Abstract
Disseminated intravascular coagulation (DIC) is the most common complication of solid tumours. In this study, the effectiveness of three polysaccharide anticoagulants (PSAs), at therapeutic doses, at inhibiting solid tumour growth was investigated.
Mice with tumour xenografts were subcutaneously injected with either unfractionated heparin (UFH; 200 units kg−1 day−1), dalteparin (75 units kg−1 day−1) or danaparoid (50 units kg−1 day−1). At these concentrations, these PSAs are equieffective at inhibiting blood coagulation activated factor X. In mice with Lewis lung carcinoma (LLC) tumours dalteparin and, to a lesser extent, UFH inhibited both tumour growth and angiogenesis, whereas danaparoid did not. In contrast, in mice with KLN205 tumours, all the PSAs inhibited tumour growth and angiogenesis.
All the PSAs significantly inhibited proliferation, migration of endothelial cells and vessel formation in matrigel plugs containing vascular endothelial growth factor (VEGF) and there were no significant differences between these effects of the PSAs. The PSAs had no effect on endothelial cell tubular formation in vitro.
Although all the PSAs inhibited VEGF production in KLN205 tumours in vivo and cells in vitro, in LLC tumours and cells only UFH and dalteparin inhibited VEGF production, whereas danaparoid did not.
In both LLC and KLN205 tumours in vivo, heparanase activity was inhibited by UFH and dalteparin, but not by danaparoid.
Hence, UFH and dalteparin may be more effective than danaparoid at inhibiting cancer progression in DIC patients with solid tumours, due at least in part to their ability to suppress VEGF and heparanase in tumours.
Keywords: Heparins, angiogenesis, vascular endothelial growth factor, heparanase, cancer
Introduction
Disseminated intravascular coagulation (DIC) is one of the most common and often fatal complications of solid tumours (Sallah et al., 2001). Conversely, solid tumours are the most common cause of DIC (Okajima et al., 2000). Although the mechanism of the derangement of the coagulation system in patients with cancer is unclear, the inhibition of blood coagulation activated factor X (FXa) is a standard treatment for DIC in cancer as well as for DIC generally. There is accumulating evidence from both animal and human studies that polysaccharide anticoagulants (PSAs) such as unfractionated heparin (UFH) or low molecular weight heparin (LMWH) can affect cancer progression (Bijsterveld et al., 1999; Hettiarachchi et al., 1999; Smorenburg & van Noorden, 2001). Although many mechanisms have been postulated for the effects of PSAs on cancer, they are mostly independent of their anticoagulant effects. Since there is no doubt that the best way of managing DIC is to treat the underlying disorder (Levi & ten Cate, 1999), the possible effects of PSAs on cancer should be investigated to elucidate their mechanism of control of the growth of solid tumours. Blood supply is essential for solid tumours and tumour growth is highly dependent on angiogenesis, the formation of new capillaries from pre-existing blood vessels (Carmeliet & Jain, 2000). Much evidence has been obtained suggesting that UFH and LMWH can affect angiogenesis (Smorenburg & van Noorden, 2001). In order to treat DIC in patients with solid tumours, it is important to know which PSAs are effective at inhibiting tumour angiogenesis and, hence, reducing tumour growth. Therefore, in this study, we compared the effects of three PSAs, at therapeutic concentrations equieffective at inhibiting FXa, on various aspects of tumour growth, in order to elucidate their mechanisms of action and determine which PSA is most suitable for use in cancer patients.
Methods
Animals
Male specific-pathogen-free C57Bl/6 or BDF1 mice (Clea Japan Inc., Tokyo, Japan) 6–9 weeks of age were used throughout the study. All procedures were performed according to Animals (Scientific Procedures) Act 1986 and approved by the local ethics panel at Tohoku University School of Medicine. The animals were killed with an overdose of urethane (20 g kg−1).
FXa evaluation
Blood was collected into 0.13 M trisodium citrate from C57Bl/6 mice subcutaneously injected with either UFH (200 units kg−1 day−1), dalteparin (75 units kg−1 day−1) or danaparoid (50 units kg−1 day−1) for 3 days. The doses of PSAs used were similar to those given initially to patients with DIC. The FXa coagulation factor in the plasma was evaluated using a coagulation analyzer (Coagulation Analyzer, SYSMEX CA 1500, Dade Behring, Kobe, Japan). The plasma of a patient with factor X (Biopool International, Venture, CA, U.S.A.) was used as a test reagent for the FXa. Results are expressed as a percentage of the mean value of the FXa in blood from control mice that were injected with 100 μl PBS for 3 days. UFH (heparin sodium), dalteparin (fragmin®) and danaparoid (orgaran®) were purchased from Takeda Co. (Osaka, Japan), Kissei (Nagano, Japan) and Organon (Molenstraat, Netherland), respectively.
Bleeding time
C57Bl/6 mice were subcutaneously injected with either UFH (200 units kg−1 day−1), dalteparin (75 units kg−1 day−1) or danaparoid (50 units kg−1 day−1) for 2 weeks. The mice were then anaesthetized with sodium thiopental and their tails transected 5 mm from the tip. Bleeding was checked with filter paper every 30 s and bleeding time was determined by measuring the time until no blood was detected on the filter paper.
