Abstract
Cardiac contractility in cirrhosis is normal at baseline but hyporesponsive to stimuli, a phenomenon known as ‘cirrhotic cardiomyopathy'. The pathogenesis remains unclear. Endocannabinoids are vasoactive, but have not previously been examined in the cirrhotic heart. We therefore aimed to systematically clarify a possible role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy.
Cirrhosis was induced in Sprague–Dawley rats by bile duct ligation; controls underwent a sham operation. At 4 weeks after operation, isolated left ventricular papillary muscle contractility was studied.
Dose–response curve for a β-adrenergic agonist isoproterenol was constructed in the presence and absence of a CB-1 antagonist AM251 (1 μM). Cirrhotic muscles had a blunted response to isoproterenol, which was completely restored by AM251.
Dose-response curves to anandamide, and CB-1 and CB-2 protein and mRNA expression in Western blot and reverse transcriptase–polymerase chain reaction experiments were not significantly different between cirrhotic and sham muscles.
Force–frequency relationship studies were performed in cirrhotic and normal muscles. At higher frequencies, anandamide reuptake blockers (VDM11 and AM404) significantly enhanced muscle relaxation in cirrhotic muscles, but not in controls. This effect was completely blocked by AM251 and pertussis toxin, whereas tetrodotoxin partially reversed it.
Taken together, these results indicate a pathogenic role for increased local (neuronal) production of endocannabinoids, mediated by a Gi-protein-dependent CB-1-responsive pathway in cirrhotic cardiomyopathy. The increased tachycardia-stress-induced release of endocannabinoids may help explain why contractility is normal at baseline but attenuated with stress.
Keywords: Cirrhosis, heart, papillary muscle, force–frequency, endocannabinoids, rat
Introduction
Although baseline cardiac output is increased in cirrhosis, ventricular contractile responsiveness to stimuli such as hemorrhage, exercise, volume expansion and inotropic drugs is blunted (Caramelo et al., 1986; Ramond et al., 1986; Bernardi et al., 1991; Ingles et al., 1991; Grose et al., 1995). This attenuated ventricular responsiveness in the face of an increased resting cardiac output has been termed cirrhotic cardiomyopathy (Ma & Lee, 1996). We have previously explored a number of possible pathogenic mechanisms for cirrhotic cardiomyopathy from cardiac adrenergic and cholinergic signal transduction pathways to cell membrane fluidity (Ma et al., 1996; 1997; Liu et al., 2000; 2001). However, the exact mechanism of this phenomenon is not yet completely clarified, in particular, the reasons for the depressed responses despite baseline hypercontractility.
Cannabinoids are derived from a tropical plant Cannabis sativa. The active substance in these compounds is Δ9-tetrahydrocannabinol. Cloning of peripheral and central receptors for exogenous cannabinoids suggested the existence of endogenous ligands for these receptors. These natural ligands are lipid-like substances called endocannabinoids, which include arachidonoyl ethanolamide or anandamide, and 2-arachidonoylglycerol (Petrocellis et al., 2004).
It has been shown that anandamide dose-dependently decreases the contractile force of electrically driven isolated rat heart and human atrial muscle strips (Ford et al., 2002; Bonz et al., 2003). There is also evidence supporting local production of endocannabinoids in mammals (Moesgaard et al., 2002), but to our knowledge alterations in local cardiac production of endocannabinoids in pathologic states have not been studied yet.
On the other hand, Batkai et al. (2001) and Ros et al. (2002) reported increased anandamide levels and expression of cannabinoid receptor-1 (CB-1) in hepatic vascular endothelial cells of cirrhotic rats and patients. Since mechanisms in peripheral vessels and cardiac tissue are often similar, we were interested in a possible role of the endocannabinoid system in the pathogenesis of cirrhotic cardiomyopathy in a rat model.
Methods
Animal model
The study protocols were approved by the Animal Care Committee of the University of Calgary Faculty of Medicine, under the guidelines of the Canadian Council on Animal Care. Male Sprague–Dawley rats (Charles River, St Laurent, QC, Canada) weighing 200–250 g were used in this study. Rats had free access to rat chow and water and maintained in a 12-h light and dark cycle. Common bile duct ligation was performed to induce cirrhosis. The surgical procedures for this cirrhotic model have been described in detail previously (Ma et al., 1999). Briefly, the common bile duct was exposed by a midline abdominal incision under general anesthesia. The duct was doubly ligated and sectioned between the ligatures. Sham-operated rats were treated in the same manner as the above-mentioned group, except that the bile duct was visually inspected but not ligated. All studies were performed 4 weeks after the bile duct ligation or sham operation.
Isolated papillary muscle contractility study
A previously published method was used to study the contractile function of isolated left ventricular papillary muscles (Ma et al., 1996). Briefly, under general anesthesia hearts were quickly dissected and placed in an oxygenated modified Tyrode's buffer that contained the following (in mM l−1): NaCl, 122.5; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.1; NaHCO3, 24; and glucose, 10; at pH 7.4. Papillary muscles from left ventricles were dissected out and suspended in an organ bath containing the above-mentioned buffer at 37°C saturated with 95% O2 and 5% CO2.
After 30 min equilibration, the papillary muscles were subjected to electrical field stimulation with a square pulse at frequency of 1.5 Hz, a voltage of 1.5-fold of threshold (40–45 V), and duration of 5 ms. The electrical signal was generated by a Grass 88 Stimulator (Quincy, MA, U.S.A.). The isometric tension or contractile force was detected with a force displacement transducer and recorded on a Grass Model 75 Polygraph connected to a Grass PolyView computerized data acquisition system. The initial tension was set at 500 mg, as previous studies had demonstrated that at this initial tension maximum contractile force could be obtained. In another set of experiments, force–frequency relationship was determined by starting the field stimulation at 0.1 Hz followed by a stepwise increase in frequency every 2 min up to 2 Hz. Dose–response curve of isolated papillary muscles to anandamide were constructed at frequencies of 0.5, 1.5 and 2 Hz. Contractile function of papillary muscles is presented as dP/dT in all experiments.
