Abstract
Ethyl caffeate, a natural phenolic compound, was isolated from Bidens pilosa, a medicinal plant popularly used for treating certain inflammatory syndromes. The purpose of this study was to investigate the structural activity, and the anti-inflammatory functions and mechanism(s) of ethyl caffeate.
Ethyl caffeate was found to markedly suppress the lipopolysaccharide (LPS)-induced nitric oxide (NO) production (IC50=5.5 μg ml−1), mRNA and protein expressions of inducible nitric oxide synthase (iNOS), and prostaglandin E2 (PGE2) production in RAW 264.7 macrophages.
Transient gene expression assays using human cox-2 promoter construct revealed that ethyl caffeate exerted an inhibitory effect on cox-2 transcriptional activity in 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated MCF-7 cells.
Immunohistochemical studies of mouse skin demonstrated that TPA-induced COX-2 expression was significantly inhibited by ethyl caffeate with a superior effect to that of celecoxib, a nonsteroidal anti-inflammatory drug.
The phosphorylation and degradation of inhibitor κB (IκB) and the translocation of nuclear transcription factor-κB (NF-κB) into the nucleus, as well as the activation of mitogen-activated protein kinases (MAPKs) induced by LPS in macrophages, were not affected by ethyl caffeate. Ethyl caffeate, however, could inhibit NF-κB activation by impairing the binding of NF-κB to its cis-acting element. These results suggest that ethyl caffeate suppresses iNOS and COX-2 expressions partly through the inhibition of the NF-κB·DNA complex formation.
Structure–activity relationship analyses suggested that the catechol moiety and α,β-unsaturated ester group in ethyl caffeate are important and essential structural features for preventing NF-κB·DNA complex formation. This study provides an insight into the probable mechanism(s) underlying the anti-inflammatory and therapeutic properties of ethyl caffeate.
Keywords: Ethyl caffeate, Bidens pilosa, NF-κB, COX-2, iNOS, PGE2
Introduction
The nuclear factor-κB (NF-κB) is essential for host defense and inflammatory responses to microbial and viral infections (Li & Verma, 2002). In response to extracellular stimuli, such as bacterial lipopolysaccharide (LPS), tumor-necrosis factor-α (TNF-α), or other inflammatory mediators, the transcription factor NF-κB is often activated and subsequently facilitates the transcription of a number of genes involved in inflammation, such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and specific cytokines (Bayon et al., 2003). The inhibitor of κB (IκB) kinase (IKK) complex is a central element of NF-κB-related signaling. IKK phosphorylates NF-κB-bound IκB complexes at two conserved serine residues within the IκB N-terminal regulatory domain. This targets IκB for ubiquitin-dependent degradation and allows the liberated NF-κB dimers to be translocated to the nucleus and bind to cognate DNA enhancer sequences that lead to the transcription of various genes (Karin et al., 2002). The other major extracellular signal transduction pathway stimulated by inflammatory mediators is the mitogen-activated protein kinase (MAPK) pathway (Guha & Mackman, 2001). MAPKs are a family of serine/threonine protein kinases composed of the p44 and p42 isoforms (also known as extracellular signal receptor-activated kinase ERK1 and ERK2), p38, and c-Jun NH2-terminal kinase (JNK) (Nishida & Gotoh, 1993; Han et al., 1994). It has been found that LPS, the key mediator in the inflammation response, can induce activation of these MAPK proteins in macrophage and other cell types (Hambleton et al., 1996; Chen & Wang, 1999; Guha & Mackman, 2001). Activation of p38, but not p44/p42 MAPK, by LPS resulted in the stimulation of NF-κB-specific DNA–protein binding and the subsequent expression of iNOS and nitric oxide (NO) release in RAW 264.7 macrophages (Chen & Wang, 1999). NF-κB and MAPKs are therefore known as important targets for anti-inflammatory molecules.
Improper activation or upregulation of iNOS or COX-2 has been shown to be associated with the pathophysiologies of certain types of human cancers as well as inflammatory disorders (Beyaert, 2003). Therefore, the identification of naturally occurring phytocompounds that can suppress or downregulate the functions of iNOS or COX-2, or the activation of their upstream transcriptional factor NF-κB, may lead to the discovery of important anti-inflammatory therapeutics. Since inflammation is closely linked to the promotion of certain tumors, substances with potent anti-inflammatory activities are anticipated to exert chemopreventive effects on carcinogenesis (Surh et al., 2001). For instance, phenolic compounds, particularly those present in edible and medicinal plants, have been reported to possess substantial anticancer or cancer chemopreventive properties (Park & Pezzuto, 2002).
Bidens pilosa (Asteraceae), commonly known as ‘hairy beggar-ticks' or ‘Spanish needles', is widely distributed in tropical and subtropical regions, and has been reported to possess antihyperglycemic (Ubillas et al., 2000), antihypertensive (Dimo et al., 2001; 2002), antiulcerogenic (Tan et al., 2000), hepatoprotective (Chin et al., 1996), immunosuppressive and anti-inflammatory (Pereira et al., 1999), antileukemic (Chang et al., 2001), antimalarial (Brandão et al., 1997), and antimicrobial (Khan et al., 2001) properties. B. pilosa has traditionally or anecdotally been used for the management of inflammatory diseases, and stomach and liver disorders. However, the cellular and molecular mechanisms underlying the anti-inflammatory properties of B. pilosa extract and its derived active compound are currently not well defined. In our previous study, we identified an ethyl acetate (EA) fraction partitioned from the ethanolic extract of fresh whole B. pilosa plants that significantly inhibited the LPS-induced NO production in RAW 264.7 cells (IC50=36 μg ml−1) (Chiang et al., 2004). In the present study, a bioactive phytocompound, ethyl caffeate that exhibits potent inhibitory effects on NO production in macrophages was identified from the bioactive EA fraction using bioactivity-guided fractionation. Ethyl caffeate was then investigated for its anti-inflammatory mechanisms in vitro in LPS-stimulated macrophages, and in vivo using TPA-treated mouse skin system. The effects of ethyl caffeate on the activation of NF-κB, MAPKs, as well as on the downstream mediators of inflammation, such as iNOS, COX-2 and prostogladin E2 (PGE2), were investigated.
Methods
Materials
IR spectra were recorded on a Perkin-Elmer 983G spectrophotometer. 1H and 13C NMR spectra were obtained from a Varian Unity Plus 400 spectrometer. EIMS were obtained on a JEOL JMS-HX 300 mass spectrometer. The chemicals and reagents: aspirin, curcumin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ethyl cinnamate, ethyl 3,4-dihydroxyhydrocinnamate, catechol, 12-O-tetradecanoylphorbol-13-acetate (TPA), LPS, indomethacin, and DTT were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Celecoxib (CELEBREX™) was from PHARMACIA ((Packer) Pharmacia Ltd., Northumberland, U.K.). Silica gel (230–400 mesh) and silica gel 60 F254 TLC and RP-18 F254S TLC plates were purchased from Merck (Germany). RP-18 silica gel (75C18-OPN) was purchased from Cosmosil (Kyoto, Japan). All other chemicals and solvents used in this study were of HPLC grade.
Isolation and elucidation of structure of ethyl caffeate from B. pilosa extract
B. pilosa Linn. var. radiata (Asteraceae) was collected on the campus of Academia Sinica, Taiwan, in 2001 and a voucher specimen (No. 11519) deposited at the herbarium of the Institute of Botany, Academia Sinica, Taiwan. The fresh whole plant of B. pilosa was crushed to give 2.5 kg of raw material, which was extracted with 25 l of 70% (v v−1) ethanol at room temperature. The total crude extract was evaporated in vacuo to yield a residue (130 g) that was then suspended in water (1 l) and successively partitioned with ethyl acetate (1 l × 3 times) and n-butanol (1 l × 3 times), yielding EA, BuOH, and Water fractions. Each fraction was evaporated on a rotary evaporator under reduced pressure to remove organic solvent and then lyophilized until dry and weighed to determine the yields. The yields of the EA, BuOH, and Water fractions were 14.1, 15.2, and 69.0%, respectively, of the total crude extract in dry weight. On the basis of bioactivity-guided fractionation, a portion of the EA fraction (18.0 g) was subjected to column chromatography on silica gel, eluted with a CH2Cl2/MeOH gradient solvent system, to give a total of eight fractions (Fractions A–H). The Fraction E (4.3 g eluted from EA fraction with 5% CH2Cl2/MeOH) was further chromatographed on a silica gel column and eluted in a hexane/ethyl acetate gradient solvent system to give a total of nine fractions (Fractions E1–E9). Ethyl caffeate (372.8 mg) was isolated from the Fraction E4 (0.7 g) by RP-18 silica gel column elution with 50% MeOH/H2O. Ethyl caffeate: colorless solid; MP 147–149°C; IR νmax: 3448, 1673, 1595 cm−1; EI-MS m/z (rel intensity): 208 [M]+ (80), 180 (20), 163 (100); 1H and 13C NMR data (acetone-d6) in agreement with published data (Lamidey et al., 2002).
