Abstract
We examined the mechanisms underlying anion secretion mediated by protease-activated receptor 2 (PAR2) and its role in the regulation of ion transport, using polarized human airway Calu-3 cells.
PAR2 stimulation by trypsin and a PAR2-activating peptide (PAR2AP), especially from the basolateral aspect, caused transient Cl− secretion due to cytosolic Ca2+ mobilization.
Antagonists of PI-PLC (U73122, ET-18-OCH3) and inositol 1,4,5-triphosphate (xestospongin C (Xest C)) were without effect on the PAR2AP-mediated Cl− secretion, whereas it was attenuated by D609 (a PC-PLC inhibitor) and phorbol 12-myristate 13 acetate (PMA, a PKC activator).
Even 30 min after removal of PAR2AP after a 10-min-exposure, cells were still poorly responsive to PAR2 stimulation, but the reduced responsiveness was upregulated by a PKC inhibitor, GF109203X (GFX).
Pretreatment with PAR2AP did not affect responses to anion secretagogues, such as isoproterenol, forskolin, thapsigargin, 1-ethyl-2-benzimdazolinone, and adenosine, but ATP-induced responses were significantly reduced. Nystatin permeabilization studies revealed that the presence of PAR2AP prevented ATP-induced increments in basolateral membrane K+ conductance without affecting apical membrane Cl− conductance.
ATP-elicited Ca2+ mobilization, which was sensitive to D609 and PMA, was inhibited by the pretreatment with PAR2AP, and this inhibition was blunted by the presence of GFX.
Collectively, stimulation of PAR2 generates a brief response of Cl− secretion through PC-PLC-mediated pathway, followed by not only auto-desensitization of PAR2 itself but also cross-desensitization of a PC-PLC-coupled purinoceptor. The two types of desensitization seem likely to have PKC-mediated downregulation of PC-PLC in common.
Keywords: Protease-activated receptor 2, airway ion transport, desensitization, Cl− secretion, phosphatidylcholine-phospholipase C, purinoceptor, protein kinase C
Introduction
Protease-activated receptors (PARs) belong to a family of seven transmembrane G protein-coupled receptors that are activated by extracellular proteases (Macfarlane et al., 2001). These receptors are activated by the proteolytic unmasking of a receptor-bound, NH2-terminal-tethered ligand domain, which is then able to interact with the extracellular loop of the receptor itself and initiate signaling. To date, four PARs (PAR1–4) have been characterized (Macfarlane et al., 2001). Thrombin activates PAR1, -3, and -4, whereas trypsin and mast cell tryptase activate PAR2 and possibly PAR4 (Macfarlane et al., 2001). Synthetic peptides based on the receptor-activating sequence of the tethered ligand can directly bind and activate the receptors (Dery et al., 1998). Various branches of science have been paying growing attention to the functional relevance of PARs, because of their systemic distribution and potential roles in numerous systems, including homeostasis, inflammation, and the regulation of smooth muscle tone (Dery et al., 1998; Cocks et al., 1999; Cocks & Moffatt, 2001; Macfarlane et al., 2001). PAR2 is the PAR predominantly expressed in human airway epithelia (Cocks & Moffatt, 2000). Recently, upregulation of PAR2 in the respiratory epithelium of patients with bronchial asthma was reported (Knight et al., 2001). Further, Northern blotting analysis of human tissues shows that trypsin-like protease mRNA is most prominently expressed in tracheal tissue, suggesting that this protease is localized in the airway (Yamaoka et al., 1998). These reports implicate PAR2 in the pathophysiology of chronic obstructive airway diseases.
It is well known that the chronic obstructive airway diseases share aspects of mucous congestive diseases (Kellerman, 2002), in which excessive and tenacious mucus secretion causes airway obstruction and is involved in the morbidity and mortality of these diseases (Lundgren & Shelhamer, 1990; Aikawa et al., 1992). In particular, bronchial gland cells predominantly contribute to airway electrolyte secretion, which is accompanied by fluid movement, thereby forming low viscosity mucus to maintain efficient mucociliary clearance (Shimura, 2000). Despite the significance of the electrolyte transport system in airway conductivity, little is known regarding the roles of PAR2 in this system. Thus, the aim of the present study was to establish how Cl− secretion is regulated by PAR2-mediated signals, using polarized Calu-3 epithelia, which is a model of human airway submucosal gland serous cells (Devor et al., 1999). Through the investigations, we also found that PAR2 stimulation caused not only auto-desensitization but also cross-desensitization of a purinoceptor that is well known as a key molecule promoting mucociliary clearance in the airway (Ramminger et al., 1999; Inglis et al., 2000; Ito et al., 2004a).
Methods
Cell culture
Calu-3 human airway cells (American Type Culture Collection, Manassas, VA, U.S.A.) at passages 29–35 were grown in a 1 : 1 mixture of Dulbecco's Modified Eagle's Medium and Ham's F-12 (Invitrogen, Carlsbad, CA, U.S.A.) containing 10% fetal bovine serum (Invitrogen), 100 μg/ml streptomycin, and 100 U/ml penicillin (Invitrogen). The cells were maintained in tissue-culture flasks (T75) at 37°C in a 95% air–5% CO2 humidified incubator. After reaching 80–90% confluence, cells were detached using a solution of PBS, 0.04% EDTA, and 0.25% trypsin. The collected cells were passaged with a 1 : 4 dilution of the same solution and seeded onto porous polyester membranes (0.4 μm pore size on Snapwell or Transwell inserts, 1.1 cm2, Costar, Cambridge, MA, U.S.A.) at a density of 106 cells/well. The inserts had been collagen-coated overnight with 0.2% human placental collagen type VI (Sigma-Aldrich, St Louis, MO, U.S.A.). The day after seeding the cells on the filters, the medium remaining on the apical side was removed to establish an air interface, which markedly improves the differentiation of human airway epithelia in a well-polarized fashion (Shen et al., 1994). The cells were fed by replacement of the basolateral medium every 48 h. Experiments were carried out after 7–13 days in culture.
Normal human bronchial epithelial cells (NHBE; BioWhittaker, Walkersville, MD, U.S.A.) seeded into T75 flasks were grown for detection of PAR2 mRNA in bronchial epithelial cell growth medium (BEGM; BioWhittaker) supplemented with bovine pituitary extract (52 μg ml−1), hydrocortisone (0.5 μg ml−1), human recombinant epidermal growth factor (0.5 ng ml−1), epinephrine (0.5 μg ml−1), transferrin (10 μg ml−1), insulin (5 μg ml−1), retinoic acid (0.1 μg ml−1), triiodothyronine (6.5 μg ml−1), gentamycin (50 μg ml−1), and amphotericin B (50 μg ml−1). The medium was changed every 48 h until cells were 90% confluent.
RT–PCR for detection of PAR2 mRNA
Total RNA was extracted from Calu-3 and NHBE cells, which had been grown to near confluence on T75 flasks, using Trizol reagent (Invitrogen). First-strand cDNA was generated using SuperScript III (Invitrogen) and random primers (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. Reverse transcriptase–polymerase chain reaction (RT–PCR) was performed with a primer set of PAR2 forward 5′-CCTGAGTGGCACCATCC and PAR2 reverse 5′-CAGGGAGATGCCAATGGC. The size of the expected PCR product was 527 bp. PCR conditions consisted of 5 min at 97°C, followed by 40 cycles of 30 s of denaturing at 96°C, 30 s of annealing at 57°C, 30 s of extension at 72°C, with a final extension at 72°C for 10 min. The PCR product was visualized by loading a 10 μl sample on a 2% agarose gel.
