Abstract
This study analyzed the expression of muscarinic acetylcholine receptors (mAChRs) in the rat cultured skeletal muscle cells and their coupling to G protein, phospholipase C and adenylyl cyclase (AC).
Our results showed the presence of a homogeneous population of [3H]methyl-quinuclidinyl benzilate-binding sites in the membrane fraction from the rat cultured muscle (KD=0.4 nM, Bmax=8.9 fmol mg protein−1). Specific muscarinic binding sites were also detected in denervated diaphragm muscles from adult rats and in myoblasts isolated from newborn rats.
Activation of mAChRs with carbachol induced specific [35S]GTPγS binding to cultured muscle membranes and potentiated the forskolin-dependent stimulation of AC. These effects were totally inhibited by 0.1–1 μM atropine.
In addition, mAChRs were able to stimulate generation of diacylglycerol (DAG) in response to acetylcholine, carbachol or selective mAChR agonist oxotremorine-M.
The carbachol-dependent increase in DAG was inhibited in a concentration-dependent manner by mAChR antagonists atropine, pirenzepine and 4-DAMP mustard.
Finally, activation of these receptors was correlated with increased synthesis of acetylcholinesterase, via a PKC-dependent pathway.
Taken together, these results indicate that expression of mAChRs, coupled to G protein and distinct intracellular signaling systems, is a characteristic of noninnervated skeletal muscle cells and may be responsible for trophic influences of acetylcholine during formation of the neuromuscular synapse.
Keywords: Muscarinic acetylcholine receptors, diacylglycerol, cyclic AMP, skeletal muscle, G protein, development
Introduction
The primary skeletal muscle culture has been used as an experimental model to investigate the molecular events involved in the myogenic differentiation, muscle plasticity and regulation of synaptic protein expression. Despite the lack of synaptic contact, differentiated cultured skeletal muscle fibers contract spontaneously (Walker & Wilson, 1975) and preserve several properties of in vivo multinucleated muscle fiber, including the expression of specific biochemical markers such as myogenin, Myo-D and myf5 (Sassoon et al., 1989; Braun et al., 1990; Li & Olson, 1992; reviewed in Sabourin & Rudnicki, 2000), as well as the compartmentalized synthesis of synaptic proteins, such as nicotinic acetylcholine receptors (nAChRs) and acetylcholinesterase (AChE), around individual nuclei (Rotundo, 1990; Gordon et al., 1992).
In contrast to innervated adult skeletal muscles, which exclusively express nAChRs, cultured muscle fibers also express muscarinic acetylcholine receptors (mAChRs) (Godinho & Rotundo, 1995; Reyes & Jaimovich, 1996; Liu et al., 2002). These cell surface receptors, with seven transmembrane-spanning domains, regulate a variety of intracellular signaling pathways by coupling to distinct heterotrimeric G proteins (Felder, 1995). Molecular cloning studies have evidenced at least five subtypes of mAChR (M1–M5). Whereas M2 and M4 mAChRs are primarily associated to Gi-dependent inhibition of adenylyl cyclase (AC), M1, M3 and M5 subtypes are preferentially coupled to Gq/11 protein–phospholipase C β (PLC β), and subsequent generation of inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), an endogenous activator of protein kinase C (PKC; for reviews, see Caulfield & Birdsall, 1998; Alexander et al., 2004).
In vertebrate skeletal muscles, activation of PKC has been linked to regulation of physiological processes, including glucose transport (Braiman et al., 1999), synaptic elimination (Lanuza et al., 2002) and expression of nAChRs and AChE (Klarsfeld et al., 1989; Choi et al., 2003; Miles & Wagner, 2003). Although activation of mAChRs has been associated to mobilization of intracellular Ca+2, via an IP3-dependent pathway in the cultured muscle cells (Reyes & Jaimovich, 1996), little is known about their connection to activation of muscle PKC. Owing to the critical role that DAG-dependent (PKC) isoforms play in muscle differentiation (Huang et al., 1992; Mendelzon et al., 1994), in the present study, we evaluated the expression of mAChRs at developing skeletal muscle cells, focusing on their ability to stimulate the G protein/DAG/PKC signaling cascade and AChE synthesis. Besides, considering that activation of mAChRs may interfere with G protein/AC transduction, we analyzed the effect of the muscarinic agonist oxotremorine-M on the intracellular cAMP content.
Methods
Rat skeletal muscle cultures
Primary skeletal muscle cultures were obtained from the hindlimb muscle of newborn rats (Godinho & Costa-Jr 2003). Briefly, newborn Wistar rats (0–12-h-old) were killed under CO2 anesthesia and the hindlimb muscles were dissected, minced and incubated for 2 h at 37°C in Hank's balanced salt solution (HBSS) containing collagenase (200 U ml−1). After mechanical dissociation, the cell suspension was centrifuged at 500 × g for 5 min and the resulting pellet was resuspended in Dulbecco's modified Eagle's medium (D-MEM) supplemented with 15% fetal calf serum (FCS) and 40 mg l−1 gentamicin. To avoid fibroblast contamination, cells were preincubated in culture flasks at 37°C for 30 min. Nonattached mononucleate myoblasts (2 × 105 cells ml−1) were seeded on 35 or 100 mm collagen-coated dishes in 2 or 8 ml final volume, respectively, and maintained in a humidified atmosphere of 90% air and 10% CO2, at 37°C. The culture medium was renewed after 24 h and replaced every other day with D-MEM containing 10% horse serum (HS) and 2% FCS (complete medium). All the experiments were performed using 7–8-day-old cultures.
Unilateral denervation of diaphragm muscle (Diap)
Denervation of the left Diap from 3-month-old Wistar rats was carried out under ether anesthesia by cutting the phrenic nerve about 10 mm from its entrance. To prevent muscle reinnervation, a 5–10 mm distal segment of nerve end was removed. After 7 days, at the time of the experimental procedure, muscle denervation was confirmed by the absence of response to repetitive stimulation of the nerve stump. All protocols were in accordance with the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by National Institute of Health and approved by the Ethical Committee at the UNIFESP-EPM (#108-01).
