Abstract
Schizophrenia is considered to be a neurodevelopmental disorder with origins in the prenatal or neonatal period. Brains from subjects with schizophrenia have enlarged ventricles, reduced cortical thickness (CT) and increased neuronal density in the prefrontal cortex compared with those from normal subjects. Subjects with schizophrenia have reduced pain sensitivity and niacin skin flare responses, suggesting that capsaicin-sensitive primary afferent neurons might be abnormal in schizophrenia.
This study tested the hypothesis that intrinsic somatosensory deprivation, induced by neonatal capsaicin treatment, causes changes in the brains of rats similar to those found in schizophrenia. Wistar rats were treated with capsaicin, 50 mg kg−1 subcutaneously, or vehicle (control) at 24–36 h of life. At 5–7 weeks behavioural observations were made, and brains removed, fixed and sectioned.
The mean body weight of capsaicin-treated rats was not significantly different from control, but the mean brain weight of male, but not female, rats, was significantly lower than control.
Capsaicin-treated rats were hyperactive compared with controls. The hyperactivity was abolished by haloperidol.
Coronal brain sections of capsaicin-treated rats had smaller cross-sectional areas, reduced CT, larger ventricles and aqueduct, smaller hippocampal area and reduced corpus callosum thickness, than brain sections from control rats. Neuronal density was increased in several cortical areas and the caudate putamen, but not in the visual cortex.
It is concluded that neonatal capsaicin treatment of rats produces brain changes that are similar to those found in brains of subjects with schizophrenia.
Keywords: Capsaicin, neonatal capsaicin, sensory deprivation, schizophrenia, neuronal density, rat brain, rat behaviour
Introduction
Schizophrenia is a chronic psychotic disorder, the aetiology of which remains unknown. Although the overt signs and symptoms of schizophrenia do not usually manifest until early adulthood, epidemiological studies have suggested that it is a neurodevelopmental disorder that has its origins in the prenatal or neonatal period (see Harrison, 1997; Schultz & Andreasen, 1999; Lewis & Lieberman, 2000; Ashe et al., 2001).
Studies on human brain have shown that the brains of subjects with schizophrenia are reduced in volume compared with those of healthy individuals (Schlaepfer et al., 1994; McDonald et al., 2002; Selemon et al., 2002), the frontal lobe being the more severely affected of all four lobes (Selemon et al., 2002). Furthermore, the brains of subjects with schizophrenia have larger ventricles and thinner cortices, particularly in the prefrontal and temporal regions, than those of normal subjects (Shenton et al., 1992; McCarley et al., 1999).
An important observation was that by Selemon et al. (1995; 1998), who found increased neuronal density in the prefrontal cortex of subjects with schizophrenia. This finding led to the ‘reduced neuropil hypothesis' that the symptoms of schizophrenia result from reduced cortical connectivity rather than a reduction in neuron numbers (Selemon & Goldman-Rakic, 1999). Reduced interneuronal space (Buxhoeveden et al., 2000), mean cell-spacing abnormalities (Casanova et al., 2005) and reduced neuronal size (Rajkowska et al., 1998; Chana et al., 2003) have also been found in the neocortex of subjects with schizophrenia. Recently, the concept has arisen that schizophrenia might result from aberrations in the neuroplasticity phenomena that govern normal brain development and function (Frost et al., 2004; McCullumsmith et al., 2004; Arnold et al., 2005).
A major impediment to schizophrenia research has been the lack of validated animal models (De Hert & Ellenbroek, 2000; Gerrits et al., 2002). The present study was prompted by the observations that deficits in pain sensation are present in subjects with schizophrenia (Kudoh et al., 2000; Blumensohn et al., 2002) and their relatives (Hooley & Delgado, 2001), and vascular responsiveness is altered, as shown by a reduced niacin skin flare in many subjects with the disorder (Waldo, 1999; Messamore et al., 2003). These observations suggested that capsaicin-sensitive primary afferent neurons might be abnormal in schizophrenia.
Capsaicin acts on transient receptor potential vanilloid 1 (TRPV1) receptors, which are calcium-permeable ion channels gated by reduced pH and high temperature (Caterina et al., 1997; Caterina & Julius, 2001). These receptors are located on a population of neuropeptide-containing unmyelinated primary afferent neurons which mediate nociception, axon reflex flare and neurogenic inflammation (Holzer, 1991; Szallasi & Blumberg, 1999). Capsaicin activates primary afferent neurons bearing TRPV1 receptors to produce release of neuropeptides, in particular calcitonin gene-related peptide and tachykinins, which produce flare and increased vascular permeability (see Holzer, 1991). On repeated application, desensitization occurs to these actions of capsaicin, with resultant loss of sensitivity of the sensory neurons to activation. Thus, agonists acting on TRPV1 receptors, including capsaicin and resiniferatoxin, may be used to reduce the sensitivity of sensory neurons, and are currently in clinical use in humans for a number of syndromes involving sensory neurons such as neuropathic pain and bladder hypersensitivity (see Valenzano & Sun, 2004). Capsaicin also has a neurotoxic effect, and, if given to neonatal rats, it produces life-long loss of capsaicin-sensitive primary afferent neurons (Jancsó et al., 1977).
TRPV1 receptors have recently been found to be widely distributed in the brain (Mezey et al., 2000; Tóth et al., 2005). Although less is known of the effects of activation of TRPV1 on central neurons, recent evidence indicates that they may mediate excitatory actions in the hypothalamus (Hori et al., 1988; Sasamura et al., 1998), locus coeruleus (Marinelli et al., 2002), ventral tegmental area (Marinelli et al., 2005) and substantia nigra (Marinelli et al., 2003), but potentiate depression in the cornu ammonis 1 (CA1) region of the hippocampus (Al-Hayani et al., 2001). Interestingly, neonatal capsaicin treatment has been reported not to affect the expression or distribution of TRPV1 receptor messenger ribonucleic acid (mRNA) in rat brain (Mezey et al., 2000).
Models of neonatal somatosensory deprivation, such as the mouse whisker barrel model, have shown that neonatal sensory deprivation induced by whisker trimming results in reduced synaptic density in the barrel cortex (Sadaka et al., 2003). If ‘reduced neuropil' in schizophrenia is the result of reduced synaptic density, it is possible that it might result from intrinsic somatosensory deprivation during development. The aim of the following experiments was to test the hypothesis that intrinsic somatosensory deprivation during development causes changes in the brains of rats similar to those found in the brains of subjects with schizophrenia. Since neonatal capsaicin treatment produces loss of a population of primary afferent neurons, it would be expected to give rise to an intrinsic somatosensory deprivation. Thus, the effects of neonatal capsaicin treatment on the brain were examined in the present study. Rats were used in these studies since there is considerable knowledge of the effects of capsaicin treatment in this species.