Cell line and cell culture
RAW 264.7 cells, a murine macrophage cell line, were obtained from the American Type Culture Collection (ATCC, MD, U.S.A.) and cultured as recommended by ATCC at 37°C in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin in a 5% CO2 incubator. Cells between 5 and 20 passages were used for experiments at a density of 1 × 106 cells ml−1 except for the NO production, PGE2 production, and MTT assays. MCF-7 cells obtained from ATCC (MD, U.S.A.) were grown in RPMI 1640 media, supplemented with 10% FBS, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin.
Measurement of NO and cell viability assays
RAW 264.7 cells were seeded in 96-well plates at a density of 2 × 105 cells well−1. The cells were treated with test compounds (0.1–20 μg ml−1) for 1 h and then incubated for 24 h in fresh DMEM with or without 1 μg ml−1 of LPS. Nitrite (a stable oxidative end product of NO) accumulation in the medium of RAW 264.7 cells was determined by the Griess reaction (Schmidt & Kelm, 1996). Briefly, 100 μl cell culture supernatants were reacted with 100 μl of Griess reagent (1 : 1 mixture of 0.1% N-(1-naphthyl)ethylenediamine in H2O and 1% sulfanilamide in 5% phosphoric acid) in a 96-well plate, and absorbance at 540 nm was recorded using an ELISA reader (Labsystems Multiskan MS). Results were expressed as total μM nitrite produced in vehicle control (untreated cells) or in cells treated with LPS over the 24 h period in the absence or presence of phytocompounds.
In parallel to the Griess assays, cell viabilities were determined using the MTT-based colorimetric assay as described elsewhere (Scudiero et al., 1988). Briefly, 1 × 104 RAW 264.7 cells treated for 24 h with vehicle or phytocompound were examined for cell viability. Viability of the macrophages treated with vehicle (0.5% DMSO) only was defined as 100% viable. Survival of macrophage cells after treatment with phytocompounds was calculated using the following formula: viable cell number (%)=OD570 (treated cell culture)/OD570 (vehicle control) × 100.
RT−PCR analysis
RAW 264.7 cells were seeded in six-well plates at a density of 3 × 106 cells well−1. The cells were treated with test compounds (0.5–5 μg ml−1) for 1 h and then incubated for 6 h in fresh DMEM with or without 1 μg ml−1 of LPS. After two washes with ice-cold PBS, the cells were harvested and total cellular RNA was isolated using TRIZOL® Reagent (Invitrogen) according to the manufacturer's instructions. For each RT−PCR reaction, 2 μg of total RNA was used to synthesise first-strand cDNA using SuperScript™ II Reverse Transcriptase (Invitrogen). For amplification of the iNOS gene, a pair of primers were used:
iNOS, 5′-CAGAAGCAGAATGTGACCATC-3′ (sense),
5′-CTTCTGGTCGATGTCATGAGC-3′ (antisense).
The cDNA sequence of GA3PDH as an internal control was also amplified using the following primers:
5′-CCATCAATGACCCCTTCATTGACC-3′ (sense),
5′-GAAGGCCATGCCAGTGAGCTTCC-3′ (antisense).
Parameters of PCR reactions were: 94°C for 2 min for one cycle, and then 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min for 30 cycles. The amplified PCR products were analyzed with 1% agarose gel electrophoresis, and visualized with ethidium bromide staining.
Western blot analysis
Total cellular proteins (for detecting iNOS, GA3PDH, actin, IκBα, p-SAPK/JNK, p-p38 MAPK, and p-p44/42 MAPK proteins) were prepared according to Lo et al. (2002). Cytosolic fraction and nuclear fraction (for PARP, α-tubulin, and NF-κB p65) of total proteins were prepared according to Natarajan et al. (1996) with minor modifications. Briefly, 1.0 × 107 cells were washed with cold phosphate-buffered saline (PBS) and suspended in 250 μl of hypotonic buffer (10 mM Hepes pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 2.0 μg ml−1 leupeptin, 2.0 μg ml−1 aprotinin, and 0.5 mg ml−1 benzamidine). The cells were allowed to swell on ice for 10 min, after which 20 μl of 2% Nonidet P-40 was added. The cells were then vortexed vigorously for 10 s, and the homogenate was centrifuged at 12,000 × g for 5 min at 4°C. The supernatants containing cytosolic proteins were collected. The nuclear pellets were resuspended in 50 μl ice-cold hypertonic buffer (20 mM Hepes, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, 2.0 μg ml−1 leupeptin, 2.0 μg ml−1 aprotinin, and 0.5 mg ml−1 benzamidine), and incubated on ice for 40 min with intermittent mixing. Samples were centrifuged at 12,000 g for 10 min at 4°C, and the supernatant (nuclear extract) was either used immediately or stored at −70°C. The protein content was measured by a dye-binding assay (Bio-Rad Protein Assay). Proteins (20 μg) were separated on a 5−20% gradient sodium dodecyl sulfate–polyacrylamide gel and then electrotransferred to polyvinylidene difluoride (PVDF) membrane (Immobilon, Millipore, Bedford, MA, U.S.A.). The blot was incubated in blocking buffer (3% w v−1 skim milk in TBS buffer) for 30 min, and then incubated, respectively, with monoclonal antibodies against PARP (Transduction Laboratories, Lexingtons, KY, U.S.A.), GA3PDH (Biogenesis, England, U.K.), actin, α-tubulin (Oncogene Science, Cambridge, U.K.), phosphorylated form of MAPK family (p42/44, SAPK/JNK, and p38) (New England Biolabs, Bevery, MA, U.S.A.), iNOS, IκBα, or NF-κB p65 (RelA) (Santa Cruz Biochemicals, Santa Cruz, CA, U.S.A.) overnight at 4°C. After incubation with the appropriate anti-rabbit, anti-goat, or anti-mouse IgG conjugated to horseradish peroxidase, the immunoreactive bands were visualized with the enhanced chemiluminescence reagents (ECL, Amersham Pharmacia Biotech, Amersham Place, U.K.).
Transfection of reporter gene constructs and luciferase assay
Chimeric luciferase reporter genes, pPGL3-Basic vector (Promega, Madison, WI, U.S.A.) containing the full length or a series of deleted 5′ flanking promoter regions of human cox-2 gene were constructed. A specific reverse primer (antisense), −1/−28,
5′-TATATAAGCTTCGCAGCGGCGGGCAGGG-3′ and four forward primers (sense),
5′-GGCGCGGTACCGGAGAGGAGGGAAAAAT-3′;
5′-GGCGCGGTACCCTGGGTTTCCGATTTTC-3′;
5′-ATTCGGGTACCCCCGACGTGACTTCCTC-3′; and
5′-ACCATGGTACCTTCCAGCTGTCAAAATC-3′
were used for the amplification of −247/−1, −320/−1, −646/−1, and −1334/−1 (full length) bp regions of human cox-2 promoter, respectively, flanked by KpnI and HindIII restriction sites from human lymphocyte genomic DNA using PCR and cloning into pPGL3-Basic vector. The PCR products were verified by DNA sequencing. The promoterless pPGL3-Basic vector was used as a negative control plasmid for luciferase assays.
MCF-7 cells were seeded in 24-well plates at a density of 1.2 × 105 cells well−1 and cotransfected with pCOX-2-Luc and pRL-TK-Luc (Promega, Madison, WI, U.S.A.) plasmids using LipofectAMINE reagent (Invitrogen) as suggested in the manufacturer's protocol. Following transfection (after 4 h), the medium was replaced with fresh RPMI medium and cells were allowed to recover for 16 h. The transfected cells were then treated with vehicle (0.1% DMSO), 50 ng ml−1 TPA alone, or TPA mixed with test compound at indicated concentrations for 6 h. Cell lysates were prepared using Passive Lysis Buffer (Promega) and then employed for luciferase activity assays. The promoter activity in arbitrary unit (AU) was obtained from the ratio of firefly luciferase activity (pCOX-2-Luc) to that of Renilla luciferase (pRL-TK-Luc).
