Abstract
Oregonin isolated from Alnus formosana is a diarylheptanoid derivative, which appears to have antioxidative and anti-inflammatory activities. In this study, our data demonstrated inhibitory actions of oregonin on the LPS-induced iNOS protein in RAW264.7 macrophages and BV-2 microglial cells. We also suggested that HO-1 induction by oregonin might contribute to this action.
Oregonin is able to dose-dependently reduce NO production, iNOS protein and iNOS promoter activity stimulated by LPS in RAW264.7 and BV-2 cells.
Oregonin also showed inhibition of LPS-mediated NF-κB promoter activity and DNA-binding ability, as well as p65 nuclear translocation and phosphorylation. However, oregonin had no effect on IKK activity. AP-1 promoter activity and p38 MAPK activation but not PKC, ERK and JNK activation induced by LPS were attenuated by oregonin.
Accompanying with iNOS protein reduction, moreover, we found that oregonin was able to induce HO-1 protein level. Results using a CO donor, [Ru(CO)3Cl2]2 further showed the ability of CO in reduction of iNOS protein level induced by LPS through the blockade of NF-κB and AP-1.
Taken together, these results provide new evidences into the anti-inflammatory actions of oregonin, which include the inhibition of iNOS gene transcription via suppressing transcriptional activity of NF-κB and AP-1, as well as the upregulation of anti-inflammatory molecule HO-1. The HO-1-derived CO may also be involved in the suppressive effect on iNOS gene regulation.
Keywords: Oregonin, iNOS, HO-1, LPS, macrophage, microglia
Introduction
Nitric oxide (NO) is a free radical produced from L-arginine in a reaction catalyzed by nitric oxide synthase (NOS) (Nathan, 1992). NO derived from inducible NOS (iNOS) in lipopolysaccharide (LPS)-activated macrophages and microglia is implicated in inflammation and cytotoxicity (Nathan & Xie, 1994). Many different transcription factors participate in the regulation of the iNOS promoter (Baeuerle & Baltimore, 1988; 1996). While iNOS induction by LPS, the binding of nuclear factor-κB (NF-κB) to its specific cognate κB sites has been shown to be functionally important. In addition, the participation of activator protein-1 (AP-1) in iNOS promoter activation was also reported (Marks-Konczalik et al., 1998). Accumulating lines of evidences indicate that IκB kinase (IKK)-dependent IκB degradation is prerequisite for NF-κB activation, and p38 MAPK, ERK and PKC were also involved in NF-κB activation and iNOS expression in response to LPS (Lee et al., 1994; Da Silva et al., 1997; Lahti et al., 2000; Chen & Lin, 2001).
Heme oxygenase (HO) is the rate-limiting enzyme in the catabolism of excess heme and generation of biliverdin, iron, and carbon monoxide (CO) (Maines, 1988). As a redox-sensitive inducible protein, HO-1 has been implicated in serving as a protective gene due to its abilities in antioxidant, anti-inflammatory and antiapoptotic actions (Maines, 1997; Morse & Choi, 2002; Otterbein et al., 2003). Accumulating study indicated that both mice and human deficiency in HO-1 expression have a phenotype of an increased inflammatory state (Poss & Tonegawa, 1997; Yachie et al., 1999; Slebos et al., 2003). In addition, it has been reported that HO-1 induction negatively regulates iNOS expression (Lin et al., 2003). Several lines of evidence also demonstrated that downregulation of iNOS-dependent NO production by HO-1 appears to be mediated by the product CO (Colville-Nash et al., 1998; Sumi & Ignarro, 2004; Yang et al., 2004).
Oregonin [(5S)-1,7-bis-(3,4-dihydroxyphenyl)-heptane-3-one-5-O-b-D-xy-lopyranoside], a diarylheptanoid derivative, was found to be the main ingredient in leaves of Alnus formosana (Priyadarsini et al., 2003; Youssef et al., 2004). Many evidences indicated that these naturally occurring diarylheptanoids retain antioxidative and anti-inflammatory properties, which appear to contribute to their chemopreventive activity (Surh, 1999; Cho et al., 2002; Priyadarsini et al., 2003; Youssef et al., 2004). Regarding to anti-inflammation using cell model to investigate the action on NO, conflict results, however, were obtained. In murine thioglycolate-induced inflammatory macrophages oregonin was able to increase NO production (Joo et al., 2002), while in murine RAW264.7 macrophages stimulated with LPS plus interferon-γ (IFN-γ), NO synthesis was inhibited (Lee et al., 2000). The latter effect was due to the suppression of iNOS mRNA expression. Up to date the precise mechanisms responsible for the inhibition of iNOS by oregonin remain unclear.
In this study, we examined the effects of oregonin on LPS-stimulated iNOS gene expression in murine RAW264.7 macrophages and BV-2 microglia, and demonstrated that it could inhibit LPS-induced NF-κB, AP-1, and p38 MAPK activation, thereby inhibiting iNOS gene expression. Furthermore, our present data indicated that HO-1 induction in response to oregonin might also contribute to the reduction of iNOS gene expression.
Methods
Materials
Dulbecco's modified Eagle's medium (DMEM) with high glucose, FBS, penicillin, and streptomycin were obtained from Life Technologies (Grand Island, NY, U.S.A.). Rabbit polyclonal antibodies for β-actin, iNOS, IκBα, IKKα, IKKβ, p38 MAPK, ERK, JNK, PKCα, and HO-1, and protein A/G agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Rabbit polyclonal antibodies against active phosphorylated ERK1/2, p38 MAPK, JNK, p65 and PKC (pan) were purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). Horseradish peroxidase-coupled anti-mouse and anti-rabbit antibodies and the ECL detection agent were purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.). [γ-32P]dATP (3000 Ci mmol−1) and [α-32P]ATP (5000 Ci mmol−1) were obtained from NEN (Boston, MA, U.S.A.). The double-stranded oligonucleotide specific for NF-κB binding was synthesized as we previously reported (Huang et al., 2003). Phenol-extracted LPS (L8274) from Escherichia coli, and other chemicals were obtained from Sigma-Aldrich (St Louis, MO, U.S.A.). All materials for SDS–PAGE were obtained from Bio-Rad (Hercules, CA, U.S.A.). The iNOS promoter–luciferase reporter plasmid containing binding sites for AP-1 and NF-κB, which are required for maximal responses to LPS, was provided by Dr C.K. Glass (Department of Medicine, University of California, San Diego, CA, U.S.A.). The pGL2–ELAM–luciferase construct (κB-Luc) under the control of three NF-κB-binding sites was constructed. The AP-1-luciferase construct was provided by Dr G. Haegeman (Flanders Interuniversity Institute for Biotechnology and University of Gent, Gent, Belgium). β-Galactosidase expression vector (pCR3lacZ) was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden).
