Abstract
The in vivo effect of inhibitors of fatty acid amide hydrolase (FAAH) upon oedema volume and FAAH activity was evaluated in the carrageenan induced hind paw inflammation model in the mouse. Oedema was measured at two time points, 2 and 4 h, after intraplantar injection of carrageenan to anaesthetised mice.
Intraperitoneal (i.p.) injections of the FAAH inhibitor URB597 (0.1, 0.3, 1 and 3 mg kg−1) 30 min prior to carrageenan administration, dose-dependently reduced oedema formation. At the 4 h time point, the ED50 for URB597 was ∼0.3 mg kg−1. Indomethacin (5 mg kg−1 i.p.) completely prevented the oedema response to carrageenan.
The antioedema effects of indomethacin and URB597 were blocked by 3 mg kg−1 i.p. of the CB2 receptor antagonist SR144528. The effect of URB597 was not affected by pretreatment with the peroxisome proliferator-activated receptor γ antagonist bisphenol A diglycidyl ether (30 mg kg−1 i.p.) or the TRPV1 antagonist capsazepine (10 mg kg−1 i.p.), when oedema was assessed 4 h after carrageenan administration. The CB1 receptor antagonists AM251 (3 mg kg−1 i.p.) and rimonabant (0.5 mg kg−1 i.p.) gave inconsistent effects upon the antioedema effect of URB597.
FAAH measurements were conducted ex vivo in the paws, spinal cords and brains of the mice. The activities of FAAH in the paws and spinal cords of the inflamed vehicle-treated mice were significantly lower than the corresponding activities in the noninflamed mice. PMSF treatment almost completely inhibited the FAAH activity in all three tissues, as did the highest dose of URB597 (3 mg kg−1) in spinal cord samples, whereas no obvious changes were seen ex vivo for the other treatments.
In conclusion, the results show that in mice, treatment with indomethacin and URB597 produce SR144528-sensitive anti-inflammatory effects in the carrageenan model of acute inflammation.
Keywords: Endocannabinoid, fatty acid amide hydrolase, URB597, indomethacin, SR144528, carrageenan, inflammation
Introduction
There is evidence to suggest that compounds affecting the endogenous cannabinoid (‘endocannabinoid') system may have therapeutic usefulness in a number of areas, including stroke, multiple sclerosis, pain and inflammation (see Pertwee, 2002; Rice et al., 2002; Croxford, 2003; Fowler, 2004 for recent reviews). The endocannabinoids, of which the two most well investigated are anandamide (arachidonoylethanolamide, AEA) and 2-arachidonoyl glycerol, are synthesised on demand (see Bisogno et al., 2005; Lambert & Fowler, 2005), and exert most of their effects by actions upon cannabinoid CB1 and CB2 receptors (Devane et al., 1988; Munro et al., 1993) and (for AEA) the transient receptor potential vanilloid type 1 receptors (TRPV1) (Zygmunt et al., 1999). The actions of the endocannabinoids are terminated by their rapid removal through uptake and intracellular degradation (see Bisogno et al., 2005; Lambert & Fowler, 2005). Inhibition of endocannabinoid metabolism is considered a promising therapeutic target on its own, since it presumably would increase the endocannabinoid level only at the location where their synthesis has been stimulated, but also in combination with exogenous AEA, prolonging its availability.
AEA is metabolised mainly by the enzyme fatty acid amide hydrolase (FAAH), producing arachidonic acid and ethanolamine (Deutsch & Chin, 1993) and also by other enzymes, including cyclooxygenase-2 (COX-2), producing prostaglandin-ethanolamides, also called prostamides (Yu et al., 1997). Although a number of compounds have been shown to inhibit FAAH, including the serine protease inhibitor phenylmethylsulphonyl fluoride (PMSF, Deutsch & Chin, 1993) and a number of nonsteroidal anti-inflammatory drugs (NSAIDs) including indomethacin and flurbiprofen (Paria et al., 1996; Fowler et al., 2003), it is only in recent years that selective compounds have been identified. Thus, for example, the carbamate compound URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate) shows good selectivity for FAAH vs other targets such as CB receptors and monoacylglycerol lipase, the main catabolic enzyme for 2-arachidonoyl glycerol in the brain (Kathuria et al., 2003). These authors demonstrated further that this compound produced potentially important effects in a model of anxiety (the elevated plus maze in the rat) as well as antinociceptive effects in the mouse hotplate test (Kathuria et al., 2003). URB597 also reduces, in a manner blocked by a CB1 (but not a CB2) receptor antagonist, established inflammatory pain in the rat produced by prior intraplantar (i.pl.) injection of Freund's complete adjuvant (Wilson et al., 2005). Other selective FAAH inhibitors have been reported to produce analgesic effects (Lichtman et al., 2004a) and to have potentially beneficial effects upon tumour cell growth (Bifulco et al., 2004).
In two recent studies, it was reported that genetic deletion of either all FAAH (Lichtman et al., 2004b) or only peripheral FAAH (Cravatt et al., 2004) produced mice that were less sensitive to the inflammatory effects of local administration of carrageenan into the hind paw. Genetic ablation of FAAH also produces protection against intestinal inflammation (Massa et al., 2004). It would be useful to know whether such effects can also be produced by pharmacological inhibition of FAAH. In the present study, the abilities of URB597 and PMSF to inhibit carrageenan-induced oedema have been investigated and compared with indomethacin, a standard NSAID known to produce robust effects in this model (see e.g. Siqueira-Junior et al., 2003). In addition, CB receptor antagonists have been used in an attempt to elucidate whether the endocannabinoid system is involved in the anti-inflammatory effects of these compounds. A report of some of the present data was presented to the 2004 Meeting of the International Cannabinoid Research Society in June of that year (abstract published, Holt et al., 2004a).
Methods
Compounds
Radiolabelled arachidonoylethanolamide [ethanolamine 1-3H] ([3H]AEA, 60 Ci mmol−1) was obtained from American Radiolabelled Chemicals, Inc. (St Louis, MO, U.S.A.), λ-carrageenan, BADGE (bisphenol A diglycidyl ether), capsazepine, and PMSF from Sigma-Aldrich, indomethacin and URB597 from the Cayman Chemical Co., Ann Arbor, MI, U.S.A. and AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) from Tocris Cookson (Bristol, U.K.). SR144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methyl-benzyl)-pyrazole-3-carboxamide) and rimonabant (SR141716, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride) was kindly supplied by Sanofi-Aventis, Montpellier, France.
Animals
Male C57BL/6J mice (9 weeks old, Harlan, Italy) were housed under controlled temperature (22±1°C), humidity (60±10%) and light (12 h/day) and allowed to acclimatise for at least 3 days before used in the experiments. Standard food and water was available ad libitum. Animal care was in accordance with Italian State regulations governing the care and treatment of laboratory animals (Permission no. 94/2000A).