Measurement of prostaglandin E2 (PGE2) production in macrophages
PGE2 production was determined according to a modified procedure of Hwang et al. (2002). Briefly, RAW 264.7 cells were seeded in 96-well plates at a density of 1 × 104 cells well−1 and incubated for 18 h. The cells were pretreated with 500 μM of aspirin for 3 h to inactivate endogenous COX-1, washed twice with PBS and then incubated with the test compounds (0.1–20 μg ml−1) for 1 h. The cells were then incubated for 16 h in fresh DMEM with or without 1 μg ml−1 of LPS. After incubation, culture media were recovered for PGE2 measurement. The amount of PGE2 was determined using the ACE™ Competitive Enzyme Immunoassay according to the supplier's instructions (EIA, Cayman Chemical, Ann Arbor, MI, U.S.A.).
Immunohistochemical study of COX-2 expression in mouse skin
The immunohistochemical study was performed according to Chun et al. (2004) with minor modifications. Female ICR mice were supplied from the Laboratory Animal Center (College of Medicine, National Taiwan University, Taipei, Taiwan). Mice were topically treated on their shaven backs with vehicle (acetone, 200 μl site−1) or TPA (10 nmol 200 μl−1 site−1) for 4 h. For the compound treatments, mice were treated with compound at the indicated doses first for 30 min, then further treated with TPA for 4 h, and finally killed by cervical dislocation. Sections (4 μm) of formalin-fixed, paraffin-embedded tissue were cut onto silanized glass slides and deparaffinized three times with xylene for 5 min each prior to rehydration through a graded alcohol bath. For antigen retrieval, the deparaffinized sections were heated and boiled in 10 mM citrate buffer (pH 6.0) for 10 min and then rinsed with PBS containing 0.05% Tween-20 (PBST) buffer for 5 min. Each section was treated with 3% hydrogen peroxide in methanol for 15 min to diminish nonspecific staining. The sections were then washed with blocking solution (PBS–1% BSA, PBA) for 30 min and then PBST twice for 5 min each. The slides were incubated with 2% normal goat serum in PBA for 30 min to minimize the immunological binding of antibody, and then incubated with a 1 : 500 dilution of polyclonal COX-2 antibody (Cayman Chemical, Ann Arbor, MI, U.S.A.) at room temperature for 1–2 h. The slides were developed using the HPR EnVisionTM system (Dako, Glostrup, Denmark), and the peroxidase-binding sites were detected by staining with 3,3′-diaminobenzidine tetrahydrochloride (Dako). Finally, counterstaining was performed using Mayer's hematoxyline.
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed (with a commercially available kit, LightShift™ Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL, U.S.A.)) according to the manufacturer's instructions. The binding reaction was optimized in binding buffer containing 10% glycerol, 100 mM KCl, 1.5 mM MgCl2, and 0.3 mM EDTA. Nuclear extracts (4 μg) were incubated with biotin end-labeled, 22-mer double-stranded NF-κB oligonucleotide, 5′-ATGTGAGGGGACTTTCCCAGGC-3′ (underlining indicates NF-κB-binding site) for 20 min at room temperature. The DNA–protein complexes were separated from nonbound oligonucleotides on a native 5−10% gradient polyacrylamide gel. The specificity of NF-κB DNA binding was examined by supershifting of the DNA–protein complex band by anti-p65 antibody, and by competition with the unlabeled NF-κB binding oligonucleotide. An antibody against Ref-1 (Santa Cruz Biochemicals, Santa Cruz, CA, U.S.A.) was employed as a negative control.
For in vitro binding assays, nuclear extracts from LPS-stimulated RAW 264.7 cells (1 μg ml−1, 30 min) were treated with different concentrations of test compounds at 37°C for 30 min and analyzed for NF-κB binding by EMSA as described above.
Results
Ethyl caffeate significantly inhibits NO production, and iNOS gene and protein expressions
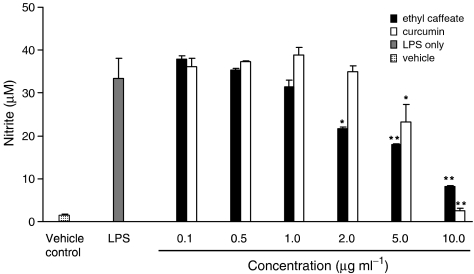
NO is a critical signaling molecule produced at inflammatory sites by iNOS, which is often expressed in response to LPS, interferon-γ, and a variety of proinflammatory cytokines (MacMicking et al., 1997). In this study, the effects of ethyl caffeate on NO production in RAW 264.7 cells stimulated by LPS were investigated. Curcumin, the major yellow pigment of turmeric (Curcuma longa L., Zingiberaceae) known for its anti-inflammatory and anticancer activities (Lin, 2004), was used in parallel as a positive control in this study. As shown in Figure 1, the nitrite level (equivalent to the NO level) in culture supernatants was markedly elevated from 1.5±0.3 μM to 33.0±4.6 μM after a 24 h treatment with LPS. Ethyl caffeate significantly inhibited LPS-induced NO production in a dose-dependent manner. At 2 μg ml−1, NO production was 35% inhibited, relative to LPS only (gray bar) in ethyl caffeate-treated cells, whereas no detectable effect was observed in the curcumin-treated cells (open bar) (Figure 1). The IC50 values for inhibiting NO production in ethyl caffeate- and curcumin-treated cells were 5.5 μg ml−1 and 6.5 μg ml−1, respectively (Figure 1).
Figure 1.
Effects of ethyl caffeate and curcumin on LPS-induced NO production in activated RAW 264.7 cells. Cells were treated with or without the indicated concentrations of test compounds for 1 h, then followed by the addition of LPS (1 μg ml−1) for 24 h. Vehicle controls were obtained from cells treated with 0.5% DMSO only. NO in the culture medium was measured as described in the Methods. The data are representative of three experiments and expressed as mean±s.d. Statistical analyses between LPS and ethyl caffeate or curcumin treatment were performed using Student's t-test. Significant inhibition is indicated by * and **, with a P-value <0.05 and <0.01, respectively.
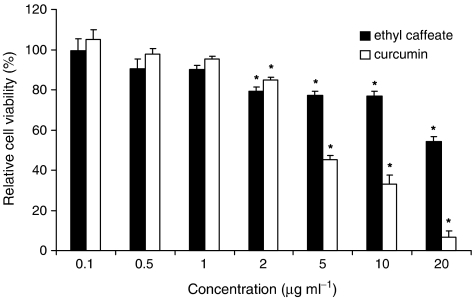
To evaluate further whether inhibition of NO production in RAW 264.7 cells was affected by the cytotoxic effects of ethyl caffeate and curcumin, viabilities of test cells were determined using MTT assays. We found that ethyl caffeate had little or no cytotoxicity to RAW 264.7 cells at concentrations of 10 μg ml−1 or below, whereas curcumin had a significant cytotoxic effect at 5 μg ml−1, as less than 50% of tested cells were detected as viable after treatment with 5 μg ml−1 curcumin for 24 h (Figure 2). These results indicate that the inhibition of NO production induced by LPS in macrophages by ethyl caffeate (Figure 1) was not due to the toxicity of the compound to the tested cells.
Figure 2.
Effect of ethyl caffeate and curcumin on cell viability of RAW 264.7 cells. Cells were treated with or without the indicated concentrations (0.1−20 μg ml−1) of test compounds for 24 h. Percentage of viable cells was determined using MTT assay. Data representative of three experiments were expressed as mean±s.d. The statistical analyses between vehicle control and ethyl caffeate or curcumin treatment were performed using Student's t-test. P<0.01 (*) is considered statistically significant.