Isolation of oregonin from leaves of A. formosana
The methaolic extract of the dry powder of leaves of A. formosana Burk (Betulaceae) (4.1 kg) was divided into fractions soluble in hexane, chloroform, n-butanol (293 g) and water by liquid–liquid partitioning. Part of n-BuOH soluble fraction (10 g) was fractionated via a Sephadex LH-20 column (MeOH) to give an oregonin-rich fraction (2.10 g), which was repeatedly purified via centrifugal partition chromatography (CPC, model CPC-LLN, Sanki Engineering Ltd, Kyoto, Japan). The mobile phases for CPC were the organic layer of CHCl3-MeOH-H2O (2 : 2 : 1) or CHCl3-MeOH-H2O-i-PrOH (10 : 10 : 5 : 1) initially to remove the less polar constituents and then the aqueous layer to separate the polar ingredients, which contained oregonin. Through this procedure, oregonin (165 mg), which was TLC and 1H NMR essentially pure and showed identical physical data to those reported (Aoki et al., 1990), was obtained.
Cell culture
Murine RAW264.7 macrophages and BV-2 microglia were grown at 37°C in 5% CO2 using DMEM as the culture medium. DMEM was supplemented with 10% FBS, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. In most conditions for NO measurement and iNOS protein assay, cells were 30 min pretreated with 10–100 μM oregonin, followed by 0.1 μg ml−1 (in BV-2) or 1 μg ml−1 (in RAW264.7) LPS for 24 h, unless otherwise indicated.
Nitrite assay
Nitrite production, as an assay of NO release, was measured. Accumulation of nitrite in the medium was determined by a colorimetric assay with Griess reagent. Cells were treated with vehicle or LPS at the indicated concentrations for different intervals. Aliquots of conditioned medium were mixed with an equal volume of Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine in 5% phosphoric acid). Nitrite concentrations were determined by comparison with the OD550 using standard solutions of sodium nitrite prepared in cell culture medium. Each experiment was performed in duplicate and repeated at least three times.
Western blot analysis
After incubation with agents, cells were washed twice in ice-cold PBS and then solubilized in buffer containing 1% Triton X-100, 125 mM NaCl, 1 mM MgCl2, 25 mM β-glycerophosphate, 50 mM NaF, 100 μM sodium orthovanadate, 1 mM PMSF, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, and 20 mM Tris-HCl, pH 7.5. Samples of equal amounts of protein (80 μg) were separated through 8–12% SDS–polyacrylamide gels. After electrophoresis, proteins were transferred to nitrocellulose paper by semidry electrophoretic transfer. Membranes were blocked in TBS containing 0.1% Tween 20 (TBST) and 5% nonfat milk for 1 h. Proteins were visualized by specific primary antibodies, followed by peroxidase-labeled secondary antibodies. Immunoreactivity was detected by ECL reagents following the manufacturer's instructions, with exposure to X-ray film and quantification by densitometer.
Preparation of nuclear extracts and electromobility gel shift assay
Nuclear extracts were prepared as we previously described (Huang et al., 2003). Equal protein amount of nuclear extracts was incubated in binding reaction mixtures (15 μl) contained 0.25 μg of poly(dI-dC) (Amersham Pharmacia Biotech) and 20,000 dpm of 32P-labeled DNA probe in binding buffer consisting of 10 mM Tris (pH 7.5), 1 mM EDTA, 4% Ficoll, 1 mM DTT, and 75 mM KCl. The binding reaction was started by the addition of cell extracts (10 μg) and was continued for 30 min at room temperature. The DNA–protein complex was resolved from free oligonucleotide by electrophoresis in a 5% polyacrylamide gel. The gels were dried and exposed to X-ray films.
Transfection and reporter gene assay
Using Lipofectamine 2000 (LF2000; Life Technologies), cells were cotransfected with 0.5 μg plasmids of iNOS promoter–luciferase reporter, pGL2–ELAM–luciferase (κB-Luc), or AP-1-luciferase, together with 1 μg β-galactosidase-expression vector. After a 24-h incubation, transfection was complete, and cells were incubated with the indicated agents. After another 24-h incubation, the media were removed, and cells were washed once with cold PBS. To prepare lysates, 50 μl of reporter lysis buffer (Promega, Madison, WI, U.S.A.) was added to each well, and cells were scraped from dishes. The supernatant was collected after centrifugation at 13,000 rpm for 30 s. Aliquots of cell lysates (5 μl) containing equal amounts of protein (10–20 μg) were placed into wells of an opaque black 96-well microplate. An equal volume of luciferase substrate (Promega) was added to all samples, and luminescence was measured in a microplate luminometer (CT, Meriden, U.S.A.). The luciferase activity value was normalized to transfection efficiency monitored by the cotransfected β-galactosidase-expression vector. The level of induction of luciferase activity was determined as a ratio compared with control response without tested compound treatment.
Immunoprecipitation and kinase assay
After stimulation, cells were washed twice in ice-cold PBS, lysed in 1 ml of lysis buffer containing 20 mM Tris (pH 7.5), 1 mM MgCl2, 125 mM NaCl, 1% Triton X-100, 1 mM PMSF, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 25 mM β-glycerophosphate, 50 mM NaF, and 100 μM sodium orthovanadate. After centrifugation, the supernatant was collected, and then anti-IKKα/β and protein A/G-agarose beads (20 μl; Santa Cruz Biotechnology) were added at 4°C overnight. The precipitates were washed three times with lysis buffer and twice with kinase buffer (25 mM HEPES pH 7.5, 20 mM MgCl2, 100 μM sodium orthovanadate, and 2 mM DTT). The immune complex kinase assay of one-half of the immunoprecipitates was performed at 30°C for 30 min in 20 μl kinase reaction buffer containing 25 μM ATP, 5 μCi [γ-32P]ATP and 1 μg GST-IκBα as a substrate. Laemmli's-loading buffer was added to stop the reaction, and samples were resolved on SDS–PAGE followed by autoradiography. The other half of the immunoprecipitates was subjected to SDS–PAGE and immunoblotting to verify that an equal amount of kinase was undergoing the kinase reaction.