Skeletal muscle membrane preparation
Muscle cultures grown on 100 mm culture dishes were rinsed three times with ice-cold phosphate-buffered saline (PBS), pH 7.4, dissociated using 0.5 ml PBS plus 10 mM EDTA (PBS–EDTA), homogenized using a Dounce tissue-grinder and centrifuged twice at 20,000 × g for 10 min. The final pellet was resuspended in 20 mM Tris-HCl, pH 7.4, containing 1 mM MnCl2 (Tris-Mn).
In another set of experiments, membranes were obtained from control and denervated diaphragms, from adult male Wistar rats. The muscles were removed, dissected out and homogenized in ice-cold PBS–EDTA containing 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin A and 5 mM bacitracin. The samples were centrifuged at 1000 × g for 10 min and the supernatant was centrifuged two times at 70,000 × g, for 1 h at 4°C. The final pellet was suspended in 10 volumes (w v−1) of 20 mM Tris-HCl, pH 7.4, containing 0.1 mM EDTA and kept at −70°C for up to 7 days. Protein concentration was determined according to the method described by Bradford (1976), using bovine serum albumin as standard.
[3H]methyl quinuclidinyl benzilate ([3H]mQNB)-binding assays
Membrane fractions were diluted in Tris-Mn, pH 7.4, to reach 10 mg protein ml−1, and incubated with 0.05–10 nM [3H]mQNB, for 60 min at 30°C, in a final volume of 100 μl. The reaction was stopped by adding 1 ml of ice-cold Tris-Mn, and the samples centrifuged for 30 min at 20,000 × g at 4°C. The pellets were rinsed with 1.5 ml Tris-Mn and centrifuged twice in similar conditions. The final pellet was suspended in 200 μl of 2% sodium dodecyl sulfate, transferred into vials containing 4 ml Insta-Gel XF and the radioactivity determined by scintillation counting. Nonspecific binding, defined as the binding obtained in the presence of 1 μM unlabeled QNB, was not statistically different from that obtained in the presence of 1 μM atropine (data not shown). The values were expressed as fmol of [3H]mQNB-binding sites per mg of protein, from four independent experiments.
[35S]GTPγS-binding assay
The functional binding of [35S]GTPγS was performed using a modified method originally described by Gonzalez-Maeso et al. (2000). Membrane fraction (50 μg) obtained from 8-day-old cultured skeletal muscle was resuspended in 50 mM Tris-HCl buffer, pH 7.4, containing 3 mM MgCl2, 100 mM NaCl and 0.2 mM EGTA (reaction buffer) and 50 μM GDP. Then, in order to analyze the effect of mAChR activation on [35S]GTPγS binding, membranes were incubated with carbachol (1–100 μM) and 1 nM [35S]GTPγS, in the presence or absence of 100 nM atropine, at 37°C in 200 μl final volume. After 2 h, the reaction was terminated by adding 1 ml of ice-cold reaction buffer and centrifuged for 20 min at 15,000 × g at 4°C. The pellets were rinsed twice with 1.0 ml reaction buffer and recentrifuged in similar conditions. The final pellet was suspended in 200 μl of 2% sodium dodecyl sulfate and the radioactivity determined by scintillation counting. Nonspecific binding was obtained in the presence of 10 μM GTPγS. The carbachol effect was expressed as percentage of basal [35S]GTPγS binding (100%) obtained in unstimulated membranes.
DAG production by skeletal muscle cultures
The effect of cholinergic agonists on the generation of DAG was evaluated using a modified method originally described by Godfrey (1989) that measures the incorporation of [3H]cytidine into cytidine diphospho-DAG. Seven-day-old cultures grown on 35 mm dishes were rinsed two times with Krebs-bicarbonate buffer (Krebs, pH 7.4), and preincubated with 1 ml Krebs containing 2 mg ml−1 glucose, 10 mM LiCl and 1 μCi ml−1 [3H]cytidine, at 37°C. After 15 min, cultures were incubated with increasing concentrations of acetylcholine or carbachol or selective muscarinic receptor agonist (oxotremorine-M) for 1 h, at 37°C.
To evaluate the effect of mAChR inhibition, cultured muscle fibers were preincubated at 37°C with atropine, pirenzepine or 4-DAMP mustard for 15 min and subsequently treated with 30 μM carbachol. After 1 h, the medium was aspirated and the cell lipids were immediately extracted, using 1 ml ice-cold chloroform : methanol (1 : 2 v v−1). The samples were transferred to microtubes containing chloroform : water (4 : 5 v v−1), vigorously mixed and centrifuged for 5 min at 500 × g. The lower organic phase was rinsed with methanol : 1 N HCl, centrifuged for 5 min at 500 × g and transferred to a scintillation vial containing 4 ml of scintillation fluid. The radioactivity was measured by scintillation counting and the DAG produced was expressed as DPM per culture dish. The incubation of 20 mM myo-inositol to the culture medium completely blocked the carbachol-dependent accumulation of DAG, showing the direct correlation between the radioactive counts and the cellular levels of DAG (data not shown).
cAMP production by skeletal muscle cultures
Muscle cultures grown on 35 mm dishes were preincubated with 1 mM 3-isobutyl-1-methylxanthine (IBMX) in Krebs solution, pH 7.4, for 15 min and incubated with 10 μM forskolin and/or 10 μM oxotremorine-M, in the presence or absence of 1 μM atropine, at 37°C. After 10 min, the medium was aspirated and the reaction stopped by adding 500 μl of cold Krebs solution containing 4 mM EDTA, as described by Da Costa et al. (2001). The cells were transferred to microfuge tubes, boiled for 10 min and centrifuged at 20,000 × g, for 10 min. cAMP from the supernatant (50 μl) was determined using the cAMP kit [3H]-assay system, and the results were expressed as pmol of cAMP per mg of protein.
Synthesis of catalytically active AChE
Skeletal muscle cultures grown on 35 mm dishes were treated at 25°C with HBSS (pH 7.4) containing diisopropylfluorophosphate (100 μM, DFP) to irreversibly inhibit all AChE molecules. After 10 min, the cultured fibers were rinsed with HBSS to remove the unreacted DFP, treated with 1 μM carbachol or vehicle, and allowed to synthesize new AChE in complete medium, for 10–120 min, as described by Da Costa et al. (2001).