Methods
Animals
Wistar rats were used in this study. All handling of animals and procedures were carried out in accordance with the guidelines established by the Animal Care and Ethics Committee of the University of Newcastle, an accredited research institution. Litters from six time-mated pregnant female rats were used in two cohorts. Cohort 1 comprized two litters and cohort 2, four litters. The rats were housed in plastic cages (73 × 54 × 24 cm3) with shredded paper pellet bedding, and a wire mesh top cover. The animals were kept at a constant temperature of 21±1°C on a 12–12 h light–dark cycle with lights on at 0700 h. Food and water were freely available.
Drugs
The drugs used in this study were: capsaicin (Sigma-Aldrich Pty Ltd, Australia); sodium pentobarbitone (Lethabarb, Virbac (Australia) Pty Ltd, Australia); salbutamol sulphate aerosol (Ventolin, Allen & Hanburys, Australia); haloperidol (Serenace, Sigma Pharmaceuticals Pty Ltd, Australia). The stock solution of capsaicin, 10−2 M, was made in a vehicle of 10% Tween 80 and 10% ethanol in saline.
Capsaicin treatment
Within 24–36 h of birth, the mother rat was removed from the home cage and the newborn rats were individually removed for injection of capsaicin or vehicle, under ice anaesthesia. Neonates were placed in the supine position on a bed of crushed ice. Immediately upon disappearance of the righting reflex, neonates were removed from the ice and injected subcutaneously (s.c.) with either capsaicin, 50 mg kg−1, or an equivalent volume of vehicle, consisting of 10% ethanol and 10% Tween 80, into the dorsal region of the neck, using a 30-gauge needle. This dose of capsaicin was chosen since it has been shown in previous studies to produce marked loss of capsaicin-sensitive primary afferent neurons (Jancsó et al., 1977). Following injection, neonates were placed in a clear perspex observation chamber with other injected littermates, each animal being placed between the fingers of a latex glove filled with 37°C water and warmed with a heating lamp. As each neonate was added to the chamber, a measured dose (two ‘puffs') of salbutamol aerosol (Ventolin) was sprayed into the chamber to alleviate respiratory difficulty induced by capsaicin. Neonates were kept in the observation chamber until all signs of respiratory distress had disappeared and they had regained their righting reflex. They were then placed in a small, warm holding cage until all littermates had been injected and had recovered. They were then transferred to a clean cage with their mother. Shreds of bedding from the original home cage were also placed in the new cage. The mother's behaviour was observed to ensure that she accepted the neonates. The rat pups were checked and weighed twice weekly. Rats were weaned 21–23 days after birth by placement into new cages, with one or two other animals of the same sex. One animal in cohort 1 died within the first week following capsaicin treatment, but all animals in cohort 2 survived for the duration of the experiment.
Behavioural observations
For assessment of behaviours, cohort 1 rats and half of cohort 2 rats were observed once only between 36 and 49 days of age and the data combined. On observation days, rats were removed from the home cage and placed in a perspex observation chamber (height 32 cm × depth 44 cm × length 72 cm) located in the home room and containing two foam objects placed identically for each animal. Measurement of behaviours was commenced immediately without giving the animals time to become familiar with the observation cage. Each animal was observed for 10 min by the same trained observer who manually quantified behaviours, including the number of circuits of the cage (locomotor activity), number of climbs of an object or sides (climbing), number of rears (rearing), face washing, scratching and chewing (stereotypy). On several occasions during the course of the experiments, behavioural observations were validated by a second independent observer who was blind to the treatment of the rats, and who was located in an adjacent room behind a glass wall. Use of strict criteria for the assessment of behaviours ensured consistent ratings by the observer. Animals were then weighed and euthanased with a lethal dose of sodium pentobarbitone, 100 mg kg−1, intraperitoneally. The behaviours of the other rats in cohort 2 were measured twice weekly over a 3-week period, that is, 23–26, 34–37 and 39–42 days, alternating between day and night observations, so that at each age there were results from a day and a night observation session. These animals were also observed on three additional occasions, without any treatment (session 1 – baseline activity), 30 min following administration of 0.9% saline (session 2) and haloperidol 1 mg kg−1 s.c. (session 3). Animals were weighed after each session and euthanased immediately after the observation session following administration of haloperidol. It was observed during the course of the first experiment that the tails of capsaicin-treated rats appeared shorter than those of control rats. Therefore, tail lengths of cohort 2 animals were measured following euthanasia.
Histology
Following euthanasia, the heads of the rats were removed using a guillotine. Brains were removed from the skulls, weighed and placed in 10% formalin solution for 2 weeks. Serial coronal sections, 50 μm, were cut on a cryostat. Every third section was mounted on gelatin chrom alum-coated slides, air-dried and Nissl-stained with 5% cresyl violet solution for 1 min and washed in running water until the water became clear (cohort 1), or with thionine using the method of Tolivia & Tolivia (1985) (cohort 2). In the latter method, slides were placed for 18 h at room temperature in a solution of 0.003% thionine, 1.7% ethanol and 0.13% formalin, adjusted to pH 3.5 with glacial acetic acid. This method yielded superior staining for neuronal counting since the cells were stained blue and the white matter pink. Following staining, all sections were dehydrated in ascending concentrations of ethanol (70, 95, 2 × 100%), cleared with histolene (Fronine, Riverstone, NSW, Australia) and coverslipped with Ultramount (Fronine).
Microscopy
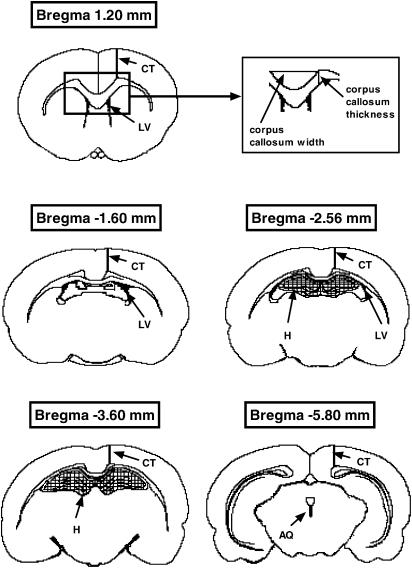
Closely matched sections from each brain at several levels were selected with the aid of the rat brain atlas of Paxinos & Watson (1998). The sections were observed under bright-field microscopy using a Zeiss Axioskop light microscope with a motorized stage and a miniature monitor (Lucivid – Microbrightfield Inc., U.S.A.) attached to a camera lucida. Neurolucida software (Microbrightfield) was used to trace outlines of the sections and count neurons using a two-dimensional counting method without correction for cell splitting. The total area of sections was measured at Bregma 2.70, 1.20, −1.60, −2.56, −3.60 and −5.80 mm, hippocampal area at Bregma −2.56 and −3.60 mm, lateral ventricle (LV) area at Bregma 1.20, −1.60, and −2.56 mm, and aqueduct area at Bregma −5.80 mm (Figure 1). Cortical thickness (CT) was measured at all levels except Bregma 2.70 mm. Corpus callosum thickness and width between the dorsal tips of the left and right cingulum were also measured at Bregma 1.20, −1.60 and −2.56 mm (Figure 1).