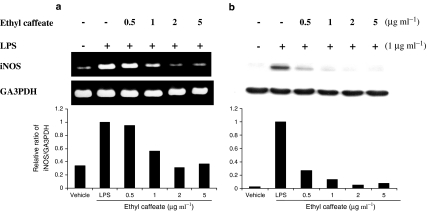
The effects of ethyl caffeate on iNOS gene and protein expression, which mediate the synthesis of NO in LPS-stimulated RAW 264.7 cells, were also investigated. RT−PCR analysis for specific mRNA in total RNA samples extracted from RAW 264.7 cells was performed. The result (Figure 3a) shows that ethyl caffeate (1 μg ml−1) significantly inhibited the iNOS mRNA expression in LPS-treated macrophages; approximately 56% reduction was observed when compared to the macrophages treated with LPS only, as determined using densitometry analysis. At a dose of 2 μg ml−1, the level of iNOS mRNA in ethyl caffeate-treated cells was similar to that in vehicle control (non-LPS or phytocompound treated) cells. Western blot analysis further demonstrated that a more significant inhibition at the protein level of iNOS was observed in ethyl caffeate-treated (0.5 μg ml−1) macrophages, with only approximately 30% of the iNOS protein level seen in the cells treated with LPS alone (Figure 3b). These results indicate that the inhibition of NO production in LPS-stimulated macrophages by ethyl caffeate is mediated through the transcriptional as well as translational downregulation of iNOS.
Figure 3.
Effects of ethyl caffeate on LPS-induced iNOS expression in RAW 264.7 cells. (a) Cells were treated with the indicated concentrations of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 6 h. Total cellular RNA (2 μg) was subjected to RT–PCR and the final PCR product resolved using 1% agarose gel electrophoresis. (b) Cells were treated with the indicated concentrations of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 18 h. Total cellular proteins (20 μg) were resolved by SDS–PAGE, then transferred to PVDF membrane and detected with specific antibodies as described in the Methods. Quantification of iNOS expression was normalized to GA3PDH using a densitometer.
Effect of ethyl caffeate on COX-2 expression and PGE2 production in MCF-7 cells or macrophages
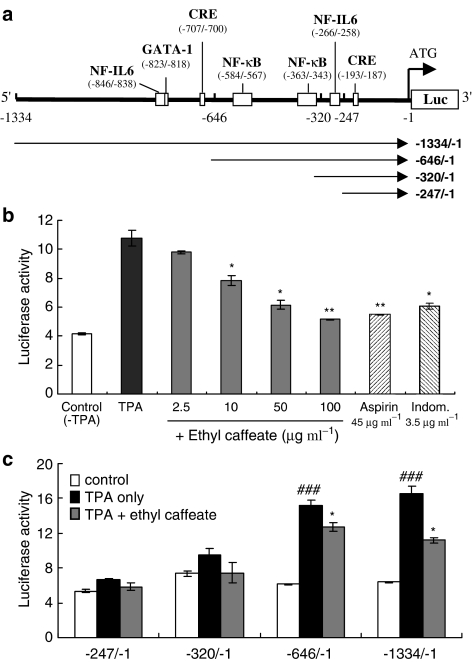
Tumor promoter TPA was reported to act as a potent inducer of COX-2 expression in various cell types, for example, macrophages and monocytic cells (Mestre et al., 2001). In order to verify whether the anti-inflammatory properties of ethyl caffeate contribute to its antitumor promoting activity, we examined the effect of ethyl caffeate on COX-2 gene (cox-2) activity in TPA-treated MCF-7 cells, a human mammary adenocarcinoma cell line. Transient transfection experiments using a cox-2 promoter-luciferase reporter assay were performed. One full length (−1334/−1), as well as a series of deleted variants of cox-2 promoter-luciferase constructs (−646/−1, −320/−1 and −247/−1) were constructed for this study (Figure 4a). As shown in Figure 4b, TPA significantly induced the transcriptional activity of the −1334/−1 (full length) cox-2 promoter in MCF-7 cells (a 2.7-fold increase, black bar), as compared to that of the control cells (without TPA treatment, white bar). The TPA-induced cox-2 promoter activity in MCF-7 cells was downregulated by ethyl caffeate in a dose-responsive manner. The addition of 10, 50, and 100 μg ml−1 ethyl caffeate to TPA-treated MCF-7 cells resulted in the decrease of full-length cox-2 promoter (−1334/−1) activities, by 72, 57, and 48% (gray bars), respectively, as compared to that of the cells treated with TPA only (black bar). No cytotoxic effects on MCF-7 cells treated with ethyl caffeate were observed at the same dosages (10−100 μg ml−1) over 6 h, as determined using MTT assay (data not shown). Aspirin (45 μg ml−1=∼250 μM) and indomethacin (3.5 μg ml−1=∼10 μM), two well-known nonsteroidal anti-inflammatory drugs (NSAID), used as reference controls in this experiment, exhibited comparable inhibitory effects to that of ethyl caffeate (at concentrations between 50 and 100 μg ml−1=∼240−480 μM) on the −1334/−1 cox-2 promoter activity in MCF-7 cells.
Figure 4.
Characterization the effect of ethyl caffeate on transcriptional activities of cox-2 promoter. (a) Map of full-length cox-2 promoter. Arrows indicate the full-length and three serial deletion cox-2 promoter constructs. (b) MCF-7 cells transfected with full-length of pCOX-2-Luc constructs (−1334/−1) and internal control plasmid pRL-TK-Luc were left unstimulated (Control) or stimulated with TPA in the absence (black bar) or presence (gray bars) of the indicated concentrations of ethyl caffeate, aspirin, and indomethacin for 6 h. Total lysate (10 μg) was subjected to dual luciferase reporter assay. The promoter activity in arbitrary unit (AU) was obtained from the ratio of firefly luciferase activity (pCOX-2-Luc) to that of Renilla luciferase (pRL-TK-Luc). Significant inhibition is indicated by * and **, with a P-value <0.05 and <0.01, respectively. (c) Sequential 5′-deletions of the full-length cox-2 promoter was cloned as described in the Methods. Specific promoter activities of −1334/−1, −646/−1, −320/−1, and −247/−1 constructs were obtained as described in (b). The data are representative of three experiments and expressed as mean±s.d. Statistical analyses between control and TPA only (#) or control and TPA+ethyl caffeate (*) were performed using Student's t-test. Significant inhibition is indicated by * and ###, with a P-value <0.05 and <0.01, respectively.
An IL-10 promoter-driven luciferase reporter gene assay was also employed in parallel in this study to examine whether the regulation of cox-2 promoter activity by ethyl caffeate is highly specific. The same doses of ethyl caffeate and experimental conditions used in the cox-2 promoter assay were employed in this IL-10 reporter assay. We found that ethyl caffeate did not affect TPA-induced IL-10 promoter activity even in the presence of 100 μg ml−1 ethyl caffeate (data not shown). This result suggests that ethyl caffeate does not regulate transcriptional activity of IL-10, and that inhibition of cox-2 promoter activity by ethyl caffeate is highly specific.
A comparative study of the transfection experiments with full-length cox-2 promoter (−1334/−1) or deletion constructs, containing NFκB-, NF-IL6-, and CRE-binding sites (−646/−1), NF-IL6, and CRE-binding sites (−320/−1), and a single CRE site (−247/−1), respectively, (Figure 4c) was performed in MCF-7 cells with or without the TPA treatment. Similar luciferase activities (a 2.5–2.6-fold higher activity than that of the basal COX-2 reporter activity in control cells without TPA treatment, white bars) were observed for the −1334/−1 and −646/−1 promoter constructs in TPA-treated MCF-7 cells (black bars) (Figure 4c). These results indicate that the −646/−1 promoter region of cox-2 promoter, containing NFκB-, NF-IL6-, and CRE-binding sites, was able to act as the minimal responsive element for TPA to induce luciferase activity in MCF-7 cells. The −320/−1 and −247/−1 deletion promoter constructs, harboring NF-IL6- and CRE-binding sites, and a single CRE site, respectively, were observed to be slightly or not responsive to the TPA treatment in MCF-7 cells (black bars), as they exhibited similar luciferase activities to the controls (white bars).
We further examined the effect of ethyl caffeate on the transcriptional activity of cox-2 in TPA-treated MCF-7 cells. In the presence of 20 μg ml−1 of ethyl caffeate (gray bars), 67 and 80% of luciferase activities were found in TPA-treated cells transfected with −1334/−1 and −646/−1 promoter constructs, respectively, compared to those of the test cells harboring the same constructs treated with TPA only (100%, black bars). Little or no effects were observed for the −320/−1 and −247/−1 promoter activities when MCF-7 cells were cotreated with TPA and ethyl caffeate (20 μg ml−1).