Ussing-chamber experiments
Snapwell inserts on which Calu-3 cells had grown confluent were rinsed with physiological saline solution (PSS) and transferred to the modified Ussing chambers (EasyMount Chamber; Physiologic Instruments, San Diego, CA, U.S.A.) that contained PSS at 37°C. The PSS was composed of (in mM) NaCl 115, KCl 5, MgCl2 1, CaCl2 2, glucose 10, HEPES 10, and NaHCO3 25. The pH of the solution was adjusted to 7.4 (at 37°C) using NaOH before addition of NaHCO3. The pH of the solution was kept at pH 7.4 when gassed with a mixture of 5% CO2 and 95% O2. Transepithelial potential differences (PD) from the basolateral to the apical side of the monolayer were continuously measured under open-circuit conditions by a high-impedance millivoltmeter that could function as a voltage clamp with automatic fluid resistance compensation (VCC MC2, Physiologic Instruments). Transepithelial conductance (Gt) was determined by imposing short (0.5 s) current pulses (ΔI=2–10 μA), and the corresponding changes in transepithelial voltage (ΔV) were calculated using Ohm's law (Gt=ΔI ΔV−1). The equivalent short-circuit current (Ieq) was calculated as Gt PD. Ieq represents net charge movement across the monolayer.
Permeabilized monolayers
To investigate apical membrane Cl− conductance, the basolateral membrane was permeabilized with the pore-forming antibiotic nystatin (100 μM) for more than 30 min. This level of nystatin was determined as the concentration at which bumetanide, an inhibitor of the basolateral Na+–K+–2Cl− cotransporter, had no effect on the ion current (Son et al., 2004). This procedure avoids the complexities associated with basolateral ion transporters and permits analyses of apical membrane Cl− conductance (Devor et al., 1999; Ito et al., 2002; Son et al., 2004). Apical membrane Cl− conductance was estimated as apical membrane Cl− current (ICl) in the apical-to-basolateral Cl− concentration gradient under closed-circuit conditions. The Cl− concentration gradient was established by replacing NaCl with equimolar Na-gluconate in the basolateral PSS. ICl reflects the apical Cl− permeability through cystic fibrosis transmembrane conductance regulator (CFTR) in Calu-3 cells because the apical anion conductance is almost completely derived from CFTR channels in Calu-3 cells (Haws et al., 1994; Devor et al., 1999). To completely eliminate the component of basolateral K+ conductance in this ICl measurement, these experiments were performed in the presence of clotimazole, an inhibitor of the human intermediate conductance Ca2+-activated K+ (hIK-1) channel. In this basolateral solution, CaCl2 was increased to 4 mM to compensate for the Ca2+-chelating capacity of the gluconate.
The basolateral membrane K+ conductance was estimated by measuring the basolateral membrane K+ current (IK) after permeabilization of the apical membrane with nystatin (50 μM) for 30 min and establishment of an apical-to-basolateral K+ concentration gradient (Devor et al., 1999; Son et al., 2004). Complete permeabilization of the apical membrane with 50 μM nystatin was confirmed by apical application of phlorizin, an inhibitor of the Na+-glucose transporter located on the apical membrane of this cell line (Ito et al., 2001; Son et al., 2004). Apical NaCl was replaced by equimolar K-gluconate, while basolateral NaCl was substituted with equimolar Na-gluconate. Cl− was removed from these solutions to completely eliminate the component of apical Cl− conductance. On the basolateral membrane, the major K+ conductance was produced by the hIK-1 channel in Calu-3 cells (Devor et al., 1999; Cowley & Linsdell, 2002).
Cytosolic Ca2+ mobilization assay
Calu-3 cells grown on the porous membranes of Transwell inserts (Costar) were rinsed twice with PSS and incubated for 1.5 h at 37°C in the same buffer containing 5 μM fluo-3/AM (Dojindo, Kumamoto, Japan) and 0.01% pluronic F127 (Molecular Probes, Eugene, OR, U.S.A.). After the loading, cell monolayers were rinsed twice with PSS to wash off residual dyes outside the cells, and then 0.5 ml and 1 ml PSS were added to the apical and basolateral membranes, respectively. Fluorescence signals were collected for 20 ms at 6 s intervals using a fluorometer (Fluoroskan Ascent CF; Labsystems, Helsinki, Finland) at the excitation wavelength of 485 nm and the emission wavelength of 538 nm. The maximum signal (Fmax) was obtained by addition of 10 μM ionomycin, and the minimum signal (Fmin) was obtained by adding 10 mM EGTA to the cell monolayer. The cytosolic Ca2+ concentration ([Ca2+]i) was calculated according to the following formula:
in which the Kd was assumed to be 390 nM (Minta et al., 1989). In the present study, maximum [Ca2+]i changes (Δ[Ca2+]i) were compared between groups.
Chemicals
Forskolin, isoproterenol, 1-ethyl-2-benzimdazolinone (1-EBIO), bumetanide, clotrimazole, ATP, ADPβS, adenosine, D609, nystatin, trypsin, thrombin, thapsigargin, U73122, and GF109203X were obtained from Sigma-Aldrich Co. Xestospongin C and ET-18-OCH3 were purchased from Calbiochem (La Jolla, CA, U.S.A.). Soybeans trypsin inhibitor was from Worthington (Lakewood, NJ, U.S.A.). SLIGKV-NH2 (a PAR2-activating peptide: PAR2AP), was a product of Bachem (Bubendorf, Switzerland). VKGILS-NH2 (a reverse sequence peptide of PAR2AP: revPAR2AP) was synthesized by Sawady Technology Co., Ltd (Tokyo, Japan). Stock solutions of ATP, ADPβS, trypsin, thrombin, and the soybean trypsin inhibitor were prepared by dissolution in distilled water. All other drugs were dissolved in DMSO. Nystatin stock solution (100 mM) was made and sonicated for 30 s just before use.
Data analysis
Numerical data are presented as means±s.e.m., where n refers to the number of experiments. Statistical differences were determined by Student's t-test for comparison of data between two groups or one-way analysis of variance for multiple group comparison. A value of P<0.05 was considered statistically significant.
Results
Bioelectric responses to PAR-related activators
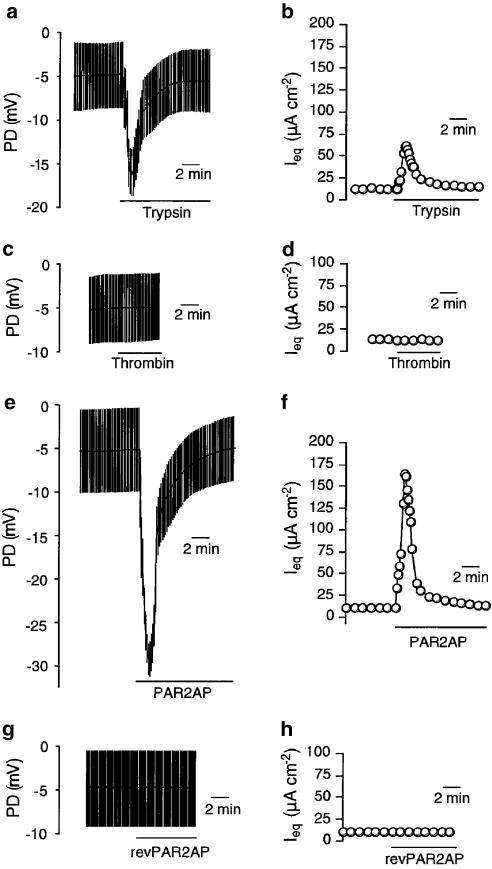
Previous investigations have found that PAR2 are unilaterally located on the basolateral membrane in native airway epithelial tissues (Kunzelmann et al., 2002) and cultured bronchial epithelial cells (Danahay et al., 2001). Thus, we first applied various PAR activators to the basolateral face of the Calu-3 monolayer (Figure 1). Application of a PAR2, -4 activator, trypsin (50 U ml−1), led to a rapid increase in apical side-negative PD from the baseline (−5.0±0.2 mV, n=41) to a peak (−15.8±0.5 mV), followed by a decay back to the baseline in a couple of minutes (Figure 1a). The corresponding values of Ieq are shown in Figure 1b. The peak values of Ieq (54.2±1.8 μA cm−2) minus basal ones (11.4±0.3 μA cm−2), which are expressed as ΔIeq, were 42.8±1.8 μA cm−2. The effects of trypsin (basolateral) were interrupted by the basolateral presence of a soybean trypsin inhibitor (data not shown). In contrast to the trypsin-induced responses, thrombin (50 U ml−1), a PAR1, -3, -4 activator, failed to induce any bioelectric effect on the monolayer (Figure 1c and d), suggesting expression of PAR2 exclusively on the basolateral membrane. The trypsin-induced responses were mimicked by PAR2AP: SLIGKV-NH2, which is a short synthetic peptide with proteolytically revealed PAR2 sequences (Figure 1e and f). Basolateral application of PAR2AP (50 μM) induced a transient increase in apical side-negative PD (ΔPD=22.1±0.8 mV, n=77; Figure 1e), resulting in transient elevation of Ieq (ΔIeq=147.0±6.9 μA cm−2; Figure 1f). Also, the trypsin-induced responses were almost completely suppressed in the presence of PAR2AP (ΔIeq=5.5±0.8 μA cm−2, n=4, P<0.001), suggesting that trypsin and PAR2AP share a common receptor. Further, PAR2 specificity in the bioelectric responses was confirmed by the finding that the reverse sequence peptide of PAR2AP (revPAR2AP, 100 μM) had no effect on the PD and Ieq (Figure 1g and h).