Infection with HO-1 adenovirus
The recombinant adenovirus carrying human HO-1 gene (Adv-HO-1) and replication-defective empty adenovius (Adv) were prepared as previously described (Juan et al., 2001) and provided by Dr L.Y. Chau (Division of Cardiovascular Research, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan). Upon infection of indicated titers of adenoviral vectors, cells were cultured in serum-free DMEM. Following 2 h incubation, an equal volume of complete medium was added to each culture and the incubation continued for an additional 22 h.
Statistical evaluation
Values were expressed as the mean±s.e.m. of at least three experiments, which were performed in duplicate. Analysis of variance was used to assess the statistical significance of the differences, and a ‘P'-value of less than 0.05 was considered statistically significant.
Results
Inhibitory effects of oregonin on NO production and iNOS gene transcription in activated macrophages and microglia
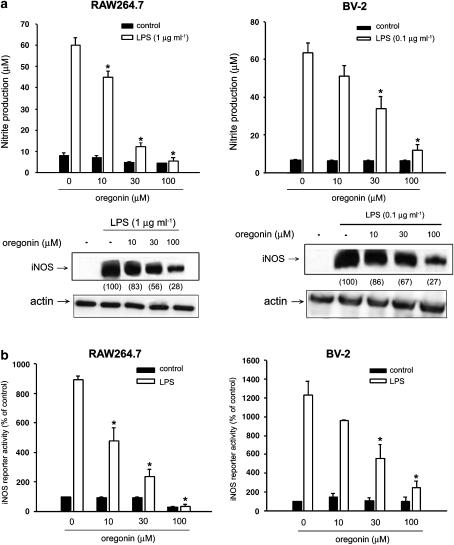
Prior to our studies on the effects of oregonin in RAW264.7 macrophages and BV-2 microglia, MTT assay was performed to determine if oregonin has cytotoxicity in these two cell lines. Results showed that oregonin at concentration up to 100 μM had no effects on cell viability (data not shown). LPS at 1 μg ml−1 and 0.1 μg ml−1 was, respectively, used in RAW264.7 and BV-2 cells, because of the maximal NO response and without cell toxicity. In RAW264.7 macrophages and BV-2 microglia, cotreatment with oregonin (10–100 μM) led to a concentration-dependent inhibition of both NO production and iNOS protein level stimulated by LPS (Figure 1a). To further identify whether the oregonin inhibition of iNOS protein induction occurs at the transcriptional level, promoter activity of iNOS as reflected by the reporter assay was determined. Results revealed that oregonin was able to decrease LPS-induced iNOS reporter activity in both cell types (Figure 1b), suggesting the inhibitory action on gene transcription. Also, given that serum may alter cell response to several agents including LPS, we tested the effects of oregonin (10–100 μM) on NO production in response to LPS in serum-deprived macrophages. We observed that the NO inhibitory action of oregonin was not affected (data not shown).
Figure 1.
Oregonin inhibits LPS-induced NO production and iNOS expression in RAW264.7 macrophages and BV-2 microglia. (a) RAW264.7 macrophages and BV-2 microglia were pretreated with oregonin at concentrations indicated for 30 min followed by the stimulation with LPS (1 μg ml−1 in RAW264.7 and 0.1 μg ml−1 in BV-2) for 24 h. The stable NO product nitrite in the culture medium and iNOS protein expression in cell lysates were measured. The numbers in parentheses express the percentages of iNOS protein abundance measured by densitometry and calculated as compared to the LPS-induced response without oregonin treatment. Actin protein abundance was considered internal control. (b) Cells transfected with iNOS-luciferase reporter gene and pCR3-LacZ plasmid for internal control were treated with indicated concentrations of oregonin and LPS. After 24 h, cells were harvested and luciferase assays were performed. Data represented the mean±s.e.m. of at least three independent experiments. *P<0.05, significant inhibition of LPS response by oregonin.
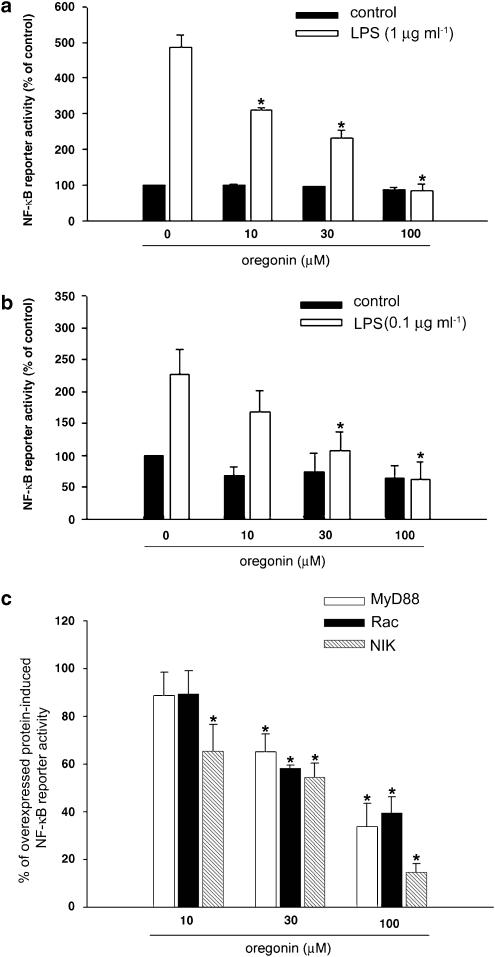
Oregonin inhibits NF-κB, but does not affect IKK activity
Previous studies have demonstrated that the transcription factor NF-κB is essential for iNOS transcription stimulated by LPS, thus we tested whether oregonin could interfere LPS-induced NF-κB activity. First, using luciferase reporter gene driven by NF-κB binding, our results indicated that the NF-κB activation induced by LPS in RAW264.7 (Figure 2a) and BV-2 (Figure 2b) cells was both attenuated by oregonin treatment. Second, to determine which signaling step for NF-κB activation in response to LPS is modulated by oregonin, we determined the effects on NF-κB activation initiated by MyD88, Rac, and NIK. When RAW264.7 cells were transfected with plasmids expressing above proteins, NF-κB activation were, respectively, increased to 267, 347, and 970% of control. As shown in Figure 2c, oregonin treatment was able to attenuate the responses of MyD88, Rac, and NIK in a dose-dependent manner.
Figure 2.