Western blot for CB-1, CB-2 and fatty acid amide hydrolase (FAAH)
Western blots for CB-1 and CB-2 cannibinoid receptor subtype proteins, and FAAH, the endocannabinoid degrading enzyme, were performed as described previously (Liu et al., 2001). Equal amounts (200 μg) of the denatured proteins per lane were loaded and separated on sodium dodecyl sulfate–10% polyacrylamide gels by electrophoresis. Proteins were then transferred to nitrocellulose by wet electroblotting at 4°C overnight. Blots were blocked for 2 h at room temperature with 10% bovine serum albumin in 0.1% Tween Tris-buffered saline buffer (TBS-T) at pH 7.5 containing 20 mM Tris base, 137 mM NaCl and 0.1% Tween-20. The membranes were then incubated at room temperature for 2 h with rabbit polyclonal anti-CB-1 antibody (1 : 1000, Affinity Bioreagents, Golden, CO, U.S.A.), goat polyclonal anti-CB-2 antibody (1 : 100, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), or rabbit polyclonal anti-FAAH antibody (1:100, Chemicon International Inc., Temecula, CA, U.S.A.). The membranes were washed with TBS-T (three times, 15 min each) and subsequently incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobin (for CB-1 and FAAH; 1 : 1000, Calbiochem, San Diego, CA, U.S.A.) or alkaline phosphatase-conjugated rabbit anti-goat immunoglobin (for CB-2; 1 : 1000, Sigma, St Louis, MO, U.S.A.). The relative expression of CB-1, CB-2 and FAAH protein in ventricular tissue of BDL and sham-operated control rats was quantified by computerized optical densitometric scanning of the membranes using a Hewlett-Packard Scan Jet IIc scanner and the NIH Image program.
Quantification of CB-1 and CB-2 mRNA expression by reverse transcriptase–polymerase chain reaction (RT–PCR)
Total RNA from the rat ventricular tissue was extracted using the acid guanidinium isothiocyanate method (Wong et al., 1994). Contaminating DNA was removed from the isolated RNA by using TURBO DNA-free kit purchased from Ambion (Austin, TX, U.S.A.). The cDNA was obtained through RT–PCR by the method of Wong et al. (1994). The previously published primer sequences are presented in Table 1. The primers were synthesized by Gibco-BRL Life Technologies (Burlington, ON, Canada). A set of (36 cycles for CB-1; 32 cycles for beta-actin) and (37 cycles for CB-2; 34 cycles for beta-actin) were chosen to ensure that the amplification of PCR products was in the exponential range according to preliminary cycle test experiments. In each PCR cycle, heat denaturation was set at 94°C for 1 min, primer annealing at 60°C for 30 s and polymerization at 72°C for 1 min. Control samples were used without reverse transcriptase to detect a possible DNA contamination. PCR product (10 μl) was electrophoresed in 1.5% agarose gels containing 0.2 μg ml−1 of ethidium bromide. The gels were scanned by computerized ultraviolet densitometric scanning of the images using a Hewlett-Packard Scan Jet IIc scanner, DeskScan II software and the NIH Image program. The results are expressed in arbitrary densitometry units normalized to internal control expression.
Table 1.
Primers used for RT–PCR
| Primer | Sequence | Product (bp) |
|---|---|---|
| CB-1 | S: 5′-GGCATCTCTTTCTCAGTCAC-3′ | 350 |
| A: 5′-ATCAGGTAGGTCTCGTCAAT-3′ | ||
| CB-2 | S: 5′-TGCTGTTGACCGATACCTATG-3′ | 470 |
| A: 5′-CCAACCTCACGTCTAGCCG-3′ | ||
| β-actin | S: 5′-TTGGCCTTAGGGTTCAGGGGG-3′ | 243 |
| A: 5′-CGTGGGCCGCCCTAGGCACCA-3′ |
CB-1=cannabinoid receptor-1; CB-2=cannabinoid receptor-2; β-actin=beta-actin; S=sense; A=antisense.
Chemical reagnts
Water-soluble forms of anandamide, and the anandamide reuptake blockers VDM11 (De Petrocellis et al., 2000) and AM404 (Beltramo et al., 1997) dissolved in Tocrisolve (1 : 4 soya oil : water emulsion) were purchased from Tocris Cookson Ltd (Elisville, MO, U.S.A.). The CB-1 antagonist AM251 and CB-2 antagonist AM630 from Tocris Cookson were dissolved in a mixture of DMSO and 100% ethanol, then subsequently diluted in Tyrode's buffer by 10 min of vortexing. The final concentrations of DMSO and ethanol in the organ bath were less than 0.01%. Preliminary studies in isolated papillary muscles confirmed that neither the DMSO–ethanol mixture nor the Tocrisolve solutions at the concentrations used in the protocols affected contractility. Isoproterenol, carbachol, tetrodotoxin (TTX) and pertussis toxin (PTX) were purchased from Sigma (St Louis, MO, U.S.A.). Other reagents were purchased from Sigma, Bio-Rad (Hercules, CA, U.S.A.), or Fisher Scientific (Pittsburgh, PA, U.S.A.) and were the highest available grade.
Statistical analysis
The results are expressed as mean±s.e.m. An unpaired Student's t-test was used to compare the differences between two groups, and for more than two groups, comparisons were analyzed by one-way ANOVA followed by Newman–Keuls post hoc test. A P-value <0.05 was considered to be significantly different.