Figure 1.
Representative traces of bioelectric responses to PAR activators in Calu-3 monolayer. Potential differences from the basolateral to apical side (PD) were monitored under an open-circuit condition with applications of repeated short (0.5 s) current pulses (10 μA). From the changes in PD, the equivalent short-circuit current (Ieq) was caluculated by Ohm's law. The cells were exposed to trypsin (50 U ml−1, a and b), thrombin (50 U ml−1, c and d), a PAR2-activating peptide (PAR2AP, 50 μM, e and f), and the reverse sequence peptide of PAR2AP (revPAR2AP, 50 μM, g and h) from the basolateral side.
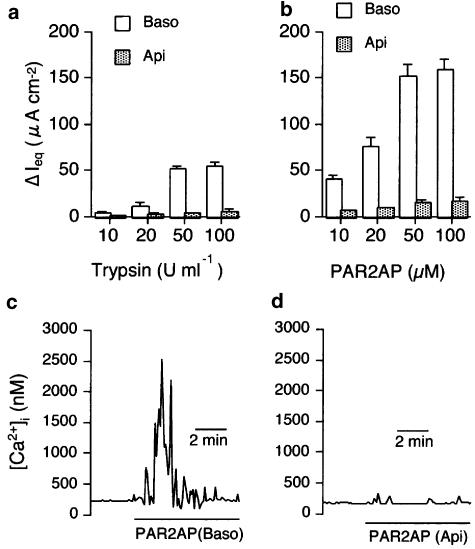
Compared with the responses to basolateral trypsin, those to apical trypsin were small (Figure 2a). The small change made by apical trypsin was not due to its leakage to the basolateral side because a soybean trypsin inhibitor (50 U ml−1) applied to the basolateral solution made no significant difference in responses to apical trypsin at 50 U ml−1: ΔIeq=5.1±0.8 μA cm−2 (n=6) without and 5.4±0.3 μA cm−2 (n=4) with 50 U ml−1 soybean trypsin inhibitor. Similarly, apically applied PAR2AP induced only a small effect on the responses (ΔIeq=14.7±3.2 μA cm−2, n=4, Figure 2b), as was the case of trypsin. Figure 2c and d show cytosolic Ca2+ changes induced by basolateral and apical PAR2AP applications, indicating the consistency of PAR2-mediated ion transport with Ca2+ mobilization.
Figure 2.
Comparison of effects of basolateral (Baso) and apical (Api) applications of PAR2 agonists, trypsin (a) and PAR2AP (b), at various concentrations. The ΔIeq was obtained by calculating the difference between peak values after the addition of PAR2 agonists and values just before their addition. Data are means±s.e.m. (n=4). (c, d) Representative tracings of [Ca2+]i changes during exposure to PAR2AP from the basolateral (c) and apical side of the monolayer (d).
The PAR2-mediated Ieq almost completely comprises the Cl− secretory component because PAR2AP-induced responses were abolished by removal of Cl− from the extracellular solutions (ΔIeq=13.3±1.9 μA cm−2, n=4) and the presence of basolateral bumetanide (50 μM), a Na+–K+–2Cl− cotransport inhibitor (ΔIeq=23.2±0.8 μA cm−2, n=4).
Molecular evidence of PAR2 expression
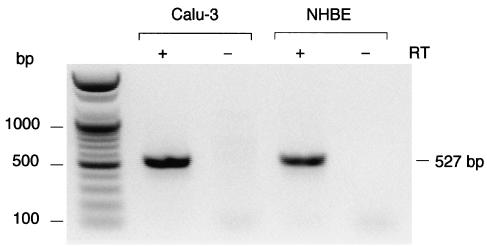
To examine PAR2 mRNA expression in Calu-3 cells, we analyzed total RNA via RT–PCR, which was performed using primers specific to the sequence of human PAR2. A 527-bp fragment was obtained, which was verified to be a PAR2 fragment by sequencing (Figure 3). PAR2 expression was also detected in normal human airway epithelial cells (NHBE; see Figure 3).
Figure 3.
Products of RT–PCR of RNA isolated from Calu-3 human airway cell line and normal human bronchial epithelial cells (NHBE), using gene-specific primer pairs targeted to PAR2. A 527-bp fragment of PAR2 was obtained from these two types of cells with reverse transcription (RT) of total RNA (+) but not without RT (–).
Potential mechanisms underlying PAR2-induced Cl− secretion
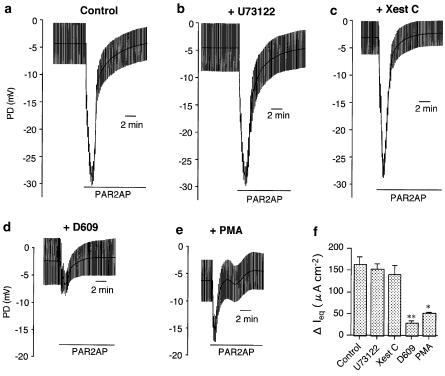
G protein-coupled phospholipase C (PLC) is a major upstream enzyme to cause [Ca2+]i mobilization and diacylglycerol (DAG)-mediated PKC activation (Hurley & Grobler, 1997). To examine the involvement of PLC in the PAR2-mediated Cl− secretion, PD changes in response to PAR2 activators were observed in the presence of specific PLC inhibitors. Compared with the control (Figure 4a), PAR2AP (50 μM)-induced bioelectric responses in cells that had been exposed beforehand to phosphatidylinositol-phospholipase C (PI-PLC) inhibitors U73122 (100 μM Figure 4b) or ET-18-OCH3 (100 μM) were still intact. Namely, ΔPD and ΔIeq values generated by the application of PAR2AP in the control (22.3±1.4 mV and 162.3±19.0 μA cm−2, n=7) were unaffected by U73122 (21.4±1.2 mV and 153.0±12.0 μA cm−2, n=5) and ET-18-OCH3 (22.4±1.6 mV and 163.6±26.5 μA cm−2, n=5), respectively (Figure 4f). Further, PAR2-induced responses were also insensitive to (Xest C, 10 μM), a selective antagonist of inositol 1,4,5-triphosphate (Ins P3; ΔPD=21.2±2.3 mV; ΔIeq=140.5±21.2 μA cm−2, n=5, Figure 4c). Similar pharmacological properties were reproduced in the cells stimulated by trypsin (data not shown). In contrast to these observations, pretreatment with a specific phosphatidylcholine-phospholipase C (PC-PLC) antagonist, D609 (100 μM), markedly prevented PAR2AP-mediated PD changes (ΔPD=3.9±0.5 mV, n=5) and the resultant Ieq (ΔIeq=28.1±5.3 μA cm−2, P<0.001), as demonstrated in Figure 4d. Figure 4e shows that the cells also became less responsive to PAR2AP in the presence of a PKC activator, PMA (1 μM): ΔPD=9.6±0.9 mV; ΔIeq=51.0±2.8 μA cm−2 (n=4, P<0.005). Summarized data of each ΔIeq value is shown in Figure 4f.