Induction and evaluation of inflammation
The animals were weighed and thereafter anaesthetised by intraperitoneal (i.p.) injection of pentobarbital (60 mg kg−1). Acute inflammation was induced by i.pl. injection of 20 μl of λ-carrageenan (2% w v−1 in saline) into the right hind paw. Control animals received a corresponding i.pl. injection of vehicle. The paw volume of the injected paw as well as the contralateral paw was then measured by a plethysmometer (Ugo Basile, Varese, Italy), before and 2 and 4 h after the carrageenan injection. At the 2 h time point, most of the animals had recovered from the anaesthesia, and at the 4 h time point all animals had recovered. The volume of the contralateral paw was subtracted from the volume of the injected paw, to obtain the oedema volume. The animals were decapitated 4 h after induction of inflammation and spinal cord, brain, and the inflamed paw were removed, immediately frozen in liquid nitrogen, and kept at −80°C.
Drug treatment
Indomethacin (2 or 5 mg kg−1), URB597 (0.3 mg kg−1), PMSF (30 mg kg−1), BADGE (30 mg kg−1), and capsazepine (10 mg kg−1) were all administered i.p. (0.1 ml 10 g−1 body weight) 30 min prior to carrageenan treatment, while the CB-receptor antagonists SR144528 (3 mg kg−1) and AM251 (1 mg kg−1) were administered i.p. 45 min prior to carrageenan treatment. In an additional experiment, SR144528 (1 mg kg−1) and rimonabant (0.5 mg kg−1) were administered i.p. 40 min prior to carrageenan. The rationale for the doses used is taken up in detail in the discussion of this paper. All substances were dissolved in ethanol and diluted with 1 : 20 ethanol : carboxymethylcellulose (1.5% w v−1 in saline) except capsazepine (dissolved in 1 : 1 : 8 ethanol: tween-80: saline) and rimonabant (dissolved in 1 : 2 : 7 tween-80: DMSO: H2O). All solutions were preheated, vortexed and sonicated before administration, and in all cases control animals received corresponding i.p. injections of vehicle.
FAAH activity assay
FAAH measurements were undertaken after transport of the samples to Sweden. Tissues were thawed, weighed and homogenised in 5 ml Tris-HCl buffer gram wet weight−1 (50 mM Tris-HCl, 1 mM EDTA, 3 mM MgCl2, pH 7.4). Paws were centrifuged at 500 r.p.m. (Beckman GPR) for 5 min, and all homogenates were sonicated and stored at −80°C until analysed. The FAAH activity of each sample was measured by monitoring the release of [3H]ethanolamine after incubation of homogenate with AEA labelled in this part of the molecule. Homogenised tissue (spinal cord: 15 μg protein assay−1 (second series 4 μg protein assay−1), brain: 10 μg protein assay−1, paw: 25 μg protein assay−1, unless otherwise stated) in assay buffer (116 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 25 mM HEPES, 1 mM NaH2PO4, 0.8 mM MgSO4, pH 7.0) was incubated at 37°C (brain and spinal cord, 4 min; paw, 15 min), with 0.5 μM [3H]AEA in the presence of 10 mg ml−1 fatty acid-free bovine serum albumin (total assay volume 100 μl), and the reaction stopped by addition of 40 μl charcoal in 160 μl 0.5 M HCl (Boldrup et al., 2004). Protein contents per assay were chosen on the basis of preliminary experiments using some of the samples to establish optimal conditions. The samples were vortexed and left to sediment for 30 min before centrifugation at 2500 r.p.m. (Beckman GPR) for 10 min. Aliquots of the supernatant were counted for tritium content by liquid scintillation spectroscopy with quench correction.
Statistics
Statistical differences between groups were determined using one-way ANOVA measures with post hoc Tukey's multiple comparison test using the GraphPad Prism software (GraphPad software Inc., San Diego, CA, U.S.A.). The initial study (summarised in Table 1) was undertaken on several different experimental days, with different groups, which were not randomised. However, there were no significant differences between the observed levels of oedema in response to the carrageenan from day to day (data not shown). In addition, when groups from each experimental day were analysed per se, in most cases, the level of significance was the same as shown in Table 1, the only exceptions being in cases where the n values were very low (such as comparison between the carrageenan control and AM251 treated mice at the 2 h time point). Most importantly for the study, the significance values for the comparisons vs SR144528 were the same for the individual experimental days as when the total data from all experimental days was used.
Table 1.
Effect of PMSF, URB597 and indomethacin upon carrageenan paw oedema in the mouse
| Treatment | Oedema (μl) | ||
|---|---|---|---|
| 2 h | 4 h | n | |
| Noninflamed control | 8±4*** | 5±2*** | 27/25 |
| Vehicle control | 117±3 | 109±4 | 34/30 |
| PMSF (30 mg kg−1) | 49±5*** | 56±7*** | 8/8 |
| URB597 (0.3 mg kg−1) | 54±6*** | 55±6*** | 13/13 |
| Indomethacin (5 mg kg−1) | 13±7*** | 0±5*** | 11/6 |
| AM251 (1 mg kg−1) | 88±6* | 75±4** | 6/6 |
| URB597 (0.3 mg kg−1)+AM251 (1 mg kg−1) | 78±8*** | 77±9* | 6/6 |
| Indomethacin (5 mg kg−1)+AM251 (1 mg kg−1) | 83±5** | 62±10*** | 6/6 |
| SR144528 (3 mg kg−1) | 105±8 | 92±13 | 6/6 |
| URB597 (0.3 mg kg−1)+SR144528 (3 mg kg−1) | 105±12 | 92±8 | 6/6 |
| Indomethacin (5 mg kg−1)+SR144528 (3 mg kg−1) | 107±8 | 103±4 | 6/6 |
Means±s.e.m. of the carrageenan-induced oedema volume (μl for the treated paw minus corresponding volume of the contralateral paw). The n values refer to the sample sizes for the 2 and 4 h time points, respectively. ***P<0.001, **P<0.01, *P<0.05 vs vehicle control, Tukey's multiple comparison following significant one-way ANOVA. The treatment groups AM251, URB597+AM251 and indomethacin+AM251 were not significantly different from each other. Similarly, the treatment groups SR144528, URB597+SR144528 and indomethacin+SR144528 were not significantly different from each other (P>0.05, Tukey's test).