To investigate a possible coupling of mAChRs to activation of DAG-dependent PKC isoforms, the ongoing synthesis of AChE was evaluated in cultures pretreated with DFP and incubated with 30 μM oxotremorine-M, 10 nM phorbol 12,13 didecanoate (PDD) or vehicle solution, in the presence or absence of the PKC inhibitor chelerythrine (Chel; 2 μM). After 2 h, the enzyme was extracted using 500 μl of 20 mM borate buffer (containing 1 M NaCl, 5 mM EDTA, 0.5% Triton X-100, 5 mM N-ethylmaleimide, 2 mM benzamidine and 0.7 mM bacitracin). The samples were centrifuged for 30 min at 20,000 × g at 4°C and the supernatant used for AChE activity assays.
Total AChE activity was assayed by radiometric procedure using [3H]ACh as substrate. Briefly, samples (20 μl) were transferred to a scintillation vial and incubated with 24 mM [3H]ACh (0.1 μCi) in the presence of the butyrylcholine inhibitor tetraisopropyl pyrophosphoramide (Iso-OMPA, 10 μM) in a final volume of 25 μl. The reaction was stopped by adding 2.5 ml of glycine-HCl buffer, pH 2.5, and the radioactivity (DPM h−1) was determined in the presence of 4 ml of scintillation fluid (Insta-Fluor Plus : butanol 1 : 4). The [3H]acetate formation was expressed as arbitrary units per mg of protein.
Drugs
Acetylcholine chloride, atropine sulfate, bacitracin, bovine serum albumin (fraction V), carbamylcholine chloride, Chel chloride, 4-DAMP mustard, DFP, IBMX, Iso-OMPA, forskolin, oxotremorine-M, pepstatin A, PMSF pirenzepine and PDD were from Sigma Chemical Co., St Louis, MO, U.S.A.; D-MEM, gentamicin, HS donor herd and FCS were from Invitrogen, Life Technologies, Carlsbad, CA, U.S.A.; acetylcholine iodide [acetyl-3H] (specific activity=55.2 μCi mmol−1), [3H]cytidine (specific activity=28.4 Ci mmol−1) and [3H]mQNB (specific activity=84.0 Ci mmol−1) were from Perkin-Elmer Life Sciences, Boston, MA, U.S.A. CAMP kit [3H]-assay system and [35S]GTPγS (specific activity=1065 Ci mmol−1) were from Amershan Biosciences, U.K.
Statistical analysis
The results were expressed as mean values±s.e.m. of determinations. The apparent dissociation constants (KD) and the maximum number of [3H]mQNB-binding sites (Bmax) were calculated by fitting saturation curves to a nonlinear regression equation using the GraphPad Prism (GraphPad Software Inc., San Diego, CA, U.S.A.). Differences between means were analyzed by Student's t-test or one-way analysis of variance, followed by Newman–Keuls multiple comparison test. The level of significance was set at P<0.05.
Results
Noninnervated rat skeletal muscle fibers express mAChRs
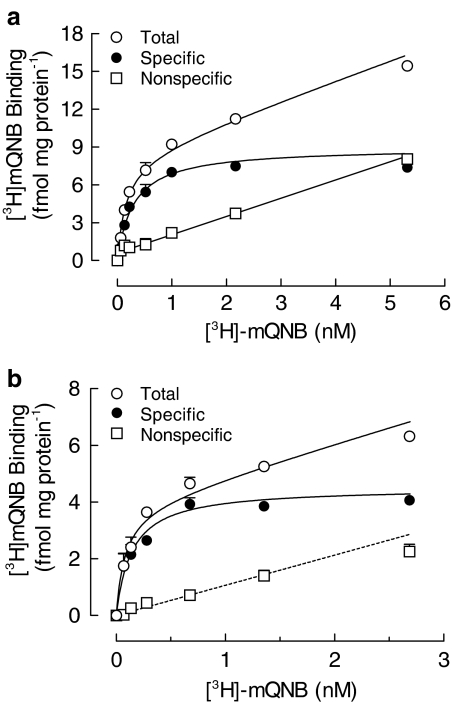
The presence of mAChRs in the rat cultured skeletal muscle membrane was analyzed using the radiolabeled antagonist [3H]mQNB. The nonlinear regression analysis of [3H]mQNB-specific saturation curves indicated a single class of high-affinity binding sites. The dissociation constant (KD) was 0.4±0.1 nM and the maximal number of binding sites (Bmax) was 8.9±1.0 fmol mg protein−1 (Figure 1a). The nonspecific binding of [3H]mQNB increased linearly throughout the range of 0.05–10 nM, accounting for less than 5% of total binding at concentrations near the KD value.
Figure 1.
Rat skeletal muscle cells express mAChRs. Saturation curves obtained by incubation of 0.05–10 nM [3H]mQNB with membranes from 7-day-old rat cultured skeletal muscles (a) or from hindlimb skeletal muscles of newborn rat (b), for 60 min, at 25°C. Nonspecific binding was determined in the presence of 1 μM unlabeled QNB. Specific [3H]mQNB binding was the difference between total and nonspecific binding. Each point is the mean±s.e.m. value from a representative experiment performed in triplicate.
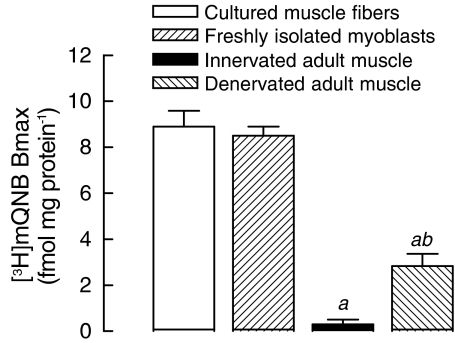
A comparable [3H]mQNB saturation curve was obtained when skeletal muscle membranes from newborn rats were used (KD=0.2±0.1 nM and Bmax=4.5±0.2 fmol mg protein−1, Figure 1b). Besides, muscarinic binding sites were also detected in myoblasts isolated from newborn rats (Bmax=8.5±0.4 fmol mg protein−1) (Figure 2), indicating that mAChR expression might be an attribute of developing muscle fibers.
Figure 2.