Oregonin inhibits NF-κB promoter activity. RAW264.7 (a) and BV-2 (b) cells transfected with NF-κB reporter gene and pCR3-LacZ were pretreated with oregonin at the indicated concentrations for 30 min followed by LPS (1 μg ml−1 in RAW264.7 and 0.1 μg ml−1 in BV-2) for 24 h. Luciferase activity was determined, normalized with LacZ expression, and represented as percentages of control response without LPS and oregonin treatment. In (c) RAW264.7 macrophages were cotransfected with NF-κB reporter gene, pCR3-LacZ and either MyD88, Rac, or NIK-expression plasmid. After 24 h transfection, cells were treated with oregonin for another 24 h. Net increase of reporter activity induced by overexpressed protein in the absence of oregonin was considered as 100%. Data represented the mean±s.e.m. of at least three independent experiments. *P<0.05, significant inhibition of LPS or overexpressed protein response by oregonin.
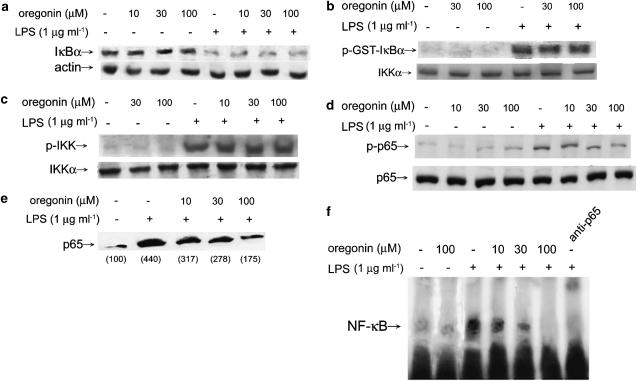
Given that the ability of activated IKK to enhance IκB phosphorylation and subsequently leading to its degradation plays an important role in LPS-mediated NF-κB activation, we examined the effects of oregonin on IκB degradation and IKK activation in RAW264.7 macrophages. Immunobloting results in Figure 3a revealed that oregonin incubation did not decrease IκB degradation caused by LPS. To confirm this result, we tested IKK activity by in vitro phosphorylation of the specific substrate, GST-IκBα, and we found that oregonin did not change IKK activity (Figure 3b). Moreover, since IKK activation leads to IKK autophosphorylation, we performed immunobloting with specific antibody for phosphorylated IKK. Consistently, the result showing no effect of oregonin in this action was observed (Figure 3c). To address the mechanism of oregonin on NF-κB inhibition, we further investigated whether oregonin could perturb nuclear translocation and DNA-binding ability of NF-κB. For this purpose, immunobloting of phosphorylated p65, nuclear p65 level and gel mobility shift assay analyzing DNA binding of transcription factor were performed. Data shown in Figure 3d–f indicate that the LPS-mediated these three events were, respectively, diminished by oregonin within 10–100 μM in a concentration-dependent manner.
Figure 3.
Effects of oregonin on LPS-induced IκBα degradation, IKK activation, p65 nuclear translocation, p65 phosphorylation, and NF-κB activation in RAW264.7 cells. Cells were treated with LPS (1 μg ml−1) with or without oregonin for 30 min. Protein levels of IκBα (a), phosphorylated IKK (c) and phosphorylated p65 (d) was determined by immunoblot using specific antibodies. The numbers in parentheses express the percentages of p65 measured by densitometry and calculated as compared to the LPS-induced response without oregonin treatment. In some experiments in vitro IKK activity assay was determined in immunoprecipitation complex of IKK using GST-IKKα as a substrate (b). In (e), nuclear fraction was prepared to determine p65 protein level. In (f), NF-κB-DNA-binding assay was performed using nuclear extracts probing with specific oligonucleotide containing conserved binding sequences for NF-κB. Specific antibody for p65 was included in the binding solution to indicate the specific binding. Results are representative of three independent experiments.
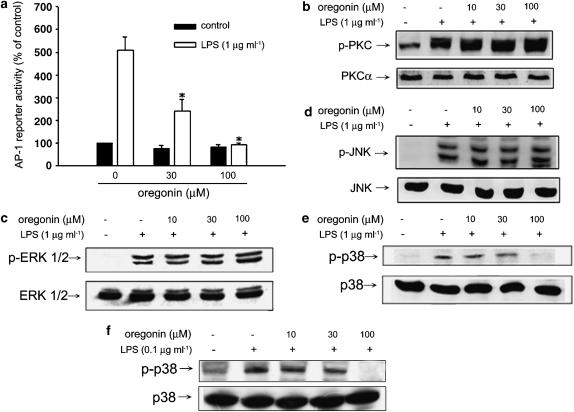
Oregonin attenuates LPS-mediated AP-1 and p38 MAPK activation
Since AP-1 participates in the upregulation of iNOS gene transcription, we determined the effect of oregonin in this event. Reporter assay driven by AP-1 transactivation in RAW264.7 macrophages revealed the ability of oregonin in inhibition of AP-1 (Figure 4a). In additional experiments, we tested the effects of oregonin on activation of PKC and MAPKs, which are well-known upstream-signaling kinases for the induction of AP-1 transcription. Determining kinase phosphorylation by immunoblot as an index of enzyme activity, we found that oregonin at concentrations up to 100 μM did not inhibit LPS-stimulated PKC (Figure 4b), ERK (Figure 4c), and JNK (Figure 4d) phosphorylation in RAW264.7 macrophages, but significantly inhibited p38 MAPK phosphorylation at 100 μM (Figure 4e). Similar inhibition of p38 MAPK phosphorylation was observed in BV-2 cells (Figure 4f).
Figure 4.
Oregonin reduces LPS-induced AP-1 activation via p38 inhibition. (a) RAW264.7 cells were transfected with AP-1 reporter gene and pCR3-LacZ plasmid as internal control. After incubation overnight, cells were treated with indicated concentrations of oregonin 30 min prior to the stimulation with LPS for another 24 h. Luciferase activity assay was determined and normalized by LacZ. Data represented the mean±s.e.m. of at least three independent experiments. *P<0.05, significant inhibition of LPS response by oregonin. In some experiments, RAW264.7 (b–e) and BV-2 cells (f) were treated with oregonin and/or LPS as indicated for 20 min. Immunobloting was carried out to measure total and phosphorylated PKC (b), ERK (c), JNK (d), and p38 MAPK (e, f). Results are representative of three independent experiments.