Results
Effect of anandamide and AM251 on papillary muscle contractility
Isoproterenol-stimulated papillary muscle contractile force was significantly lower in BDL rats compared to that of the sham group (Figure 1a). Preincubation of BDL muscles with AM251 alone (1 μM) for 30 min completely restored this blunted responsiveness to isoproterenol in BDL rats, while it had no significant effect in the sham-control group (Figure 1a).
Figure 1.
(a and b), Effect of 30 min preincubation with a CB-1 antagonist (AM251, 1 μM) and anandamide (0.3 μM) on isoproterenol dose–response curves of isolated papillary muscles. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated group; ANM, anandamide. *P<0.05 compared to maximum response of sham group. #P<0.05 compared to maximum response of BDL group.
In another set of experiments, preincubation of sham papillary muscles with anandamide (0.3 μM) for 30 min significantly blunted their contractile responsiveness to isoproterenol. This dose of anandamide was chosen based on the preliminary results that showed the maximum effect at this dose. This effect of anandamide was blocked by AM251 preincubation (Figure 1b).
Anandamide dose-dependently decreased the contractile force in electrically driven sham and BDL papillary muscles after stimulation with a submaximal dose (10 μM) of isoproterenol. There was no significant difference between the response of sham and BDL groups to anandamide in three different frequencies of contraction (0.5, 1.5 and 2 Hz). Only the data obtained at 1.5 Hz are shown in Figure 2. The relaxant effect of anandamide was significantly blocked by preincubation with the CB-1 antagonist AM251 (Figure 2).
Figure 2.
Dose–response curves to anandamide in the presence and absence of a CB-1 antagonist (AM251, 1 μM) in isolated papillary muscles. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated group. *P<0.05 compared to maximum response of sham group. #P<0.05 compared to maximum response of BDL group.
Ventricular CB-1 and CB-2 mRNA and protein expression
Representative RT–PCR photographs of CB-1 and CB-2 mRNA expression in sham and BDL ventricles are shown in Figure 3. There was no significant difference in CB-1 or CB-2 mRNA expression between sham and BDL groups. Control samples without reverse transcriptase showed no band in these experiments.
Figure 3.
mRNA expression of CB-1 and CB-2 receptors in ventricular homogenates by RT–PCR. BDL, bile duct-ligated group; PC, positive control (cerebellum for CB-1 and spleen for CB-2). There were no significant differences in band density of CB-1 or CB-2 between BDL and shams.
CB-1, CB-2 and FAAH protein expression in sham and BDL ventricles are shown in Figure 4. There were no significant differences in CB-1, CB-2 or FAAH protein expression between the BDL and sham-control groups.
Figure 4.
Western blot protein expression of CB-1 and CB-2 receptors and FAAH in ventricular homogenates. BDL, bile duct-ligated group. There were no significant differences between BDL and sham groups in these proteins.
Effects of VDM11, AM404, PTX, TTX and N-nitro-L-arginine-methyl ester (L-NAME) on papillary muscle contractility
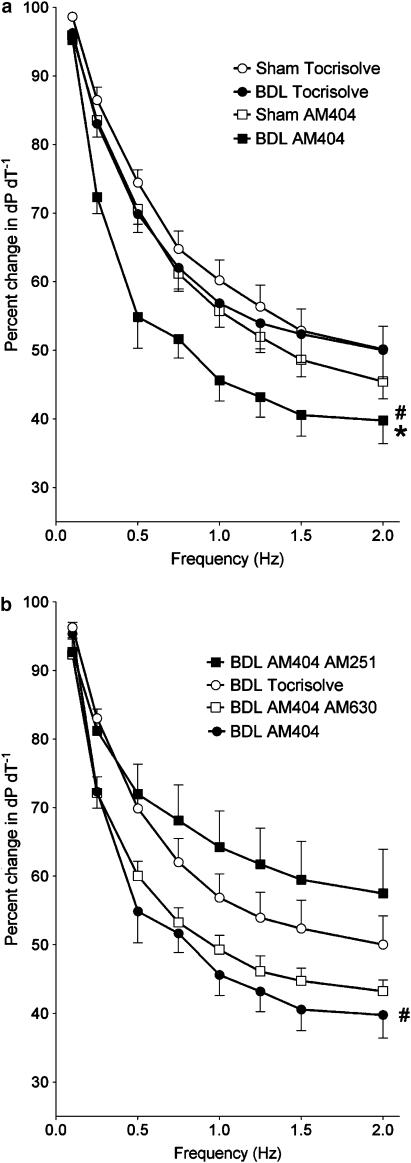
The baseline force–frequency relationship in BDL papillary muscles was not significantly different from that of the sham-control group (Figure 5a). Sham and BDL muscles were preincubated with different doses of an anandamide reuptake blocker (VDM11) and the optimal dose was found at 10 μM. In BDL muscles, administration of VDM11 significantly reduced the contractile force at higher frequencies, while it had no significant effect in sham papillary muscles (Figure 5a). This effect in BDL papillary muscles could be completely blocked by AM251 (1 μM) preincubation and partially restored by pretreatment with the CB-2 blocker AM630 (1 μM) (Figure 5b).
Figure 5.
(a and b), Effect of 30 min preincubation with an anandamide reuptake blocker (VDM11, 10 μM) and a CB-1 antagonist (AM251, 1 μM) or a CB-2 antagonist (AM630, 1 μM) on contractility of isolated papillary muscles at different frequencies of contraction. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated group. Tocrisolve, solvent used for VDM11. *P<0.05 compared to maximum response of sham-Tocrisolve group. #P<0.05 compared to maximum response of BDL-Tocrisolve group. +P<0.05 compared to maximum response of BDL-VDM11 group.