Figure 1.
Diagrams of coronal brain sections at Bregma 1.20, −1.60, −2.56, −3.60, and −5.80 mm, showing the location of structures measured in this study (adapted from Paxinos & Watson, 1998). Cross-sectional area was calculated from the area of the entire section. Location of measurements of CT, and corpus callosum height and width (see inset) are shown as thick lines, areas of the LVs and AQ are shown in black, and the hippocampus (H) is shown cross-hatched (Bregma 2.70 mm not shown).
Neurons were counted in six specific regions: anterior cingulate (AC), the primary somatosensory cortex jaw (SIJ), the secondary motor cortex (M2) and caudate putamen (CPu) at Bregma 1.20 mm, the primary auditory cortex (Au1) at Bregma −3.60 mm and the primary visual cortex monocular (VIM) at Bregma −5.80 mm. For each region, a counting box of appropriate size was used and all neurons in which the nucleolus was visible were counted in the box. Neuronal density was calculated from the total neuronal count divided by the area of the counting box measured by the Neurolucida software. The neuronal density for each region for each animal was calculated as the average results for the left and right sides of the brain and expressed as cells per μm2.
Statistical analyses
Data that did not involve repeated measures of the same parameter on the same animals were analysed by two-way analysis of variance using GraphPad Prism 4.0 (GraphPad Software Inc.). Where analysis showed a significant gender effect, data for males and females were analysed separately using Bonferroni post-tests. For data where measures on the same animals were repeated (behavioural data), two-way analysis of variance with repeated measures was performed using SPSS 12.0 for Windows, since the occasional missing data point could not be handled by GraphPad Prism 4.0. Growth rate was analysed using linear regression. Results are shown in figures and tables as means and standard errors of the means (s.e.m.'s). For all data analyses, P=0.05 was used.
Results
Capsaicin-treated rats from both cohorts developed normally, except that some animals groomed their faces excessively to the extent that the skin became damaged. It was also noted by the investigators and the animal house staff that the cages of capsaicin-treated rats were dirtier than those of control animals.
Body weight
At 36–43 days of age, the mean body weights of male and female capsaicin-treated rats in cohorts 1 and 2 were not significantly lower than those of vehicle-treated rats (Figure 2). Linear regression of mean body weights measured every 3–4 days from the day of weaning (21–23 days of age) until euthanasia at 36–43 days over time showed that growth rates of the male and female capsaicin- and vehicle-treated rats did not differ significantly (Figure 2). Therefore, except where specified otherwise, data from cohorts 1 and 2 were combined.
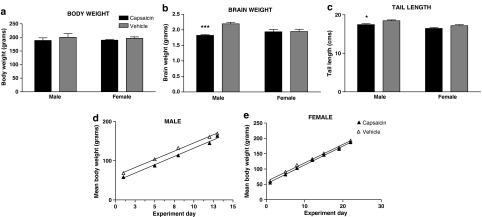
Figure 2.
Effect of neonatal capsaicin treatment on body weight (a), brain weight (b), tail length (c), and growth rate of male (d) and female (e) rats. Histograms show mean±s.e.m. measurements obtained from rats at age 36–49 days. Solid histograms show data from 13 capsaicin-treated male rats and 10 capsaicin-treated female rats for body weight and brain weight data, and cross-hatched histograms show data from 13 vehicle control male rats and 7 vehicle control female rats. For tail length data, there were five rats in each treatment and gender group. Growth rates from weaning at age 21–23 days until euthanasia at 36–43 days of male and female rats are shown as linear regression lines (▴, capsaicin; ▵, vehicle). Asterisks show significant differences from vehicle control. ***P<0.001; *P<0.05.
Brain weight
Two-way analysis of variance showed that there was a significant effect of capsaicin treatment on brain weight (F1,41=10.95, P=0.002), and a significant interaction between capsaicin treatment and gender (F1,41=9.94, P=0.003). Bonferroni post-tests following two-way analysis of variance showed that the mean brain weights of capsaicin-treated male rats were significantly lower than those of vehicle-treated male rats (P<0.001), whereas the mean brain weights of capsaicin-treated female rats were not significantly different from those of vehicle-treated female rats (Figure 2).
Tail length
Tail lengths of cohort 2 rats only were measured. The mean tail lengths of capsaicin-treated male rats were significantly less than those of vehicle-treated male rats (P<0.05, Bonferroni post-test following two-way analysis of variance), whereas the mean tail lengths of female rats did not differ significantly from those of vehicle-treated animals (Figure 2).
Behaviour
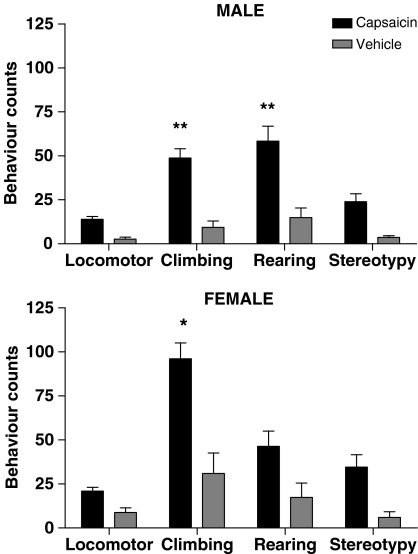
Since the behaviours were measured over a short period of time immediately after the rats were placed in the observation cage, the behavioural measures gave an indication of the ability of the animals to become familiar with a novel environment rather than a general background level of activity. For assessment of behaviours, cohort 1 rats and half of cohort 2 rats were observed once only between 36 and 49 days of age and the data combined. Repeated-measures two-way analysis of variance showed that the capsaicin-treated male and female rats in both cohorts were hyperactive overall compared with vehicle controls (F1,42=44.26, P<0.0001). Bonferroni post-tests showed that climbing and rearing behaviours were significantly greater in the capsaicin-treated male rats than in male vehicle controls (both P<0.01), and climbing behaviour was significantly greater in capsaicin-treated female rats than in female controls (P<0.05) (Figure 3).
Figure 3.
Effect of neonatal capsaicin treatment on the behaviours of male and female rats at age 36–49 days observed over 10 min. Solid histograms show mean±s.e.m. data from 13 capsaicin-treated male rats and 10 capsaicin-treated female rats and cross-hatched histograms show data from 13 vehicle control male rats and seven vehicle control female rats. Asterisks show significant difference from vehicle control. **P<0.01; *P<0.05.