Oregonin-induced HO-1 protein expression participates in the reduction of iNOS gene expression
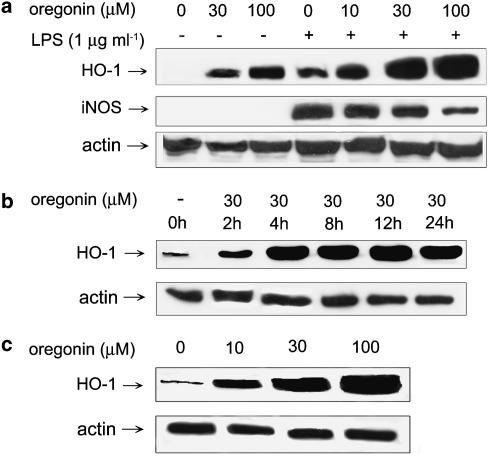
As HO-1 induction participates in the negative regulation of iNOS expression, we attempted to determine whether oregonin has such action. Figure 5a and c showed that in oregonin (10–100 μM)-treated RAW264.7 and BV-2 cells, HO-1 protein was dose-dependently induced. LPS also induced a moderate increase of HO-1 protein level and this response was additive to oregonin. Time course analysis in RAW264.7 cells revealed that the onset for HO-1 induction by 30 μM oregonin was around 2 h and this response achieved maximum at 4 h (Figure 5b). Along with the concentration-dependent increase of HO-1, LPS-induced increase of iNOS protein level was accordingly attenuated by oregonin.
Figure 5.
Oregonin induces HO-1 expression in RAW264.7 macrophages and BV-2 microglia. RAW264.7 (a, b) and BV-2 (c) cells were treated with oregonin at concentrations indicated and/or LPS (1 μg ml−1) for 24 h (a, c) or indicated periods (b). Cell lysates were harvested to determine the protein level of HO-1 and iNOS. Actin protein level was considered an internal control. Results are representative of three independent experiments.
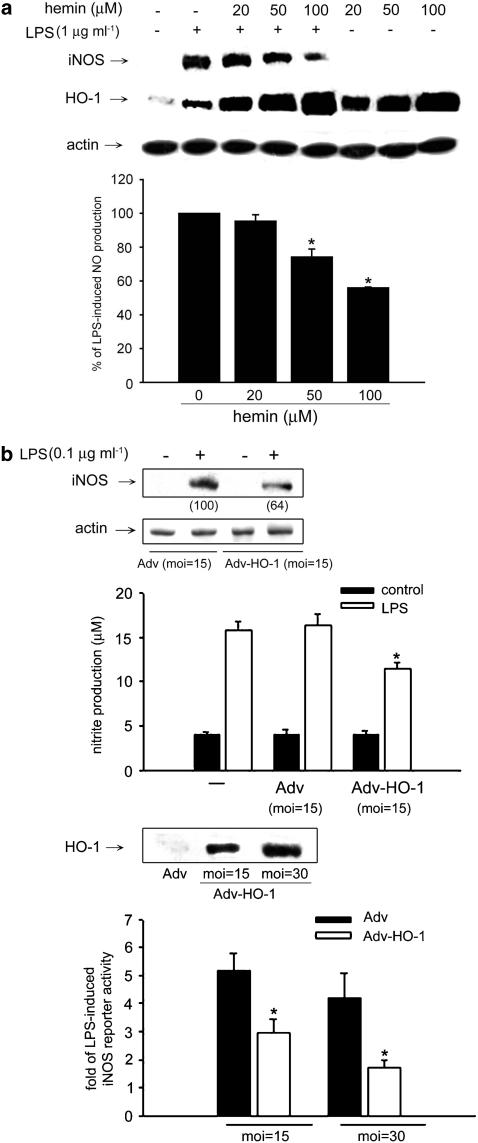
In order to further clarify the contribution of HO-1 in oregonin-mediated iNOS regulation, we tested the action of hemin, which is a potent HO-1 inducer. Results in Figure 6a showed a concentration-dependent effect of hemin (20–100 μM) on inhibition of LPS-induced NO production. Immunoblotting with HO-1 antibody also confirmed the strong effect of hemin on HO-1 induction. Accordingly LPS-induced iNOS protein level was reduced by hemin, which is in parallel with the high expression of HO-1 in hemin plus LPS-stimulated condition. Second, we used adenovirus-mediated gene transfer method to overexpress HO-1 protein (Figure 6b). Compared to empty vector, we found LPS-induced NO production and iNOS protein expression were inhibited in cells infected with HO-1 adenovirus (Figure 6b). iNOS promoter assay further showed the MOI-dependent inhibition (Figure 6b). All these results raise the possibility that HO-1 induction might participate in the action of oregonin for iNOS reduction. Since HO-1 inhibitor ZnPPIX alone would interrupt LPS-induced NO response independent of HO-1 inhibition (our unpublished data), this compound is not suitable for use in the purpose of blocking HO-1 activity and evaluating the role of HO-1 activation in oregonin inhibition of iNOS induction by LPS.
Figure 6.
Increased HO-1 expression leads to the inhibition of iNOS gene expression in RAW264.7 macrophages. (a) Cells were treated with hemin at the concentrations indicated and LPS (1 μg ml−1) for 24 h. (b) Cells infected with or without indicated MOI of empty Adv or Adv-HO-1 for 24 h were stimulated by LPS (0.1 μg ml−1) for 24 h. Protein abundance of iNOS, HO-1, and β-actin, nitrite production, and promoter activity of iNOS were, respectively, determined by immunoblotting, Griess reaction and reporter assay, respectively. The numbers in parentheses in (b) (upper panel) express the percentages of iNOS protein abundance measured by densitometry and calculated as compared to the LPS-induced response with empty Adv infection. *P<0.05, significant inhibition of LPS response by hemin or Adv-HO-1.