Results
Antioedema effects of PMSF, URB597 and indomethacin
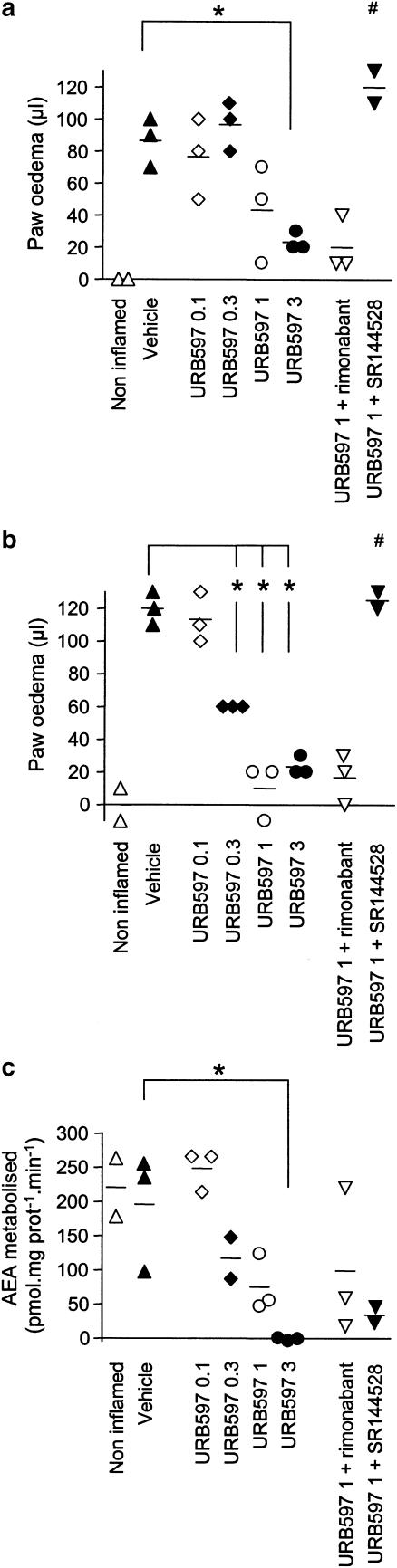
The volume of the carrageenan injected hind paw was measured by a plethysmometer and compared to the volume of untreated contralateral paw to obtain the oedema volume (Table 1). A robust oedema response could be seen 2 and 4 h after the injection (compare the noninflamed (i.e. no carrageenan in either paw) and vehicle (i.e. carrageenan treated ipsilateral paw) data in Table 1). Consistent with the literature (see e.g. Siqueira-Junior et al., 2003 for mouse data) prophylactic treatment with indomethacin (5 mg kg−1) completely inhibited the oedema produced by carrageenan. A lower dose (2 mg kg−1) was also investigated and found to reduce the oedema by about half (data not shown). PMSF (30 mg kg−1) and URB597 (0.3 mg kg−1) both significantly decreased the oedema. In an additional experiment, URB597 (0.1, 0.3, 1 and 3 mg kg−1) dose-dependently inhibited the oedema formation (Figure 1a and b), the two highest concentrations to a level that was not significantly different (P>0.05) from the noninflamed control animals when measured 4 h after the exposure to carrageenan (Figure 1b). In this additional experiment, only a small sample size was used, which was possible due to the limited data spread with this model. In consequence, we have elected to show the data as a scattergram to allow the reader to see the individual data.
Figure 1.
Effect of different doses of URB597 (0.1, 0.3, 1 and 3 refer to the doses in mg kg−1 i.p.) upon carrageenan-induced oedema and its sensitivity to CB-receptor antagonists. The oedema was measured 2 h (a) and 4 h (b) after the exposure to carrageenan, and expressed as μl for the treated paw minus corresponding volume of the contralateral paw. Ex vivo FAAH activity (c) was measured in spinal cord (4 μg protein assay−1) from the same animals. The values for the individual animals are displayed in scattergrams and the group means are indicated by a line. *P<0.05 vs the vehicle treated controls. #P<0.05 for the animals treated with URB597 (1 mg kg−1)+SR144528 (1 mg kg−1 i.p.) vs the animals treated with 1 mg kg−1 URB597. The dose of rimonabant used was 0.5 mg kg−1 i.p.
Pretreatment with the CB1-receptor antagonist AM251 (1 mg kg−1) reduced the oedema per se at both time points (Table 1), thereby complicating interpretation of the data and precluding determination as to whether the antioedema effect of URB597 could be prevented by this compound. However, the combination of rimonabant (0.5 mg kg−1) and 1 mg kg−1 URB597 produced a reduction of oedema similar to that seen with URB597 alone (Figure 1a and b). The antioedema effect produced by indomethacin, on the other hand, was significantly reduced by AM251 treatment (Table 1). The CB2-antagonist SR144528 (3 mg kg−1) lacked significant effect per se (Table 1), but completely blocked the effect of both URB597 and indomethacin (Table 1). The blockade of the effect of URB597 was also seen with a higher dose of the FAAH inhibitor (1 mg kg−1) and a lower dose of the antagonist (1 mg kg−1) (Figure 1a and b).
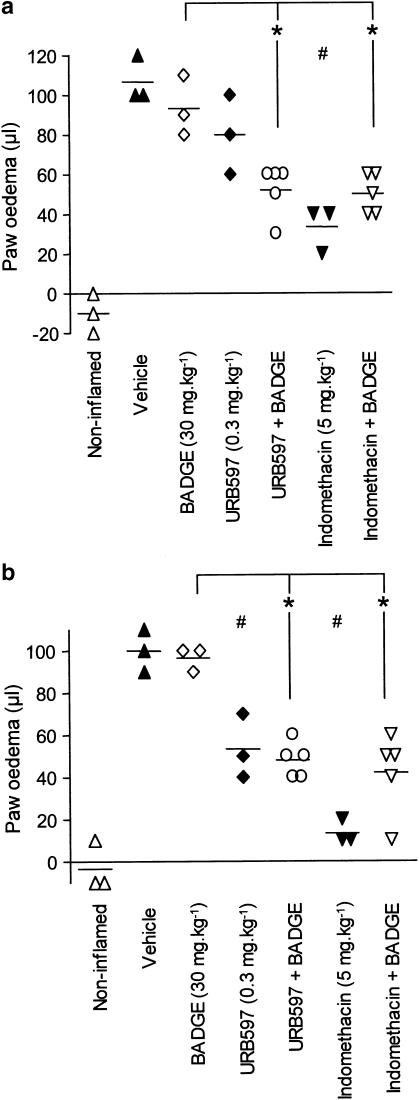
In a second series of experiments, the effect of BADGE (30 mg kg−1) on the carrageenan-induced oedema was measured after 2 h (Figure 2a) and 4 h (Figure 2b). BADGE pretreatment was without effect per se, and did not significantly alter the antioedema effect of URB597 or indomethacin at the 4 h time point. At the 2 h time point, the effect of URB597 alone had not reached significance (in contrast to the data shown in Table 1 but consistent with the experiment shown in Figure 1), but a significant antioedema effect was seen for the combination of BADGE+URB597.
Figure 2.
Effect of BADGE (30 mg kg−1 i.p.), URB597 (0.3 mg kg−1 i.p.) and indomethacin (5 mg kg−1 i.p.) upon the oedema response to carrageenan. BADGE was administered 15 min prior to URB597 or indomethacin and the carrageenan was injected into the hind paw 30 min later. The oedema volume was measured after 2 h (a) and 4 h (b) and expressed as μl for the treated paw minus corresponding volume of the contralateral paw. The values for the individual animals are displayed in scattergrams and the group means are indicated by a line. *P<0.05 vs the animals treated with BADGE alone. For the animals not treated with BADGE, #P<0.05 vs the vehicle treated controls. The oedemas in the presence of BADGE were not significantly different from the corresponding vehicle, URB597 and indomethacin oedemas (P>0.05).