Figure 4.
Mechanisms underlying PAR2-mediated anion secretion in Calu-3 cells. (a–e) Representative traces of potential differences from the basolateral to the apical side (PD) before and after application of the PAR2-activating peptide (PAR2AP, 50 μM, in the presence of the vehicle (0.1% DMSO: Control, a), U73122 (100 μM, b), xetospongin C (Xest C, 10 μM, c), D609 (100 μM, d), and PMA (1 μM, e). PD was monitored under open-circuit condition with applications of repeated short (0.5 s) current pulses (10 μA). (f) Summary of the PAR2AP-induced elevation of the equivalent short-circuit current (ΔIeq), which was calculated by PD data. U73122, Xest C, D609, and PMA were applied 30 min prior to addition of PAR2AP. Data are means±s.e.m. (n=4–7). *P<0.005, **P<0.001.
Auto-desensitization induced by PAR2 activation
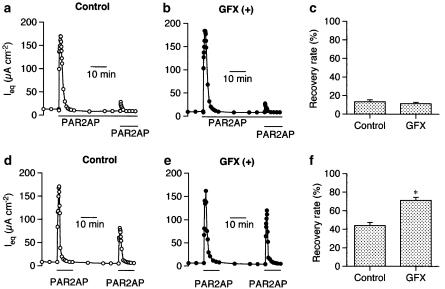
After completion of transient reactions due to PAR2AP (50 μM, basolateral), additional PAR2 stimulation (PAR2AP, 50 μM, basolateral) in the continuous presence of PAR2AP hardly generated responses (Figure 5a). The desensitization was not counteracted by pretreatment with the PKC inhibitor GFX (1 μM, Figure 5b). Figure 5c is a summary of the experiments shown in Figure 5a and b, indicating that the Ieq induced by the second PAR2AP application was 13.3±2.2% (n=4) of the first one in the control, and 11.3±1.4% (n=4) in the presence of GFX. When PAR2AP was removed after being present for 10 min, cells had only partially recovered their responsiveness to PAR2AP even 30 min later (Figure 5d), suggesting the desensitization remained for a long time after the wash-out. In contrast to the results shown in Figure 5a–c, however, the recovery in this case was further potentiated by the presence of GFX (Figure 5e): the responses to the second application was 43.9±3.5% (n=4) of the response to the first in the control and 71.1±3.4% (n=4, P<0.005) in the presence of GFX (Figure 5f).
Figure 5.
Auto-desensitization of PAR2-mediated signals in Calu-3 cells. (a–c) In the continuous presence of PAR2AP (50 μM, basolateral), Ieq responses to additional PAR2AP (50 μM, basolateral) were severely suppressed (a), and the suppression was not counteracted by the presence of the PKC inhibitor GF109203X (GFX, 1 μM, b). (c) Summary of the experiments shown in (a) and (b). Recovery rates (ordinates) were obtained by dividing the second ΔIeq by the first one. (d–f) PAR2AP was removed after 10 min exposure, and PAR2AP was reapplied 30 min after its removal in the absence (d) and presence of GF109203X (GFX, 1 μM, e). (f) Summary of the experiments shown in (d) and (e). GF109203X was applied 30 min before the first application of PAR2AP. Data are means±s.e.m. (n=4). *P<0.005, significantly different from the control values.
Cross-desensitization caused by PAR2 activation
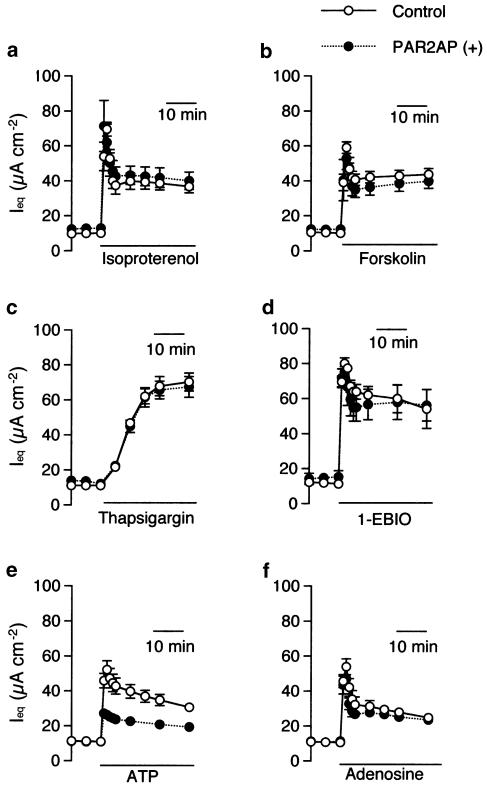
Next, we observed the effect of PAR2 stimulation on responses to various anion secretagogues. As shown in Figure 6a–d, Ieq responses to isoproterenol (10 nM, a β-adrenergic agonist), forskolin (10 μM, an adenylate cyclase (AC) activator), thapsigargin (1 μM, a Ca2+ mobilizing agent due to inhibition of Ca2+-ATPase and stimulation of capacitative Ca2+ entry), 1-EBIO (1 mM, a direct opener of human intermediated conductance, inward-rectifying Ca2+-activated K+ [hIK-1] channels) were unaffected by pretreatment with PAR2AP (50 μM, for 30 min). After PAR2 pretreatment, however, the cells became less responsive only to ATP (Figure 6e) and ADPβS. Namely, ΔIeq produced by ATP (100 μM, apical) and ADPβS (100 μM, apical) were reduced from 46.6±3.7 μA cm−2 (n=10) and 32.9±1.4 μA cm−2 (n=19) to 16.6±1.5 μA cm−2 (n=8, P<0.001) and 19.4±1.6 μA cm−2 (n=4, P<0.001), respectively. In contrast, responses to an A2B agonist, adenosine, a metabolite of ATP through ectonucleotidases, were intact in the presence of PAR2AP (Figure 6f).
Figure 6.
PAR2-induced desensitization of the subsequent heterologous responses. Ieq responses to isoproterenol (10 nM, basolateral, a), forskolin (10 μM, basolateral, b), thapsigargin (1 μM, apical, c), and 1-ethyl-2-benzimdazolinone (1-EBIO, 1 mM, bilateral, d) were unaffected by pretreatment with PAR2AP (PAR2AP (+), 50 μM, basolateral) applied 30 min prior to these anion secretagogues. In contrast, ATP-induced Ieq was inhibited by the presence this PAR2 agonist (e), although the responses to adenosine, a metabolite of ATP through ectonucleotidases, were intact under this condition (f). Data are means±s.e.m. (n=4–12).