Effects of CO on LPS-induced iNOS, NF-κB and AP-1 activation
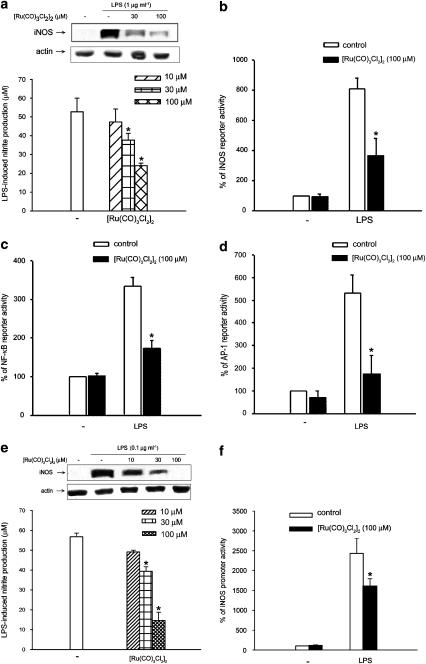
We examined the effect of CO on LPS-stimulated NO production and iNOS protein increase by using the tricarbonyldichororuthenium(II) dimer, [Ru(CO)3Cl2]2, a CO-releasing compound (Motterlini et al., 2002), to serve as the CO donor. As shown in Figure 7a, the LPS-induced nitrite production after 24 h incubation was inhibited by [Ru(CO)3Cl2]2 in a concentration-dependent manner. In addition, the accompanied iNOS protein induction was inhibited in parallel. To explore whether the suppressive action of [Ru(CO)3Cl2]2 on iNOS protein is resulting from the reduced iNOS gene transcription, we measured the effects of [Ru(CO)3Cl2]2 on LPS-induced iNOS promoter activity and on essential transcription factors of iNOS gene expression, NF-κB and AP-1. We found that [Ru(CO)3Cl2]2 treatment at 100 μM suppressed LPS-stimulated iNOS (Figure 7b), NF-κB (Figure 7c) and AP-1 (Figure 7d) promoter activities by 58, 70, and 78%, respectively. In BV-2 cells, [Ru(CO)3Cl2]2 still was able to reduce LPS-induced NO production, iNOS protein level (Figure 7e) and iNOS promoter activity (Figure 7f).
Figure 7.
CO donor inhibits LPS-induced iNOS expression, NF-κB, and AP-1 promoter activity. RAW264.7 (a–d) and BV-2 (e, f). Cells were pretreated with [Ru(CO)3Cl2]2 at the indicated concentrations for 30 min followed by the stimulation with LPS (1 μg ml−1 in RAW264.7 cells and 0.1 μg ml−1 in BV-2 cells) for 24 h. Nitrite production, iNOS protein expression (a, e), iNOS promoter activity (b), NF-κB reporter activity (c) and AP-1 reporter activity (d) were determined as previously described. Data represented the mean±s.e.m. of at least three independent experiments. *P<0.05, significant inhibition of LPS response by [Ru(CO)3Cl2]2.
Discussion
Although previous studies have shown that oregonin, a natural diarylheptanoid compound isolated from A. formosana, acts as an inhibitor of NO production in RAW264.7 macrophages, the mechanism by which oregonin regulates iNOS expression is still unknown. In this study, we explored the effect of oregonin in the regulation of iNOS gene expression in RAW264.7 macrophages and BV-2 microglia. Here we not only confirm the inhibitory effect of oregonin on NO production and iNOS expression induced by LPS in two cell types, but also provide data supporting its inhibition on transcriptional level via NF-κB and AP-1 inhibition as well as HO-1 induction.
It is well known that LPS positively regulates NF-κB for iNOS gene expression (Zhang et al., 2003). In unstimulated cells, NF-κB exists in the cytosol in a quiescent form bound to its inhibitory protein, IκB (Baeuerle & Baltimore, 1988). After stimulating cells with various agents, IκB becomes phosphorylated and goes to subsequent proteolytic degradation. Releasing IκB from NF-κB allows the nuclear accumulation and activation of NF-κB subunits. To investigate the mechanism underlying inhibition of NF-κB activity by oregonin in RAW264.7, we individually transfected construct plasmids encoding MyD88, Rac, and NIK. In consistent with notion that these molecules sequentially transduce the signaling pathways elicited by LPS for NF-κB activation (Sanlioglu et al., 2001; Chang et al., 2002; Chen et al., 2002), we herein detected a significant stimulation of NF-κB reporter activity. Under these conditions, the inhibitory results of oregonin on NF-κB activation suggest that the target of oregonin is located downstream of NIK. Afterwards we began to dissect which downstream event(s) for NF-κB activation is affected by oregonin, that is, IKK activation, IκB degradation, p65 phosphorylation, p65 nuclear translocation, and DNA-binding ability of NF-κB. Our data ruled out the inhibition on IKK activation and IκBα degradation induced by LPS. However, we identified that p65 phosphorylation, activated nuclear translocation of p65, and binding of free NF-κB subunit to specific DNA element were inhibited. Although currently we do not have sufficient evidence to explain how oregonin inhibits p65 translocation, we suggest that p38 MAPK inhibition might contribute to p65 phosphorylation and subsequent NF-κB activation. Previous studies have proved the role of p38 MAPK in p65 phosphorylation, and thus increasing the transcriptional activity of NF-κB (Vanden Berghe et al., 1998; Jefferies & O'Neill, 2000).
AP-1 transcription factor is also involved in the induction of iNOS gene by LPS (Marks-Konczalik et al., 1998). In this study, we reported that oregonin treatment also significantly reduced AP-1 promoter activity. For AP-1 activation, signalings through three types of MAPKs and PKC were implicated in LPS-mediated AP-1 activation (Karin, 1995; Marks-Konczalik et al., 1998). Our current data showed that oregonin could inhibit p38 phosphorylation, but leave ERK, JNK, and PKC unaffected.
HO-1 expression induced by stress provides protective effect. The enzyme HO-1 has been shown to have potent anti-inflammatory effects. Cellular defense against NO-induced injury mediated by HO-1 has been demonstrated (Mosley et al., 1998; Datta et al., 1999). Actually, LPS-stimulated macrophages can protect themselves from triggered overproduction of superoxide anion and NO by enhancing HO-1 expression (Srisook & Cha, 2004). In addition, some studies indicated that HO-1 induction is involved in the inhibitory effects on LPS-induced NO production (Oh et al., 2004; Lin et al., 2005). Here we have found the ability of oregonin to induce HO-1 expression in RAW264.7 and BV-2 cells. Moreover, our current experiments using HO-1 inducer hemin as well as adenovirus-mediated gene transfer further proved that HO-1 overexpression in RAW264.7 cells stimulated with LPS suppressed the induction of iNOS protein. Based on present and previous results, we suggest that HO-1 induction contributes to the anti-inflammatory action of oregonin in iNOS inhibition.
Given that CO may play a critical role in protective effect of HO-1 (Otterbein et al., 2000; Fujita et al., 2001; Slebos et al., 2003), we examined the effect of CO on iNOS-derived NO production in RAW264.7 and BV-2 cells, indicating that CO suppressed NO production, iNOS protein, and iNOS promoter activity. Moreover, our data demonstrated that CO is an effective molecule to inhibit NF-κB and AP-1 activation upon LPS stimulation, leading to downregulation of iNOS gene expression and NO production. Supporting this data are previous findings showing the inhibition of NF-κB (Lee et al., 2003; Soares et al., 2004) and AP-1 (Morse et al., 2003) by HO-1.