The effect of the TRPV1-antagonist capsazepine (10 mg kg−1) on the ability of URB597 (0.3 mg kg−1) to inhibit oedema formation was also investigated, but the oedema was not significantly different from treatment with URB597 (0.3 mg kg−1) alone at either time point (P>0.05, data not shown).
FAAH activity
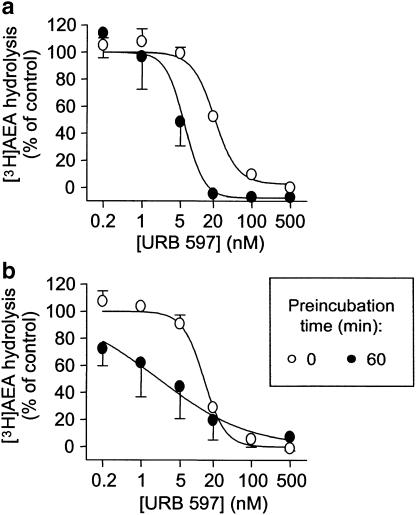
In their paper, Kathuria et al. (2003) reported that URB597 inhibited rat brain FAAH with an IC50 value of 4.6±1.6 nM, corresponding to a pI50 value of 8.34. However, data on the in vitro potency of this compound towards mouse brain FAAH were not presented. In consequence, we investigated the potency of this compound towards FAAH in three of the control mouse brain samples that were generated in this study. In the absence of a preincubation between inhibitor and enzyme, URB597 inhibited the hydrolysis of 0.5 μM [3H]AEA with pI50 values of 7.65±0.05 and 7.87±0.05 being found at assay buffer pH of 7 (Figure 3a) and 9 (Figure 3b), respectively. Preincubation of the inhibitor and enzyme for 60 min prior to addition of substrate increased the inhibitory potency of the compound to give pI50 values of 8.31±0.72 and 8.68±0.28 at assay buffer pH values of 7 and 9, respectively (Figure 3). Parallel experiments conducted using rat brain homogenates gave similar results, with pI50 values of 7.76±0.07 and 7.78±0.06 (no preincubation) and 8.20±0.07 and 8.68±0.07 (60 min preincubation), being obtained at pH 7 and 9, respectively (data not shown).
Figure 3.
Inhibition of mouse brain FAAH by URB597. Control (i.e. noninflamed) brain homogenates were incubated at an assay pH of either 7 (a) or 9 (b), and 3 (pH 7) or 1.5 (pH 9) μg protein assay−1 were incubated with [3H]AEA for 10 min. Shown are means and s.e.m., n=2–3.
FAAH activity was also measured ex vivo in membrane preparations of brain, spinal cord, and paws of the carrageenan exposed animals (Table 2), where a significant difference between the FAAH activities in the noninflamed and vehicle control animals was seen (Table 2). The process of homogenisation naturally involves a considerable dilution of free inhibitor, so that any noncovalent FAAH inhibition will be lost. Consistent with its action as an irreversible inhibitor, PMSF treatment resulted in a complete loss of FAAH activity measured ex vivo in all three tissues examined. In contrast, none of the other treatments resulted in a decreased FAAH activity (Table 2). However, in the additional series of experiments, the increasing i.p. doses of URB597 (0.1, 0.3, 1 and 3 mg kg−1) produced an increasing ex vivo inhibition of FAAH activity in spinal cord, with the highest dose showing complete inhibition (Figure 1c). It should be noted that the observed activity in these samples was generally higher than in the first set of experiments. The samples from the first set of experiments were frozen for a longer period than those from the second series, and in our view the most likely explanation is that the homogenisation process used in the second series of experiments gave a higher recovery of FAAH than in the first series of experiments, hence the use of a lower protein concentration per assay (4 μg vs 15 in the first series). Whatever the explanation, the most important factor is that all the samples within a given experiment were homogenised and analysed at the same time, so that such differences in homogenisation efficiencies would not affect the intraexperimental variation and hence comparison of ex vivo treatment effects.
Table 2.
Effect of FAAH inhibitors and NSAIDs upon FAAH activity ex vivo in the mouse
| Treatment | FAAH activity (pmol min−1 mg protein−1) | |||
|---|---|---|---|---|
| Brain | Sp. cord | Paw | n | |
| Noninflamed control | 175±8 | 80±3** | 1.94±0.14*** | 25 |
| Vehicle control | 202±6 | 57±4 | 1.17±0.09 | 30 |
| PMSF (30 mg kg−1) | 2±1*** | 6±0.3*** | -0.07±0.03*** | 8 |
| URB597 (0.3 mg kg−1) | 188±11 | 37±6 | 0.81±0.05 | 13 |
| Indomethacin (5 mg kg−1) | 171±7 | 44±12 | 1.90±0.16* | 6 |
| AM251 (1 mg kg−1) | 153±32 | 37±15 | 1.37±0.18 | 6 |
| URB597 (0.3 mg kg−1)+AM251 (1 mg kg−1) | 172±8 | 45±10 | 1.21±0.08 | 6 |
| Indomethacin (5 mg kg−1)+AM251 (1 mg kg−1) | 183±11 | 53±13 | 1.76±0.22 | 6 |
| SR144528 (3 mg kg−1) | 200±6 | 58±12 | 1.09±0.14 | 6 |
| URB597 (0.3 mg kg−1)+SR144528 (3 mg kg−1) | 205±10 | 66±6 | 0.97±0.08 | 6 |
| Indomethacin (5 mg kg−1)+SR144528 (3 mg kg−1) | 192±9 | 79±6 | 0.91±0.16 | 6 |
Means±s.e.m. of the FAAH activity (pmol [3H]AEA metabolised min−1 mg protein−1) measured ex vivo in animals killed 4 h after carrageenan administration is shown. ***P<0.001, **P<0.01, *P<0.05 vs vehicle control, Tukey's multiple comparison following significant one-way ANOVA. Sp. Cord, spinal cord.
Discussion
In the present study, the effect of FAAH inhibitors on the ability of carrageenan to elicit an oedema in the mouse paw has been investigated and compared with the effect of indomethacin. In experiments of the type undertaken here, a limited number of doses can be investigated, thereby rendering it essential that the doses used are relevant, that is that sufficient drug is given to produce a complete inhibition of the target protein, but not at the cost of selectivity. Thus, for example, the dose of 30 mg kg−1 of PMSF used here was chosen since it has been reported to produce complete inhibition of brain FAAH and a robust potentiation of the effects of AEA in vivo, whereas higher doses of this compound produce effects per se that are not related to effects on the endocannabinoid system (Compton & Martin, 1997; Quistad et al., 2002). As expected, the dose of PMSF completely and irreversibly inhibited FAAH activity in the brain (Table 2). In a recent study we found that this dose of PMSF produces complete inhibition of brain FAAH but only partial inhibition of lung AEA hydrolysis (Holt et al., 2004b). The finding here that complete inhibition was also seen in the spinal cord and paw samples, would suggest that the partial inhibition in the lung is unlikely to reflect tissue-dependent differences in PMSF distribution.