The effect of the anandamide reuptake blocker AM404 on the force–frequency relationship of BDL and sham papillary muscles was very similar to that of VDM11. The same solvent (Tocrisolve) was used for both AM404 and VDM11. AM404 (10 μM) significantly decreased the contractile force of BDL muscles at higher frequencies, while it had no significant effect in the sham group (Figure 6a). This effect of AM404 was completely restored after preincubation of muscles with AM251 (1 μM) and partially blocked by AM630 (1 μM) (Figure 6b).
Figure 6.
(a and b) Effect of 30 min preincubation with an anandamide reuptake blocker (AM404, 10 μM) and a CB-1 antagonist (AM251, 1 μM) or a CB-2 antagonist (AM630, 1 μM) on contractility of isolated papillary muscles at different frequencies of contraction. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated group. Tocrisolve, solvent used for AM404. *P<0.05 compared to maximum response of sham-Tocrisolve group. #P<0.05 compared to maximum response of BDL-Tocrisolve group.
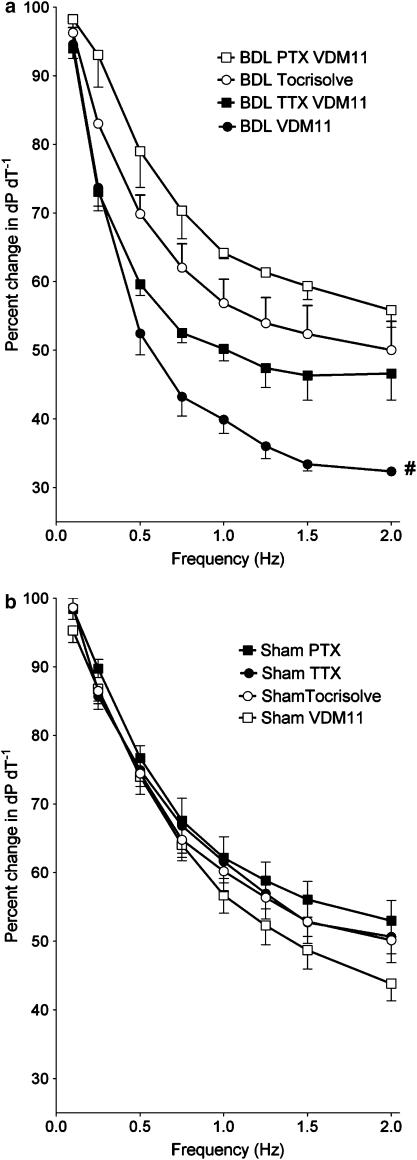
PTX is known to block inhibitory Gi-protein activity. In pilot studies, PTX (40 μg kg−1, intravenously (i.v.), 48 h before the experiment) significantly blocked the relaxant effect of the cholinergic agonist carbachol on sham and BDL papillary muscles after precontraction with isoproterenol (0.1 μM), thus confirming effective Gi-protein inhibition of that dose. PTX blocked the effect of VDM11 on force–frequency relationship in BDL muscles, while it had no significant effect in sham papillary muscles (Figure 7).
Figure 7.
(a and b) Effect of 30 min preincubation with an anandamide reuptake blocker (VDM11, 10 μM) and TTX (1 μM) or treatment with PTX (40 μg kg−1 body weight, i.v., 48 h before the experiment) on contractility of isolated papillary muscles at different frequencies of contraction. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated group; TTX, tetrodotoxin; PTX, pertussis toxin; Tocrisolve, solvent used for VDM11. *P<0.05 compared to maximum response of sham-Tocrisolve group. #P<0.05 compared to maximum response of BDL-Tocrisolve group.
In order to inactivate local neurons as possible sources of anandamide production, sham and BDL papillary muscles were preincubated with different doses of the neurotoxin TTX (0.1, 1 and 10 μM) for 30 min. The effects of these three doses of TTX were identical in blocking the effect of VDM11 on force-frequency relationship of BDL papillary muscles (Figure 7, only the dose of 1 μM is shown), while they did not affect the response of sham muscles.
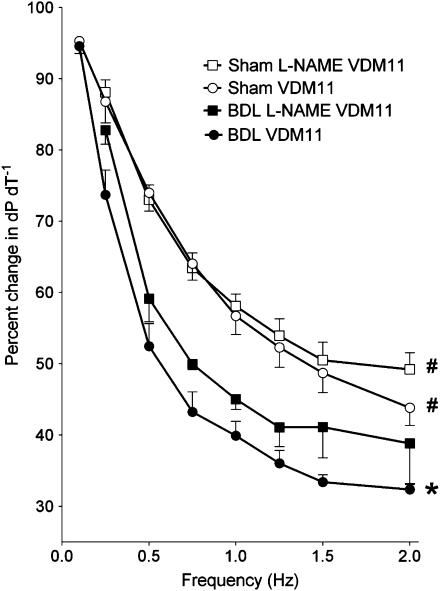
Preincubation of muscles with the non-specific nitric oxide (NO) synthase (NOS) inhibitor L-NAME (10 μM) did not induce any significant change in the effect of VDM11 (10 μM) on either sham or BDL papillary muscles (Figure 8).
Figure 8.
Effect of 30 min preincubation with an NOS blocker (L-NAME, 10 μM) on contractility of isolated papillary muscles at different frequencies of contraction. Values are the mean±s.e.m. of four to five rats per group. BDL, bile duct-ligated; L-NAME, N-nitro-L-arginine-methyl ester. *P<0.05 compared to maximum response of sham-VDM11 group. #P<0.05 compared to maximum response of BDL-VDM11 group.