The behaviours of the other rats in cohort 2 were measured twice weekly over a 3-week period, that is, 23–26, 34–37 and 39–42 days, alternating between day and night observations, so that at each age there were results from a day and a night observation session. The results are shown in Table 1. Two-way repeated-measures analysis of variance showed that capsaicin-treated rats in this group were also significantly more active overall than vehicle controls (F1,16=26.17, P<0.0001), and that there was a significant effect of age (F1.95, 31.15=16.39, P<0.0001), and time of day (F1,16=22.20, P<0.0001). Analysis at the three ages showed that capsaicin-treated male and female rats were not more active than vehicle controls at age 23–26 days. Indeed, at this age, male vehicle controls were more active on rearing behaviour (P<0.05, Bonferroni post-test) than capsaicin-treated rats. However, at age 34–37 days, both male and female capsaicin-treated rats were more active during the day, but not at night, on locomotor (male, P<0.05; female, P<0.01), climbing (male, P<0.05; female, P<0.05), rearing (male, P<0.015; female P<0.05) and stereotypy (male, P<0.05; female P<0.05) behaviours than controls. At 39–42 days, male capsaicin-treated rats were more active than controls during the day on locomotor (P<0.05) and stereotypy (P<0.01) behaviours, and were more active on climbing at both day (P<0.05) and night (P<0.05) observations than controls. At age 39–42 days, female capsaicin-treated rats were more active during the day than controls on locomotor activity (P<0.05), and more active during the night on stereotypy (P<0.01) behaviour than controls (Table 1). Analysis of the data for time of day showed that although capsaicin-treated male and female rats were more active at both day and night observation sessions than controls (F1,56=30.65, P<0.0001), there was no effect of gender on day or night behaviour. Analysis of the combined behavioural data from all animals in both cohorts showed no significant effect of gender on behaviour.
Table 1.
Effect of neonatal capsaicin treatment on behaviours of male and female rats during day and night 10-min observation sessions at ages 23–26, 34–37 and 39–42 days
| Age | Capsaicin | Vehicle | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Day | Night | Day | Night | Day | Night | Day | Night | |
| 23–26 days | ||||||||
| Locomotor | 6.40±2.20 | 3.00±1.34 | 4.60±1.29 | 5.80±1.59 | 3.60±0.51 | 1.60±0.51 | 4.20±0.37 | 2.40±0.93 |
| Climbing | 15.40±6.27 | 6.40±4.48 | 11.20±4.58 | 19.00±7.87 | 24.20±4.21 | 3.80±1.24 | 15.60±2.71 | 7.40±2.56 |
| Rearing | 10.40±2.16* | 2.20±1.74 | 8.60±2.20 | 4.40±1.94 | 21.00±3.55 | 1.00±0.77 | 12.60±3.85 | 1.60±0.68 |
| Stereotypy | 9.80±2.75 | 5.80±3.28 | 2.60±1.60 | 3.20±0.73 | 4.80±1.16 | 1.80±1.11 | 4.00±1.18 | 2.00±1.05 |
| 34–37 days | ||||||||
| Locomotor | 8.00±1.14** | 7.80±1.69 | 9.00±1.38** | 3.20±1.36 | 1.40±0.75 | 1.40±0.51 | 3.20±0.58 | 4.60±1.60 |
| Climbing | 37.60±6.50* | 24.40±5.61 | 41.60±6.34* | 7.60±3.74 | 4.60±4.35 | 1.20±0.73 | 7.00±3.13 | 15.00±4.71 |
| Rearing | 13.80±2.40** | 9.40±3.64 | 12.80±4.76* | 6.00±4.00 | 0.40±0.24 | 0.80±0.80 | 1.80±1.36 | 6.00±3.08 |
| Stereotypy | 11.00±3.36* | 8.20±1.46 | 9.60±1.54* | 4.80±1.53 | 0.40±0.25 | 1.80±0.20 | 1.80±1.11 | 1.00±0.45 |
| 39–42 days | ||||||||
| Locomotor | 9.20±1.80* | 9.60±1.72 | 9.00±1.05* | 10.40±0.87 | 2.20±0.73 | 3.60±0.68 | 4.40±0.68 | 6.60±0.51 |
| Climbing | 37.80±9.84* | 40.20±7.16* | 39.80±3.17 | 38.40±5.46 | 5.60±1.89 | 15.00±4.02 | 15.40±4.32 | 26.60±1.63 |
| Rearing | 6.40±2.06 | 7.20±2.50 | 8.60±3.36 | 11.00±1.18 | 2.80±1.24 | 1.60±0.93 | 3.00±1.45 | 4.80±1.28 |
| Stereotypy | 21.40±5.16** | 12.20±3.51 | 9.00±1.58* | 16.80±3.65* | 2.60±0.93 | 2.80±1.02 | 3.80±1.50 | 4.40±0.93 |
Values show mean±s.e.m. behaviour counts obtained from five rats. Asterisks show significant differences from vehicle control.
P<0.01;
P<0.05.
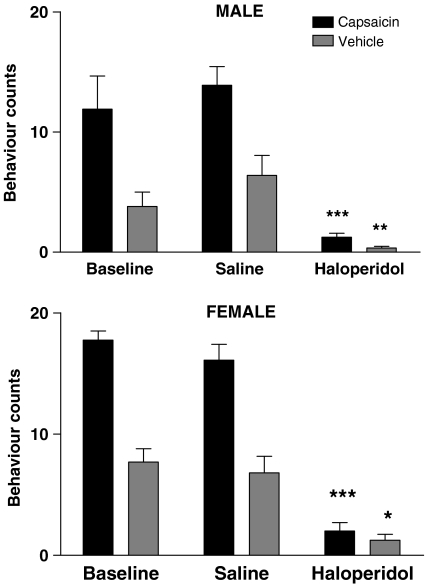
The effect of haloperidol, 1 mg kg−1 s.c. given 30 min before, was assessed on the behaviours of the rats at age 46–49 days. Haloperidol produced a marked inhibition of behaviours of both capsaicin male and female rats (both P<0.001) and vehicle male (P<0.01) and female (P<0.05) rats (Figure 4).
Figure 4.
Effect of haloperidol 1 mg kg−1 s.c. on the behaviour of male and female capsaicin- and vehicle-treated rats at age 39–42 days observed over 10 min. Histograms show mean±s.e.m. behaviour counts obtained from five rats. Solid histograms show data for capsaicin-treated rats and cross-hatched histograms show data from vehicle control rats. Asterisks show significant differences between saline and haloperidol treatments. ***P<0.001; **P<0.01; *P<0.05.