Cell culture
Lewis lung carcinoma cells (LLCs: ATCC, Manassas, VA, U.S.A.) were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal calf serum (FCS), 100 μg ml−1 kanamycin. KLN205 (ATCC, Manassas, VA, U.S.A.) cells, a cell line of mouse squamous cell lung cancer, were cultured in minimum essential medium (MEM) containing 10% FCS, 1% nonessential amino acids and 100 μg ml−1 kanamycin. Primary human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA, U.S.A.), and were cultured in endothelial cell growth medium (EGM-2 Bullet kit, Clonetics). To determine the effects of the PSAs on the proliferation of LLC, KLN205 and HUVEC in vitro, 4 × 104 cells were seeded into six-well dishes, and then washed with PBS 12 h later. Subsequently, the adherent cells were stimulated by the addition of culture medium containing various concentrations of the PSAs. To estimate the proliferation of HUVECs, after 72 h of treatment with PSAs, the number of HUVECs was counted. To quantify VEGF in the supernatant of cultured LLC and KLN205 cells, 2 × 105 cells were seeded into 100-mm dishes containing each of the PSAs and, 72 h later, the supernatant was collected and stored at −80°C.
In vivo tumour models
LLCs were injected (3 × 105 cells per animal) subcutaneously into the centre of the back of 6- to 9-week-old C57BL/6 male mice on day 0. KLN 205 cells were injected (5 × 105 cells per animal) subcutaneously into the centre of the back of 6- to 9-week-old BDF1 male mice on day 0. On day 5, when each tumour became palpable, inoculated mice were randomly allocated into four groups; mice in each group received a subcutaneous injection of either PBS (100 μl), UFH (200 units kg−1), dalteparin (75 units kg−1) or danaparoid (50 units kg−1). Injections were started from day 5 and given daily until the mice were killed. Tumour size was quantified daily as width2 × length × 0.52.
Immunohistochemistry and determination of microvessel density (MVD)
When the diameter of the tumour became ∼1 cm, tumour tissues were fixed in 10% formalin, embedded in paraffin and sectioned. The sections were blocked with 10% normal goat serum and incubated with polyclonal anti-human factor VIII-related antigen antibody (DAKO, Carpinteria, CA, U.S.A.). Subsequently, the sections were incubated with biotinylated goat anti-rabbit IgG (Vector, Burlingame, CA, U.S.A.), and then with ABC kit (Vector), and were detected by 3-amino-9-ethylcarbazole (Vector), counterstained with haematoxylin.
The intratumoral MVD was determined as described previously (Weidner et al., 1991; Kanda et al., 2003). In brief, the vessels inside the tumour were stained with anti-human factor VIII-related antigen antibody. The image that contained the highest number of microvessels was chosen for each section from an initial scan at × 100 magnification. Then, the vessels were counted in the selected image at × 200 magnification. At least four fields were counted for each section, and the highest count was used. Two independent investigators evaluated the number of vessels.
Endothelial cell migration assay
To elucidate the effect of the PSAs on VEGF-induced endothelial cell migration, we used a modified Boyden chamber (Chemotaxicell, Kurabo, Osaka, Japan). Human recombinant VEGF (20 ng ml−1), in the presence of each PSA or PBS, was added to the bottom well of the chamber at a volume of 750 μl of EBM-2 (San Diego, CA, U.S.A.) containing 0.4% FCS. Polycarbonate membranes with 3 μm pores were coated with the attachment factor containing 0.1% gelatin (Cascade Biologics, Portland, OR, U.S.A.) and placed between the test substances and the upper chambers. HUVECs were harvested at appropriate cell densities. Endothelial cells (1 × 105) were seeded to the inside of the upper chamber suspended in 250 μl of EBM containing 0.4% FCS. The cells were incubated at 37°C in 5% CO2 for 12 h. After incubation, all the nonmigrated cells were removed from the upper side of the filters with a cotton ball. The filters were fixed with methanol, stained with Dif-Quick solution and mounted onto microscope slides. The migrated cells were counted at × 40 magnification using a microscope.
Endothelial cell tubular formation assay
Confluent HUVECs were detached with trypsin-EDTA and suspended in EBM containing 0.5% FCS, 10 ng ml−1 human recombinant VEGF (R&D Systems) and 1 or 10 units ml−1 of each PSA, to investigate the effect of the PSAs on VEGF-induced morphogenesis in vitro (Kanda et al., 2003). The cells were seeded into matrigel-coated 12-well tissue culture plates at a density of 3 × 104 well−1 and incubated at 37°C. After 8 h, the cells were observed using an inverted phase-contrast microscope (Nikon Eclipse TE300). Images were captured with a laser scanning confocal imaging system (Bio-Rad, Hercules, CA, U.S.A.). Tubular formation was quantified by measuring the length of tubes in randomly selected fields using the NIH image program.
In vivo matrigel plug assay
The angiogenic effect of each PSA within 0.5 ml of matrigel was studied in 6- to 9-week-old C57Bl/6 mice. A mixture of matrigel (Becton Dickinson Labware) with either PBS or one of the PSAs (10 units ml−1) was injected into the abdominal subcutaneous tissue of mice along the peritoneal midline. The matrigel rapidly forms a solid plug at body temperature. After 10 days, excised plugs were photographed and their haemoglobin content was determined using Hemoglobin Test Wako (Wako Pure Chemical Industries, Osaka, Japan).