The doses of URB597 were chosen to straddle 0.3 mg kg−1 for which a pharmacological profile has been established in rodents (increased levels of AEA and potentiation of the effects of exogenous AEA on hypothermia) (Kathuria et al., 2003; Fegley et al., 2005). In contrast to PMSF, the residual inhibition seen ex vivo (upon homogenisation and assay, and thereby dilution, of the samples) was not obvious for URB597 in the first experiment. However, 0.3 mg kg−1 appears to be a threshold dose for this compound, since a degree of ex vivo inhibition was seen at this dose in the small additional experiment. Similarly, the ability of this dose of URB597 to reduce oedema at the 2 h time point was seen in the first, but not the additional experiments, whereas a robust effect was seen in all experiments at the 4 h time point. Higher concentrations (1 and 3 mg kg−1) of URB597 produced both a clear ex vivo inhibition of FAAH and a robust effect on oedema at both time points. It should be pointed out that although the URB597 was obtained from the same supplier, a different sample was used for the initial experiments than for the subsequent experiments, and thus small differences in the degree of solubility from experiment to experiment may affect the observed results for threshold doses.
A report that uterine FAAH activity is reduced ex vivo following administration of indomethacin (100 μg mouse−1) notwithstanding (Paria et al., 1996), the lack of observed irreversible inhibition ex vivo was expected for indomethacin, given that its inhibition of FAAH is presumably reversible. The data with URB597, however, deserve some comment, in view of the hypothesis that carbamate inhibitors of this type exert their inhibition secondary to covalent attachment to a key serine residue of FAAH (Basso et al., 2004), and in view of the recent finding that 0.3 mg kg−1 of URB597 produces a reduced mouse brain FAAH activity when measured ex vivo 2 h after i.p. administration (Fegley et al., 2005). In their original paper, Kathuria et al. (2003) reported that this dose of URB597 gives a robust inhibition of rat brain FAAH measured ex vivo for a least 6 h after treatment. However, inspection of their data (Figure 2b of Kathuria et al., 2003) indicates that the inhibition of rat brain peaks at 1 h after treatment and then decreases thereafter. Indeed, in their follow-up paper, Fegley et al. (2005) reported that rat brain FAAH activity measured ex vivo had returned to normal by 24 h, which is rather quicker than would be expected for a stable irreversibly inhibited enzyme. For comparison, the t1/2 for recovery of mouse brain FAAH following treatment with octylsulphonyl fluoride is in the range 2–3 days (Quistad et al., 2002). Although there is a risk of overinterpreting these results, the time-dependency of the inhibition measured ex vivo is consistent with the hypothesis that inhibition of FAAH by URB597 may be either covalent but instable, or alternatively tight binding, but not covalent. Our finding here that the inhibitor is as potent in the mouse as in the rat and also shows time-dependent inhibition in vitro can simply be interpreted by the suggestion that the inhibition in the mouse is also tight binding, but that the ‘tightness' of binding is lower (or alternatively, that the stability of the covalent binding is lower), thereby allowing a threshold for loss of inhibition at a dose of 0.3 mg kg−1 when measured ex vivo after 4.5 h, but not after 2 h. It may be possible to compare the different molecular structures of mouse and rat FAAH in molecular modelling studies of the type recently reported for URB597 by Mor et al. (2004) to shed further light on this issue.
With respect to the CB receptor antagonists, ‘standard' doses of AM251 (1 mg kg−1), rimonabant (0.5 mg kg−1) and SR144528 (1 and 3 mg kg−1) were used. AM251 is preferable to rimonabant in view of the reports of putative non-CB1 receptors that are rimonabant-selective but AM251-resistant (Ford et al., 2002; Bátkai et al., 2004), although non-CB1 receptor effects of AM251 have been reported in the literature (Jin et al., 2004). SR144528 at a dose of 3 mg kg−1 i.p. blocks the ability of the CB2 receptor agonist GW405833 (1-(2,3-dichlorobenzoyl)-5-methoxy-2-methyl-(2-morpholin-4-yl) ethyl-1H indole) to reduce carrageenan-induced oedema in the rat paw (Clayton et al., 2002) and the antihyperalgesic effect of WIN55,212-2 in a model of carrageenan hyperalgesia in the mouse (Kehl et al., 2003). In contrast, an oral dose of SR144528 of 10 mg kg−1 (a dose producing an almost complete block of binding of [3H]CP-55,940 to mouse spleen CB2 receptors measured ex vivo, Rinaldi-Carmona et al., 1998) was ineffective in preventing the LPS-induced increase in plasma levels of TNF-α, an effect mediated by CB1 receptors (Croci et al., 2003). Thus, it is reasonable to conclude that effects seen with URB597 and PMSF at the doses used reflect FAAH inhibition, that a blockade of effects by SR144528 would point to mediation via CB2 receptors, but that blockade by AM251 is not necessarily an indication of a CB1 receptor-mediated effect.
The main findings of the present study are discussed below.
FAAH activity is affected by the carrageenan treatment
In the spinal cord and the paw, but not in the brain samples, the carrageenan treatment appeared to result in a reduction in FAAH activity at the 4 h time point when the noninflamed and inflamed controls were compared. The word ‘appeared' is used here, since although the result was highly significant, some of the other treatments resulted in similar levels of FAAH activity. Thus, for example, in the spinal cord, the combination of SR144528+indomethacin gave a similar ex vivo level of FAAH activity as seen for the noninflamed controls. In addition, in the small second experiment, the decrease was not seen, and higher specific activities were found for the spinal cords. Nonetheless, the large group sizes of the non-inflamed and inflamed controls in the first experiment (25 and 30 animals, respectively) instil confidence in the finding. Rat paw skin FAAH activity is not affected by formalin injection (Beaulieu et al., 2001), and croton oil-induced inflammation of the small intestine increases FAAH activity (Izzo et al., 2001) while lipopolysaccharide induced pulmonary inflammation does not affect lung FAAH activity (Holt et al., 2004b), leading to the conclusion that inflammation per se does not automatically lead to a consistently changed FAAH activity. There is evidence, however, that carrageenan administration results in an increased level of oxidative stress (Wang et al., 2004). Such events would be expected to cause severe damage, which has been shown in many systems to increasee the rate of synthesis of AEA and other N-acyl-ethanolamines (see e.g. Berger et al., 2004, for a recent example), and to decrease the activity of FAAH (Gubellini et al., 2002). If such mechanisms are operative here, a reduced rate of hydrolysis of AEA (and of other endogenous N-acylethanolamines), together with an increased rate of synthesis of these agents following the carrageenan injection, would result in higher circulating levels of AEA and palmitoylethanolamide in the spinal cord and/or the paw.