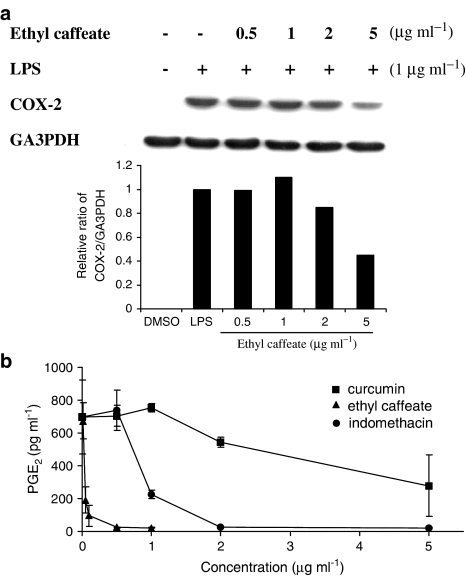
The COX-2 protein expression in LPS-stimulated macrophages was further examined using Western blot analysis. Figure 5a shows that LPS-mediated COX-2 expression (100%) in RAW 264.7 cells was effectively inhibited by the ethyl caffeate treatment (45%) at a dose of 5 μg ml−1, as quantitatively determined using densitometry. We next determined the amount of PGE2, a critical inflammatory mediator, which is one of the major products produced by the enzymatic reaction of COX-2. Figure 5b shows that ethyl caffeate at 1 μg ml−1 markedly suppressed the PGE2 production in LPS-stimulated RAW 264.7 cells. Ethyl caffeate (2–5 μg ml−1) treatment caused total inhibition of PGE2, whereas only 40−78% was inhibited by curcumin at the same doses. Indomethacin used as a positive control in this study exhibited total inhibition of PGE2 production at a concentration between 0.5 and 1 μg ml−1.
Figure 5.
Effects of ethyl caffeate on LPS-induced COX-2 expression and PGE2 production in RAW 264.7 cells. (a) Cells were treated with the indicated concentration of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 18 h. Total cellular proteins (20 μg) were resolved by SDS–PAGE, then transferred to PVDF membrane and detected with specific antibodies as described in the Methods. Quantification of COX-2 expression was normalized to GA3PDH using a densitometer. (b) Aspirin-pretreated RAW 264.7 cells were treated with the indicated concentrations of ethyl caffeate for 1 h and then stimulated with LPS for 16 h. PGE2 in the culture medium was measured as described in the Methods. Indomethacin was used as a positive control. The data are representative of three experiments and expressed as mean±s.d.
Effects of ethyl caffeate on TPA-induced COX-2 expression in mouse skin
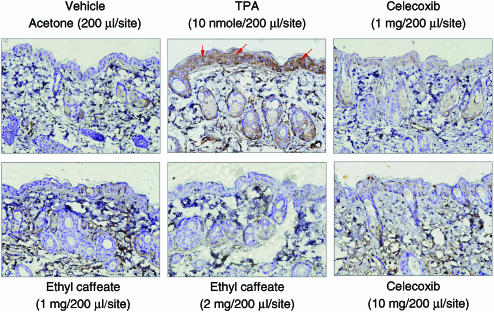
In order to examine whether ethyl caffeate can inhibit COX-2 expression in vivo, we topically applied ethyl caffeate or celecoxib, a well-known NSAID with potent COX-2 inhibitory activity, on TPA-treated mouse skin and conducted immunohistochemical analyses. Upon treatment with acetone (vehicle control) for 4 h, specific COX-2 immunostaining was detectable in the dermal sebaceous gland (Figure 6). In contrast, COX-2 expression increased dramatically in the epidermal layer upon TPA treatment (10 nmol) for 4 h. TPA-induced COX-2 expression was significantly abolished by ethyl caffeate in a dose-dependent manner. An inhibition of COX-2 protein expression by 1 mg 200 μl−1 site−1 ethyl caffeate (24 mM) was comparable to the inhibitory effect of 1 mg 200 μl−1 site−1 celecoxib (13 mM), and, strikingly, 48 mM ethyl caffeate (2 mg 200 μl−1 site−1) was observed to be more effective than 10 mg 200 μl−1 site−1 celecoxib treatment (131 mM) (Figure 6). Similar results were obtained in three independent repeated experiments.
Figure 6.
Immunohistochemical study on the inhibitory effect of ethyl caffeate on COX-2 expression in TPA-treated murine skin. Dorsal skins of female ICR mice were treated topically with acetone (vehicle control) or TPA only (10 nmol) for 4 h, or treated with the indicated concentrations of ethyl caffeate or celecoxib for 30 min and then treated with TPA for 4 h. Paraffin-embedded tissues from vehicle, TPA only, and compound/TPA-treated mice were immunostained for specific COX-2 protein and counter-stained with hematoxylin, as described in the Methods. Immunohistograms were taken with an Olympus DP-70 camera on a Nikon ECLIPSE E800 microscope (mangnification: × 200).
Ethyl caffeate does not affect LPS-induced activation of MAPK in macrophages
The activation of SAPK/JNK, p38, and p42/44 (ERK 1/2) MAPK proteins known to be involved in the regulation of iNOS or COX-2 expression were further investigated to determine if there was any link between these signaling molecules and the anti-inflammatory mechanism(s) of ethyl caffeate. Since phosphorylation is required for the activation of MAPKs, immunoblotting analyses using phospho-specific antibodies against MAPK proteins were performed. After 30 min of LPS stimulation, the phospho-MAPK proteins were significantly induced; however, no significant inhibition on the protein levels of phospho-MAPK proteins were observed upon ethyl caffeate treatment of murine macrophages at concentrations between 1 and 10 μg ml−1 (ca., 4.8−48 μM) (Figure 7). Recently, it has been shown that octyl caffeate, a semisynthesized compound from caffeic acid, at 50 μM, can suppress LPS/INF-γ-induced expressions of SAPK/JNK, p38, and p42/44 proteins in rat aortic smooth muscle cells (Hsiao et al., 2003). It is not clear whether the differences in cell type (murine macrophages vs rat aortic smooth muscle cells) with respect to specific inducing conditions (i.e., LPS induction vs LPS and INF-γ coinduction) between the previous (Hsiao et al., 2003) and this study could have resulted in the different effects on the activation of MAPK proteins by the two structurally similar compounds, that is, octyl caffeate and ethyl caffeate.
Figure 7.
Effects of ethyl caffeate on MAPKs phosphoration in RAW 264.7 cells. Cells were treated with various concentrations of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 30 min. Total cellular proteins were prepared for Western blot analysis of phospho-MAPKs proteins by specific anti-phospho-MAPKs antibodies.
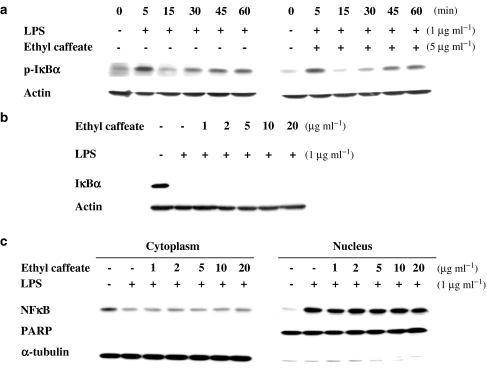
Ethyl caffeate does not affect NF-κB translocation
The translocation of NF-κB to the nucleus is preceded by phosphorylation, ubiquitination, and proteolytic degradation of IκB (Karin & Lin, 2002). Western blot analysis was performed to determine whether ethyl caffeate can abolish the NF-κB translocation in macrophages. In this regard, we determined the protein distribution of NF-κB (Rel A) and phospho-IκBα in cytosolic and nuclear proteins as well as the protein level of IκBα in cytosolic proteins (correlated to the degradation of IκBα protein) of LPS-stimulated RAW 264.7 cells treated with ethyl caffeate or vehicle control. First, we observed that the phosphorylation as well as the degradation of IκB induced by LPS in macrophages was not affected by treatment with ethyl caffeate (Figure 8a and b). We further examined the appearance of the p65 subunit (Rel A) of NF-κB in the cytosolic and nuclear extracts of control and ethyl caffeate-treated cells. Results shown in Figure 8c revealed that ethyl caffeate treatment for 1 h, at concentrations between 1 and 20 μg ml−1, did not reduce nuclear localization of the Rel A protein, since equal levels of RelA proteins were observed in the nuclear proteins. Poly(ADP-ribose) polymerase (PARP), a nuclear protein, and α-tubulin, a cytosolic protein, were used as internal controls to ensure that there was no cross contamination during protein extractions of the cellular and nuclear fractions.
Figure 8.