In a parallel experiment, VEGF-induced angiogenesis in vivo was assessed as the growth of blood vessels from subcutaneous tissue into a solid matrigel plug that contained 500 ng ml−1 VEGF. Growth factor-reduced matrigel (Becton Dickinson Labware), in liquid form at 4°C, was mixed with 500 ng ml−1 mouse recombinant VEGF (R&D Systems) and injected (0.5 ml). To investigate the effect of PSA treatment on VEGF-induced angiogenesis in vivo, mice were subcutaneously injected with either PBS or each PSA, at the same doses used in the in vivo tumour experiments, into their backs every day for 10 days. The plugs were cut out by retaining the peritoneal tissues, fixed in 10% formalin and embedded in paraffin. Sections stained with haematoxylin and eosin were studied by light microscopy. The vessel area and the total matrigel area were planimetrically calculated from the stained sections using the NIH image program. Only those structures possessing a patent lumen and containing erythrocytes were considered to be vessels. Results are expressed as a percentage, calculated as the ratio of the vessel area to the total matrigel area.
Quantification of VEGF protein by ELISA
To quantify serum VEGF proteins, the inferior vena cava of the mouse was punctured and peripheral blood was collected. To quantify tumour VEGF proteins, 0.3 g of the frozen tumour tissues were homogenized in 3 ml PBS, centrifuged for 20 min at 10,000 × g at 4°C and the supernatant collected. The concentration of VEGF in each sample was determined using a murine VEGF ELISA kit (R&D). Simultaneously, the total amount of protein in each sample was measured by using a Bio-Rad protein assay (Bio-Rad, Hercules, Ca, U.S.A.). The VEGF concentration in tumour samples is expressed as pg mg−1 protein.
Heparanase activity assay
Frozen tumours were homogenized in extraction buffer (0.1 M PBS, 0.15 M NaCl, 1 mM PMSF, 10 g ml−1 leupeptin, 1% NP-40) and centrifuged at 10,000 × g for 15 min at 4°C. The protein concentration of the supernatant was measured by use of a Bradford assay (Bio-Rad, Richmond, CA, U.S.A.). The heparanase activity in the supernatant was determined by measuring heparan sulphate-degrading enzyme activity in the sample using a Heparan Degrading Enzyme Assay Kit (Takara Bio Inc., Otsu, Japan) (Takahashi et al., 2004).
Quantification of VEGF and heparanase RNA in LLCs
LLCs were cultured in growth media with or without PSAs for 72 h and RNA was isolated using RNAzol B reagent (Tel-test, Inc., Friendswood, TX, U.S.A.). RNA expression of VEGF and heparanase in LLCs was determined by quantitative RT–PCR using specific oligonucleotide primers and TaqMan probes purchased from Applied Biosystems (Foster City, CA, U.S.A.) (Assay-on-Demand Products: Mm00437304 for mouse VEGF and Mm00461768 for mouse heparanase). Mouse β-actin RNA was simultaneously assessed as a housekeeping gene using the following primers and probes: forward, 5′-ACGGCCAGGTCATCACTATTG-3′; reverse, 5′-CCAAGAAGGAAGGCTGGAAAA-3′; TaqMan probe, 5′-FAM-CAACGAGCGGTTCCGATGCCC-TAMRA-3′. Real-time RT–PCR application was carried out in duplicate in 50 μl reaction volumes containing 4 ng total RNA for each specific reaction using TaqMan One-Step RT–PCR Master Mix Reagents Kit (Applied Biosystems, CA, U.S.A.).
Data analysis and statistics
Data are presented as mean±s.e. Unless otherwise indicated, statistical comparisons between groups were performed using Student's t-test or one-way ANOVA with Fisher's least-significant-difference test as a post hoc test, as appropriate.
Results
FXa inhibition and bleeding time in mice
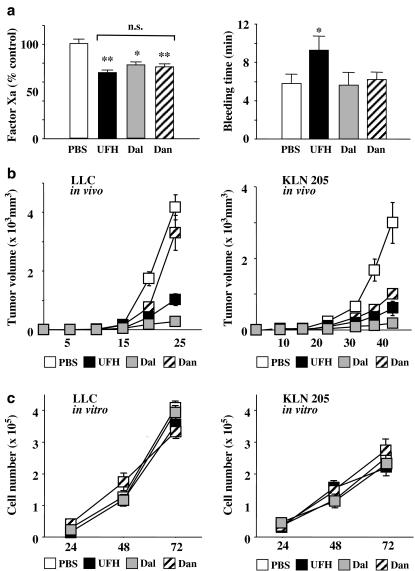
As shown in Figure 1a, the levels of FXa in mice injected with UFH, dalteparin and danaparoid were significantly lower than those in PBS-treated mice (P<0.01, 0.05 and 0.01, respectively). There was no significant difference in FXa activity between mice injected with UFH, dalteparin and danaparoid. However, the mice injected with UFH had significantly longer bleeding times than those in the other three groups. There was no significant difference in bleeding times between the mice injected with PBS, dalteparin and danaparoid.
Figure 1.
(a) Effects of PSAs on factor Xa activities (left) and bleeding time (right). Mice were injected with PBS (100 μl), UFH (200 units kg−1), dalteparin (Dal; 75 units kg−1) or danaparoid (Dan; 50 units kg−1) for 3 days. Factor Xa activities are expressed as the percentage of the mean of PBS-injected mice (control). *P<0.05, **P<0.01 vs control by Student's unpaired t-test. N.s. (not significant) between PSAs by one-way ANOVA with Fisher's least-significant-difference test. The values represent the mean±s.e. (n=5–7 per group). (b) The tumour volumes in LLC inoculated mice (left) and KLN205 inoculated mice (right). The values represent the mean±s.e. (n=7–10 per group). (c) Proliferation curves for LLCs (left) and KLN205s (right) in vitro in the mediums supplemented with PSAs (10 units ml−1). The values represent mean±s.e. of triplicate wells.