To address whether the guanylate cyclase/cGMP pathway mediates effect of CO, we treated RAW264.7 cells with dBcGMP, an analogue of cGMP, following LPS treatment. We found that LPS-induced NO production was not significantly altered (data not shown), suggesting that the GC/cGMP-dependent signaling pathway was not involved in the downregulation of NO production induced by oregonin. Our findings were consistent with previous study (Otterbein et al., 2000), however, other models studied indicated that actions of CO were through GC/cGMP-dependent mechanism (Duckers et al., 2001; Song et al., 2003).
Modulation of gene transcription is the principal mechanism for HO-1 protein induction. Currently, numbers of pathways have been implicated in transmitting the extracellular signals to the nuclei for HO-1 gene expression. In general, HO-1 gene expression can be induced through signaling pathways, such as MAPKs (ERK, JNK, and p38 MAPK) (Immenschuh & Ramadori, 2000; Zhang et al., 2002; Wu et al., 2004), and cGMP/PKG (Polte et al., 2000; Eguchi et al., 2001; Chen et al., 2005). Currently, we are attempting to elucidate the signaling pathways involved in oregonin-mediated HO-1 protein induction, and clarify whether intracellular oxidative and nitrosative stresses might regulate this event. The latter point is based on the finding with LPS, which can through superoxide anion and NO production to upregulate HO-1 in RAW264.7 macrophages (Srisook & Cha, 2004).
In conclusion, in this study we demonstrate that natural product oregonin with antioxidant activity can inhibit LPS-induced iNOS gene transcription and upregulate HO-1 expression. Both actions are involved in the anti-inflammatory action of oregonin. p38 MAPK inhibition and CO production from HO-1 upregulation lead to the diminished NF-κB and AP-1 transactivation, and account for the anti-inflammation action.
Acknowledgments
This work was supported by the Grants (NSC92-2320-B-002-082; NSC93-2320-B-002-020; CCMP94-RD-030).
Abbreviations
- Adv-HO-1
recombinant adenovirus carrying human HO-1 gene
- AP-1
activator protein-1
- CO
carbon monoxide
- DMEM
Dulbecco's-modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- HO-1
heme oxygenase-1
- IFN-γ, interferon-γ; IKK
IκB kinase
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun-N-terminal kinase
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- NO
nitric oxide
References
- AOKI T., OHTA S., SUGA T. Triterpenoids, diarylhepanoids and their glycosides in the flowers of Alnus species. Phytochemistry. 1990;29:3611–3614. [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- CHANG Y.H., HSIEH S.L., CHEN M.C., LIN W.W. Lymphotoxin β receptor induces interleukin 8 gene expression via NF-κB and AP-1 activation. Exp. Cell Res. 2002;278:166–174. doi: 10.1006/excr.2002.5573. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LIN W.W. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br. J. Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN B.C., WU W.T., HO F.M., LIN W.W. Inhibition of interleukin-1β-induced NF-κB activation by calcium/calmodulin-dependent protein kinase kinase occurs through Akt activation associated with interleukin-1 receptor-associated kinase phosphorylation and uncoupling of MyD88. J. Biol. Chem. 2002;277:24169–24179. doi: 10.1074/jbc.M106014200. [DOI] [PubMed] [Google Scholar]
- CHEN J.C., HUANG K.C., LIN W.W.HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways Cell. Signal. 2005. in press [DOI] [PubMed]
- CHO S.M., KWON Y.M., LEE J.H., YON K.H., LEE M.W. Melanogenesis inhibitory activities of diarylheptanoids from Alnus hirsuta Turcz in B16 mouse melanoma cell. Arch. Pharm. Res. 2002;25:885–888. doi: 10.1007/BF02977009. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., QURESHI S.S., WILLIS D., WILLOUGHBY D.A. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J. Immunol. 1998;161:978–984. [PubMed] [Google Scholar]
- DA SILVA J., PIERRAT B., MARY J.L., LESSLAUER W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J. Biol. Chem. 1997;272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- DATTA P.K., KOUKOURITAKI S.B., HOPP K.A., LIANOS E.A. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J. Am. Soc. Nephrol. 1999;10:2540–2550. doi: 10.1681/ASN.V10122540. [DOI] [PubMed] [Google Scholar]
- DUCKERS H.J., BOEHM M., TRUE A.L., YET S.F., SAN H., PARK J.L., CLINTON WEBB R., LEE M.E., NABEL G.J., NABEL E.G. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- EGUCHI D., WEILER D., ALAM J., NATH K., KATUSIC Z.S. Protective effect of heme oxygenase-1 gene transfer against oxyhemoglobin-induced endothelial dysfunction. J. Cereb. Blood Flow Metab. 2001;21:1215–1222. doi: 10.1097/00004647-200110000-00010. [DOI] [PubMed] [Google Scholar]
- FUJITA T., TODA K., KARIMOVA A., YAN S.F., NAKA Y., YET S.F., PINSKY D.J. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by depression of fibrinolysis. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- HUANG K.C., CHEN C.W., CHEN J.C., LIN W.W. HMG-CoA reductase inhibitors inhibit inducible nitric oxide synthase gene expression in macrophages. J. Biomed. Sci. 2003;10:396–405. doi: 10.1007/BF02256431. [DOI] [PubMed] [Google Scholar]
- IMMENSCHUH S., RAMADORI G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- JEFFERIES C.A., O'NEILL L.A. Rac1 regulates interleukin 1-induced nuclear factor kappaB activation in an inhibitory protein kappaBalpha-independent manner by enhancing the ability of the p65 subunit to transactivate gene expression. J. Biol. Chem. 2000;275:3114–3120. doi: 10.1074/jbc.275.5.3114. [DOI] [PubMed] [Google Scholar]
- JOO S.S., KIM H.J., KWON H.S., LEE D.I. Augmentation of macrophage antitumor activities and nitric oxide production by oregonin. Arch. Pharm. Res. 2002;25:457–462. doi: 10.1007/BF02976602. [DOI] [PubMed] [Google Scholar]
- JUAN S.H., LEE T.S., TSENG K.W., LIOU J.Y., SHYUE S.K., WU K.K., CHAU L.Y. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- KARIN M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- LAHTI A., LAHDE M., KANKAANRANTA H., MOILANEN E. Inhibition of extracellular signal-regulated kinase suppresses endotoxin-induced nitric oxide synthesis in mouse macrophages and in human colon epithelial cells. J. Pharmacol. Exp. Ther. 2000;294:1188–1194. [PubMed] [Google Scholar]