Measurements on coronal brain sections
The data for measurements made on coronal sections at six brain levels are shown in Table 2. Two-way analysis of variance showed significant reductions in cross-sectional area in capsaicin-treated rats at Bregma 1.20 mm (F1,15=4.62, P<0.05), Bregma −1.60 mm (F1,15=4.38, P<0.05), Bregma −2.56 mm (F1,16=4.52, P<0.05), Bregma −3.60 mm (F1,16=8.68, P<0.01) and Bregma −5.80 mm (F1,14=4.69, P<0.05). CT was significantly less in capsaicin-treated rats at Bregma 1.20 mm (F1,15=7.10, P<0.05), Bregma −3.60 mm (F1,16=5.40, P<0.05) and Bregma −5.80 mm (F1,12=23.46, P<0.001). Ventricle area was significantly greater in capsaicin-treated rats at Bregma −1.60 mm (F1,14=4.73, P<0.05). Hippocampal area was significantly less in capsaicin-treated rats at Bregma −3.60 mm (F1,16=7.48, P<0.05). Corpus callosum thickness at Bregma 1.20 mm was also significantly less (F1,15=18.11, P<0.001). There was a significant effect of gender for the corpus callosum thickness (Bregma 1.20 mm: F1,15=9.94, P<0.01; Bregma −1.60 mm: F1,14=12.99, P<0.01). Bonferroni post-tests showed that the corpus callosum thickness of male rats, rather than female rats, was significantly affected by capsaicin treatment at both of these brain levels (Bregma 1.20 mm: P<0.01; Bregma −1.60 mm: P<0.05). The width between the left and right cingulum was not different at any brain level. The area of the cerebral aqueduct (AQ) was significantly larger in capsaicin-treated rats (F1,15=6.39, P<0.05) (Table 2).
Table 2.
Effect of neonatal capsaicin treatment on brain measurements of male and female rats
| Capsaicin | Vehicle | Significance of capsaicin treatment | |||
|---|---|---|---|---|---|
| Brain measurement | Male | Female | Male | Female | |
| Cross-sectional area (mm2) | |||||
| 2.70 | 81.69±2.54 | 81.31±3.28 | 84.68±3.28 | 83.09±2.54 | |
| 1.20 | 97.47±2.30 | 99.72±2.97 | 102.58±2.97 | 103.51±2.30 | * |
| −1.60 | 119.61±2.65 | 123.74±3.43 | 132.99±3.43 | 130.39±2.65 | * |
| −2.56 | 127.06±3.19 | 126.12±4.11 | 126.27±4.11 | 132.25±3.19 | * |
| −3.60 | 125.30±3.72 | 123.54±4.81 | 139.65±4.81 | 135.68±3.72 | ** |
| −5.80 | 116.41±4.31 | 110.72±5.56 | 127.09±5.56 | 128.44±4.31 | * |
| Cortical thickness (mm) | |||||
| 1.20 | 2.15±0.07 | 2.06±0.06 | 2.35±0.07 | 2.16±0.05 | * |
| −1.60 | 1.72±0.06 | 1.82±0.05 | 1.79±0.06 | 1.72±0.04 | |
| −2.56 | 1.55±0.07 | 1.55±0.06 | 1.65±0.07 | 1.65±0.05 | |
| −3.60 | 1.40±0.04 | 1.50±0.04 | 1.62±0.04 | 1.50±0.03 | * |
| −5.80 | 1.27±0.04 | 1.29±0.04 | 1.41±0.04 | 1.40±0.03 | *** |
| Ventricle area (mm2) | |||||
| 1.20 | 0.54±0.09 | 0.38±0.07 | 0.47±0.08 | 0.32±0.07 | |
| −1.60 | 0.97±0.21 | 1.15±0.17 | 0.82±0.18 | 0.58±0.17 | * |
| −2.56 | 0.40±0.09 | 0.51±0.07 | 0.53±0.08 | 0.52±0.07 | |
| Hippocampal area (mm2) | |||||
| −2.56 | 10.05±1.07 | 8.73±0.96 | 8.86±0.96 | 7.83±0.96 | |
| −3.60 | 12.494±1.20 | 10.59±1.08 | 14.97±1.08 | 13.96±1.08 | * |
| Corpus callosum thickness (mm) | |||||
| 1.20 | 0.90±0.03## | 0.88±0.02 | 1.09±0.04 | 0.98±0.03 | *** |
| −1.60 | 0.94±0.04# | 0.83±0.03 | 1.06±0.05 | 0.84±0.04 | |
| −2.56 | 0.77±0.04 | 0.66±0.04 | 0.86±0.06 | 0.73±0.04 | |
| Corpus callosum width (mm) | |||||
| 1.20 | 3.36±0.09 | 3.43±0.08 | 3.44±0.18 | 3.51±0.09 | |
| −1.60 | 2.32±0.10 | 2.50±0.09 | 2.68±0.19 | 2.43±0.10 | |
| −2.56 | 2.43±0.06 | 2.50±0.05 | 2.58±0.11 | 2.58±0.06 | |
| Aqueduct (mm2) | |||||
| −5.80 | 0.18±0.02 | 0.15±0.02 | 0.09±0.02 | 0.08±0.02 | * |
Brain levels are mm from Bregma (see text and Figure 1 for description of measurements). Values shown are means±s.e.m. obtained from five rats. Asterisks show the overall significance of capsaicin treatment, ***P<0.001; **P<0.01; *P<0.05. Hatches show the significance of capsaicin treatment on male rats for comparisons where there was a significant effect of gender, ##P<0.01; #P<0.05.
Neuronal density
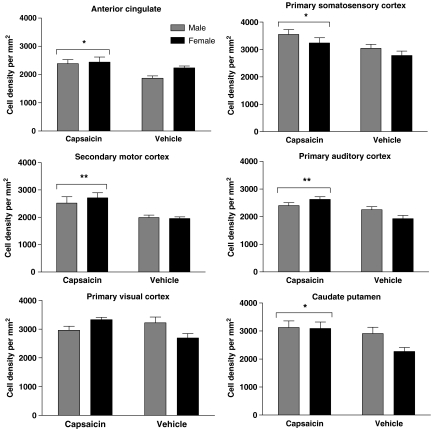
The mean results for neuronal density for each group of rats for each of the six regions counted are shown in Figure 5. Two-way analysis of variance showed that there was no significant effect of gender on neuronal density in any of the brain areas investigated. However, capsaicin treatment produced a significant increase in neuronal density in the AC (F1,15=8.03, P<0.05), SIJ (F1,16=8.13, P<0.01), M2 (F1,15=14.54, P<0.01), Au1 (F1,16=15.56, P<0.01) and CPu (F1,16=5.96, P<0.05), but not in the primary VIM. Although there was no significant difference in neuronal density in the primary visual cortex, there was a trend towards an increase in the female animals. An overall increase of 17.5±6.6% was found in the anterior cingulate, 16.5±6.0% in the primary somatosensory cortex, 32.6±8.2% in the M2, 20.2±5.8% in the Au1 and 20.1±8.9% in the CPu (s.e.m. of ratios calculated according to the method of Motulsky, 1995). The neurons in the anterior cingulate and motor cortex, in particular, appeared to be bunched and arranged in beaded columns.