Expression of mAChRs is detected only in noninnervated skeletal muscle cells. Membranes from 8-day-old cultured muscle fibers, freshly isolated myoblasts of newborn rat and control, or denervated diaphragms of adult rats (3-month-old) were incubated with [3H]mQNB for 60 min, at 25°C. Nonspecific binding was determined in the presence of 1 μM unlabeled QNB. The [3H]mQNB-specific binding was expressed as fmol per mg of protein. Each baris the mean±s.e.m. value (n=3–5). aSignificantly different from cultured skeletal muscles; bsignificantly different from adult innervated muscles, P<0.05.
On the other hand, [3H]mQNB-binding sites were almost undetectable in diaphragm membranes from adult rats (Figure 2). In this group, the nonspecific binding accounted for 84% of total [3H]mQNB binding. Interestingly, mAChR expression was remarkably upregulated in denervated muscle from adult rats (2.8 fmol mg protein−1), suggesting that neural signals might be involved in repression of mAChRs at adult innervated muscle fiber.
Activation of mAChRs induces the binding of [35S]GTPγS to the rat cultured skeletal muscle membranes
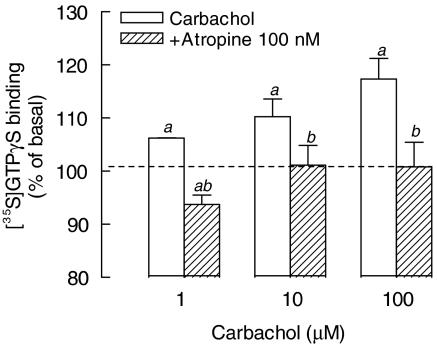
To assess the functional coupling between activated mAChRs and G proteins, [35S]GTPγS-binding assays were performed using membrane from the rat cultured muscles. As shown in Figure 3, carbachol increased the specific [35S]GTPγS binding in a concentration-dependent manner. This effect was related to stimulation of mAChRs, since it was completely prevented by preincubation of membranes with 100 nM atropine.
Figure 3.
Carbachol stimulates [35S]GTPγS-specific binding to membranes from rat cultured skeletal muscles. Membranes from 8-day-old cultures were incubated for 2 h, at 30°C, with [35S]GTPγS plus carbachol (1–100 μM) or vehicle (100%), in the presence or absence of muscarinic receptor antagonist atropine (100 nM). Nonspecific binding was obtained in the presence of 10 μM unlabeled GTPγS. Each bar is the mean±s.e.m. value (n=4). aSignificantly different from the control group (100%); bsignificantly different from the carbachol-stimulated group, P<0.05.
Effect of activation of mAChRs on cAMP production in cultured skeletal muscle
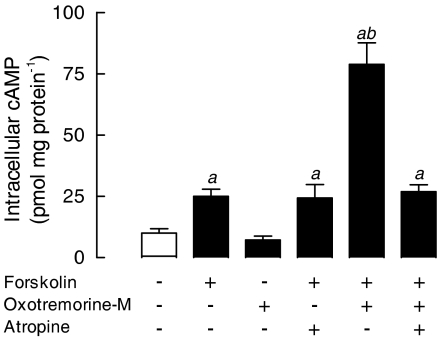
One of the predicted consequences of stimulation of M2 and M4 mAChRs would be a decreased accumulation of intracellular cAMP induced by Gi-dependent inhibition of AC. To determine whether activation of mAChRs could modify the accumulation of cAMP in skeletal muscle cells, the effect of oxotremorine-M on the intracellular cAMP content was evaluated in control and forskolin-treated rat muscle cultures. As shown in Figure 4, the basal cAMP content (10.3±1.8 pmol mg protein−1) was increased by 143% after direct activation of AC with forskolin (25.0±2.9 pmol mg protein−1). Although oxotremorine-M alone did not modify the intracellular cAMP, it potentiated by 3.2-fold (78.9±8.8 pmol mg protein−1) the accumulation of cAMP induced by forskolin. Atropine did not modify the forskolin effect. Conversely, potentiation elicited by oxotremorine-M was completely abolished by preincubation of cells with atropine, supporting the involvement of mAChR in the oxotremorine-M-dependent effect.
Figure 4.
Oxotremorine-M potentiates the forskolin-dependent increase of intracellular cAMP in cultured skeletal muscles. Cultured skeletal muscles (7-day-old), preincubated with 1 mM IBMX, were treated for 10 min, at 37°C, with 10 nM oxotremorine-M and/or 10 μM forskolin, in the presence or absence of 10 nM atropine. The intracellular cAMP was extracted and measured using a cAMP [3H]-assay kit. Each bar is the mean±s.e.m. value (n=4 cultures). aSignificantly different from the untreated control group; bsignificantly different from the forskolin-stimulated group, P<0.05.
Effect of activation of mAChRs on DAG production in cultured skeletal muscle
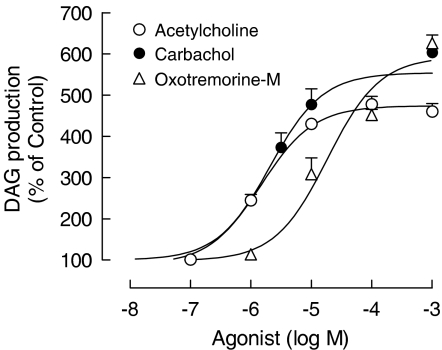
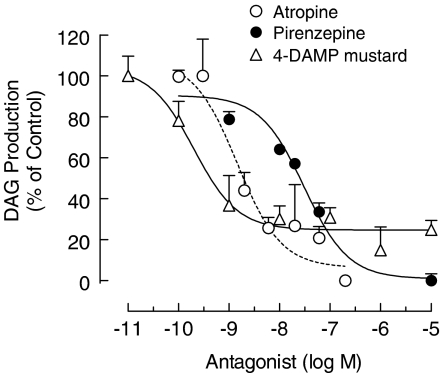
Assuming that M1, M3 and M5 mAChRs are primarily coupled to Gq/11 protein – PLC β, the following experiments analyzed the effect of muscarinic receptor agonists on the accumulation of DAG. As shown in Figure 5, acetylcholine, carbachol and selective mAChR agonist oxotremorine-M increased the basal DAG (100%), in a concentration-dependent manner. Moreover, carbachol-induced accumulation of DAG was proportionally inhibited by increasing concentrations of mAChR antagonists atropine (IC50=1.5±0.2 nM), pirenzepine (IC50=31±0.2 nM) and 4-DAMP mustard (IC50=0.2±0.1 nM) (Figure 6), strengthening the view of functional coupling of mAChRs to the Gq/11-PLC β cascade, in skeletal muscle fibers.