Effect of PSAs on tumour growth and cancer cell proliferation
In mice inoculated with LLCs or KLN205s, all the PSAs, at the doses used, had similar inhibitory effects on FXa. Whereas in LLC-inoculated mice, the tumour growth was markedly inhibited by dalteparin and, to a lesser extent, by UFH, but danaparoid did not inhibit tumour growth (Figure 1b). In KLN205-inoculated mice, all the PSAs inhibited tumour growth, the potency order being dalteparin>UFH>danaparoid. The proliferation curves of LLCs and KLN205s were investigated in vitro. None of the PSAs affected either LLC or KLN205 proliferation, suggesting that PSAs do not inhibit tumour growth by directly affecting the proliferation of cancer cells.
Effects of PSAs on tumour angiogenesis
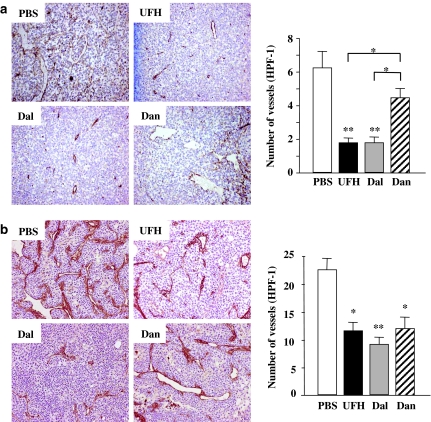
The immunostaining of factor VIII-related antigen in tumours of similar size (∼1 cm diameter) showed that there were decreased densities of vessels in UFH- and dalteparin-treated tumours compared with those treated with PBS and danaparoid (Figure 2a, left). The MVDs in UFH- and dalteparin-treated tumours were also significantly lower than those in PBS- and danaparoid-treated tumours (Figure 2a, right). There was no difference in MVD between PBS- and danaparoid-treated LLC tumours, and between UFH- and dalteparin-treated LLC tumours.
Figure 2.
(a) Immunohistochemical staining for factor VIII-related antigen of the LLC tumours. Left: representative photographs from each group are shown (magnification, × 100). Right: the effects of the PSAs on the tumour microvessel densities. Each value is the mean±s.e. (n=5–7 per group) of vessel counts observed with a high-power field (magnification, × 200). *P<0.05 among PSAs by one-way ANOVA with Fisher's least-significant-difference test. **P<0.01 compared with PBS group by Student's unpaired t-test. (b) Immunohistochemical staining for factor VIII-related antigen of KLN205 tumours. Left: representative photographs from each group are shown (magnification, × 200). Right: the effects of the PSAs on the tumour microvessel densities. Each value is the mean±s.e. (n=5–7 per group) of vessel counts observed with a high-power field (magnification, × 200). *P<0.05, **P<0.01 compared with the PBS group by the Student's unpaired t-test. There was no significant difference between the PSAs by one-way ANOVA with Fisher's least-significant-difference test.
In KLN205 tumours, the immunostaining showed decreased densities of vessels in all PSA-treated tumours compared with PBS-treated tumours (Figure 2b, left). The microvascular densities in PSA-treated KLN205 tumours were significantly lower than those in PBS-treated KLN205 tumours (Figure 2b, right).
Effect of PSAs on in vivo angiogenesis in matrigel plugs
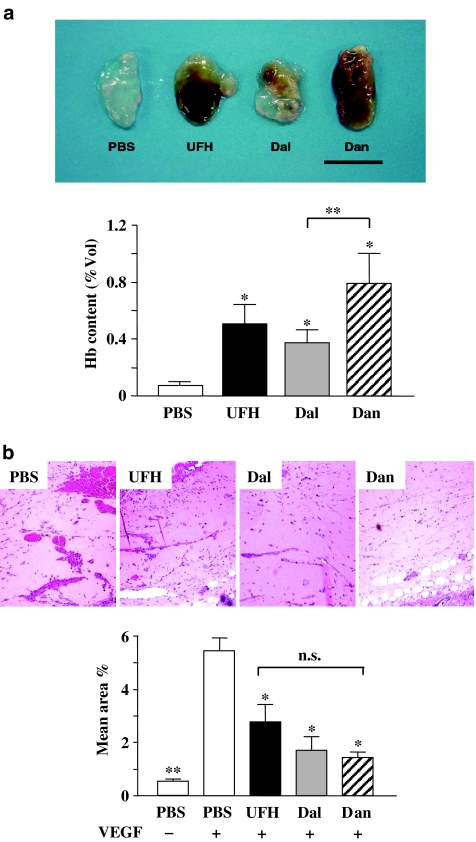
To investigate the interaction between PSAs and angiogenesis, we performed a matrigel plug assay using matrigels containing 10 units ml−1 of each of the PSAs. PSA-containing matrigels showed a bloody appearance, suggesting vascularization inside the plug, whereas PBS-containing matrigels did not (Figure 3a, upper). Estimation of haemoglobin content in the matrigel plug showed that PSAs themselves are able to induce angiogenesis (Figure 3a, lower).
Figure 3.
(a) Matrigel containing either PBS or each PSA (10 units ml−1). Upper: representative plugs from each group are shown (bar, 1 cm). Lower: the haemoglobin content of the matrigels containing the PSAs. The values represent the mean±s.e. (n=8–10 per group). *P<0.05 vs PBS by Student's unpaired t-test. **P<0.05 between the PSAs by one-way ANOVA with Fisher's least-significant-difference test. (b) Vessel formation in matrigels containing VEGF. Upper: representative microphotographs of matrigel plugs containing VEGF treated with PBS or PSAs as indicated (magnification, × 200). Lower: the vessel area compared to the total matrigel area (n=5–7 per group). *P<0.05, **P<0.01 vs PBS+VEGF by Student's unpaired t-test. There was no significant difference between the PSAs by one-way ANOVA with Fisher's least-significant-difference test.