Figure 5.
Mean neuronal densities expressed as cells per μm2 in brain regions of male and female capsaicin- and vehicle-treated rats. AC, anterior cingulate; SIJ, primary somatosensory cortex jaw region; M2, secondary motor cortex; Au1, primary auditory cortex; VIM, primary visual cortex monocular; CPu, caudate putamen. Histograms show mean±s.e.m. behaviour counts obtained from five rats. Single-hatched histograms show data from male rats and cross-hatched histograms data from female rats. Asterisks show significant differences from vehicle control. **P<0.01; *P<0.05.
Discussion
In this study, neonatal capsaicin treatment of rats was used to cause permanent destruction of a population of capsaicin-sensitive primary afferent fibres, thereby presumably reducing the somatosensory input into the brain for the life of the animal. Although the possibility of a permanent effect of neonatal capsaicin treatment on central TRPV1 receptors cannot be excluded, it is considered unlikely in light of the report that TRPV1 receptor mRNA expression was unaffected in rat brain (Mezey et al., 2000). In the first weeks of life, the capsaicin-treated rats apparently developed normally and, at 5–7 weeks of age, they had body weights similar to those of vehicle-treated rats. However, the male capsaicin-treated rats had shorter tails, indicating that development, at least of male rats, was slightly affected.
Shortly after weaning (23–26 days), the behaviour of capsaicin-treated rats in a novel environment was similar to that of vehicle controls. However, at 5 and 7 weeks of age, capsaicin-treated rats were found to be more active in a novel environment than vehicle control animals. There was no gender difference in the effect of capsaicin treatment on behaviour. In this study, the behaviour of rats was observed in a novel environment for only 10 min, and thus no basal level of activity was established. Therefore, the findings might indicate either that the capsaicin-treated rats did not adapt as rapidly to the novel environment as control rats, or that they had a higher basal level of activity than controls. Long-term behaviour monitoring would be required to distinguish between these possibilities. Other aspects of behaviour were not investigated in the present study. However, monitoring of the social interactions of capsaicin-treated rats would clearly be of interest.
The D2 receptor antagonist, haloperidol, greatly reduced the activity of all rats and abolished the hyperactivity of capsaicin-treated rats, indicating that the general basal activity of rats and the hyperactivity of capsaicin-treated rats were mediated by dopaminergic activity. Hyperactivity associated with increased dopaminergic activity has been reported in several other animal models of schizophrenia, such as the neonatal ventral hippocampal lesion model (Lipska et al., 2003). However, hyperactivity is not a feature of schizophrenia. Despite the long-standing dopaminergic hypothesis of schizophrenia and the effectiveness of dopamine receptor antagonists in treatment of psychosis, further work is still required to determine the role of dopamine in schizophrenia.
An important finding was that the male, but not female, capsaicin-treated rats had significantly lower brain weights than vehicle controls. Capsaicin-treated rats also had smaller coronal section areas at several brain levels, thinner cortices, larger LV and aqueduct areas, and smaller hippocampal area than vehicle-treated animals, but there was no significant effect of gender on these parameters. However, there was a significant effect of gender on corpus callosum thickness, male rats being more affected than female rats. The brain changes in capsaicin-treated rats bear a strong resemblance to the changes that have been reported in the brains of subjects with schizophrenia (Shenton et al., 1992; Schlaepfer et al., 1994; McCarley et al., 1999; Keshavan et al., 2002; McDonald et al., 2002; Selemon et al., 2002).
It is noteworthy that, in the present study, male rats were more affected by capsaicin treatment on a number of parameters, including tail length, brain weight and corpus callosum thickness, suggesting that intrinsic sensory deprivation induced by capsaicin had a greater effect on male rats than female rats. Several studies have demonstrated that the number of male sufferers of schizophrenia is greater than females. In a recent study on brain volumes, it was found that increased ventricle size was predominately demonstrated only in male sufferers of schizophrenia (McDonald et al., 2002). Elucidation of the mechanism of the gender difference in the action of capsaicin might provide useful information on the gender-specific aspects of neurodevelopment and their relevance to schizophrenia.
Rats treated as neonates with capsaicin were found to have significantly greater neuronal densities than control rats, in the somatosensory cortex, motor cortex, Au1 and CPu, but not in the primary visual cortex. Interestingly, there was no significant effect of gender on neuronal density. Since nociceptive pathways ascend to the thalamus and thus to the somatosensory cortex, the loss of capsaicin-sensitive primary afferent neurons in rats treated as neonates with capsaicin might be expected to result in reduced connectivity in the somatosensory cortex. However, the changes seen in other cortical areas and in the CPu are important observations that require consideration.
Each brain region is a complex of intrinsic and extrinsic feedforward and feedback neural networks. Thus, changes in sensory input might be expected to produce changes in the cortical association areas, as well as the motor areas and the basal ganglia, which receive glutamatergic projections from the motor cortex. An interesting observation was that the neurons in the anterior cingulate and motor cortex appeared to be bunched and arranged in beaded columns. It is noteworthy that mean cell-spacing abnormalities have recently been reported in the neocortex of subjects with schizophrenia (Casanova et al., 2005).
There have been several reports of aberrant processing of somatosensory information at thalamic and cortical levels in schizophrenia (Reite et al., 2003; Waberski et al., 2004). Functional magnetic resonance imaging studies by Schroder et al. (1999) have shown reduced sensorimotor cortex activation during motor performance in subjects with schizophrenia. In a study using cognitive-habit learning, Weickert et al. (2002) concluded that the abnormality in schizophrenia was in the cortical input in subjects with schizophrenia, whereas the striatal circuitry was normal. However, no studies on the neuronal density in the CPu of subjects with schizophrenia have been reported.
The finding of a significant increase in cell density in the Au1 in capsaicin-treated rats is particularly noteworthy, since it has been demonstrated that a deficit in somatosensory input can effect changes in the auditory cortex. Auditory hallucinations and thought disorder are cardinal symptoms of schizophrenia, the severity of which have been correlated with reductions in auditory cortex grey matter volume (Kasai et al., 2003). Recently, anatomic evidence of pathology in the Au1 of subjects with schizophrenia has been reported. Sweet et al. (2004) found a reduction in the mean pyramidal cell somal volume in layer 3 neurons of the auditory cortex, consistent with abnormalities in auditory feedforward projection neurons in schizophrenia. Beasley et al. (2005) also found that neuronal size was reduced in layer 3 of the planum temporale, an auditory association region within the superior temporal gyrus. Since pyramidal cell somal volume is correlated with the extent of dendritic arborization and the number of dendritic spines (Jacobs et al., 1997), it is tempting to speculate that neuronal density might also be increased in the auditory cortex in schizophrenia. However, Beasley et al. (2005) did not find increased neuronal density in the planum temporale. Both neuronal density and neuron somal volume are indirect indicators of the extent of dendritic arborization, and it is possible that in some brain areas deficits in dendritic arborization may be present without increased neuronal density. Further studies are required to elucidate the neuropathology in the auditory cortex in schizophrenia.