Discussion
Interest in cirrhotic cardiomyopathy has been burgeoning recently. In particular, the increasing use of procedures that stress the heart, such as transjugular intrahepatic portosystemic stent-shunts and liver transplantation, has highlighted the clinical significance of this syndrome. Cardiac failure accounts for 7–15% of postoperative mortality following liver transplantation (Rayes et al., 1995; Myers & Lee, 2000). Moreover, cirrhotic cardiomyopathy may contribute to the pathogenesis of hepatorenal syndrome (Lee, 2003; Ruiz-del-Arbol et al., 2003).
A number of possible mechanisms have been investigated to explain the inability of the cirrhotic heart to respond properly to inotropic stimuli, but it is likely that several mechanisms are involved (Liu & Lee, 1999). Previous studies on endocannabinoids and cardiac function in cirrhosis are practically nonexistent. Ross et al. reported an increase in the circulating level of anandamide and increased expression of CB-1 receptors in hepatic vascular endothelial cells of cirrhotic rats. They showed that i.v. administration of a CB-1 antagonist, SR141716, increases the blood pressure without affecting the cardiac output. From these findings they concluded that CB-1 antagonism abrogates the hyperdynamic circulation in cirrhosis by eliminating the vasodilatory effect of circulating endocannabinoids (Ros et al., 2002). However, no firm conclusions about ventricular function can be drawn from that study because cardiac output is affected by many other factors such as body temperature, cardiac contractile force, blood volume, heart rate, peripheral vascular resistance (afterload) and central venous pressure (preload) (Hayes et al., 1984; Kissling et al., 1993).
We therefore decided to investigate systematically the effects of the endocannabinoid system in the pathogenesis of blunted cardiac function of cirrhosis. We studied isolated left ventricular papillary muscles in vitro in order to eliminate the confounding effects of the autonomic nervous system and peripheral vascular tone that are inevitable in an in vivo study.
The inotropic response of BDL papillary muscles to isoproterenol was significantly blunted compared to that of sham-control animals. This finding agrees with previous studies on BDL-cirrhotic rats (Ma et al., 1997) as well as cirrhotic patients (Moller et al., 2001).
Preincubation of sham papillary muscles with anandamide blunted their contractile response to isoproterenol. This effect was completely blocked by preincubation with AM251, confirming that this effect of anandamide was mediated by CB-1 receptors. These results indicate that increased endocannabinoid signaling through the CB-1 receptor can blunt the ventricular responsiveness to β-adrenergic stimuli. In other words, it suggests that ventricular exposure to endocannabinoids might be partly responsible for the blunted function of the cirrhotic heart.
Incubation of BDL papillary muscles with AM251 restored their blunted responsiveness to the sham-control level. This finding further suggests a role for overactivity of the endocannabinoid system, mediated via CB-1 receptors in the cirrhotic heart. CB-1 receptors may show some degree of constitutional activity even in the absence of any ligand in the environment (Gifford et al., 2000; Christopoulos et al., 2001; Wilson & Nicoll, 2001). Considering that our experiments were performed in an organ bath where circulating endocannabinoids could not affect the cardiac tissue, this suggested an increased density of CB-1 receptors in the cirrhotic heart.
However, expression of CB-1 and CB-2 mRNA and protein were not significantly different between the sham and BDL groups. Moreover, dose–response curves to anandamide were similar in sham and BDL muscles, suggesting no alteration in the density or sensitivity of CB-1 and CB-2 receptors in the cirrhotic heart. Blockade of the relaxant effect of anandamide in sham and BDL muscles after AM251 incubation confirmed that anandamide had a receptor-mediated effect.
The above-mentioned experiments convinced us that although blocking CB-1 receptors could restore the blunted function of cirrhotic papillary muscles, upregulation of ventricular cannabinoid receptors was not the responsible mechanism. An alternative explanation could be increased local production of endocannabinoids in the heart. Anandamide is synthesized in cardiac tissue of mammals including cats, dogs and rats (Moesgaard et al., 2002). We hypothesized that local anandamide production in response to stimuli such as tachycardia or β-adrenergic agonists might be increased in cirrhotic hearts.
Anandamide is normally rapidly transported or diffuses across the cell membrane and then degraded by the enzyme FAAH. In order to discern the effect of any possible difference in local anandamide production, two structurally different anandamide reuptake blockers (VDM11 and AM404) were used. These compounds prolong the effect of locally released endocannabinoids by inhibiting their cellular reuptake and subsequent hydrolysis by FAAH (Bifulco et al., 2004). In our preliminary studies, a standard dose–response curve to VDM11 at a frequency of 1 Hz showed a nonsignificant tendency toward increased relaxation in BDL papillary muscles (data not shown). If our hypothesis of increased local endocannabinoid release in response to stress was to be adequately tested, a stronger stress stimulus would be required. We therefore examined higher contraction frequencies with the idea that relative tachycardia might be sufficiently stressful. Therefore, the effect of the anandamide reuptake blockers was examined using force–frequency response plots. There was no significant difference between the response of sham and BDL muscles to higher frequencies of contraction. However, VDM11 and AM404 significantly increased the relaxation of BDL papillary muscles at higher frequencies, whereas the reuptake blockers had no significant effect in the sham controls. These findings strongly support the hypothesis of enhanced stress-induced endocannabinoid release in the cirrhotic heart.
It has been reported that AM404 also blocks brain FAAH activity (Jarrahian et al., 2000), which may complicate the interpretation of our study. On the other hand, it is shown that in most cases, but not all, VDM11 is a weak inhibitor of FAAH (Fowler et al., 2004). The similarity of effect of these two compounds and their ineffectiveness in sham-control muscles suggest that anandamide reuptake blockade was the main mechanism responsible for these effects, rather than inhibition of FAAH enzymatic activity. This idea is further supported by equal expression of FAAH in sham and BDL ventricles in our experiments.