Effects of ethyl caffeate on IκBα phosphorylation and degradation, and NF-κB translocation in RAW 264.7 cells. (a) Cells were treated with 1 μg ml−1 of LPS in the absence or presence of 5 μg ml−1 ethyl caffeate for different time courses as indicated. Total cellular proteins were prepared for Western blot analysis of phospho-IκBα protein by specific anti-phospho-IκBα antibody. (b) Cells were treated with various concentrations of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 11 min. Total cellular proteins were prepared for Western blot analysis of IκBα protein by specific anti-IκBα monoclonal antibody. (c) Cells were treated with various concentrations of ethyl caffeate for 1 h followed by the addition of LPS (1 μg ml−1) for 30 min. Cytosolic and nuclear extracts (20 μg) were prepared for Western blot analysis of p65 of NF-κB protein by specific anti-p65 NF-κB monoclonal antibody. PARP, a nuclear protein, and α-tubulin, a cytosolic protein, were used as controls to confirm that there was no contamination during extraction of each fraction.
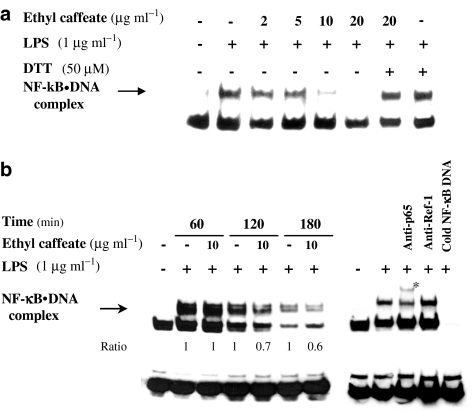
Ethyl caffeate inhibits LPS-induced NF-κB activation through prevention of NF-κB binding to DNA
LPS has been reported to act as a potent inducer of NF-κB activation in macrophages (Bayon et al., 2003). We used an in vitro binding assay to examine the effect of ethyl caffeate to perturb the ability of active NF-κB to bind to a biotin-labeled oligonucleotide containing κB DNA elements using EMSA. Nuclear extracts from LPS-stimulated cells were incubated with increasing concentrations of ethyl caffeate for 30 min at 37°C. Inhibition of NF-κB binding to DNA by ethyl caffeate was observed in a dose-dependent manner in that the compound significantly inhibited the NF-κB binding to DNA at 10 μg ml−1 with virtually a complete inhibition at 20 μg ml−1 (Figure 9a). It has been shown that reducing agents (e.g., DTT) can reverse the inhibitory effect of a number of compounds, for example, caffeic acid phenethyl ester, L-1-tosylamido-2-phenylethyl chloromethyl ketone (a serine protease inhibitor), and herbimycin A, on NF-κB activation (Natarajan et al., 1996). We, therefore, further examined the ability of DTT to reverse the effect of ethyl caffeate in preventing the formation of NF-κB DNA complex. As shown in Figure 9a, DTT at 50 μM had no significant effect on LPS-dependent activation of NF-κB, but it completely reversed the inhibition induced by ethyl caffeate. This result is in agreement with the previous reports (Natarajan et al., 1996), suggesting the important role of sulfhydryl groups in the TNF- or LPS-dependent activation of NF-κB.
Figure 9.
Inhibition of LPS-induced NF-κB activation by ethyl caffeate in vitro and in vivo. (a) Nuclear extracts from LPS-stimulated RAW 264.7 cells were treated with different concentrations of ethyl caffeate for 30 min at 37°C in vitro and analyzed for NF-κB binding by electrophoretic mobility shift assays (EMSA) as described in the Methods. The arrow indicates the gel location of NF-κB bound to DNA. (b) Cells were treated with various concentrations of ethyl caffeate for 1 h followed by treatment with LPS (1 μg ml−1) for 60, 120, and 180 min. Nuclear extracts were prepared and assayed for activation of NF-κB by EMSA. Specificity of NF-κB band was analyzed by incubation with anti-p65 antibody, anti-Ref-1 antibody, and unlabeled NF-κB oligonucleotide. The arrow indicates the gel location of NF-κB bound to DNA and the asterisk indicates the supershift band.
We next investigated the effect of ethyl caffeate on inhibition of NF-κB activation in macrophages by EMSA. As shown in Figure 9b, the induction of specific NF-κB DNA binding activity by LPS in RAW 264.7 cells was moderately inhibited by 10 μg ml−1 ethyl caffeate. The relative level of NF-κB DNA binding complex in ethyl caffeate-treated macrophages activated by LPS at 60, 120, and 180 min were 1.0, 0.7, and 0.6, respectively, compared to those (1.0) in the cells activated with LPS only (without compound treatment). The NF-κB DNA complex in the LPS-treated cells was supershifted (band labeled with asterisk) when coincubated with specific antibody against p65 (Rel A protein of NF-κB) and was unaffected by the addition of a nonspecific antibody against Ref-1 (redox factor 1, a cellular redox/DNA repair protein). Moreover, the specific binding of NF-κB DNA to nuclear protein could be completely prevented with the addition of excess, unlabeled consensus NF-κB oligonucleotide. Taken together, these results demonstrate that NF-κB DNA binding can be very specifically inhibited by ethyl caffeate.
Structure–activity relationship studies of ethyl caffeate inhibition of NF-κB DNA complex formation
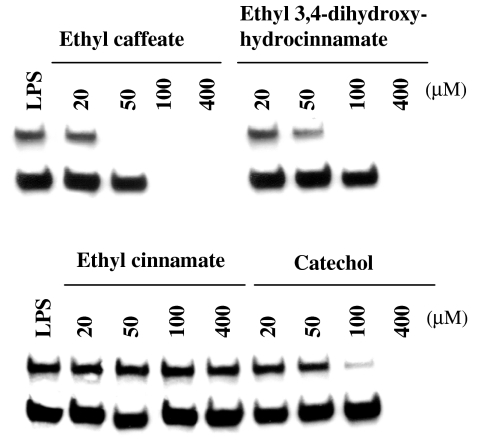
To delineate the role of ethyl caffeate in the inhibition of NF-κB·DNA complex formation, ethyl caffeate and its structural analogs, ethyl 3,4-dihydroxyhydrocinnamate, ethyl cinnamate, and catechol, were used for in vitro NF-κB DNA-binding assays. Figure 10 shows that ethyl caffeate and ethyl 3,4-dihydroxyhydrocinnamate (containing no α,β-unsaturated ester group) completely inhibited NF-κB DNA binding at 50 and 100 μM, respectively. Catechol totally blocked the binding at 400 μM, whereas ethyl cinnamate, which contains an α,β-unsaturated ester group but lacks the catechol moiety, did not inhibit NF-κB-binding activity even at a concentration of 400 μM. These results therefore suggest that the catechol moiety and the α,β-unsaturated ester group together may play important roles in the inhibition of binding of NF-κB to DNA.
Figure 10.
In vitro inhibition of NF-κB binding to DNA by different structural analogs of ethyl caffeate. Nuclear extracts from LPS-stimulated RAW 264.7 cells were treated with different concentrations (μM) of test compounds for 30 min at 37°C and analyzed for NF-κB binding by EMSA.
Discussion
Many lines of evidence have indicated that nitric oxide (NO) is a potent proinflammatory mediator and may have a multifaceted role in mutagenesis and carcinogenesis (Surh et al., 2001). iNOS catalyzes the oxidative deamination of L-arginine to produce NO. Therefore, aberrant or excessive expression of iNOS is often implicated in the oncogenesis and pathogenesis of cancer. Compounds that can selectively inhibit the undesirable expression of iNOS may serve as potential cancer chemoprevention candidates. Here, we demonstrate that a natural phenolic compound, ethyl caffeate, isolated from a medicinal plant, B. pilosa, exhibits potent inhibitory effects on NO production as well as iNOS expression in LPS-activated murine macrophages RAW 264.7 cells. Two signaling pathways, the MAPK and NF-κB activation mechanisms, known to be involved in the iNOS expression mediated by LPS in mouse macrophages, were examined to elucidate the possible molecular actions of ethyl caffeate on inhibiting the production of NO and iNOS. Although phosphorylation plays an important role in activating MAPK in LPS signaling, we found that the activated phosphorylated forms of MAPKs including SAPK/JNK, p38, and p42/44 induced by LPS were not affected by ethyl caffeate treatment. In addition, the translocation of NF-κB to the nucleus, mediated by the phosphorylation, ubiquitination, and proteolytic degradation of IκB, was also not responsive to ethyl caffeate. However, we did observe that the activity of NF-κB binding to the consensus sequence of κB was inhibited by ethyl caffeate in activated macrophage cells and in cell-free binding systems. We thus propose here that the significant inhibition of NO production observed was likely due to the downregulation of transcriptional and translational activities of iNOS caused by the interference of NF-κB activity by ethyl caffeate.