The parallels between the findings in the present study in capsaicin-treated rats and those of Selemon et al. (1995; 1998) and Selemon & Goldman-Rakic (1999) in postmortem human brains from subjects with schizophrenia are striking. These workers found increases in neuronal density in frontal cortical areas in subjects with schizophrenia, of magnitudes similar (17% in Brodmann area 9 and 21% in Brodmann area 46) to those found in capsaicin-treated rats. The finding of relative sparing of the visual cortex is also similar to the findings of Selemon et al. (1995) in human brain from subjects with schizophrenia.
It is unlikely that any animal model of schizophrenia could ever replicate all of the characteristics of this complex human disorder. However, the results from the present study suggest that the neonatal capsaicin-treated rat is a useful new model that might shed light on the aetiology of schizophrenia. Thus, it is proposed that schizophrenia results from an intrinsic somatosensory deprivation that causes subnormal dendritic arborization during development, particularly in the cortex and perhaps other areas such as the thalamus. The abnormality remains silent until synaptic pruning in adolescence results in a loss of connectivity below the threshold for normal signal processing. One of the characteristic features of schizophrenia is impairment of the ability to inhibit irrelevant environmental stimuli with consequent sensory overload. It is possible that abnormality in any part of the sensory or associated systems might lead to deficits in sensory gating mechanisms.
Considerable further work is required to validate the intrinsic somatosensory deprivation hypothesis of schizophrenia and this new animal model. Nevertheless, validation would be of great importance to the field of schizophrenia research. Future studies including tests of sensory gating, as measured by the pre-pulse inhibition paradigm, as well as tests of learning and memory, are required. The importance of the present study is that it has demonstrated the extensive influence of the peripheral somatosensory system on brain development.
Acknowledgments
Funding from the Neuroscience Institute of Schizophrenia and Allied Disorders, New South Wales, Australia, is gratefully acknowledged.
Abbreviations
- AC
anterior cingulate cortex
- AQ
cerebral aqueduct
- Au1
primary auditory cortex
- CA1
cornu ammonis 1 region of the hippocampus
- CPu
caudate putamen
- CT
cortical thickness
- H
hippocampus
- LV
lateral ventricle
- M2
secondary motor cortex
- mRNA
messenger ribonucleic acid
- s.e.m.
standard error of the mean
- SIJ
primary somatosensory cortex jaw
- TRPV1
transient receptor potential vanilloid 1 receptor
- VIM
primary visual cortex monocular
References
- AL-HAYANI A., WEASE K.N., ROSS R.A., PERTWEE R.G., DAVIES S.N. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. doi: 10.1016/s0028-3908(01)00145-9. [DOI] [PubMed] [Google Scholar]
- ARNOLD S.E., TALBOT K., HAHN C.G. Neurodevelopment, neuroplasticity, and new genes for schizophrenia. Prog. Brain Res. 2005;147:319–345. doi: 10.1016/S0079-6123(04)47023-X. [DOI] [PubMed] [Google Scholar]
- ASHE P.C., BERRY M.D., BOULTON A.A. Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25:691–707. doi: 10.1016/s0278-5846(01)00159-2. [DOI] [PubMed] [Google Scholar]
- BEASLEY C.L., CHANA G., HANOVER M., LANDAU S., EVERALL I.P., COTTER D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophrenia Res. 2005;73:69–78. doi: 10.1016/j.schres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- BLUMENSOHN R., RINGLER D., ELI I. Pain perception in patients with schizophrenia. J. Nerv. Mental Dis. 2002;190:481–483. doi: 10.1097/00005053-200207000-00011. [DOI] [PubMed] [Google Scholar]
- BUXHOEVEDEN D., ROY E., SWITALA A. Reduced interneuronal space in schizophrenia. Biol. Psychiatry. 2000;47:681–683. doi: 10.1016/s0006-3223(99)00275-9. [DOI] [PubMed] [Google Scholar]
- CASANOVA M.F., DE ZEEUW L, SWITALA A., KRECZMANSKI P., KORR H., ULFIG N., HEINSEN H., STEINBUSCH H.W.M., SCHMITZ C. Mean cell spacing abnormalities in the neocortex of patients with schizophrenia. Psychiatry Res. 2005;133:1–12. doi: 10.1016/j.psychres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., JULIUS D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- CHANA G., LANDAU S., BEASLEY C., EVERALL I.P., COTTER D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol. Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- DE HERT M., ELLENBROEK B. Animal models of schizophrenia. Neurosci. Res. Commun. 2000;26:279–288. [Google Scholar]
- FROST D.O., TAMMINGA C.A., MEDOFF D.R., CAVINESS V., INNOCENTI G., CARPENTER W.T. Neuroplasticity and schizophrenia. Biol. Psychiatry. 2004;56:540–543. doi: 10.1016/j.biopsych.2004.01.020. [DOI] [PubMed] [Google Scholar]
- GERRITS M.A.F.M., WOLTERINK G., DAENEN E.W.P.M., VAN REE J.M. Animal models of schizophrenia as a neurodevelopmental disorder. Eur. Neuropsychopharmacol. 2002;12 Suppl 3:112–113. [Google Scholar]
- HARRISON P.J. Schizophrenia: a disorder of neurodevelopment. Curr. Opin. Neurobiol. 1997;7:285–289. doi: 10.1016/s0959-4388(97)80018-9. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOOLEY J.M., DELGADO M.L. Pain insensitivity in the relatives of schizophrenia patients. Schizophrenia Res. 2001;47:265–273. doi: 10.1016/s0920-9964(00)00064-5. [DOI] [PubMed] [Google Scholar]
- HORI T., SHIBATA M., KIYOHARA T., NAKASHIMA T., ASAMI A. Responses of anterior hypothalamic-preoptic thermosensitive neurons to locally applied capsaicin. Neuropharmacology. 1988;27:135–142. doi: 10.1016/0028-3908(88)90162-1. [DOI] [PubMed] [Google Scholar]
- JACOBS B., DRISCOLL L., SCHALL M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative golgi study. J. Comp. Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- JANSCÓ G., KIRÁLY E., JANSCÓ-GÁBOR A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature (London) 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- KASAI K., SHENTON M.E., SALISBURY D.F., HIRAYASU Y., ONITSUHA T., SPENCER M.H., YURGELUN-TODD D.A., KIKINIS R., JOLESZ F.A., MCCARLEY R.W. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESHAVAN M.S., DICK E., MANKWOSKI I., HARENSKI K., MONTROSE D.M., DIWADKAR V., DEBELLIS M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophrenia Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- KUDOH A., ISHIHARA H., MATSUKI A. Current perception thresholds and postoperative pain in schizophrenic patients. Region. Anaesth. Pain Med. 2000;25:475–479. doi: 10.1053/rapm.2000.7617. [DOI] [PubMed] [Google Scholar]