In order to investigate whether the observed effects were mediated through CB-1 receptors, papillary muscles were pretreated with AM251 before adding VDM11 or AM404. AM251 completely blocked the effect of these compounds, indicating that this was indeed a selective receptor-mediated effect.
It is known that anandamide can act on vanilloid TRPV1 receptors under pathological conditions (Di Marzo et al., 2001; Ross, 2003). Domenicali et al. (2005) have recently reported that the effect of anandamide in mesenteric arteries of CCl4-induced cirrhotic rats is partly mediated by vanilloid TRPV1 receptors. Moreover, Pan & Chen (2004) have recently demonstrated a pathophysiological role for these receptors in the ischemic heart. Considering the depressant effect of TRPV1 receptors on cardiac contractility, it is possible that the relaxant effect of anandamide reuptake blockers in our experiments was mediated through vanilloid receptors. However, complete restoration of the observed effects with AM251, which does not have an antagonistic effect on TRPV1 receptors, makes such an involvement unlikely. Furthermore, AM404, which is also a TRPV1 agonist, and VDM11, which is not (De Petrocellis et al., 2000), appeared to be equally effective in BDL rats.
AM404 and VDM11 in high concentrations may exert a direct agonist effect on CB-1 receptors (De Petrocellis et al., 2000). However, it is unlikely responsible for their effect in our experimental setting as they did not have a similar relaxant effect in sham control rats. Specifically, the dose–response curve to anandamide constructed at different frequencies of contraction (0.5, 1.5 and 2 Hz) were identical in sham and BDL rats, while there was a significant difference in their response to VDM11 and AM404. These findings further ruled out a direct CB-1 action as the mechanism responsible for the effect of reuptake blockers in our experiments.
Further experiments were performed to confirm that the effect of VDM11 was mediated through CB-1 receptors. The cardiac effects of CB-1 receptors are thought to be mediated by inhibitory G-proteins (Randall et al., 2002). PTX treatment blocks Gi-protein activity (Otani et al., 2001) and therefore should attenuate the signaling through cannabinoid receptors. PTX pretreatment blocked the effect of VDM11 on BDL papillary muscles, while it had no significant effect on sham muscles. This suggests that the effect of anandamide reuptake blockers was mediated through Gi-protein. This finding together with the result of the AM251 experiments strongly suggests that the effect of VDM11 and AM404 was selectively mediated through CB-1 receptors and also suggests increased local endocannabinoid production in the cirrhotic papillary muscles.
In order to examine the role of CB-2 receptors, muscles were incubated with AM630 (1 μM) before VDM11 or AM404 administration. This manipulation partially restored the effect of VDM11, but failed to significantly affect the response of BDL muscles to AM404. AM630 (1 μM) not only blocks CB-2 receptors but also inhibits the activity of CB-1 receptors by 23% (Ross et al., 1999). Considering the above-mentioned observations and complete blockade of the effect of VDM11 and AM404 by AM251, which is a selective CB-1 antagonist, we believe that CB-1 and not CB-2 is the main receptor responsible for these effects; however, further experiments are required to elucidate the role of CB-2 receptors in this situation.
The next step was to determine the source of increased endocannabinoid production in the cirrhotic papillary muscle. This local endocannabinoid could originate from cardiomyocytes, neurons or trapped immune cells in the tissue. To rule out a neuronal source, papillary muscles were preincubated with TTX to paralyze the neurons without affecting the function of cardiomyocytes (Campbell et al., 2004). TTX blocks neuronal voltage-dependent Na+ channels, but has no effect on voltage-sensitive Ca2+ channels, which are critical for cardiomyocyte excitation. TTX blocked the effect of VDM11 by approximately 70%, suggesting a predominant neuronal source for increased endocannabinoid production, and also a contribution from other sources.
It has been shown that TTX at 100 nM concentration can completely block cardiac neuronal elements but only weakly inhibits myocardial Na+ channels (Honerjager, 1982; Lewis & Endean, 1986). Based on these findings, we used this dose in our protocol. It was possible that the concentration of TTX in the inner regions of the papillary muscle might be less than that of the organ bath, suggesting an even greater neuronal origin of endocannabinoids. In order to investigate this possibility, higher doses of TTX were tested as well (1 and 10 μM). These higher concentrations did not have any additional effect confirming that non-neuronal sources also contribute, in a minor way, to local endocannabinoid production.
The above-mentioned experiments strongly suggest an increase in local endocannabinoid release in cirrhotic ventricles in response to stress. The enhanced levels could be explained by either an increase in local anandamide synthesis or a decrease in anandamide degradation rate. However, FAAH protein expression in the ventricles did not differ between control and BDL rats, suggesting that the anandamide degradation process was intact in the cirrhotic heart.
Shah (2001) has previously suggested that endocannabinoid effects in the peripheral vasculature of cirrhosis are mediated by an NO-dependent mechanism. Moreover, we have previously demonstrated a significant negative-inotropic role of NO in the cirrhotic rat heart (Liu et al., 2000). We therefore used the NOS inhibitor L-NAME to investigate a possible interaction between the cannabinoid and NO systems. Intact force–frequency relationship in sham papillary muscles after L-NAME pretreatment agrees with previous reports in normal rats (Prabhu et al., 1999). Furthermore, L-NAME did not have a significant effect on the responsiveness of BDL muscles to VDM11. This agrees with previous studies in noncirrhotic rat hearts showing that NOS blockers do not affect anandamide responses (Bonz et al., 2003). These results rule out an involvement of NO-mediated mechanisms in the cardiac endocannabinoid effect and further support a selective and independent CB-1 effect.