FAAH inhibitors reduce carrageenan-induced inflammation that in the case of URB597 is blocked by SR144528 but not by capsazepine or rimonabant
Consistent with the finding that a dose of 3 mg kg−1 URB597 s.c. gave a greater effect than a dose of 0.3 mg kg−1 s.c. upon established Freund's complete adjuvant-induced hypersensitivity in the rat (Wilson et al., 2005), a clear dose–response relationship was seen in the present study for the antioedema effect of URB597 (Figure 1a and b), where a dose of 1 mg kg−1 decreased the oedema production to a level undistinguishable from the noninflamed animals. Time course data are, however, also required to determine the ‘window of opportunity' for administration of FAAH inhibitors after the onset of the inflammation. Of course, the finding that the FAAH inhibitors used produce effects in the carrageenan model does not conclusively prove that it is their action upon FAAH that is the key to their efficacy here. PMSF is far from selective, and URB597 also has actions upon other serine hydrolases (Lichtman et al., 2004a). Nevertheless, the finding that pretreatment with URB597 and PMSF reduce the oedema response to carrageenan is in accordance with recent data that FAAH knockout mice are less sensitive to oedema produced by carrageenan treatment (see below) and would be consistent with an increased local concentration of AEA and/or other potentially relevant endogenous compounds that are substrates for FAAH, such as palmitoylethanolamide (Natarajan et al., 1984), 2-arachidonoylglycerol (Di Marzo et al., 1998; Goparaju et al., 1998) and arachidonoylglycine (Huang et al., 2001).
On the basis of the data shown in Table 1 and Figure 1, we conclude that the effect of URB597 can be blocked by SR144528 but not by either rimonabant or capsazepine at the doses used, while the effects of AM251 per se obfuscate interpretation of the data with this antagonist. Whether or not these effects of AM251 per se point to some sort of endocannabinoid tone involved in the inflammatory response requires further investigation. A note of caution should also be made with respect to capsazepine – although it has been reported to be very effective in the rat at the dose used (see e.g. Costa et al., 2004), it is less efficaceous in the mouse (see e.g. Di Marzo et al., 2000; Ikeda et al., 2001) and possibly higher doses are required. With respect to the effects of SR144528, there is good evidence in the literature that CB2 receptors may regulate oedema and hyperalgesia in response to carrageenan. Thus, in the rat, both responses are reduced following administration into the paw of the CB2-selective agonist AM1241 (Quartilho et al., 2003), and a similar antioedema effect of the CB2-selective agonist JTE-907 (N-(benzo [1,3]dioxol-5-ylmethyl)-7-methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3-carboxamide) has been reported in the mouse (Iwamura et al., 2001). Furthermore, i.pl. administration of AEA inhibits mechanically evoked responses of spinal neurones in carrageenan-inflamed rat paws in a manner that is blocked by SR144528 but not by rimonabant (Sokal et al., 2003).
With respect to studies on genetically modified mice, Lichtman et al. (2004b) measured the oedema after a 5-h period, and found that the increase in paw diameter for the FAAH−/− mice was ∼40% of that seen in the wild-type animals. More recently, these authors have also investigated mice lacking peripheral, but not central FAAH, and found that their oedema response to carrageenan is also reduced compared to heterozygote FAAH+/− mice, and that the reduction was to the level seen for FAAH−/− mice (Cravatt et al., 2004). This may mean that the local component of FAAH inhibition by URB597 and PMSF is sufficient to produce the effect upon oedema. In the data reported in Lichtman et al. (2004b), the reduction in oedema in the FAAH−/− mice was partially reversed by i.p. treatment with 3 mg kg−1 SR144528, in line with the present findings with URB597. However, no such reversal was seen with this dose of SR144528 in the FAAH−/− mice in their other report (Cravatt et al., 2004). This variation in SR144528 sensitivity between the two experimental series may reflect differences in relative local amounts of palmitoylethanolamide and AEA produced during the treatments, since the former exerts effects upon inflammation via both SR144528-dependent (Conti et al., 2002) and independent (Lo Verme et al., 2005) mechanisms.
The inhibition of carrageenan-induced oedema by indomethacin can be blocked by SR144528
It is of course well established that the primary mechanism of action of NSAIDs is the blockade of prostaglandin production. Thus, for example, i.p. pretreatment of rats with a neutralising monoclonal antibody against prostaglandin E2 (PGE2) reduces carrageenan induced oedema to the level seen following indomethacin (30 mg kg−1 p.o.) treatment (Portanova et al., 1996). Intrathecally given indomethacin can also prevent the oedema in rats, an effect reversed by i.t. administered PGE2 (Daher & Tonussi, 2003). Wallace et al. (1998) have argued that the antioedema effect of NSAIDs is correlated to the abilities of the compounds to inhibit COX-1 rather than COX-2. In the mouse, the level of carrageenan-induced oedema and its reduction by indomethacin (3 mg kg−1 p.o.) was similar in wild-type and COX-2 deficient mice (Wallace et al., 1998). In our hands, the COX-2 selective inhibitor nimesulide (which does not affect FAAH activity in vitro, Fowler et al., 2003) was less efficacious than indomethacin in the mouse model used here, doses of 2 and 10 mg kg−1 producing 25 and 42% reductions in the inflammation response to carrageenan at the 4 h time point (S. Holt & B. Costa, unpublished data), a result consistent with the main role of COX-1 as suggested by Wallace et al. (1998).
Against this background, the ability of SR144528 completely to block the antioedema effects of indomethacin are at first sight somewhat surprising, particularly since no such blockade was seen using rats (Conti et al., 2002). In that study, SR144528 had no effect per se, whereas other studies have shown both increased (Clayton et al., 2002) and decreased (Iwamura et al., 2001) oedema following carrageenan administration to rodents. However, there are reports in the literature that indomethacin may have effects upon the endocannabinoid system. Thus, Gühring et al. (2002) reported that the antinociceptive effect of spinally administered indomethacin in the formalin test in the mouse was inhibited with a CB1-antagonist, and that CB1 knockout mice were insensitive to indomethacin treatment (Gühring et al., 2002). On the basis of these, and other experiments, the authors suggested that the action of spinally administered indomethacin and flurbiprofen may be to increase the synthesis of endocannabinoids secondary to a build up of spinal arachidonic acid (Gühring et al., 2002; Ates et al., 2003). It is possible that in the carrageenan-model of oedema in the mouse (but not the rat), the action of indomethacin requires both inhibition of the production of COX-derived proinflammatory products and an increase in the local levels of endocannabinoids, and that blockade of either pathway may be sufficient to negate its antioedema effects. However, much more work is required to test this conjecture.
SR144528 as an antagonist at PPARγ?
In addition to its inhibition of COX, indomethacin has been reported to activate peroxisome proliferator-activated receptor γ (PPARγ) (at concentrations similar to those required to inhibit FAAH in vitro), and it has been suggested that this effect may contribute to the pharmacological actions of this compound (Lehmann et al., 1997). However, the literature is by no means clear for indomethacin, since an antagonist action of this compound at PPARγ has also been reported (Bishop-Bailey & Warner, 2003). Be that as it may, the notion that PPARγ may be involved raises the possibility that the action of SR144528 to prevent the antioedema effects of indomethacin and URB597 may reflect a hitherto unreported action of this compound as a PPARγ antagonist. In order to investigate this notion further, we pretreated animals with BADGE at a dose (30 mg kg−1 i.p.) previously reported completely to block the effect of the PPARγ activator rosiglitazone upon carrageenan-induced oedema in the rat (Cuzzocrea et al., 2004). It is clear from the 4 h time point data that the combination of BADGE and either indomethacin or URB597 still gives an inflammation, albeit lower than the level for indomethacin alone. This does not conclusively prove or disprove the involvement of PPARγ in the actions of indomethacin, but the total lack of effect against URB597 at the 4 h time point would suggest at the very least that the antagonism produced by SR144528 is not a hitherto unreported action of this compound at PPARγ.