We investigated the effect of subcutaneous injections of the PSAs on VEGF-induced angiogenesis using matrigel plugs containing VEGF. Vessel formation in matrigels in PSA-injected mice did not look like that in matrigels in PBS-injected mice (Figure 3b, upper). Since the matrigel itself did not contain the PSAs, it had little haemoglobin, so angiogenesis was estimated by calculating the area of vessels. All PSAs significantly inhibited VEGF-induced vessel formation in matrigel plugs compared with PBS. There were no significant differences between the inhibitory effects of the PSAs on VEGF-induced vessel formation.
Direct effects of PSAs on endothelial cells in vitro
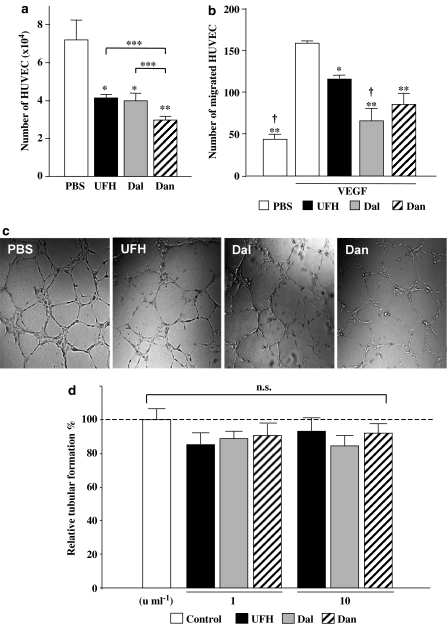
In contrast to in vivo angiogenesis, 1 U ml−1 of each of the PSAs significantly suppressed HUVEC proliferation (Figure 4a). Moreover, all the PSAs significantly inhibited VEGF-induced migration of HUVEC in vitro (Figure 4b) and there were no significant differences between the inhibitory effects of the PSAs on migration.
Figure 4.
(a) Number of HUVECs after 72 h treatment with PSAs (10 units ml−1). The values represent the mean±s.e. of triplicate wells. *P<0.05, **P<0.01 vs PBS by Student's unpaired t-test. N.s. (not significant), ***P<0.05 between PSAs by one-way ANOVA with Fisher's least-significant-difference test. (b) VEGF-induced migration of HUVEC. *P<0.05, **P<0.01 vs VEGF+PBS, and †P<0.05 vs VEGF+UFH by ANOVA with Fisher's least-significant-difference test. (c) The effect of PSAs on HUVEC tubular formation in vitro. Representative microphotographs of HUVEC morphogenesis to form capillary-like structures in culture medium containing PBS or PSAs (× 100). (d) Quantitative analysis of the extent of tubular formation. Each bar indicates the mean±s.e. of data from four experiments performed in duplicate wells. There were no significant differences between control (PBS) and PSA-treated HUVEC by Student's unpaired t-test.
In addition, the effect of the PSAs on the morphogenesis of endothelial cells was investigated by estimating the tubular formation of HUVEC. VEGF-induced tubular formation in the matrigel was not significantly affected by the addition of the PSAs (Figure 4c).
Effect of PSAs on VEGF expression and production
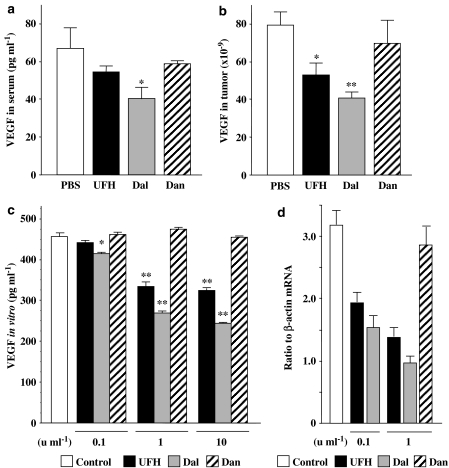
Since VEGF is known to have a pivotal role in tumour angiogenesis (Ferrara et al., 2003) and the angiogenesis induced by VEGF (Figures 3b and 4b) was inhibited by PSAs, we investigated the possible modulation of VEGF by PSAs. In mice with LLC tumours, the average serum VEGF protein levels in the four groups were in the order of PBS>danaparoid>UFH>dalteparin. The serum VEGF protein level in dalteparin-treated mice was significantly lower than the other three groups (Figure 5a), but there were no significant differences between the PBS, UFH and danaparoid groups. The concentrations of VEGF protein in LLC tumours were in the same order as those in serum (Figure 5b). The concentration of VEGF protein in tumours in dalteparin-treated mice was significantly decreased compared with that in the other three groups (Figure 5b). In vitro, VEGF protein levels in culture medium containing LLCs were reduced by UFH and dalteparin in a dose-dependent manner, but not by danaparoid (Figure 5a). The inhibitory effect of dalteparin was always greater than that of UFH. We assessed VEGF mRNA in LLCs quantitatively by real-time PCR. Both UFH and dalteparin dose-dependently inhibited VEGF mRNA (Figure 5d).
Figure 5.