In summary, the present study showed that local ventricular endocannabinoid production is increased in cirrhosis and this plays a significant role in blunted contractile responsiveness. These effects are mediated through CB-1 and not CB-2 receptors. These results suggest that the endocannabinoid system may contribute to the pathogenesis of cirrhotic cardiomyopathy.
Acknowledgments
We are grateful to Drs Keith Sharkey and Mark Swain for useful discussions of some results. This study was supported by an operating research grant from the Canadian Institutes of Health Research (CIHR). Dr Gaskari was supported by a CIHR and HSF Trainee award, Dr Li by a Canadian Association of Gastroenterology Research Fellowship and Dr Lee by an Alberta Heritage Foundation for Medical Research Senior Scholarship award. Dr Baik was supported by a sabbatical leave award from the Yonsei University Wonju College of Medicine, Wonju, Korea, and research scholarship awards from the Korean Association for Study of Liver and GlaxoSmithKline Korea.
Abbreviations
- BDL
bile duct ligated
- CB-1
cannabinoid receptor, subtype 1
- CB-2
cannabinoid receptor, subtype 2
- FAAH
fatty acid amide hydrolase
- L-NAME
N-nitro-L-arginine-methyl ester
- PTX
pertussis toxin
- TTX
tetrodotoxin
References
- BATKAI S., JARAI Z., WAGNER J.A., GOPARAJU S.K., VARGA K., LIU J., WANG L., MIRSHAHI F., KHANOLKAR A.D., MAKRIYANNIS A., URBASCHEK R., GARCIA N., JR, SANYAL A.J., KUNOS G. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- BELTRAMO M., STELLA N., CALIGNANO A., LIN S.Y., MAKRIYANNIS A., PIOMELLI D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- BERNARDI M., RUBBOLI A., TREVISANI F., CANCELLIERI C., LIGABUE A., BARALDINI M., GASBARRINI G. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J. Hepatol. 1991;12:207–216. doi: 10.1016/0168-8278(91)90940-d. [DOI] [PubMed] [Google Scholar]
- BIFULCO M., LAEZZA C., VALENTI M., LIGRESTI A., PORTELLA G., DI MARZO V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- BONZ A., LASER M., KULLMER S., KNIESCH S., BABIN-EBELL J., POPP V., ERTL G., WAGNER J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- CAMPBELL F., ATWELL R., FENNING A., HOEY A., BROWN L. Cardiovascular effects of the toxin(s) of the Australian paralysis tick, Ixodes holocyclus, in the rat. Toxicon. 2004;43:743–750. doi: 10.1016/j.toxicon.2004.02.025. [DOI] [PubMed] [Google Scholar]
- CARAMELO C., FERNANDEZ-MUNOZ D., SANTOS J.C., BLANCHART A., RODRIGUEZ-PUYOL D., LOPEZ-NOVOA J.M., HERNANDO L. Effect of volume expansion on hemodynamics, capillary permeability and renal function in conscious, cirrhotic rats. Hepatology (Baltimore, MD) 1986;6:129–134. doi: 10.1002/hep.1840060125. [DOI] [PubMed] [Google Scholar]
- CHRISTOPOULOS A., COLES P., LAY L., LEW M.J., ANGUS J.A. Pharmacological analysis of cannabinoid receptor activity in the rat vas deferens. Br. J. Pharmacol. 2001;132:1281–1291. doi: 10.1038/sj.bjp.0703930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DI MARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L. Anandamide: some like it hot. Trends Pharmacol. Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- DOMENICALI M., ROS J., FERNANDEZ-VARO G., CEJUDO-MARTIN P., CRESPO M., MORALES-RUIZ M., BRIONES A.M., CAMPISTOL J.-M., ARROYO V., VILA E., RODES J., JIMENEZ W. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD W.R., HONAN S.A., WHITE R., HILEY C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER C.J., TIGER G., LIGRESTI A., LOPEZ-RODRIGUEZ M.L., DI MARZO V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur. J. Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- GIFFORD A.N., BRUNEUS M., GATLEY S.J., VOLKOW N.D. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br. J. Pharmacol. 2000;131:645–650. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSE R.D., NOLAN J., DILLON J.F., ERRINGTON M., HANNAN W.J., BOUCHIER I.A.D., HAYES P.C. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J. Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- HAYES B.E., WILL J.A., ZARNSTORFF W.C., BISGARD G.E. Limitations of thermodilution cardiac output measurements in the rat. Am. J. Physiol. 1984;246:H754–H760. doi: 10.1152/ajpheart.1984.246.6.H754. [DOI] [PubMed] [Google Scholar]
- HONERJAGER P. Cardioactive substances that prolong the open state of sodium channels. Rev. Physiol. Biochem. Pharmacol. 1982;92:1–74. doi: 10.1007/BFb0030502. [DOI] [PubMed] [Google Scholar]
- INGLES A.C., HERNANDEZ I., GARCIA-ESTAN J., QUESADA T., CARBONELL L.F. Limited cardiac preload reserve in conscious cirrhotic rats. Am. J. Physiol. 1991;260:H1912–H1917. doi: 10.1152/ajpheart.1991.260.6.H1912. [DOI] [PubMed] [Google Scholar]
- JARRAHIAN A., MANNA S., EDGEMOND W.S., CAMPBELL W.B., HILLARD C.J. Structure-activity relationships among N-arachidonylethanolamine (Anandamide) head group analogues for the anandamide transporter. J. Neurochem. 2000;74:2597–2606. doi: 10.1046/j.1471-4159.2000.0742597.x. [DOI] [PubMed] [Google Scholar]