Figure 5.
Muscarinic receptor agonists stimulate the DAG production in the rat cultured skeletal muscles. Cultures (7-day-old), previously incubated with [3H]cytidine and 10 mM LiCl, were stimulated with acetylcholine, carbachol or oxotremorine-M for 60 min, at 37°C. DAG production was expressed as percentage of basal values (100%). Each bar is the mean±s.e.m. value (n=3 cultures).
Figure 6.
Effect of muscarinic receptor antagonists on the carbachol-dependent production of DAG in the rat cultured skeletal muscles. Cultures (7-day-old), previously incubated with [3H]cytidine and 10 mM LiCl, were stimulated with 30 μM carbachol in the presence or absence of atropine, pirenzepine or 4-DAPM mustard, for 60 min, at 37°C. Each point is the mean±s.e.m. value (n=3 cultures).
Effects of mAChR agonists on the synthesis of AChE
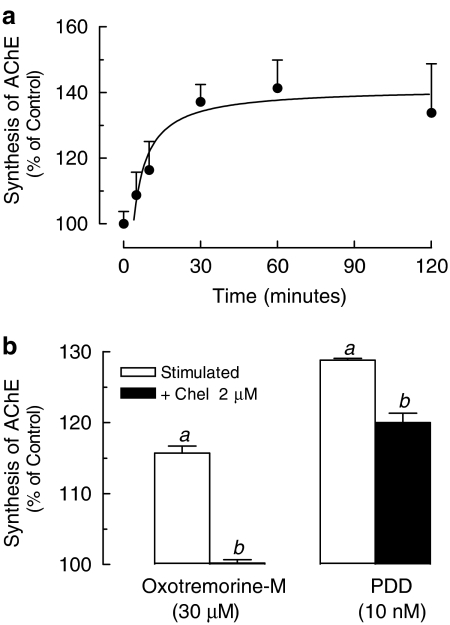
A recent study had shown that activation of Gq/11 protein-coupled receptors is capable of regulating AChE expression in skeletal muscle through a PKC-dependent signaling pathway (Choi et al., 2003). Thus, to determine whether mAChR agonists were able to modify the synthesis of AChE, after irreversible inhibition of AChE with DFP, rat muscle cultures were incubated with 1 μM carbachol for 5–120 min. Figure 7a shows that carbachol induced a time-dependent increase of newly synthesized AChE that reached 130% of control values after 120 min. An increased synthesis of AChE was also observed after incubation of cultured muscles with oxotremorine-M or the PKC activator PDD (Figure 7b). Preincubation of fibers with Chel, a specific inhibitor of PKC, reduced by 30% the effect of PDD and totally abolished oxotremorine-M-dependent increase of AChE synthesis, indicating the coupling of mAChRs to G-protein-dependent activation of the PLC/DAG/PKC system.
Figure 7.
Activation of muscarinic receptors or PKC stimulates the synthesis of AChE. (a) Rat cultured skeletal muscles (7-day-old) were pretreated with 100 μM DFP for 10 min at 25°C and incubated with 1 μM carbachol or vehicle (100%)in complete medium, for 15–120 min, at 37°C. (b) Rat cultured skeletal muscles (7-day-old) pre-exposed to DFP were treated with 2 μM Chel or vehicle for 10 min and incubated with 30 μM oxotremorine-M or 10 nM PDD, for 120 min, at 37°C. The newly synthesized AChE was extracted and total enzyme activity assayed using [3H]ACh as substrate. Each point is the mean±s.e.m. value (n=3 cultures). aSignificantly different from the untreated control group (100%); bsignificantly different from the stimulated group, P<0.05.
Discussion and conclusions
The present study provides pharmacological evidences for expression of functional mAChRs at developing muscle fibers that may contribute to postsynaptic response to ACh. Using radiolabeled mQNB, we detected high-affinity muscarinic binding sites in the differentiated rat cultured skeletal muscles. The current results extend previous findings on rat and avian cultured myotubes (Godinho & Rotundo, 1995; Reyes & Jaimovich, 1996; Jordan et al., 2003) to show that freshly isolated myoblasts also express mAChRs, which eliminates the possible artifactual mAChR expression induced by tissue-culturing manipulation. The similar density of mAChRs at multinucleated muscle fibers grown in culture (8.9±1.0 fmol mg protein−1) and at isolated myoblasts (8.5±0.4 fmol mg protein−1) also suggests that synthesis of mAChR may be a characteristic of aneural developing muscle fiber. Taking into account the absence of mAChRs at adult innervated muscles, it seems possible that, in the course of endplate formation, nerve-derived signals would inhibit the expression of mAChRs in innervated muscle fiber. This hypothesis also explains the low density of mAChRs in skeletal muscle from neonatal rats, since it is constituted by a mixed population of innervated fibers and noninnervated cells (muscle fibers and myoblasts/satellite cells) (Cardasis & Cooper, 1975).
In fact, the transitory developmental expression of mAChRs has been described in other tissues such as heart (McKinnon & Nathanson, 1995) and blood vessels (Hohmann et al., 1995). A transient occurrence of mAChR was also reported in ferret and newt retinas (Hutchins, 1994; Cheon et al., 2001) even before any retina cells synthesize acetylcholine. Moreover, during embryonic development, the expression of acetylcholine receptors in myogenic progenitor cells (nAChRs) and in the central nervous system (mAChRs or nAChRs) precedes the formation of cholinergic synaptic structure (Enna et al., 1976; Bevan & Steinbach, 1977; Role & Berg, 1996).
The expression of mAChRs at the aneural skeletal muscle cell is also supported by the upregulation of mAChRs induced after denervation of Diap of adult rats, indicating that mAChR-dependent signaling pathways might be important not only in the early developmental stages of the muscle cells, but also after denervation of adult muscle fiber. This idea is consistent with functional studies showing that carbachol and oxotremorine-M are able to induce the membrane hyperpolarization in the denervated rat diaphragm that is inhibited by muscarinic antagonists atropine and pirenzepine (Urazaev et al., 2000).