Expression of COX-2 has been related to various human cancers, including breast and colon cancers (Bing et al., 2001). In this study, therefore, we used a human mammary carcinoma cell line, MCF-7, to evaluate the effect of ethyl caffeate on transcriptional activities of COX-2. Promoter elements in cox-2 gene for NF-κB, NF-IL6, and CRE have been found to be important in regulating cox-2 gene transcription (Mestre et al., 2001). Mestre et al. reported that the maximal transcriptional activity of cox-2 induced by LPS in murine macrophages was observed as a sophisticated cooperation among NF-κB, NF-IL6, and CRE promoter sites. In other words, the isolated NF-κB, NF-IL6, and CRE promoter sites were less effective than the intact cox-2 promoter, containing the three promoter sites, in mediating the cox-2 transcription in LPS-treated macrophages. In the present study, we analyzed the cox-2 transcriptional activity mediated by TPA in MCF-7 cells using transfection experiments with cox-2 promoter-luciferase full-length and deletion constructs. Our results showed that the −646/−1 promoter construct, containing mainly the NF-κB, NF-IL6, and CRE promoter sites, exhibited significant luciferase activities, reaching a similar level as that of the −1334/−1 (full-length) construct, in TPA-treated MCF-7 cells (Figure 4c). Whereas the −320/−1 and −247/−1 deletion promoter constructs, which harbors NF-IL6 and CRE promoter sites and a single CRE site, respectively, were observed to exhibit little or no response to TPA treatment. These results indicated that the promoter element for NF-κB or the coexistence of the NF-κB, NF-IL6, and CRE promoter elements play an important role in mediating cox-2 gene transcription in response to TPA treatment in MCF-7 cells. The inhibitory effects of ethyl caffeate on cox-2 promoter were found to act upon the −1334/−1 (full length) and −646/−1 promoter constructs, inhibiting the full-length promoter the most, suggesting that the very upstream 5′ flanking region of cox-2, containing the cis-acting element(s), for example, SP-1, GATA-1, or GRE, other than the three NF-κB, NF-IL6, and CRE promoter sites, may play a role in, or be responsive to, the treatment of ethyl caffeate.
Ethyl caffeate was observed to significantly inhibit NF-κB activation not by blocking the degradation of IκBα, but by preventing the binding of NF-κB and DNA. These observations are similar to that obtained from caffeic acid phenethyl ester (CAPE), an active component of propolis from honeybee hives. CAPE is known to exhibit various bioactivities including anti-inflammatory and anticarcinogenic properties (Natarajan et al., 1996). Like CAPE, we found that ethyl caffeate-dependent inhibition of NF-κB activation was reversed by the reducing agent DTT, suggesting that ethyl caffeate may modify a critical sulfhydryl group in NF-κB protein. Structure–activity relationship studies on CAPE, as demonstrated by Natarajan et al. (1996), showed that the number and placement of hydroxyl groups in CAPE is a critical determinant of the extent of inhibition of NF-κB DNA binding. In this study, we further demonstrated that the presence of hydroxyl groups in the catechol moiety of caffeic acid derivatives are essential for their ability to inhibit NF-κB DNA complex formation, as there was no detectable inhibition found for ethyl cinnamate, which contains an α,β-unsaturated ester group but lacks the catechol moiety (Figure 10). In contrast to ethyl cinnamate, ethyl caffeate containing both catechol and an α,β-unsaturated ester group exhibited the most significant inhibitory effect (Figure 10).
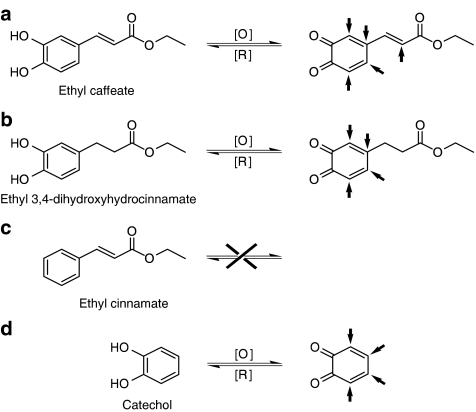
Phytocompounds containing α,β-unsaturated ester groups, for example, Avicin G, a triterpenoid saponin from Acacia victoriae (Haridas et al., 2001), or containing α,β-unsaturated carbonyl moiety, for example, sesquiterpene lactones helanalin (Lyss et al., 1998), have been suggested to prevent the binding of NF-κB to DNA by directly targeting cysteine residues of p65 subunit of NF-κB. X-ray crystallography studies have demonstrated that cysteine residues in p65 play an important role in optimal DNA–protein interactions (Kumar et al., 1992; Chen et al., 1998). Mutational and other biochemical studies further showed that these two types of structural groups can react with nucleophiles, especially the cysteine sulfhydryl groups of p65 by a Michael-type addition (Schmidt, 1997). Our structure–activity relationship study showed that the effectiveness of ethyl caffeate and its structural analogs at preventing NF-κB DNA binding were as follows: ethyl caffeate>ethyl 3,4-dihydroxyhydrocinnamate>catechol>ethyl cinnamate (Figure 10). On the basis of this result, previous reports described above, and the chemical environment of the target phytocompounds, we propose here an ‘oxidation–inhibition' mechanism for the observed effects of ethyl caffeate and its structural analogs on the inhibition of NF-κB DNA binding (Figure 11). Oxidant(s) present in the cellular environment, for example, nuclear extract, might thus have the ability to oxidize the catechol moiety, thereby generating o-benzoquinone that may in turn more effectively interact with the Cys residues of NF-κB leading to the inhibition of NF-κB binding to the DNA (Figure 11d). Ethyl 3,4-dihydroxyhydrocinnamate containing a catechol, but no α,β-unsaturated ester moiety, inhibits NF-κB DNA binding; however, the inhibition is less effective (approximately two-fold lower) than that of ethyl caffeate (Figure 10). One possible reason is that ethyl caffeate may generate five possible reaction centers and ethyl 3,4-dihydroxyhydrocinnamate may only generate four after oxidation (Figure 11a and b, as indicated by arrows), therefore, ethyl caffeate may be more effective in covalently interacting with the p65 protein than ethyl 3,4-dihydroxyhydrocinnamate, possibly via Michael-type addition (Schmidt, 1997). Ethyl cinnamate, however, which lacks the catechol moiety (Figure 11c), cannot be oxidized and thus it cannot block the Cys residues of NF-κB through the proposed ‘oxidation–inhibition' mechanism.
Figure 11.
An ‘oxidation–inhibition' mechanism proposed for the ethyl caffeate and its derivatives to prevent NF-κB binding to DNA. The arrowheads indicate the possible reaction centers of (a) ethyl caffeate, (b) ethyl 3,4-dihydroxyhydrocinnamate, (c) ethyl cinnamate and (d) catechol.
In summary, this is to our knowledge the first report to demonstrate that a naturally occurring phytocompound, ethyl caffeate, possesses potent anti-inflammatory activities. Our results reveal that ethyl caffeate exerts its anti-inflammatory effects by attenuating NF-κB activation and suppression of NF-κB-regulated proteins. This NF-κB regulation in turn affects iNOS and COX-2 expression at the gene transcriptional or translational levels. The production of PGE2, a growth-promoting factor in certain carcinoma cell lines and a mediator of inflammation (Tjandrawinata et al., 1997), was also significantly inhibited by ethyl caffeate in this study. The anti-inflammatory properties of ethyl caffeate seem to also contribute to its antitumor promoting activity as demonstrated using the TPA-stimulated mouse skin evaluation system. The TPA induced overexpression of COX-2 protein in mouse skin was found to be drastically inhibited by ethyl caffeate treatment. Since inadequate or abnormal overexpressions of COX-2 and iNOS are implicated in the pathogenesis of various types of human cancers, results obtained from this study indicate that ethyl caffeate may have therapeutic applications for inflammatory disorders or chemopreventive activities against cancers.