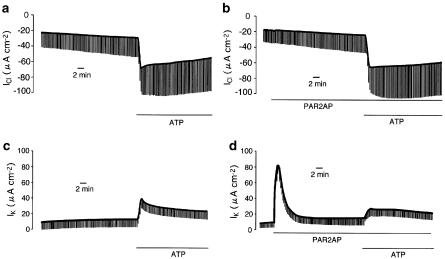
It was previously reported that extracellular ATP and ADPβS cause Cl− secretion via P2Y1 purinoceptor (P2Y1R), which is coupled both to AC and PC-PLC in Calu-3 cells (Communi et al., 1999; Son et al., 2004). Thus, there are three possible explanations for the attenuation of P2Y1R-mediated Cl− secretion: the stimulation of PAR2 inhibits (1) the AC/cAMP pathway relevant to CFTR activities, (2) the PC-PLC-Ca2+ pathway regulating the basolateral hIK-1 channel that provides a driving force for Cl− secretion, or (3) both. To rule out the involvement of cAMP-dependent signal transductions in the PAR2-induced suppression of P2Y1R signals, apical membrane Cl− conductance, which reflects CFTR (a cAMP-dependent Cl− channel)-mediated anion conductance, was followed by apical application of extracellular ATP (100 μM) under nystatin-permeabilized conditions (Figure 7a and b). However, the presence of PAR2AP (50 μM, Figure 7b) failed to affect the inward ICl across the apical membrane in the apical to basolateral Cl− gradient (Figure 7b). Indeed, there was no significant difference in the ATP-augmented ΔICl between the groups with PAR2AP (43.9±3.0 μA cm−2, n=4) and without (41.7±4.4 μA cm−2). In contrast, an ATP-induced increase in basolateral membrane IK (ΔIK=28.2±0.7 μA cm−2, n=4, Figure 7c), which is highly sensitive to charybdotoxin, a selective hIK-1 inhibitor (Son et al., 2004), was significantly reduced by the pretreatment with PAR2AP (ΔIK=14.7±0.6 μA cm−2, n=4, P<0.001, Figure 7d).
Figure 7.
(a, b) Effects of PAR2 activation on extracellular ATP-induced Cl− conductance across the apical membrane in nystatin-permeabilized Calu-3 monolayer (see Methods). To isolate apical Cl− conductance, the apical membrane Cl− current (ICl) was measured after the apical to basolateral Cl− gradient was established. An inward ICl is consistent with an absorptive Cl− flow. (a) Under this condition, Cl− conductance was continuously augmented by ATP (100 μM) added to the apical solution. (b) Even in the cells pretreated with PAR2AP (50 μM), the ATP-induced Cl− conductance was not significantly affected. (c, d) Effects of PAR2 activation on ATP-induced K+ conductance across the basolateral membrane in a nystatin-permeabilized Calu-3 monolayer (see Methods). Basolateral membrane K+ conductance was estimated through basolateral membrane K+ current after establishment of an apical-to-basolateral K+ gradient. (c) K+ conductance was increased by additon of ATP (100 μM) to the apical solution. (d) Application of PAR2AP induced a transient increase in IK. After exposure to PAR2AP (50 μM, basolateral) for 30 min, an ATP-induced increase in K+ conductance was attenuated. These experiments were performed under closed-circuit conditions.
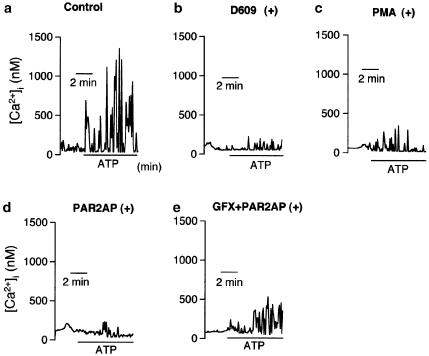
In addition, fluo-3 signal measurement revealed that apically applied ATP (100 μM) elevated [Ca2+]i in an oscillatory fashion (Figure 8a). The ATP-induced [Ca2+]i changes were markedly attenuated by the presence of D609 (a PC-PLC inhibitor) or PMA (a PKC activator), and were also suppressed by the PAR2AP pretreatment (Figure 8b–d). Namely, Δ[Ca2+]i was reduced from 1288.5±356.2 nM (n=9, Figure 8a) to 178.1±51.2 nM (n=6, P<0.05, Figure 8d) by the presence of PAR2AP. Further, the PAR2-mediated attenuation of [Ca2+]i movement was reversed by the PKC inhibitor GFX (1 μM) up to 423.8±59.9 nM (n=6, P<0.05 vs without GFX, Figure 8e).
Figure 8.
(a–e) Effects of PARAP2 on extracellular ATP-induced Ca2+ mobilization in Calu-3 cells preloaded with fluo-3. Application of ATP (100 μM, apical) on the apical side increased [Ca2+]i in a oscillatory fashion (a). The ATP-induced Ca2+ mobilization, which was significantly suppressed by D609 (100 μM, b) or PMA (1 μM, c), was also abolished by pretreatment with the PAR2-activating peptide (PAR2AP, 50 μM, basolateral, d). The PAR2-mediated attenuation was blunted by pretreatment with the PKC inhibitor GF109203X (GFX, 1 μM, e). D609, PMA, PAR2AP, and GF109203X were applied 30 min before addition of ATP.
Discussion
Thrombin (a PAR1, -3, -4 agonist) and trypsin (a PAR2, -4 agonist) are well-characterized enzymatic activators of PARs (Dery et al., 1998). We showed in human airway epithelial Calu-3 cells that trypsin, but not thrombin, applied to the basolateral face of the monolayer caused a transient change in ion transport that was mimicked by the application of PAR2AP, suggesting selective PAR2 expression. Our RT–PCR experiments to detect PAR2 mRNA verified this point in the present study. When trypsin and PAR2AP were used to determine concentration-dependent responses, apical application of these PAR2 agonists caused much smaller effects than basolateral application did. These observations indicate predominant localization of PAR2 on the basolateral membrane of Calu-3 cells, in accordance with native and cultured airway epithelial cells (Danahay et al., 2001; Kunzelmann et al., 2002). The most likely second messenger in the regulation of PAR2-mediated ion transport is cytosolic Ca2+, because PAR2-mediated Ieq changes were well correlated to those in [Ca2+]i, as has been reported in normal human airway epithelial cells (Danahay et al., 2001). Calu-3 cells display the phenotype of CFTR-rich airway epithelium without any component of amiloride-sensitive Na+ absorption, and [Ca2+]i elevation causes Cl− secretion but neither HCO3− secretion nor Na+ absorption (Moon et al., 1997; Singh et al., 1997; Devor et al., 1999). This would render it conceivable that PAR2-stimulated ion transport in Calu-3 cells essentially comprises Cl− secretion. This speculation was confirmed by the results that PAR2AP-induced responses were markedly suppressed by removal of Cl− from the extracellular solutions and the presence of basolateral bumetanide, an inhibitor of Cl− uptake across the basolateral membrane via the Na+–K+–2Cl− cotransporter. Although Calu-3 cells almost exclusively express CFTR without Ca2+-dependent Cl− channels (Haws et al., 1994), Ca2+-dependent CFTR-mediated Cl− secretion does occur. Namely, activation of basolateral Ca2+-activated K+ channels provides a driving force for Cl− efflux across the apical CFTR at rest (Moon et al., 1997). In Calu-3 cells, the majority of basolateral K+ conductance can be accounted for by the hIK-1 channel (an intermediate conductance Ca2+-activated K+ channel) because only a small cAMP-activated K+ conductance but a much larger hIK-1-dependent K+ conductance could be identified in the permeabilized monolayer (Devor et al., 1999; Cowley & Linsdell, 2002; Ito et al., 2004b; Szkotak et al., 2004). Preliminarily, we ascertained that PAR2-induced Ieq was significantly inhibited by the hIK-1 channel inhibitors charybdotoxin and clotrimazole (data not shown). Thus, it is most likely that PAR2-induced Cl− secretion via resting CFTR is generated by Ca2+-dependent activation of the basolateral hIK-1 channel (see Figure 7b and d).