More importantly, our results strongly support a functional coupling of mAChRs to the G protein/DAG/PKC cascade. First, stimulation of mAChRs with carbachol increased the binding of [35S]GTPγS to membranes from cultured fibers, evidencing the activation of heterotrimeric G proteins. Agonist stimulation of [35S]GTPγS binding in this system was concentration-dependent and antagonized by atropine. Second, activation of mAChRs by acetylcholine or carbachol induced a concentration-dependent stimulation of DAG production that was mimicked by muscarinic agonist oxotremorine-M and prevented in a competitive manner by atropine, indicating the association of these receptors with PLC signaling pathways. Whereas Reyes and Jaimovich (1996) suggested the existence of M1 mAChRs in this system, based on the ability of a single dose of pirenzepine (100 nM) to inhibit the muscarine-elicited IP3 generation, our results show that carbachol-dependent accumulation of DAG was prevented by pirenzepine (IC50=31 nM) and 4-DAMP mustard (IC50=0.2 nM) in a concentration-dependent way. While pirenzepine exhibits higher selectivity to M1mAChRs, functional studies in the rat ileum have shown that low concentrations of 4 DAMP mustard (40 nM) selectively alkylate M3 subtype (88%), reducing by 65% the oxotremorine-M-mediated phosphoinositide hydrolysis (Ehlert, 1996). Thus, it seems possible that mAChR eliciting DAG production in cultured muscles can be either M1 or M3. This hypothesis is consistent with the detection of M1 and M3 mRNA at the rat cultured muscle fibers by reverse transcription-polymerase chain reaction (RT–PCR) assays (data not shown).
The expression of mAChRs coupled to Gq/PLC β can also explain the unusual effect of oxotremorine-M on intracellular cAMP. Whereas oxotremorine-M alone was not able to significantly modify the basal cAMP content, it potentiated the forskolin-dependent activation of AC. These results are not consistent with the predicted inhibition of AC mediated by the M2 or M4 mAChR/Gi protein system (Felder, 1995); however, the implication of mAChRs on oxotremorine-M response was substantiated by the inhibitory effect of atropine. Besides, skeletal muscle fibers express only three forskolin-sensitive AC isoforms (AC2, AC6, AC7) (Suzuki et al., 1998), but AC2 and AC7 isoforms are not directly modulated by the α subunits of the Gi family (Hanoune & Defer, 2001). Interestingly, several studies have shown that M1 and M3 mAChRs can stimulate calcium/calmodulin-sensitive AC indirectly through activation of Gαq and βγ subunits (Baumgold & Fishman, 1988; Felder et al., 1989; Murthy & Makhlouf, 1997). In fact, it was recently shown that activation of M1 mAChRs transfected into HEK cells significantly enhances forskolin-induced cAMP accumulation, via activation of AC6 (Beazely & Watts, 2005). The potentiation of forskolin effect elicited by muscarinic agonists was Pertussis toxin-insensitive, dependent on intracellular Ca+2/calmodulin and mimicked by transfection of constitutive Gαq protein (Beazely & Watts, 2005), indicating the coupling of mAChRs/PLC β signaling to activation of AC. Thus, a plausible interpretation for the amplification of forskolin induced by oxotremorine-M on cultured muscle fibers may be the activation of calcium-dependent AC induced by M1 and/or M3 mAChRs subtypes.
Finally, the present study showed that activation of mAChRs with either carbachol or oxotremorine-M was able to stimulate the synthesis of AChE, a major component of the neuromuscular synapse. This effect was related to DAG-dependent activation of PKC, since it was mimicked by the phorbol ester PDD and reduced by the PKC inhibitor Chel. In fact, the involvement of DAG/PKC signaling in the regulation of AChE, presented here, is in agreement with a recent study showing the stimulation of AChE transcription, via G-protein-coupled receptors (P2Y1 receptors) and PKC signaling pathways, in mouse and chick myotubes (Choi et al., 2003). The association of mAChRs to the Gαq/PKC cascade was also reported in chicken nerve-muscle cocultures (Jordan et al., 2003), indicating that occurrence of mAChRs at skeletal muscle cells is not restricted to rodent species.
It is well known that PKCα and θ, the most abundant isoforms expressed in skeletal muscle fibers, contain binding sites for DAG (Osada et al., 1992). Whereas PKCα is a classic Ca2+/DAG-dependent isoform, PKCθ belongs to the nPKC family activated by DAG in a Ca2+-independent way (Hilgenberg & Miles, 1995). Interestingly, expression of PKCθ is found in restricted association to the neuromuscular junction, being developmentally regulated by neural influences (Hilgenberg & Miles, 1995; Hilgenberg et al., 1996). Considering that carbachol induces the translocation of both PKCθ and PKCα to the membrane of cultured myotubes (Kim et al., 2002) and that axonal growth cones, in vivo, are capable of spontaneous and evoked quantal acetylcholine release, even before contact with target myotubes (Hume et al., 1983; Sun & Poo, 1987; for a review, see Grinnell, 1995), it is possible that mAChRs may contribute to trophic effects of acetylcholine during formation of the neuromuscular synapse, stimulating the synthesis of AChE, via a DAG-dependent PKC cascade.
In conclusion, these results demonstrate the expression of mAChRs in noninnervated developing skeletal muscle, coupled to different signaling transduction pathways: PLC β/PKC and AC/cAMP. Taking these findings together, it is possible that, in vivo, one signaling pathway beginning with activation of mAChRs and including the postsynaptic generation of DAG and subsequent activation of PKC might be involved in the early events of muscle cell differentiation.
Acknowledgments
This work was supported by research grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP 01/01417-5 (R.O. Godinho). I. Furlan was a MS fellow of FAPESP 00/01841-9.