A related question concerns PPARα, which is activated by palmitoylethanolamide (Lo Verme et al., 2005), and which is also known to play a role in inflammation (see e.g. Taylor et al., 2002). FAAH inhibition by URB597 increases levels of palmitoylethanolamide in the rat (Fegley et al., 2005), and exogenously applied palmitoylethanolamide decreases carrageenan-induced paw oedema and phorbol ester-induced ear oedema in wild type, but not in PPARα−/− mice (Lo Verme et al., 2005). However, SR144528 (2 mg kg−1 i.p.) could not antagonise the effect of palmitoylethanolamide in the ear oedema model (Lo Verme et al., 2005), which would argue against involvement of PPARα in the effects seen here.
In conclusion, the present study has demonstrated that at the doses used, the selective FAAH inhibitor URB597 and the NSAID indomethacin reduce the oedema response to carrageenan in the mouse, and that these effects are blocked by SR144528. While these data would suggest that the endocannabinoid system may be involved in both these actions, further data are necessary, in particular analysis of the endogenous compounds involved, before such a conclusion can be considered proven.
Acknowledgments
We thank Britt Jacobsson for excellent technical assistance. This study was supported by grants from the Swedish Research Council (Grant No. 12158, medicine), Konung Gustav V's and Drottning Victorias Foundation, Gun and Bertil Stohne's Foundation, Stiftelsen för Gamla Tjänarinnor and the Research Funds of the Medical Faculty, Umeå University.
Abbreviations
- AEA
arachidonoylethanolamide (anandamide)
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1-H-pyrazole-3-carboxamide
- BADGE
bisphenol A diglycidyl ether
- COX
cyclooxygenase
- FAAH
fatty acid amide hydrolase
- NSAID
nonsteroidal antiinflammatory drug
- PPARγ
peroxisome proliferator-activated receptor γ
- PMSF
phenylmethylsulphonyl fluoride
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- TRPV1
transient receptor potential vanilloid type 1
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
References
- ATES M., HAMZA M., SEIDEL K., KOTALLA C.E., LEDENT C., GÜHRING H. Intrathecally applied flurbiprofen produces an endocannabinoid-dependent antinociception in the rat formalin test. Eur. J. Neurosci. 2003;17:597–604. doi: 10.1046/j.1460-9568.2003.02470.x. [DOI] [PubMed] [Google Scholar]
- BASSO E., DURANTI A., MOR M., PIOMELLI D., TONTINI A., TARZIA G., TRALDI P. Tandem mass spectrometric data-FAAH inhibitory activity relationships of some carbamic acid O-aryl esters. J. Mass Spectosc. 2004;39:1450–1455. doi: 10.1002/jms.729. [DOI] [PubMed] [Google Scholar]
- BÁTKAI S., PACHER P., JÁRAI Z., WAGNER J.A., KUNOS G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am. J. Physiol. 2004;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER C., SCHMID P.C., SCHABITZ W.-R., WOLF M., SCHWAB S., SCHMID H.H.O. Massive accumulation of N-acylethanol-amines after stroke. Cell signalling in acute cerebral ischemia. J. Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- BEAULIEU P., BISOGNO T., PUNWAR S., FARQUHAR-SMITH P., AMBROSINO G., DI MARZO V., RICE A.S.C. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur. J. Pharmacol. 2001;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- BIFULCO M., LAEZZA C., VALENTI M., LIGRESTI A., PORTELLA G., DI MARZO V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- BISHOP-BAILEY D., WARNER T.D. PPARγ ligands induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor-γ antagonist. FASEB J. 2003;17:1925–1927. doi: 10.1096/fj.02-1075fje. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., LIGRESTI A., DI MARZO V. The endocannabinoid signalling system: biochemical aspects. Pharmacol. Biochem. Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- BOLDRUP L., WILSON S.J., BARBIER A.J., FOWLER C.J. A simple stopped assay for fatty acid amide hydrolase avoiding the use of a chloroform extraction phase. J. Biochem. Biophys. Methods. 2004;60:171–177. doi: 10.1016/j.jbbm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- CLAYTON N., MARSHALL F.H., BOUNTRA C., O'SHAUGHNESSY C.T. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- COMPTON D.R., MARTIN B.R. The effect of the enzyme inhibitor phenylmethylsulfonyl fluoride on the pharmacological effect of anandamide in the mouse model of cannabimimetic activity. J. Pharmacol. Exp. Ther. 1997;283:1138–1143. [PubMed] [Google Scholar]
- CONTI S., COSTA B., COLLEONI M., PAROLARO D., GIAGNONI G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002;135:181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTA B., GIAGNONI G., FRANKE C., TROVATO A.E., COLLEONI M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br. J. Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVATT B.F., SAGHATELIAN A., HAWKINS E.G., CLEMENT A.B., BRACEY M.H., LICHTMAN A.H. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., LANDI M., GALZIN A.-M., MARINI P. Role of cannabinoid CB1 receptors and tumor necrosis factor-α in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br. J. Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROXFORD J.L. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17:179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., PISANO B., DUGO L., IANARO A., MAFFIA P., PATEL N.S.A., DI PAOLA R., IALENTI A., GENOVESE T., CHATTERJEE P.K., DI ROSA M., CAPUTI A.P., THIEMERMANN C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. Eur. J. Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- DAHER J.B., TONUSSI C.R. A spinal mechanism for the peripheral anti-inflammatory action of indomethacin. Brain Res. 2003;962:207–212. doi: 10.1016/s0006-8993(02)04056-8. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., CHIN S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., DYSARZ F.A., III, JOHNSON M.R., MELVIN L.S., HOWLETT A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., SUGIURA T., MELCK D., DE PETROCELLIS L. The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem. J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C., BISOGNO T., MELCK D., PATRICK G., TAO Q., SZALLASI A., RAZDAN R.K., MARTIN B.R. Neurobehavioral activity in mice of N-vanillyl-arachidonyl-amide. Eur. J. Pharmacol. 2000;406:363–374. doi: 10.1016/s0014-2999(00)00687-7. [DOI] [PubMed] [Google Scholar]
- FEGLEY D., GAETANI S., DURANTI A., TONTINI A., MOR M., TARZIA G., PIOMELLI D. Characterization of the fatty-acid amide hydrolase inhibitor URB597: effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- FORD W.R., HONAN S.A., WHITE R., HILEY C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER C.J. Metabolism of the endocannabinoids anandamide and 2-arachidonoyl glycerol, a review, with emphasis on the pharmacology of fatty acid amide hydrolase, a possible target for the treatment of neurodegenerative diseases and pain. Curr. Med. Chem. Central Nerv System Agents. 2004;4:161–174. [Google Scholar]
- FOWLER C.J., HOLT S., TIGER G. Acidic non-steroidal anti-inflammatory drugs inhibit rat brain fatty acid amide hydrolase in a pH-dependent manner. J. Enzyme Inhibition Med. Chem. 2003;18:55–58. doi: 10.1080/1475636021000049726. [DOI] [PubMed] [Google Scholar]
- GOPARAJU S.K., UEDA N., YAMAGUCHI H., YAMAMOTO S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- GUBELLINI P., PICCONI B., BARI M., BATTISTA N., CALABRESI P., CENTONZE D., BERNARDI G., FINAZZI-AGRÒ A., MACCARRONE M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J. Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÜHRING H., HAMZA M., SERGEJEVA M., ATES M., KOTALLA C.E., LEDENT C., BRUNE K. A role for endocannabinoids in indomethacin-induced spinal antinociception. Eur. J. Pharmacol. 2002;454:153–163. doi: 10.1016/s0014-2999(02)02485-8. [DOI] [PubMed] [Google Scholar]
- HOLT S., COSTA B., FOWLER C.J. FAAH inhibitors and indomethacin reduce carrageenan induced hind paw inflammation in the mouse – role of cannabinoid receptors. 2004 Symposium on the Cannabinoids, Burlington, Vermont. Int Cannabinoid Res Soc. 2004a. p. 51.