In most cellular systems, the key enzyme for the regulation of intracellular Ca2+ mobilization is G protein-coupled PLC. PLC is classified into PI-PLC and PC-PLC (Camina et al., 1999). PI-PLC stimulates the breakdown of phosphatidylinositol 4,5-biphosphate at the internal leaflet of the plasma membrane to form two second messengers, InsP3 and DAG (Nakamura, 1996). InsP3 triggers Ca2+ release from the endoplasmic reticulum, while DAG activates transient receptor potential channels, Ca2+ entry pathways (Kiselyov & Muallem, 1999). There are several lines of evidence that PAR-mediated Ca2+ mobilization is coupled to PI-PLC (Mule et al., 2002; Oshiro et al., 2002). Nevertheless, the results in Figure 4 revealed that the PAR2-mediated responses were unaffected by PI-PLC inhibitors, such as U73122 (Ki=4–10 μM) (Connor et al., 2001; Saeed et al., 2003; Rasheed & Saeed, 2004) or ET-18-OCH3 (Ki=2–18 μM) (Powis et al., 1992; Daniel et al., 1995; Seebeck et al., 1998), and an InsP3 inhibitor, Xest C (Ki=0.3–0.4 μM) (Gafni et al., 1997). However, recent studies have shed light on PC-PLC as an alternative pathway for production of DAG (Snetkov et al., 2001). In the present study, PAR2AP-induced Cl− secretion and Ca2+ mobilization were markedly diminished by pretreatment with a specific PC-PLC inhibitor (D609), whose Ki on PC-PLC has been reported to be 5–20 μM (Tschaikowsky et al., 1994; Amtmann, 1996; Kengatharan et al., 1996; Nakahata et al., 2000). These results, thereby, suggest the possible coupling of PAR2 with PC-PLC on the basolateral membrane of Calu-3.
PAR-mediated activation of PC-PLC and resultant generation of DAG should activate PKC as well as mobilize Ca2+ (Nystedt et al., 1994). PKC has been reported to be involved in regulating PAR2-mediated Ca2+ mobilization in kidney and intestinal epithelial cell lines (Bohm et al., 1996). To investigate the effect of PKC on PAR2-mediated Cl− secretion in Calu-3 cells, cells pretreated with PMA, a PKC activator, were exposed to PAR2AP, showing that PAR2 on Calu-3 cells seem likely to be under the regulation of PKC (see Figure 4e and f). From these observations, we predicted that PKC activation via PAR2 stimulation is involved in its auto-desensitization, and consequently we found it partially involved. To put it concretely, auto-desensitization during the continuous exposure to PAR2AP, however, was unaffected by a PKC inhibitor (Figure 5a and b), whereas auto-desensitization during the intermittent exposure to PAR2AP was mitigated by the PKC inhibition (see Figure 5d and e). In other words, PAR2-mediated PKC activation participates in extension of PAR2 auto-desensitization even after recovery from PKC-independent auto-desensitization (i.e., uncoupling from G-protein, or internalization into early endosomes) (Dery et al., 1998; Macfarlane et al., 2001).
PKC could be implicated in cross-desensitization between different receptors, supposing auto-desensitization of PAR2 involves PKC, which may act at the level of the receptor or at more distal steps in the signaling pathway (Bohm et al., 1996). Thus, we examined the effects of PAR2 activation on the anion secretion due to heterologous stimuli in Calu-3 cells. As shown in Figure 6, anion secretion generated by cAMP-related agents, such as isoproterenol, forskolin, and adenosine, were unaffected by the PAR2 activation through PAR2AP, suggesting that PAR2 activation does not influence signals via AC-coupled receptors (i.e., β2-adrenergic receptor, A2B adenosine receptor), the cAMP-PKA cascade, and cAMP-dependent channels (i.e., CFTR, cAMP-dependent K+ channels). Thapsigargin inhibits the Ca2+-ATPase that contributes to the Ca2+ uptake of intracellular Ca2+ into its store sites, leading to [Ca2+]i elevation (Kim et al., 1998). Depletion of Ca2+ in the store sites due to thapsigargin also stimulates Ca2+ entry from the extracellular space (Takemoto et al., 1998). [Ca2+]i elevation causes an opening of the basolateral hIK-1 channel, leading to Cl− secretion, as described above. Thus, to determine whether Ca2+ mobilization itself or the hIK-1 channel was a target of PAR2-mediated desensitization, we examined the effects of PAR2 activation on the thapsigargin- and 1-EBIO (a hIK-1 opener)-induced anion secretion, but found no significant effect. Notwithstanding these observations, we found in this study that ATP- and ADPβS-induced anion secretion was desensitized by the pretreatment with PAR2AP. Previous studies to detect subtypes of P2Y receptors revealed that only P2Y1R mRNA is expressed in Calu-3 cells (Communi et al., 1999), and extracellular ATP and ADPβS is well capable of stimulating this purinoceptor (Ralevic & Burnstock, 1998; Son et al., 2004). We have previously demonstrated that stimulation of P2Y1R, which is coupled to both AC and PC-PLC, simultaneously causes CFTR activation and Ca2+ mobilization, generating Cl− secretion (Son et al., 2004). The locus of the P2Y1R-mediated signal transduction affected by PAR2 activation is not at the receptor levels but in the stream of the PC-PLC-mediated pathway, because, as shown in Figures 7 and 8, CFTR-mediated apical Cl− conductance augmented by the addition of ATP was unchanged under PAR2-prestimulated conditions, while pretreatment with PAR2AP markedly attenuated ATP-mediated Ca2+ mobilization and elevation of basolateral K+ conductance that is predominantly mediated by a Ca2+-activated K+ channel hIK-1 in Calu-3 cells (Son et al., 2004). Further, the PAR2-mediated inhibition of ATP-induced Ca2+ mobilization was counteracted by the presence of a PKC inhibitor GFX. Therefore, we conclude that PC-PLC-mediated PKC activation through PAR2 causes a selective cross-desensitization of the P2Y1R–PC-PLC pathway.
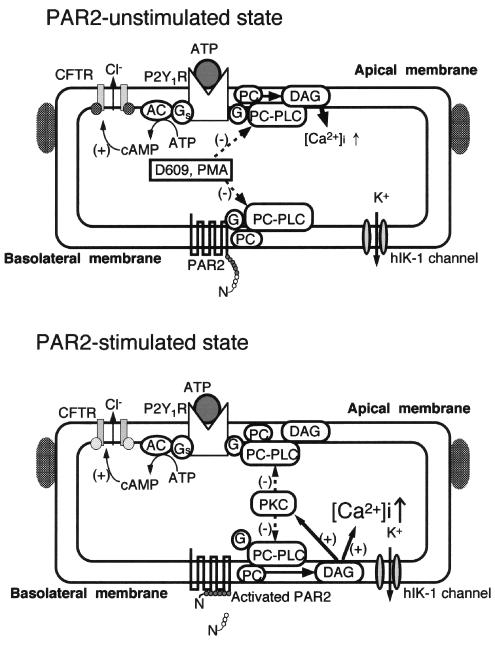
Of the PAR2-mediated auto-and cross-desensitizations, PKC activation via PC-PLC may act on G proteins, PC-PLC, or later steps in the signaling pathway. Nevertheless, it is more likely that G-proteins are not a principal target of PKC because of the previous findings that the Gqα-subunit, which is at least partially involved in PAR2- and P2Y1-transduction (Ralevic & Burnstock, 1998; Macfarlane et al., 2001), is not phosphorylated by PMA (Lounsbury et al., 1993). Further, it has been reported that thapsigargin-induced Ca2+ mobilization, which acts on distal steps in the PLC pathway, is insensitive to PMA (Munshi et al., 1993), suggesting less involvement of PKC in the distal steps of PC-PLC. Add results shown in Figure 6c is also suggestive regarding this point. Alternatively, PC-PLC seems more likely to be a target for PAR2-mediated, PKC-dependent desensitization because both PC-PLC and PI-PLC are phosphorylated in response to activation of PKC by the PKC activator PMA or through PLC-coupled receptors, preventing interaction with Gi- and Gq-proteins (Camina et al., 1999). Considering the results obtained from the present study, we propose the hypothetical scheme of PAR2-mediated regulation of P2Y1R shown in Figure 9.