Abbreviations
- AC
adenylyl cyclase
- AChE
acetylcholinesterase
- Bmax
maximal number of binding sites
- cAMP
adenosine 3′,5′-cyclic monophosphate
- Chel
chelerythrine
- DAG
diacylglycerol
- DFP
diisopropylfluorophosphate
- Diap
diaphragm muscle
- 4-DAMP mustard hydrochloride
4-diphenylacetoxy-N-(2-chloroethyl)piperidine hydrochloride
- D-MEM
Dulbecco's modified Eagle's medium
- EDTA
ethylenediaminetetraacetic acid
- FCS
fetal calf serum
- HBSS
Hank's balanced salt solution
- [3H]mQNB
[3H]methyl quinuclidinyl benzilate
- HS
horse serum
- IBMX
3-isobutyl-1-methylxanthine
- IP3
inositol 1,4,5-triphosphate
- Iso-OMPA
tetraisopropyl pyrophosphoramide
- KD
apparent dissociation constant
- Krebs
Krebs-bicarbonate buffer
- mAChRs
muscarinic acetylcholine receptors
- nAChRs
nicotinic acetylcholine receptors
- PBS
phosphate-buffered saline
- PDD
phorbol 12,13 didecanoate
- PKC
protein kinase C
- PLC β
phospholipase C β
- PMSF
phenylmethylsulfonyl fluoride
- QNB
quinuclidinyl benzilate
References
- ALEXANDER S.P., MATHIE A., PETERS J.A. Guide to receptors and channels, 1st edition. Br. J. Pharmacol. 2004;141:S1–S126. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGOLD J., FISHMAN P.H. Muscarinic receptor-mediated increase in cAMP levels in SK-N-SH human neuroblastoma cells. Biochem. Biophys. Res. Commun. 1988;154:1137–1143. doi: 10.1016/0006-291x(88)90259-8. [DOI] [PubMed] [Google Scholar]
- BEAZELY M.A., WATTS V.J. Gα(q)-coupled receptor signaling enhances adenylate cyclase type 6 activation. Biochem. Pharmacol. 2005;70:113–120. doi: 10.1016/j.bcp.2005.04.007. [DOI] [PubMed] [Google Scholar]
- BEVAN S., STEINBACH J.H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J. Physiol. (London) 1977;267:195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BRAIMAN L., ALT A., KUROKI T., OHBA M., BAK A., TENNENBAUM T., SAMPSON S.R. Protein kinase Cδ mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol. Endocrinol. 1999;13:2002–2012. doi: 10.1210/mend.13.12.0393. [DOI] [PubMed] [Google Scholar]
- BRAUN T., WINTER B., BOBER E., ARNOLD H.H. Transcriptional activation domain of the muscle-specific gene-regulatory protein myf5. Nature. 1990;346:663–665. doi: 10.1038/346663a0. [DOI] [PubMed] [Google Scholar]
- CARDASIS C.A., COOPER G.W. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J. Exp. Zool. 1975;191:347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P., BIRDSALL N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- CHEON E.W., KUWATA O., SAITO T. Muscarinic acetylcholine receptors in the normal, developing and regenerating newt retinas. Dev. Brain Res. 2001;127:9–21. doi: 10.1016/s0165-3806(01)00104-3. [DOI] [PubMed] [Google Scholar]
- CHOI R.C.Y., SIOW N.L., CHENG A.W.M., LING K.K.Y., TUNG E.K.K., SIMON J., BARNARD E.A., TSIM K.W.K. ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J. Neurosci. 2003;23:4445–4456. doi: 10.1523/JNEUROSCI.23-11-04445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DA COSTA V.L., LAPA A.J., GODINHO R.O. Short- and long-term influences of calcitonin gene-related peptide on the synthesis of acetylcholinesterase in mammalian myotubes. Br. J. Pharmacol. 2001;133:229–236. doi: 10.1038/sj.bjp.0704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNA S.J., YAMAMURA H.I., SNYDER S.H. Development of muscarinic cholinergic and GABA receptor binding in chick embryo brain. Brain Res. 1976;101:177–183. doi: 10.1016/0006-8993(76)91001-5. [DOI] [PubMed] [Google Scholar]
- EHLERT F.J. The interaction of 4-DAMP mustard with subtypes of the muscarinic receptor. Life Sci. 1996;58:1971–1978. doi: 10.1016/0024-3205(96)00187-7. [DOI] [PubMed] [Google Scholar]
- FELDER C.C. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995;9:619–625. [PubMed] [Google Scholar]
- FELDER C.C., KANTERMAN R.Y., MA A.L., AXELROD J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J. Biol. Chem. 1989;264:20356–20362. [PubMed] [Google Scholar]
- GODFREY P.P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem. J. 1989;258:621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODINHO R.O., ROTUNDO R.L. Acetylcholinesterase regulation in muscle-cells – muscarinic receptor activation and involvement of phosphatidylcholine-phospholipase-C. Mol. Biol. Cell. 1995;6:1447. [Google Scholar]
- GODINHO R.O., COSTA-JR V.L. Regulation of intracellular cyclic AMP in skeletal muscle cells involves the efflux of cyclic nucleotide to the extracellular compartment. Br. J. Pharmacol. 2003;138:995–1003. doi: 10.1038/sj.bjp.0705130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-MAESO J., RODRIGUEZ-PUERTAS R., GABILONDO A.M., MEANA J.J. Characterization of receptor-mediated [35S]GTPγS binding to cortical membranes from postmortem human brain. Eur. J. Pharmacol. 2000;390:25–36. doi: 10.1016/s0014-2999(99)00827-4. [DOI] [PubMed] [Google Scholar]
- GORDON H., RALSTON E., HALL Z.W. Cooperation between the products of different nuclei in hybrid myotubes produces localized acetylcholine receptor clusters. Proc. Nat. Acad. Sci. U.S.A. 1992;89:6595–6598. doi: 10.1073/pnas.89.14.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINNELL A.D. Dynamics of nerve–muscle interaction in developing and mature neuromuscular junctions. Physiol. Rev. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- HANOUNE J., DEFER N. Regulation and role of adenylyl cyclase isoforms. Ann. Rev. Pharmacol. Toxicol. 2001;41:145. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- HILGENBERG L., MILES K. Developmental regulation of a protein kinase C isoform localized in the neuromuscular junction. J. Cell Sci. 1995;108 Part 1:51–61. doi: 10.1242/jcs.108.1.51. [DOI] [PubMed] [Google Scholar]
