5.385. [DOI] [PubMed] [Google Scholar]
- YANG S., BUXTON I.L., PROBERT C.B., TALBOT J.N., BRADLEY M.E. Evidence for a discrete UTP receptor in cardiac endothelial cells. Br. J. Pharmacol. 1996;117:1572–1578. doi: 10.1111/j.1476-5381.1996.tb15323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]