Figure 9.
Possible mechanisms underlying PAR2-mediated desensitization related to PKC in Calu-3 cells. (+) and (−) indicate stimulatory and inhibitory effects, respectively. Both basolateral PAR2 and apical P2Y1 purinoceptor (P2Y1R) are coupled with PC-PLC, which hydrolyzes phosphatidylcholine at the plasma membrane. PC-PLC activity is inhibited by D609 and PMA. PAR2-mediated PC-PLC activation and the resultant formation of DAG are greatly responsible for Ca2+ mobilization, which activates the basolateral hIK-1 channel for Cl− secretion, and PKC activation, which inhibits PC-PLC activity. The PAR2-mediated PKC activation leads to desensitization of PAR2 itself and P2Y1R through PKC-to-PC-PLC negative feedback mechanisms. In this process, the adenylate cyclase (AC)-mediated pathway, which is also coupled with P2Y1R, is intact.
In conclusion, PAR2 stimulation generates a brief response of Cl− secretion through PC-PLC-mediated pathway, followed by not only auto-desensitization of PAR2 itself but also cross-desensitization of a PC-PLC-coupled purinoceptor (P2Y1R). In both types of desensitization, PKC-to-PC-PLC negative feedback mechanisms appear to be involved. As a corollary of these results, the auto- and cross-desensitization of PAR2 signal transduction may be one of the important systems regulating airway anion secretion.
Acknowledgments
This work was supported by Research Grant Funds from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Hibino Memorial Research Fund, and the Aichi Health Promotion Foundation to Y. Ito.
Abbreviations
- [Ca2+]i
cytosolic Ca2+ concentration
- CFTR
cystic fibrosis transmembrane conductance regulator
- DAG
diacylglycerol
- 1-EBIO
1-ethyl-2-benzimdazolinone
- GFX
GF109203X
- Gt
transepithelial conductance
- hIK-1 channel
human intermediate conductance, inward-rectifying Ca2+-activated K+ channel
- ICl
apical membrane Cl− current
- Ieq
equivalent short-circuit current
- IK
basolateral membrane K+ current
- Ins P3
inositol 1,4,5-triphosphate
- NHBE
normal human bronchial epithelial cells
- PAR2
protease-activated receptor 2
- PAR2AP
PAR2-activating peptide
- PC-PLC
phosphatidylcholine-phospholipase C
- PD
transepithelial potential difference
- PI-PLC
phosphatidylinositol-phospholipase C
- PKC
protein kinase C
- PSS
physiological saline solution
- P2Y1R
P2Y1 purinoceptor
- revPAR2AP
the reverse sequence peptide of PAR2-activating peptide
- Xest C
xestospongin C
References
- AIKAWA T., SHIMURA S., SASAKI H., EBINA M., TAKISHIMA T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- AMTMANN E. The antiviral, antitumoural xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phospholipase C. Drugs. Exp. Clin. Res. 1996;22:287–294. [PubMed] [Google Scholar]
- BOHM S.K., KHITIN L.M., GRADY E.F., APONTE G., PAYAN D.G., BUNNETT N.W. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J. Biol. Chem. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- CAMINA J.P., CASABIELL X., CASANUEVA F.F. Inositol 1,4,5-trisphosphate-independent Ca2+ mobilization triggered by a lipid factor isolated from vitreous body. J. Biol. Chem. 1999;274:28134–28141. doi: 10.1074/jbc.274.40.28134. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.M., ANDERSON G.P., FRAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., MOFFATT J.D. Protease-activated receptors: sentries for inflammation. Trends Pharmacol. Sci. 2000;21:103–108. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., MOFFATT J.D. Protease-activated receptor-2 (PAR2) in the airways. Pulm. Pharmacol. Ther. 2001;14:183–191. doi: 10.1006/pupt.2001.0285. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PAINDAVOINE P., PLACE G.A., PARMENTIER M., BOEYNAEMS J.M. Expression of P2Y receptors in cell lines derived from the human lung. Br. J. Pharmacol. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR J.D., RASHEED H., GILANI A.H., CHEEMA M., RIZVI Z., SAEED S.A. Second messengers in platelet aggregation evoked by serotonin and A23187, a calcium ionophore. Life Sci. 2001;69:2759–2764. doi: 10.1016/s0024-3205(01)01347-9. [DOI] [PubMed] [Google Scholar]
- COWLEY E.A., LINSDELL P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J. Physiol. 2002;538:747–757. doi: 10.1113/jphysiol.2001.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANAHAY H., WITHEY L., POLL C.T., VAN DE GRAAF S.F., BRIDGES R.J. Protease-activated receptor-2-mediated inhibition of ion transport in human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2001;280:C1455–C1464. doi: 10.1152/ajpcell.2001.280.6.C1455. [DOI] [PubMed] [Google Scholar]
- DANIEL L.W., CIVOLI F., ROGERS M.A., SMITHERMAN P.K., RAJU P.A., ROEDERER M. ET-18-OCH3 inhibits nuclear factor-kappa B activation by 12-O-tetradecanoylphorbol-13-acetate but not by tumor necrosis factor-alpha or interleukin 1 alpha. Cancer Res. 1995;55:4844–4849. [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., LAMBERT L.C., DELUCA A., FRIZZELL R.A., BRIDGES R.J. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J. Gen. Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAFNI J., MUNSCH J.A., LAM T.H., CATLIN M.C., COSTA L.G., MOLINSKI T.F., PESSAH I.N. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- HAWS C., FINKBEINER W.E., WIDDICOMBE J.H., WINE J.J. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl- conductance. Am. J. Physiol. 1994;266:L502–L512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- HURLEY J.H., GROBLER J.A. Protein kinase C and phospholipase C: bilayer interactions and regulation. Curr. Opin. Struct. Biol. 1997;7:557–565. doi: 10.1016/s0959-440x(97)80122-4. [DOI] [PubMed] [Google Scholar]
- INGLIS S.K., OLVER R.E., WILSON S.M. Differential effects of UTP and ATP on ion transport in porcine tracheal epithelium. Br. J. Pharmacol. 2000;130:367–374. doi: 10.1038/sj.bjp.0703324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y., NAKAYAMA S., SON M., KUME H., YAMAKI K. Protection by tetracyclines against ion transport disruption caused by nystatin in human airway epithelial cells. Toxicol. Appl. Pharmacol. 2001;177:232–237. doi: 10.1006/taap.2001.9313. [DOI] [PubMed] [Google Scholar]
- ITO Y., SATO S., SON M., KONDO M., KUME H., TAKAGI K., YAMAKI K. Bisphenol A inhibits Cl− secretion by inhibition of basolateral K+ conductance in human airway epithelial cells. J. Pharmacol. Exp. Ther. 2002;302:80–87. doi: 10.1124/jpet.302.1.80. [DOI] [PubMed] [Google Scholar]
- ITO Y., SON M., SATO S., ISHIKAWA T., KONDO M., NAKAYAMA S., SHIMOKATA K., KUME H. ATP release triggered by activation of the Ca2+-activated K+ channel in human airway Calu-3 cells. Am. J. Respir. Cell Mol. Biol. 2004a;30:388–395. doi: 10.1165/rcmb.2003-0184OC. [DOI] [PubMed] [Google Scholar]
- ITO Y., SON M., SATO S., OHASHI T., KONDO M., SHIMOKATA K., KUME H. Effects of fluoranthene, a polycyclic aromatic hydrocarbon, on cAMP-dependent anion secretion in human airway epithelia. J. Pharmacol. Exp. Ther. 2004b;308:651–657. doi: 10.1124/jpet.103.059089. [DOI] [PubMed] [Google Scholar]