- HILGENBERG L., YEARWOOD S., MILSTEIN S., MILES K. Neural influence on protein kinase C isoform expression in skeletal muscle. J. Neurosci. 1996;16:4994–5003. doi: 10.1523/JNEUROSCI.16-16-04994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHMANN C.F., POTTER E.D., LEVEY A.I. Development of muscarinic receptor subtypes in the forebrain of the mouse. J. Comp. Neurol. 1995;358:88–101. doi: 10.1002/cne.903580106. [DOI] [PubMed] [Google Scholar]
- HUANG C.F., TONG J., SCHMIDT J. Protein kinase C couples membrane excitation to acetylcholine receptor gene inactivation in chick skeletal muscle. Neuron. 1992;9:671–678. doi: 10.1016/0896-6273(92)90030-h. [DOI] [PubMed] [Google Scholar]
- HUME R.I., ROLE L.W., FISCHBACH G.D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- HUTCHINS J.B. Development of muscarinic acetylcholine receptors in the ferret retina. Dev. Brain Res. 1994;82:45–61. doi: 10.1016/0165-3806(94)90147-3. [DOI] [PubMed] [Google Scholar]
- JORDAN T., LI J., JIANG H., DIMARIO J.X. Repression of slow myosin heavy chain 2 gene expression in fast skeletal muscle fibers by muscarinic acetylcholine receptor and Gαq signaling. J. Cell Biol. 2003;162:843–850. doi: 10.1083/jcb.200303164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S., BONDEVA T., NELSON P.G. Activation of protein kinase C isozymes in primary mouse myotubes by carbachol. Brain research. Dev. Brain Res. 2002;137:13–21. doi: 10.1016/s0165-3806(02)00362-0. [DOI] [PubMed] [Google Scholar]
- KLARSFELD A., LAUFER R., FONTAINE B., DEVILLERS-THIERY A., DUBREUIL C., CHANGEUX J.P. Regulation of muscle AChR alpha subunit gene expression by electrical activity: involvement of protein kinase C and Ca2+ Neuron. 1989;2:1229–1236. doi: 10.1016/0896-6273(89)90307-3. [DOI] [PubMed] [Google Scholar]
- LANUZA M.A., GARCIA N., SANTAFE M., GONZALEZ C.M., ALONSO I., NELSON P.G., TOMAS J. Pre- and postsynaptic maturation of the neuromuscular junction during neonatal synapse elimination depends on protein kinase C. J. Neurosci. Res. 2002;67:607–617. doi: 10.1002/jnr.10122. [DOI] [PubMed] [Google Scholar]
- LI L., OLSON E.N. Regulation of muscle cell growth and differentiation by the MyoD family of helix–loop–helix proteins. Adv. Cancer Res. 1992;58:95–119. doi: 10.1016/s0065-230x(08)60292-4. [DOI] [PubMed] [Google Scholar]
- LIU T.P., YU P.C., LIU I.M., TZENG T.F., CHENG J.T. Activation of muscarinic M1 receptors by acetylcholine to increase glucose uptake into cultured C2C12 cells. Auton. Neurosci. 2002;96:113–118. doi: 10.1016/s1566-0702(01)00396-4. [DOI] [PubMed] [Google Scholar]
- MCKINNON L.A., NATHANSON N.M. Tissue-specific regulation of muscarinic acetylcholine receptor expression during embryonic development. J. Biol. Chem. 1995;270:20636–20642. doi: 10.1074/jbc.270.35.20636. [DOI] [PubMed] [Google Scholar]
- MENDELZON D., CHANGEUX J.P., NGHIEM H.O. Phosphorylation of myogenin in chick myotubes: regulation by electrical activity and by protein kinase C. Implications for acetylcholine receptor gene expression. Biochemistry. 1994;33:2568–2575. doi: 10.1021/bi00175a028. [DOI] [PubMed] [Google Scholar]
- MILES K., WAGNER M. Overexpression of nPKC theta is inhibitory for agrin-induced nicotinic acetylcholine receptor clustering in C2C12 myotubes. J. Neurosci. Res. 2003;71:188–195. doi: 10.1002/jnr.10467. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent m2-mediated inhibition via Gαi3 and m3-mediated stimulation via Gβγq. J. Biol. Chem. 1997;272:21317–21324. doi: 10.1074/jbc.272.34.21317. [DOI] [PubMed] [Google Scholar]
- OSADA S., MIZUNO K., SAIDO T.C., SUZUKI K., KUROKI T., OHNO S. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol. Cell. Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYES R., JAIMOVICH E. Functional muscarinic receptors in cultured skeletal muscle. Arch. Biochem. Biophys. 1996;331:41–47. doi: 10.1006/abbi.1996.0280. [DOI] [PubMed] [Google Scholar]
- ROLE L.W., BERG D.K. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- ROTUNDO R.L. Nucleus-specific translation and assembly of acetylcholinesterase in multinucleated muscle cells. J. Cell Biol. 1990;110:715–719. doi: 10.1083/jcb.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABOURIN L.A., RUDNICKI M.A. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- SASSOON D., LYONS G., WRIGHT W.E., LIN V., LASSAR A., WEINTRAUB H., BUCKINGHAM M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- SUN Y.A., POO M.M. Evoked release of acetylcholine from the growing embryonic neuron. Proc. Natl. Acad. Sci. U.S.A. 1987;84:2540–2544. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI Y., SHEN T., POYARD M., BEST-BELPOMME M., HANOUNE J., DEFER N. Expression of adenylyl cyclase mRNAs in the denervated and in the developing mouse skeletal muscle. Am. J. Physiol. 1998;274:C1674–C1685. doi: 10.1152/ajpcell.1998.274.6.C1674. [DOI] [PubMed] [Google Scholar]
- URAZAEV A., NAUMENKO N., MALOMOUGH A., NIKOLSKY E., VYSKOCIL F. Carbachol and acetylcholine delay the early postdenervation depolarization of muscle fibres through M1-cholinergic receptors. Neurosci. Res. 2000;37:255–263. doi: 10.1016/s0168-0102(00)00126-7. [DOI] [PubMed] [Google Scholar]
- WALKER C.R., WILSON B.W. Control of acetylcholinesterase by contractile activity of cultured muscle cells. Nature. 1975;256:215–216. doi: 10.1038/256215a0. [DOI] [PubMed] [Google Scholar]