Abstract
We report on a rapid simulation method for predicting protein orientation on a surface based on electrostatic interactions. New methods for predicting protein immobilization are needed because of the increasing use of biosensors and protein microarrays, two technologies that use protein immobilization onto a solid support, and because the orientation of an immobilized protein is important for its function. The proposed simulation model is based on the premise that the protein interacts with the electric field generated by the surface, and this interaction defines the orientation of attachment. Results of this model are in agreement with experimental observations of immobilization of mitochondrial creatine kinase and type I hexokinase on biological membranes. The advantages of our method are that it can be applied to any protein with a known structure; it does not require modeling of the surface at atomic resolution and can be run relatively quickly on readily available computing resources. Finally, we also propose an orientation of membrane-bound cytochrome c, a protein for which the membrane orientation has not been unequivocally determined.
Keywords: electric double layer, electrostatic simulations, orientation flexibility
Adsorption of proteins on solid interfaces has become an area of great theoretical and practical interest because of the recently extensive use of immobilized proteins and applications involving the catalytic potential of immobilized enzymes. Thus with the advent of protein microarrays and other solid miniaturized devices involving proteins, combined with applications in biomedical material engineering and biosensors, a greater need for defining the interactions of proteins with various surfaces has become evident. Accordingly, information on the orientation by which a protein may encounter the surface for immobilization has become an essential requirement. With such information, it would be possible to make predictions regarding details of interactions involving the participating components on the biomolecule and the reactive surface. Moreover, the residues on the biomolecule available for interaction with other proteins and ligands in solution and for binding to the reactive groups at the surface could be identified. Furthermore, customized surface chemistries resulting in a desirable immobilization orientation would become facilitated, resulting in a more targeted approach to protein immobilization.
Despite the increasing need to understand how proteins attach to cellular and artificial surfaces, experimental details of the orientation by which proteins are immobilized are, to our knowledge, available only in two cases [type I hexokinase and mitochondrial creatine kinase (MtCK)] because of the difficulty in obtaining reliable experimental data. As for membrane proteins, the structure of only a limited number of cases (e.g., bacteriorhodopsin, and microsomal P450, see refs. 1 and 2) have been determined, because of the inherent difficulties associated with their crystallization. The other main structural technique, NMR, is not amenable to structure determination for large membrane-bound protein assemblies. Use of molecular dynamics (MD) simulations to predict angular orientation of membrane-attached proteins has proven very time-consuming, particularly for large molecules. In addition, MD simulations have limited capacity for describing the electrostatic properties of protein–membrane systems because of the need to approximate long-range interactions (3, 4). Thus, given the difficulty of experimental determination and existing MD methods, we propose an alternative approach based on electrostatic interactions involving the protein structure and the interfacial electric field occurring between the charged planar surface (on which the proteins will be immobilized) and the surrounding liquid. Models based on electrostatic interactions continue to be useful because of their simplicity and relatively modest computational requirements. Currently, protein orientation is predicted by visual inspection of its electrostatic potential profile (5) or by modeling the membrane targeting domains of the protein (6). In this article, we report on a more accurate estimation of the equilibrium orientation, which has the minimum electrostatic free energy of the interaction between protein and surface. A general partial differential equation solver (PROPHET; www-tcad.stanford.edu/∼prophet) has been adapted to calculate the electrostatic free energies for all possible protein/membrane orientations. Type I hexokinase, a 100-kDa protein, and MtCK, an 89-kDa protein, were chosen as model proteins, because their orientation of interaction with mitochondrial membranes has been reported by using mutational studies (7), limited proteolytic digestion (8), and mAbs (9). In addition to these proteins, we predict the orientation of cytochrome c, a protein for which experimental findings on orientation are equivocal (10–13). Our prediction for cytochrome c remains to be confirmed or refuted experimentally.
Theoretical Models
Protein recruitment to charged surfaces such as biomembranes is facilitated by nonspecific and long-distance electrostatic interactions (6). When a protein molecule encounters the electric double layer (EDL) surrounding the membrane, in its approach to the surface, the protein finally adopts its equilibrium orientation on the surface.
Oscillation and Alignment of Proteins with Electric Field.