VEGF protein levels in serum (a) and tumours (b) in mice with LLC tumours were quantified by ELISA. The values represent the mean±s.e. (n=5–7 per group, *P<0.05, **P<0.01 compared to other groups using one-way ANOVA with Fisher's least-significant-difference test). (c) The VEGF protein levels in LLCs in culture medium. The values represent the mean±s.e. of triplicate wells (*P<0.05, **P<0.01 vs control using Student's unpaired t-test). (d) VEGF mRNA of LLCs treated with the PSAs. Results of quantitative RT–PCR are expressed as the ratio to β-actin mRNA. The values represent the mean±s.e. of triplicate wells. The data are representative results of three independent experiments.
In contrast, in mice with KLN205 tumours, all the PSAs inhibited serum VEGF to a similar extent (Figure 6a) and they significantly reduced tumour VEGF production (Figure 6b). In vitro, all the PSAs inhibited VEGF production in KLN205 cells in a concentration-dependent manner, with a potency order of UFH=dalteparin>danaparoid (Figure 6c). In addition, all the PSAs significantly inhibited the expression of VEGF mRNA in vitro (Figure 6d).
Figure 6.
VEGF protein levels in serum (a) and tumours (b) in mice with KLN205 tumours were quantified by ELISA. The values represent the mean±s.e. (n=5–7 per group, *P<0.05 compared to other groups using one-way ANOVA with Fisher's least-significant-difference test). (c) The VEGF protein levels in KLN205 cell culture medium. The values represent the mean±s.e. of triplicate wells (*P<0.05, **P<0.01 vs control using Student's unpaired t-test). (d) VEGF mRNA in KLN205 cells treated by PSAs. Results of quantitative RT–PCR are expressed as the ratio to β-actin mRNA. The values represent the mean±s.e. of triplicate wells. The data are representative results from three independent experiments.
Effects of PSAs on heparanase activity and mRNA expression
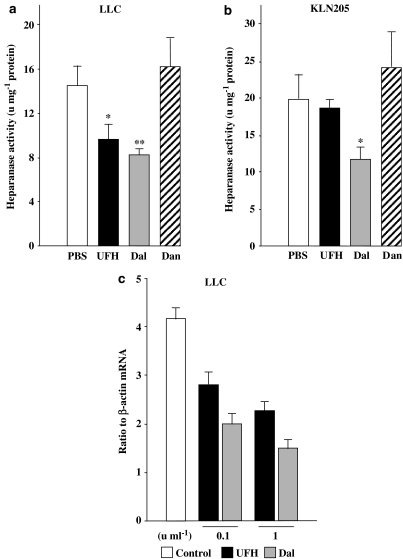
PSAs are well-known inhibitors of heparanase activity (Parish et al., 1987; Voldavsky & Friedmann, 2001) and heparanase is a potent angiogenic factor (Elkin et al., 2001; Goldshmidt et al., 2002). Hence, we investigated the effects of the PSAs on heparanase activity in LLC and KLN205 tumours in vivo. In LLC tumours, UFH and dalteparin significantly inhibited tumour heparanase activity, whereas danaparoid did not at the dose used (Figure 7a). In KLN205 tumours, dalteparin was the only PSA that inhibited heparanase activity (Figure 7b). We also assessed heparanase mRNA quantitatively in LLCs treated with UFH and dalteparin in vitro; both UFH and dalteparin inhibited heparanase mRNA in a dose-dependent manner (Figure 7c).
Figure 7.
Heparanase activity in LLC tumours (a) and KLN205 tumours (b). Each value for heparanase activity was normalized to protein concentration (units mg−1 protein) and represents the mean±s.e. (n=5–7 per group). Differences between the two groups were determined by Student's unpaired t-test (*P<0.05, **P<0.01 compared with the PBS group). (c) The heparanase mRNA expression. The values represent the mean±s.e. of triplicate wells. The data are representative results from three independent experiments.
Discussion
In this study, we showed that an LMWH, dalteparin, is more effective at inhibiting experimental tumour growth than UFH and danaparoid at concentrations that produce the same level of FXa inhibition and that this effect is mediated, at least in part, by inhibition of tumour VEGF and heparanase production. Despite the increasing availability of LMWHs, UFH is still widely used as an antithrombotic drug for the initial management of DIC because it is inexpensive (Hirsh et al., 2001) and danaparoid is sometimes chosen because of its potent anti-FXa activity (Meuleman, 1992). Since DIC is one of the most common complications of cancer, the effect of these drugs on tumour growth during DIC treatment might be an important factor in the choice of antithrombotic drug.
Although the dose of the drug used to treat DIC will vary depending on the situation, the initial doses of UFH, dalteparin, and danaparoid are about 50–100 units kg−1 day−1, 100 units kg−1 day−1, and 750 anti-FXa units kg−1 day−1, respectively. Since the half-life of UFH is about four times less than those of dalteparin and danaparoid, from the pharmacokinetics of subcutaneous injections, doses of 200 units kg−1 day−1, 75 units kg−1 day−1 and 50 anti-FXa units kg−1 day−1, respectively, were chosen for the daily injections. The anti-FXa level is the only method available for estimating clotting activities for all these drugs. At the concentrations used, UFH, dalteparin and danaparoid were equieffective at inhibiting FXa, suggesting that their potency at treating DIC should be comparable. However, bleeding time was significantly prolonged in UFH-treated mice (Figure 1).