- KELLERMAN D.J. P2Y2 receptor agonists: a new class of medication targeted at improved mucociliary clearance. Chest. 2002;121:201S–205S. doi: 10.1378/chest.121.5_suppl.201s. [DOI] [PubMed] [Google Scholar]
- KENGATHARAN M., DE KIMPE S.J., THIEMERMANN C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br. J. Pharmacol. 1996;117:1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.K., SAKONG J., CHO K.H., LEE C.O. Vanadate-sensitive microsomal ATPases and microsomal 45Ca2+ uptake in tracheal epithelial cells. J. Biochem. (Tokyo) 1998;124:1094–1100. doi: 10.1093/oxfordjournals.jbchem.a022226. [DOI] [PubMed] [Google Scholar]
- KISELYOV K., MUALLEM S. Fatty acids, diacylglycerol, Ins(1,4,5)P3 receptors and Ca2+ influx. Trends Neurosci. 1999;22:334–337. doi: 10.1016/s0166-2236(99)01426-5. [DOI] [PubMed] [Google Scholar]
- KNIGHT D.A., LIM S., SCAFFIDI A.K., ROCHE N., CHUNG K.F., STEWART G.A., THOMPSON P.J. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J. Allergy Clin. Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- KUNZELMANN K., SCHREIBER R., KONIG J., MALL M. Ion transport induced by proteinase-activated receptors (PAR2) in colon and airways. Cell Biochem. Biophys. 2002;36:209–214. doi: 10.1385/CBB:36:2-3:209. [DOI] [PubMed] [Google Scholar]
- LOUNSBURY K.M., SCHLEGEL B., PONCZ M., BRASS L.F., MANNING D.R. Analysis of Gz alpha by site-directed mutagenesis. Sites and specificity of protein kinase C-dependent phosphorylation. J. Biol. Chem. 1993;268:3494–3498. [PubMed] [Google Scholar]
- LUNDGREN J.D., SHELHAMER J.H. Pathogenesis of airway mucus hypersecretion. J. Allergy Clin. Immunol. 1990;85:399–417. doi: 10.1016/0091-6749(90)90147-v. [DOI] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G.D., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MINTA A., KAO J.P., TSIEN R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- MOON S., SINGH M., KROUSE M.E., WINE J.J. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am. J. Physiol. 1997;273:L1208–L1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- MULE F., BAFFI M.C., FALZONE M., CERRA M.C. Signal transduction pathways involved in the mechanical responses to protease-activated receptors in rat colon. J. Pharmacol. Exp. Ther. 2002;303:1265–1272. doi: 10.1124/jpet.102.041301. [DOI] [PubMed] [Google Scholar]
- MUNSHI R., DEBERNARDI M.A., BROOKER G. P2U-purinergic receptors on C6-2B rat glioma cells: modulation of cytosolic Ca2+ and cAMP levels by protein kinase C. Mol. Pharmacol. 1993;44:1185–1191. [PubMed] [Google Scholar]
- NAKAHATA N., TAKANO H., OHIZUMI Y. Thromboxane A2 receptor-mediated tonic contraction is attributed to an activation of phosphatidylcholine-specific phospholipase C in rabbit aortic smooth muscles. Life Sci. 2000;66:PL71–PL76. doi: 10.1016/s0024-3205(99)00613-x. [DOI] [PubMed] [Google Scholar]
- NAKAMURA S. Phosphatidylcholine hydrolysis and protein kinase C activation for intracellular signaling network. J. Lipid Mediat. Cell Signal. 1996;14:197–202. doi: 10.1016/0929-7855(96)00525-1. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHIRO A., OTANI H., YAGI Y., FUKUHARA S., INAGAKI C. Protease-activated receptor-2-mediated Ca2+ signaling in guinea pig tracheal epithelial cells. Life Sci. 2002;71:547–558. doi: 10.1016/s0024-3205(02)01705-8. [DOI] [PubMed] [Google Scholar]
- POWIS G., SEEWALD M.J., GRATAS C., MELDER D., RIEBOW J., MODEST E.J. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52:2835–2840. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RAMMINGER S.J., COLLETT A., BAINES D.L., MURPHIE H., MCALROY H.L., OLVER R.E., INGLIS S.K., WILSON S.M. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. Br. J. Pharmacol. 1999;128:293–300. doi: 10.1038/sj.bjp.0702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASHEED H., SAEED S.A. Involvement of thromboxane A2 and tyrosine kinase in the synergistic interaction of platelet activating factor and calcium ionophore A23187 in human platelet aggregation. Exp. Mol. Med. 2004;36:220–225. doi: 10.1038/emm.2004.30. [DOI] [PubMed] [Google Scholar]
- SAEED S.A., RASHEED H., GILANI A.U. Synergism interaction between arachidonic acid by 5-hydroxytryptamine in human platelet aggregation is mediated through multiple signalling pathways. Acta. Pharmacol. Sin. 2003;24:958–964. [PubMed] [Google Scholar]
- SEEBECK J., KRUSE M.L., SCHMIDT-CHOUDHURY A., SCHMIDTMAYER J., SCHMIDT W.E. Pituitary adenylate cyclase activating polypeptide induces multiple signaling pathways in rat peritoneal mast cells. Eur. J. Pharmacol. 1998;352:343–350. doi: 10.1016/s0014-2999(98)00372-0. [DOI] [PubMed] [Google Scholar]
- SHEN B.Q., FINKBEINER W.E., WINE J.J., MRSNY R.J., WIDDICOMBE J.H. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am. J. Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- SHIMURA S. Signal transduction of mucous secretion by bronchial gland cells. Cell Signal. 2000;12:271–277. doi: 10.1016/s0898-6568(00)00066-8. [DOI] [PubMed] [Google Scholar]
- SINGH M., KROUSE M., MOON S., WINE J.J. Most basal ISC in Calu-3 human airway cells is bicarbonate-dependent Cl− secretion. Am. J. Physiol. 1997;272:L690–L698. doi: 10.1152/ajplung.1997.272.4.L690. [DOI] [PubMed] [Google Scholar]
- SNETKOV V.A., HAPGOOD K.J., MCVICKER C.G., LEE T.H., WARD J.P. Mechanisms of leukotriene D4-induced constriction in human small bronchioles. Br. J. Pharmacol. 2001;133:243–252. doi: 10.1038/sj.bjp.0704076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SON M., ITO Y., SATO S., ISHIKAWA T., KONDO M., NAKAYAMA S., SHIMOKATA K., KUME H. Apical and basolateral ATP-induced anion secretion in polarized human airway epithelia. Am. J. Respir. Cell Mol. Biol. 2004;30:411–419. doi: 10.1165/rcmb.2003-0183OC. [DOI] [PubMed] [Google Scholar]
- SZKOTAK A.J., MURTHY M., MACVINISH L.J., DUSZYK M., CUTHBERT A.W. 4-Chloro-benzo[F]isoquinoline (CBIQ) activates CFTR chloride channels and KCNN4 potassium channels in Calu-3 human airway epithelial cells. Br. J. Pharmacol. 2004;142:531–542. doi: 10.1038/sj.bjp.0705846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO M., TAKAGI K., OGINO K., TOMITA T. Comparison of contractions produced by carbachol, thapsigargin and cyclopiazonic acid in the guinea-pig tracheal muscle. Br. J. Pharmacol. 1998;124:1449–1454. doi: 10.1038/sj.bjp.0701993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSCHAIKOWSKY K., MEISNER M., SCHONHUBER F., RUGHEIMER E. Induction of nitric oxide synthase activity in phagocytic cells inhibited by tricyclodecan-9-yl-xanthogenate (D609) Br. J. Pharmacol. 1994;113:664–668. doi: 10.1111/j.1476-5381.1994.tb17043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAOKA K., MASUDA K., OGAWA H., TAKAGI K., UMEMOTO N., YASUOKA S. Cloning and characterization of the cDNA for human airway trypsin-like protease. J. Biol. Chem. 1998;273:11895–11901. doi: 10.1074/jbc.273.19.11895. [DOI] [PubMed] [Google Scholar]