- LEWIS D.A., LIEBERMAN J.A. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- LIPSKA B., LERMAN D., KHAING Z.Z., WEINBERGER D.R. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur. J. Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- MARINELLI S., VAUGHAN C.W., CHRISTIE M.J., CONNOR M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J. Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINELLI S., DI MARZO V., BERRETTA N., MATIAS I., MACCARRONE M., BERNARDI G., MERCURI N.B. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J. Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINELLI S., PASCUCCI T., BERNARDI G., PUGLISI-ALLEGRA S., MERCURI N.B. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2005;30:864–870. doi: 10.1038/sj.npp.1300615. [DOI] [PubMed] [Google Scholar]
- MCCARLEY R.W., WILBE C.G., FRUMIN M., HIRAYASU Y., LEVITT J.J., FISCHER I.A., SHENTON M.E. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLUMSMITH R.E., CLINTON S.M., MEADOR-WOODRUFF J.H. Schizophrenia as a disorder of neuroplasticity. Int. Rev. Neurobiol. 2004;59:19–45. doi: 10.1016/S0074-7742(04)59002-5. [DOI] [PubMed] [Google Scholar]
- MCDONALD C., GRECH A., TOULOPOULOU T., SCHULZE K., CHAPPLE B., SHAM P., WALSHE M., SHARMA T., SIGMUNDSSON T., CHINTIS X., MURRAY R.M. Brain volumes in familial and non-familial schizophrenic probands and their unaffected relatives. Am. J. Med. Genet., (Neuropsychiatr. Genet.) 2002;114:616–625. doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- MESSAMORE E., HOFFMAN W.E., JANOWSKY A. The niacin skin flush abnormality in schizophrenia: a quantitative dose–response study. Schizophrenia Res. 2003;62:251–258. doi: 10.1016/s0920-9964(02)00311-0. [DOI] [PubMed] [Google Scholar]
- MEZEY E., TÓTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTULSKY H. Intuitive Biostatistics. New York, U.S.A.: Oxford University Press; 1995. [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1998San Diego, CA, U.S.A.: Academic Press; 4th edn [Google Scholar]
- RAJKOWSKA G., SELEMON L.D., GOLDMAN-RAKIC P.S. Neuronal and glial somal size in the prefrontal cortex: a post-mortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- REITE M., TEALE P., ROJAS D.C., BENKERS T.L., CARLSON J. Anomalous somatosensory cortical localisation in schizophrenia. Am. J. Psychiatry. 2003;160:2148–2153. doi: 10.1176/appi.ajp.160.12.2148. [DOI] [PubMed] [Google Scholar]
- SADAKA Y., WEINFELD E., LEV D.L., WHITE E.L. Changes in mouse barrel synapses consequent to sensory deprivation from birth. J. Comp. Neurol. 2003;457:75–86. doi: 10.1002/cne.10518. [DOI] [PubMed] [Google Scholar]
- SASAMURA T., SASAKI M., TOHDA C., KURAISHI Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. NeuroReport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- SELEMON L.D., GOLDMAN-RAKIC P.S. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- SELEMON L.D., RAJKOWSKA G., GOLDMAN-RAKIC P.S. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52:805–820. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- SELEMON L.D., RAJKOWSKA G., GOLDMAN-RAKIC P.S. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a 3-dimensional, stereologic counting method. J. Comp. Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- SELEMON L.D., KLEINMAN J.E., HERMAN M.M., GOLDMAN-RAKIC P.S. Smaller frontal gray matter volume in post-mortem schizophrenic brains. Am. J. Psychiatry. 2002;159:1983–1991. doi: 10.1176/appi.ajp.159.12.1983. [DOI] [PubMed] [Google Scholar]
- SCHRODER J., ESSIG M., BLAUDNENDISTEL K., JAHN T., GERDSEN I., STOCKERT A., SHAD L.R., KNOPP V. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9:81–87. doi: 10.1006/nimg.1998.0387. [DOI] [PubMed] [Google Scholar]
- SCHLAEPFER T.E., HARRIS G.J., TIEN A.Y., PENG L.W., LEE S., FEDERMAN E.B., CHASE G.A., BARTA P.E., PEARLSON G.D. Decreased regional cortical gray matter volume in schizophrenia. Am. J. Psychiatry. 1994;151:842–848. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S.K., ANDREASEN N.C. Schizophrenia. Lancet. 1999;353:1425–1430. doi: 10.1016/s0140-6736(98)07549-7. [DOI] [PubMed] [Google Scholar]
- SHENTON M.E., KIKINIS R., JOLESZ F.A., POLLAK S.D., LEMAY M., WIBLE C.G., HOKAMA H., MARTIN J., METCALF D., COLEMAN M., MCCARLEY R.W. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. New Engl. J. Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- SWEET R.A., BERGEN S.E., SUN Z., SAMPSON A.R., PIERRI J.N., LEWIS D.A. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol. Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- TOLIVIA J., TOLIVIA D. A new technique for differential and simultaneous staining of nerve cells and fibers. J. Neurosci. Methods. 1985;13:305–311. doi: 10.1016/0165-0270(85)90078-0. [DOI] [PubMed] [Google Scholar]
- TÓTH A., BOCZÁN J., KEDEI N., LIZANECZ E., BAGI Z., PAPP Z., ÉDES I., CSIBA L., BLUMBERG P.M. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Mol. Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- VALENZANO K.J., SUN Q. Current perspectives on the therapeutic utility of VR1 antagonists. Current Med. Chem. 2004;11:3185–3202. doi: 10.2174/0929867043363686. [DOI] [PubMed] [Google Scholar]
- WABERSKI T.D., NORRA C., KAWOHL W., THYERLEI D., HOCK D., KLOSTERMANN F., CURIO G., BUCHNER H., HOFF P., GOBBELE R. Electrophysiological evidence for altered early cerebral somatosensory signal processing in schizophrenia. Psychophysiology. 2004;41:361–366. doi: 10.1111/1469-8986.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- WALDO M.C. Co-distribution of sensory gating and impaired niacin flush response in the parents of schizophrenics. Schizophrenia Res. 1999;40:49–53. doi: 10.1016/s0920-9964(99)00031-6. [DOI] [PubMed] [Google Scholar]
- WEICKERT T.W., TERRAZAS A., BIGELOW L.B., MALLEY J.D., HYDE T., EGAN M.F., WEINBERGER D.R., GOLDBERG T.E. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn. Mem. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]