Several studies have described both stimulatory and inhibitory effects of heparins on tumour growth and metastasis (Hejma et al., 1999). These diverse effects may be due to the ability of these drugs to affect other processes, such as angiogenesis, in addition to their anticoagulant properties (Folkman & Shing, 1992). In this study, although none of the PSAs affected the proliferation of cancer cells, both LLC tumour growth and microvessel densities were significantly suppressed by UFH and dalteparin but not by danaparoid. In KLN205 tumours, all the PSAs inhibited both tumour growth and angiogenesis in vivo. These results suggest that the suppression of tumour growth can be attributed to the inhibition of tumour angiogenesis. LMWH and UFH have been found to have different effects on angiogenesis in previous studies (Norrby, 1993; Pieper et al., 2002).
It was interesting that, in the absence of cancer cells, all three PSAs induced angiogenesis in vivo when they were included in the matrigel plug (Figure 3a). In particular, the matrigel plug containing danaparoid had a higher haemoglobin content than the others. Angiogenesis in matrigel plugs has also been found to be markedly enhanced by the addition of heparin (Passaniti et al., 1992). However, when PSAs were not included in the matrigel, VEGF-induced vessel formation in the matrigel was similarly inhibited by subcutaneous injections of each of the PSAs (Figure 3b). This suggests that the PSAs are able to modulate angiogenic growth factors.
Although various studies have evaluated the effects of UFH and LMWH on the proliferation of vascular endothelial cells in vitro (Smorenburg & van Noorden, 2001), few have investigated the effect of exogenously added heparan sulphate. In vitro, all three PSAs significantly inhibited the proliferation and migration of vascular endothelial cells (Figure 3a and b). However, the PSAs had no significant effect on endothelial cell morphogenesis in vitro (Figure 3c). The results depicted in Figures 3 and 4 suggest that, in the absence of cancer cells, the effects of the PSAs on angiogenesis are similar both in vitro and in vivo. In contrast, the effect of danaparoid on tumour angiogenesis was different in LLC and KLN205 tumours. Since the induction and maintenance of tumour angiogenesis requires angiogenic growth factors produced by cancer cells (Carmeliet, 2000), the diverse effects of danaparoid on angiogenesis in LLC and KLN205 tumours could be due to differences in the effects of danaparoid on the production of these angiogenic growth factors.
Of all the angiogenic factors produced by tumours, VEGF is one of the most potent (Ferrara et al., 2003). The interaction of VEGF with its receptor is believed to play a major role in angiogenesis in human tumours (Ferrara, 2002). We found that, in LLC tumours, UFH and dalteparin suppressed VEGF production in vivo, whereas danaparoid did not. This result is consistent with those from the in vitro experiments in which VEGF release from LLCs was determined. In KLN205 tumours, all the PSAs significantly inhibited VEGF production in tumours, in vivo, and from KLN205 cells in vitro. Thus, the effect of danaparoid on VEGF production in cancer cells depends on their type. Although VEGF is bound to heparins (El-Sheikh et al., 2002; Lever & Page, 2002), our study showed that both UFH and dalteparin had the ability to suppress VEGF production in cancer cells at the mRNA level.
Owing to structural similarities with heparan sulphate, UFH and LMWH inhibited the enzyme heparanase, which hydrolyses the internal glycosidic linkage of heparan sulphate in basement membranes and ECM (Parish et al., 1987; Voldavsky & Friedmann, 2001). In cancer progression, heparanase has a role not only in invasion and metastasis but also in the induction of tumour angiogenesis (Elkin et al., 2001; Goldshmidt et al., 2002). UFH and LMWH both inhibited heparanase activity in LLC tumours, whereas danaparoid did not (Figure 7a), which is consistent with the inhibitory effects of UFH and dalteparin on heparanase mRNA expression in LLCs in vivo. On the other hand, heparanase activity in KLN205 tumours was significantly inhibited only by dalteparin, suggesting the lack of a relationship between heparanase activity and tumour angiogenesis in KLN205 cells (Figure 7b).
Taken together, our data from LLC and KLN205 tumours suggest that PSAs inhibit tumour angiogenesis, at least in part, by suppressing VEGF production rather than heparanase production in cancer cells. The effect of danaparoid on VEGF production in cancer cells might be cell type specific, resulting in different effects on tumour angiogenesis. Since there are several other angiogenic growth factors such as basic fibroblast growth factor, placenta-like growth factor, platelet-derived growth factors and interleukin-8, further studies are needed to clarify the antiangiogenic mechanism of PSAs.
Subcutaneous injections of danaparoid did not inhibit heparanase in either LLC or KLN205 tumours. Heparanase is known to have an important role in cancer metastasis as it degrades the extracellular matrix (Parish et al., 1987) and is involved in the progression of cancer (Takahashi et al., 2004). Our results suggest that, in contrast to heparins, danaparoid does not inhibit cancer metastasis.
In our experiments, although both UFH and dalteparin inhibited angiogenesis in vivo and in vitro, the effect of dalteparin was generally seen before that of UFH. Moreover, at doses that are similarly potent at inhibiting FXa, dalteparin is less likely to cause bleeding than UFH (Figure 1a). Therefore, our results suggest that LMWH may have advantages compared to other PSAs as a treatment for DIC in patients with solid tumours. Recently, danaparoid has become widely used as an anticoagulant in patients with active or past heparin-induced thrombocytopaenia (Tardy-Poncet et al., 1999). However, the precise mechanism of its selective action on FXa is unclear. Since we found that the effect of danaparoid on tumour growth and angiogenesis differs between cancer cells, it might be of clinical importance to evaluate the survival of various cancer patients treated with danaparoid.
Acknowledgments
We thank Miss Eri Fujita for her excellent technical assistance and Mr Grant Crittenden for his review of the English. This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Japan (No. 15590795), and a Grant from the Kanae Foundation for Life & Socio-Medical Science to S.E.