- HOLT S., ROCKSÉN D., BUCHT A., PETERSEN G., HANSEN H.S., VALENTI M., DI MARZO V., FOWLER C.J. Lipopolysaccharide-induced pulmonary inflammation is not accompanied by a release of anandamide into the lavage fluid or a down-regulation of the activity of fatty acid amide hydrolase. Life Sci. 2004b;76:461–472. doi: 10.1016/j.lfs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., PETROS T.J., CHANG S.Y., ZAVITSANOS P.A., ZIPKIN R.E., SIVAKUMAR R., COOP A., MAEDA D.Y., DE PETROCELLIS L., BURSTEIN S., DI MARZO V., WALKER J.M. Identification of a new class of molecules, the arachidonoyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- IKEDA Y., UENO A., NARABA H., OH-ISHI S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69:2911–2919. doi: 10.1016/s0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- IWAMURA H., SUZUKI H., UEDA Y., KAYA T., INABA T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- IZZO A.A., FEZZA F., CAPASSO R., BISOGNO T., PINTO L., IUVONE T., ESPOSITO G., MASCOLO N., DI MARZO V., CAPASSO F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN K., XIE S.H., PARMENTIER-BATTEUR S., SUN Y., MAO X.O., CHILDS J., GREENBERG D.A. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- KATHURIA S., GAETANI S., FEGLEY D., VALIÑO F., DURANTI A., TONTINI A., MOR M., TARZIA G., LA RANA G., CALIGNANO A., GIUSTINO A., TATTOLI M., PALMERY M., CUOMO V., PIOMELLI D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- KEHL L.J., HAMAMOTO D.T., WACNIK P.W., CROFT D.L., NORSTED B.D., WILCOX G.L., SIMONE D.A. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- LAMBERT D.M., FOWLER C.J. The endocannabinoid system: drug targets, lead compounds, and potential therapeuticx applications. J. Med. Chem. 2005;48:5059–5087. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]
- LEHMANN J.M., LENHARD J.M., OLIVER B.B., RINGOLD G.M., KLIEWER S.A. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., LEUNG D., SHELTON C.C., SAGHATELIAN A., HARDOUIN C., BOGER D.L., CRAVATT B.F. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: Evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 2004a;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., SHELTON C.C., ADVANI T., CRAVATT B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- LO VERME J., FU J., ASTARITA G., LA RANA G., RUSSO R., CALIGNANO A., PIOMELLI D. The nuclear receptor PPAR-α mediates the antiinflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- MASSA F., MARSICANO G., HERMANN H., CANNICH A., MONORY K., CRAVATT B.F., FERRI G.-L., SIBAEV A., STORR M., LUTZ B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOR M., RIVARA S., LODOLA A., PLAZZI P.V., TARZIA G., DURANTI A., TONTINI A., PIERSANTI G., KATHURIA S., PIOMELLI D. Cyclohexylcarbamic acid 3′- or 4′-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modelling studies. J. Med. Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- NATARAJAN V., SCHMID P.C., REDDY P.V., SCHMID H.H.O. Catabolism of N-acylethanolamine phospholipids by dog brain preparations. J. Neurochem. 1984;42:1613–1619. doi: 10.1111/j.1471-4159.1984.tb12750.x. [DOI] [PubMed] [Google Scholar]
- PARIA B.C., DEUTSCH D.D., DEY S.K. The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Mol. Reprod. Dev. 1996;45:183–192. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoids and multiple sclerosis. Pharmacol. Ther. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- PORTANOVA J.P., ZHANG V., ANDERSON G.D., HAUSER S.D., MASFERRER J.L., SEIBERT K., GREGORY S.A., ISAKSON P.C. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J. Exp. Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUARTILHO A., MATA H.P., IBRAHIM M.M., VANDERAH T.W., PORRECA F., MAKRIYANNIS A., MALAN T.P. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- QUISTAD G.B., SPARKS S.E., SEGALL Y., NOMURA D.K., CASIDA J.E. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol. Appl. Pharmacol. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- RICE A.S., FARQUHAR-SMITH W.P., NAGY L. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostagl. Leukot. Essent. Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.-M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOULABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIÈRE J.-C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SIQUEIRA-JUNIOR J.M., PETERS R.R., DE BRUM-FERNANDES A.J., RIBEIRO-DO-VALLE R.M. Effects of valeryl salicylate, a COX-1 inhibitor, on models of acute inflammation in mice. Pharmacol. Res. 2003;48:437–443. doi: 10.1016/s1043-6618(03)00188-9. [DOI] [PubMed] [Google Scholar]
- SOKAL D.M., ELMES S.J.R., KENDALL D.A., CHAPMAN V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurons via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45:404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- TAYLOR B.K., DADIA N., YANG C.B., KRISHNAN S., BADR M. Peroxisome proliferator-activated receptor agonists inhibit inflammatory edema and hyperalgesia. Inflammation. 2002;26:121–127. doi: 10.1023/a:1015500531113. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., BAK A., MCKNIGHT W., ASFAHA S., SHARKEY K.A., MACNAUGHTON W.K. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gasteroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- WANG Z.Q., PORRECA F., CUZZOCREA S., GALEN K., LIGHTFOOT R., MASINI E., MUSCOLI C., MOLLACE V., NDENGELE M., ISCHIROPOULOS H., SALVEMINI D. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- WILSON A.W., CLAYTON N.M., MEDHURST S.J., BOUNTRA C., CHESSELL I.P. The FAAH inhibitor URB597 reverses inflammatory pain through a CB1 receptor mediated mechanism. Proc. Br. Pharm. Soc. 2005.
- YU M., IVES D., RAMESHA C.S. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SØRGÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]