- KISSLING G., ROSS C., BRANDLE M. Validity of thermal dilution technique for measurement of cardiac output in rats. Am. J. Physiol. 1993;265:H1007–H1013. doi: 10.1152/ajpheart.1993.265.3.H1007. [DOI] [PubMed] [Google Scholar]
- LEE S.S. Cardiac dysfunction in spontaneous bacterial peritonitis: a manifestation of cirrhotic cardiomyopathy. Hepatology. 2003;38:1089–1091. doi: 10.1053/jhep.2003.50489. [DOI] [PubMed] [Google Scholar]
- LEWIS R.J., ENDEAN R. Direct and indirect effects of ciguatoxin on guinea-pig atria and papillary muscles. Naunyn Schmiedebergs Arch. Pharmacol. 1986;334:313–322. doi: 10.1007/BF00508787. [DOI] [PubMed] [Google Scholar]
- LIU H., LEE S. Cardiopulmonary dysfunction in cirrhosis. J. Gastroenterol. Hepatol. 1999;14:600–608. doi: 10.1046/j.1440-1746.1999.01920.x. [DOI] [PubMed] [Google Scholar]
- LIU H., MA Z., LEE S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology. 2000;118:937–944. doi: 10.1016/s0016-5085(00)70180-6. [DOI] [PubMed] [Google Scholar]
- LIU H., SONG D., LEE S.S. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- MA Z., LEE S.S. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- MA Z., LEE S.S., MEDDINGS J.B. Effects of altered cardiac membrane fluidity on beta-adrenergic receptor signalling in rats with cirrhotic cardiomyopathy. J. Hepatol. 1997;26:904–912. doi: 10.1016/s0168-8278(97)80259-0. [DOI] [PubMed] [Google Scholar]
- MA Z., MIYAMOTO A., LEE S.S. Role of altered beta-adrenoceptor signal transduction in the pathogenesis of cirrhotic cardiomyopathy in rats. Gastroenterology. 1996;110:1191–1198. doi: 10.1053/gast.1996.v110.pm8613009. [DOI] [PubMed] [Google Scholar]
- MA Z., ZHANG Y., HUET P.M., LEE S.S. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J. Hepatol. 1999;30:485–491. doi: 10.1016/s0168-8278(99)80109-3. [DOI] [PubMed] [Google Scholar]
- MOESGAARD B., PETERSEN G., MORTENSEN S.A., HANSEN H.S. Substantial species differences in relation to formation and degradation of N-acyl-ethanolamine phospholipids in heart tissue: an enzyme activity study. Comp. Biochem. Physiol. B. 2002;131:475–482. doi: 10.1016/s1096-4959(02)00003-9. [DOI] [PubMed] [Google Scholar]
- MOLLER S., BENDTSEN F., HENRIKSEN J.H. Splanchnic and systemic hemodynamic derangement in decompensated cirrhosis. Can. J. Gastroenterol. 2001;15:94–106. doi: 10.1155/2001/603012. [DOI] [PubMed] [Google Scholar]
- MYERS R.P., LEE S.S. Cirrhotic cardiomyopathy and liver transplantation. Liver Transplant. 2000;6:S44–S52. doi: 10.1002/lt.500060510. [DOI] [PubMed] [Google Scholar]
- OTANI H., OSHIRO A., YAGI M., INAGAKI C. Pertussis toxin-sensitive and -insensitive mechanisms of [alpha]1-adrenoceptor-mediated inotropic responses in rat heart. Eur. J. Pharmacol. 2001;419:249–252. doi: 10.1016/s0014-2999(01)00979-7. [DOI] [PubMed] [Google Scholar]
- PAN H.-L., CHEN S.-R. Sensing tissue ischemia: another new function for capsaicin receptors. Circulation. 2004;110:1826–1831. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- PETROCELLIS L.D., CASCIO M.G., MARZO V.D. The endocannabinoid system: a general view and latest additions. Br. J. Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRABHU S.D., AZIMI A., FROSTO T. Nitric oxide effects on myocardial function and force–interval relations: regulation of twitch duration. J. Mol. Cell Cardiol. 1999;31:2077–2085. doi: 10.1006/jmcc.1999.1038. [DOI] [PubMed] [Google Scholar]
- RAMOND M.J., COMOY E., LEBREC D. Alterations in isoprenaline sensitivity in patients with cirrhosis: evidence of abnormality of the sympathetic nervous activity. Br. J. Clin. Pharmacol. 1986;21:191–196. doi: 10.1111/j.1365-2125.1986.tb05174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., HARRIS D., KENDALL D.A., RALEVIC V. Cardiovascular effects of cannabinoids. Pharmacol. Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- RAYES N., BECHSTEIN W.O., KECK H., BLUMHARDT G., LOHMANN R., NEUHAUS P. Cause of death after liver transplantation: an analysis of 41 cases in 382 patients. Zentralbl. Chir. 1995;120:435–438. [PubMed] [Google Scholar]
- ROS J., CLARIA J., TO-FIGUERAS J., PLANAGUMA A., CEJUDO-MARTIN P., FERNANDEZ-VARO G., MARTIN-RUIZ R., ARROYO V., RIVERA F., RODES J., JIMENEZ W. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- ROSS R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., STEVENSON L.A., MURPHY V.L., TEMPLETON F., MAKRIYANNIS A., PERTWEE R.G. Agonist–inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ-DEL-ARBOL L., URMAN J., FERNANDEZ J., GONZALEZ M., NAVASA M., MONESCILLO A., ALBILLOS A., JIMENEZ W., ARROYO V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- SHAH V. Portal hypertension and the hyperdynamic circulation: nitric oxide in a haze of cannabinoid smoke. Hepatology. 2001;34:1060–1061. doi: 10.1053/jhep.2001.0341060. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- WONG H., ANDERSON W.D., CHENG T., RIABOWOL K.T. Monitoring mRNA expression by polymerase chain reaction: the primer-dropping method. Anal. Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]