Abbreviations
- DIC
disseminated intravascular coagulation
- FXa
activated coagulation factor X
- LLC
Lewis lung carcinoma
- LMWH
low molecular weight heparin
- PSA
polysaccharide anticoagulant
- UFH
unfractionated heparin
- VEGF
vascular endothelial growth factor
References
- BIJSTERVELD N.R., HETTIARACHCHI R.J.K., PETERS R., PRINS M.H., LEVINE M., BÜLLER H.R. Low-molecular weight heparins in venous and arterial thrombotic disease. Thromb. Haemost. 1999;82 Suppl 2:139–147. [PubMed] [Google Scholar]
- CARMELIET P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- CARMELIET P., JAIN R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- ELKIN M., ILAN N., ISHAI-MICHAELI R., FRIEDMANN Y., PAPO O., PECKER I., VLODAVSKY I. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- EL-SHEIKH A., LIU C., HUANG H., EDGINGTON T.S. A novel vascular endothelial growth factor heparin-binding domain substructure binds to glycosaminoglycans in vivo and localizes to tumor microvascular endothelium. Cancer Res. 2002;62:7118–7123. [PubMed] [Google Scholar]
- FERRARA N. VEGF and the quest for tumor angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- FERRARA N., GERBER H.P., LE-COUTER J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J., SHING Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv. Exp. Med. Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- GOLDSHMIDT O., ZCHARIA E., ABRAMOVITCH R., METZGER S., AINGORN H., FRIEDMANN Y., SCHIRRMACHER V., MITARI E., VLODAVSKY I. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10031–10036. doi: 10.1073/pnas.152070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEJMA M., RADERER M., ZIELINSKI C.C. Inhibition of metastases by anticoagulants. J. Natl. Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- HETTIARACHCHI R.J.K., SMORENBERG S.M., GINSBERG J., LEVINE M., PRINS M.H., BÜLLER H.R. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb. Haemost. 1999;82:947–952. [PubMed] [Google Scholar]
- HIRSH J., WARKENTIN T.E., SHAUGHNESSY S.G., ANAND S.S., HALPERIN J.L., RASCHKE R., GRANGER C., OHMAN E.M., DALEN J.E. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119 Suppl 1:64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- KANDA A., EBIHARA S., TAKAHASI H., SASAKI H. Loxoprofen sodium suppresses mouse tumor growth by inhibiting vascular endothelial growth factor. Acta Oncol. 2003;42:62–70. doi: 10.1080/0891060310002258. [DOI] [PubMed] [Google Scholar]
- LEVER R., PAGE C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- LEVI M., TEN CATE H. Disseminated intravascular coagulation. N. Engl. J. Med. 1999;19:586–592. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- MEULEMAN D.G. Orgaran (ORG 10172): its pharamacological profile in experimental models. Haemostasis. 1992;22:58–65. doi: 10.1159/000216296. [DOI] [PubMed] [Google Scholar]
- NORRBY K. Heparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis. 1993;23:141–149. doi: 10.1159/000216923. [DOI] [PubMed] [Google Scholar]
- OKAJIMA K., SAKAMOTO Y., UCHIDA M. Heterogeneity in the incidence and clinical manifestations of disseminated intravascular coagulation: a study of 204 cases. Am. J. Hematol. 2000;65:215–222. doi: 10.1002/1096-8652(200011)65:3<215::aid-ajh7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- PARISH C.R., COOMBE D.R., JAKOBSEN K.B., BENNETE F.A., UNDERWOOD P.A. Evidence that sulphated polysaccharides inhibit tumor metastasis by blocking tumor-cell-derived heparanases. Int. J. Cancer. 1987;40:511–518. doi: 10.1002/ijc.2910400414. [DOI] [PubMed] [Google Scholar]
- PASSANITI A., TAYLOR R.M., PILI R., GUO Y., LONG P.V., HANEY J.A., PAULY R.R., GRANT D.S., MARTIN G.R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- PIEPER J.S., HAFMANS T., VAN WACHEM P.B., VAN LUYN M.J., BROUWER L.A., VEERKAMP J.H., VAN KUPPEVELT T.H. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J. Biomed. Mater. Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- SALLAH S., WAN J.Y., NGUYEN N.P., HANRAHAN L.R., SIGOUNAS G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb. Haemost. 2001;86:828–833. [PubMed] [Google Scholar]
- SMORENBURG S.M., VAN NOORDEN C.J.F. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol. Rev. 2001;53:93–105. [PubMed] [Google Scholar]
- TAKAHASHI H., EBIHARA S., OKAZAKI T., SUZUKI S., ASADA M., KUBO H., SASAKI H. Clinical significance of heparanase activity in primary resected non-small cell lung cancer. Lung Cancer. 2004;45:207–214. doi: 10.1016/j.lungcan.2004.02.007. [DOI] [PubMed] [Google Scholar]
- TARDY-PONCET B., TARDY B., REYNAUD J., MAHUL P., MISMETTI P., MAZET E., GUYOTAT D. Efficacy and safety of danaparoid (ORG 10172) in critically ill patients with heparin-associated thrombocytopenia. Chest. 1999;115:1616–1620. doi: 10.1378/chest.115.6.1616. [DOI] [PubMed] [Google Scholar]
- VOLDAVSKY I., FRIEDMANN Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J. Clin. Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDNER N., SEMPLE J.P., WELCH W.R., FOLKMAN J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N. Engl. J. Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]