- LEE J.C., LAYDON J.T., MCDONNELL P.C., GALLAGHER T.F., KUMAR S., GREEN D., MCNULTY D., BLUMENTHAL M.J., HEYS J.R., LANDVATTER S.W. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- LEE M.W., KIM N.Y., PARK M.S., AHN K.H., TOH S.H., HAHN D.R., KIM Y.C., CHUNG H.T. Diarylhepatonoids with in vitro inducible nitric oxide synthesis inhibitory acitivyt from Alnus hirsute. Planta Med. 2000;66:551–553. doi: 10.1055/s-2000-8606. [DOI] [PubMed] [Google Scholar]
- LEE T.S., TSAI H.L., CHAU L.Y. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J 2. J. Biol. Chem. 2003;278:19325–19330. doi: 10.1074/jbc.M300498200. [DOI] [PubMed] [Google Scholar]
- LIN H.Y., JUAN S.H., SHEN S.C., HSU F.L., CHEN Y.C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003;66:1821–1832. doi: 10.1016/s0006-2952(03)00422-2. [DOI] [PubMed] [Google Scholar]
- LIN H.Y., SHEN S.C., CHEN Y.C. Anti-inflammatory effect of heme oxygenase 1: glycosylation and nitric oxide inhibition in macrophages. J. Cell Physiol. 2005;202:579–590. doi: 10.1002/jcp.20160. [DOI] [PubMed] [Google Scholar]
- MAINES M.D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- MAINES M.D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- MARKS-KONCZALIK J., CHU S.C., MOSS J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J. Biol. Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- MORSE D., CHOI A.M. Heme oxygenase-1: the ‘emerging molecule' has arrived. Am. J. Respir. Cell Mol. Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- MORSE D., PISCHKE S.E., ZHOU Z., DAVIS R.J., FLAVELL R.A., LOOP T., OTTERBEIN S.L., OTTERBEIN L.E., CHOI A.M. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- MOSLEY K., WEMBRIDGE D.E., CATTELL V., COOK H.T. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998;53:672–678. doi: 10.1046/j.1523-1755.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- MOTTERLINI R., CLARK J.E., FORESTI R., SARATHCHANDRA P., MANN B.E., GREEN C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- NATHAN C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- NATHAN C., XIE Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- OH G.S., PAE H.O., CHOI B.M., CHAE S.H., LEE H.S., RYU D.G., CHUNG H.T. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 2004;320:1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., BACH F.H., ALAM J., SOARES M.P., TAO LU H., WYSK M., DAVIS R.J., FLAVELL R.A., CHOI A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., SOARES M.P., YAMASHITA K., BACH F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends. Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- POLTE T., ABATE A., DENNERY P.A., SCHRODER H. Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. Arterioscler. Thromb. Vasc. Biol. 2000;20:1209–1215. doi: 10.1161/01.atv.20.5.1209. [DOI] [PubMed] [Google Scholar]
- POSS K.D., TONEGAWA S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIYADARSINI K.I., MAITY D.K., NAIK G.H., KUMAR M.S., UNNIKRISHNAN M.K., SATAV J.G., MOHAN H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free. Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- SANLIOGLU S., WILLIAMS C.M., SAMAVATI L., BUTLER N.S., WANG G., MCCRAY P.B., RITCHIE T.C., HUNNINGHAKE G.W., ZANDI E., ENGELHARDT J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J. Biol. Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- SRISOOK K., CHA Y.N. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochem. Pharmacol. 2004;68:1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- SLEBOS D.J., RYTER S.W., CHOI A.M. Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respir. Res. 2003;4:7–15. doi: 10.1186/1465-9921-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOARES M.P., SELDON M.P., GREGOIRE I.P., VASSILEVSKAIA T., BERBERAT P.O., YU J., TSUI T.Y., BACH F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- SONG R., NING W., LIU F., AMEREDES B.T., CALHOUN W.J., OTTERBEIN L.E., CHOI A.M. Regulation of IL-1beta-induced GM-CSF production in human airway smooth muscle cells by carbon monoxide. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L50–L56. doi: 10.1152/ajplung.00212.2002. [DOI] [PubMed] [Google Scholar]
- SUMI D., IGNARRO L.J. Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes. 2004;53:1841–1850. doi: 10.2337/diabetes.53.7.1841. [DOI] [PubMed] [Google Scholar]
- SURH Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- VANDEN BERGHE W., PLAISANCE S., BOONE E., DE B.K., SCHMITZ M.L., FIERS W., HAEGEMAN G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- WU W.T., CHI K.H., HO F.M., TSAO W.C., LIN W.W. Proteasome inhibitors up-regulate haem oxygenase-1 gene expression: requirement of p38 MAPK (mitogen-activated protein kinase) activation but not of NF-κB (nuclear factor κB) inhibition. Biochem. J. 2004;379:587–593. doi: 10.1042/BJ20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YACHIE A., NIIDA Y., WADA T., IGARASHI N., KANEDA H., TOMA T., OHTA K., KASAHARA Y., KOIZUMI S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG N.C., LU L.H., KAO Y.H., CHAU L.Y. Heme oxygenase-1 attenuates interleukin-1beta-induced nitric oxide synthase expression in vascular smooth muscle cells. J. Biomed. Sci. 2004;11:799–809. doi: 10.1007/BF02254365. [DOI] [PubMed] [Google Scholar]
- YOUSSEF K.M., EL-SHERBENY M.A., EL-SHAFIE F.S., FARAG H.A., AL-DEEB O.A., AWADALLA S.A. Synthesis of curcumin analogues as potential antioxidant, cancer chemopreventive agents. Arch. Pharm. 2004;337:42–54. doi: 10.1002/ardp.200300763. [DOI] [PubMed] [Google Scholar]
- ZHANG X., BEDARD E.L., POTTER R., ZHONG R., ALAM J., CHOI A.M., LEE P.J. Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia–reperfusion lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L815–L829. doi: 10.1152/ajplung.00485.2001. [DOI] [PubMed] [Google Scholar]
- ZHANG X., SHAN P., OTTERBEIN L.E., ALAM J., FLAVELL R.A., DAVIS R.J., CHOI A.M., LEE P.J. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J. Biol. Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]