Acknowledgments
We are indebted to Dr Pei-Wen Hsiao for valuable suggestions and we thank other colleagues at the Institute of BioAgricultural Sciences, Academia Sinica, for their excellent technical assistance. We thank Dr Vanisree Staniforth (Dr Ning-Sun Yang laboratory) for providing us with the plasmid pIL10-Luc carrying IL-10 promoter and luciferase reporter gene. We thank Wei-Yu Chen, M.D. (Department of Pathology, Taipei Medical University, Taiwan) for his excellent assistance on preparation of paraffin-embedded mouse skin tissue sections. This work was supported by grant (AS91IBAS1PP) from the Genomic Research Center (GRC), Academia Sinica, Taiwan, R.O.C.
Abbreviations
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PGE2
prostaglandin E2
- TPA
12-O-tetradecanoylphorbol-13-acetate
References
- BAYON Y., ORTIZ M.A., LOPEZ-HERNANDEZ F.J., GAO F., KARIN M., PFAHL M., PIEDRAFITA F.J. Inhibition of IkappaB kinase by a new class of retinoid-related anticancer agents that induce apoptosis. Mol. Cell. Biol. 2003;23:1061–1074. doi: 10.1128/MCB.23.3.1061-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEYAERT R. Nuclear Factor κB: Regulation and Role in Disease. Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- BING R.J., MIYATAKA M., RICH K.A., HANSON N., WANG X., SLOSSER H.D., SHI S.R. Nitric oxide, prostanoids, cyclooxygenase, and angiogenesis in colon and breast cancer. Clin. Cancer Res. 2001;7:3385–3392. [PubMed] [Google Scholar]
- BRANDÃO M.G., KRETTLI A.U., SOARES L.S., NERY C.G., MARINUZZI H.C. Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J. Ethnopharmacol. 1997;57:131–138. doi: 10.1016/s0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- CHANG J.S., CHIANG L.C., CHEN C.C., LIU L.T., WANG K.C., LIN C.C. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am. J. Chin. Med. 2001;29:303–312. doi: 10.1142/S0192415X01000320. [DOI] [PubMed] [Google Scholar]
- CHEN C.C., WANG J.K. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol. Pharmacol. 1999;55:481–488. [PubMed] [Google Scholar]
- CHEN Y.Q., GHOSH S., GHOSH G. A novel DNA recognition mode by the NF-kappa B p65 homodimer. Nat. Struct. Biol. 1998;5:67–73. doi: 10.1038/nsb0198-67. [DOI] [PubMed] [Google Scholar]
- CHIANG Y.M., CHUANG D.Y., WANG S.Y., KUO Y.H., TSAI P.W., SHYUR L.F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004;95:409–419. doi: 10.1016/j.jep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- CHIN H.W., LIN C.C., TANG K.S. The hepatoprotective effects of Taiwan folk medicine ham-hong-chho in rats. Am. J. Chin. Med. 1996;24:231–240. doi: 10.1142/S0192415X96000293. [DOI] [PubMed] [Google Scholar]
- CHUN K.S., CHA H.H., SHIN J.W., NA H.K., PARK K.K., CHUNG W.Y., SURH Y.J. Nitric oxide induces expression of cyclooxygenase-2 in mouse skin through activation of NF-kappaB. Carcinogenesis. 2004;25:445–454. doi: 10.1093/carcin/bgh021. [DOI] [PubMed] [Google Scholar]
- DIMO T., AZAY J., TAN P.V., PELLECUER J., CROS G., BOPELET M., SERRANO J.J. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J. Ethnopharmacol. 2001;76:215–221. doi: 10.1016/s0378-8741(01)00229-x. [DOI] [PubMed] [Google Scholar]
- DIMO T., RAKOTONIRINA S.V., TAN P.V., AZAY J., DONGO E., CROS G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 2002;83:183–191. doi: 10.1016/s0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- GUHA M., MACKMAN N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- HAMBLETON J., WEINSTEIN S.L., LEM L., DEFRANCO A.L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN J., LEE J.D., BIBBS L., ULEVITCH R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- HARIDAS V., ARNTZEN C.J., GUTTERMAN J.U. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-kappaB by inhibiting both its nuclear localization and ability to bind DNA. Proc. Natl. Acad. Sci. USA. 2001;98:11557–11562. doi: 10.1073/pnas.191363498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIAO G., SHEN M.Y., CHANG W.C., CHENG Y.W., PAN S.L., KUO Y.H., CHEN T.F., SHEU J.R. A novel antioxidant, octyl caffeate, suppression of LPS/IFN-gamma-induced inducible nitric oxide synthase gene expression in rat aortic smooth muscle cells. Biochem. Pharmacol. 2003;65:1383–1392. doi: 10.1016/s0006-2952(03)00070-4. [DOI] [PubMed] [Google Scholar]
- HWANG B.Y., LEE J.H., KOO T.H., KIM H.S., HONG Y.S., RO J.S., LEE K.S., LEE J.J. Furanoligularenone, an eremophilane from Ligularia fischeri, inhibits the LPS-induced production of nitric oxide and prostaglandin E2 in macrophage RAW264.7 cells. Planta Med. 2002;68:101–105. doi: 10.1055/s-2002-20250. [DOI] [PubMed] [Google Scholar]
- KARIN M., CAO Y., GRETEN F.R., LI Z.W. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- KARIN M., LIN A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- KHAN M.R., KIHARA M., OMOLOSO A.D. Anti-microbial activity of Bidens pilosa, Bischofiajavanica, Elmerillia papuana and Sigesbekia orientalis. Fitoterapia. 2001;72:662–665. doi: 10.1016/s0367-326x(01)00261-1. [DOI] [PubMed] [Google Scholar]
- KUMAR S., RABSON A.B., GELINAS C. The RxxRxRxxC motif conserved in all Rel/kappa B proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol. Cell. Biol. 1992;12:3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMIDEY A.M., FERNON L., POUYSEGU L., DELATTRE C., QUIDEAU S., PARDON P. A convenient synthesis of the Echinacea-derived immunostimulator and HIV-1 integrase inhibitor (−)-(2R,3R)-chicoric acid. Helv. Chim. Acta. 2002;85:2328–2334. [Google Scholar]
- LI Q., VERMA I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- LIN J.K. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch. Pharm. Res. 2004;27:683–692. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- LO A.H., LIANG Y.C., LIN-SHIAU S.Y., HO C.T., LIN J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- LYSS G., KNORRE A., SCHMIDT T.J., PAHL H.L., MERFORT I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- MACMICKING J., XIE Q.W., NATHAN C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- MESTRE J.R., MACKRELL P.J., RIVADENEIRA D.E., STAPLETON P.P., TANABE T., DALY J.M. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J. Biol. Chem. 2001;276:3977–3982. doi: 10.1074/jbc.M005077200. [DOI] [PubMed] [Google Scholar]
- NATARAJAN K., SINGH S., BURKE T.R., JR, GRUNBERGER D., AGGARWAL B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. US.A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIDA E., GOTOH Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- PARK E.J., PEZZUTO J.M. Botanicals in cancer chemoprevention. Cancer Metast. Rev. 2002;21:231–255. doi: 10.1023/a:1021254725842. [DOI] [PubMed] [Google Scholar]
- PEREIRA R.L., IBRAHIM T., LUCCHETTI L., DA SILVA A.J., GONCALVES DE MORAES V.L. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology. 1999;43:31–37. doi: 10.1016/s0162-3109(99)00039-9. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., KELM M.Determination of nitrite and nitrate by the Griess reaction Methods in Nitric Oxide Research 1996New York: John Wiley & Sons Inc; 491–497.eds. Feelisch M and Stamler JS, pp [Google Scholar]
- SCHMIDT T.J. Helenanolide-type sesquiterpene lactones – III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg. Med. Chem. 1997;5:645–653. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- SCUDIERO D.A., SHOEMAKER R.H., PAULL K.D., MONKS A., TIERNEY S., NOFZIGER T.H., CURRENS M.J., SENIFF D., BOYD M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- SURH Y.J., CHUN K.S., CHA H.H., HAN S.S., KEUM Y.S., PARK K.K., LEE S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- TAN P.V., DIMO T., DONGO E. Effects of methanol, cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J. Ethnopharmacol. 2000;73:415–421. doi: 10.1016/s0378-8741(00)00290-7. [DOI] [PubMed] [Google Scholar]
- TJANDRAWINATA R.R., DAHIYA R., HUGHES-FULFORD M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br. J. Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UBILLAS R.P., MENDEZ C.D., JOLAD S.D., LUO J., KING S.R., CARLSON T.J., FORT D.M. Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Med. 2000;66:82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]