The electrostatic interaction mechanisms at microscopic levels essentially depend on the charge assembly involved, i.e., net charge and polarization. Biomolecules can generally have both dipolar and unipolar charges formed by the spatial partial charges in the molecular structure. When an electric field is applied to a protein molecule, electrostatic interaction induces translation and rotation of the molecule. In a uniform electric field, the translation of the molecule is governed by interaction of the electric field with the net charge of the molecule, unipolar interaction, and the rotation is governed by interaction of the electric field with dipolar charge of the molecule, dipolar interaction. However, if the electric field is not spatially uniform, as is the case in the EDLs, the translatory motion is determined by the sum of all forces applied to spatial partial charges in the molecule, and the rotary motion is formed by the sum of all partial torques applied to the molecule.
The torque produces an angular acceleration, which accelerates the molecule and aids in restoring it into a stable position. In a stable position, torque is equal to zero and the electrostatic free energy of the system is at a local minimum. Unfortunately, the existence of multiple local minima makes it difficult to determine the global minimum. Energy analyses can find and quantify the global minimum among all orientations of a protein molecule with respect to the membrane. To generate all of these orientations, it is sufficient to fix the membrane and transform the protein by an isometric transformation, which moves the objects in the space while keeping their shapes and distances unchanged.
Any isometry in 3D space can be generated by combination of rotations around the x, y, z axis. In our case, because the planar surface is symmetric along its normal vector (taken to be the z axis), we can consider only rotations around the x and y axis. Let θ be the angle of rotation around x axis and φ the angle of rotation around y axis. Then values of θ between 0, 2π and values of φ between 0, π generate all possible relative positions. In each case, the electrostatic free energy of the interaction is calculated and the global minimum is derived empirically.
Calculating Electrostatic Free Energy of the Protein–Surface Interaction.
Generally, at the interface of two different phases in contact, a charge separation will be spontaneously formed. Mechanisms for this charge separation include differences in the affinity of two phases for electrons, difference in the affinity of two phases for ions of one charge or the other, physical entrapment of nonmobile charge in one phase, and finally, the ionization of surface groups. The spontaneously formed surface charge at the liquid/solid interface forms an EDL that contains a net excess of mobile ions with a polarity opposite to that of the interface. Accordingly, upon the interaction of charged protein atoms with the EDL surrounding the charged surface, in its approach for immobilization, the protein takes up an equilibrium orientation that corresponds to an orientation with the minimum electrostatic free energy by which it becomes attached to the surface. Polar groups of the protein may rearrange themselves to maximize the electrostatic attraction; nevertheless, in this simple model, we use a single conformation for the protein and thus do not account for motions caused by internal degrees of freedom, which would result in changes of conformation.
The electrostatic potential and the mean distribution of the concentration of the ions at each lattice point are calculated by solving a nonlinear Poisson-Boltzmann equation (14):
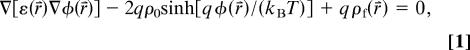 |
where φ(r⇑) is the electrostatic potential, ε(r⇑) is the dielectric constant, ρ0 is the equilibrium ion concentration in the bulk solution, q is the elementary charge, and qρf(r⇑) is the density profile of fixed charges associated with proteins and membranes. An inherent assumption in nonlinear Poisson-Boltzmann-based models is the use of the mean electrostatic potential to estimate the potential of mean force (PMF). The applicability of this assumption to biophysical and colloid chemistry was discussed in ref. 14; the error caused by the PMF estimation was found to be small for 1:1 salt.
The nonlinear Poisson-Boltzmann equation is numerically solved by using a general partial differential equation (PDE) solver, PROPHET. PROPHET provides a script-based programming framework for the assembly and solution of nonlinear PDEs in three-space dimensions and time. The PDEs that can be solved by PROPHET include the elliptic (Poisson) type, diffusion-advection-reaction type, and their combinations. Such capabilities make it a particularly suitable numerical tool for continuum-based modeling of nonequilibrium transport and equilibrium electrostatics in bioelectrical systems. Previously, PROPHET has been used extensively in the modeling and simulation of semiconductor-based processes (15) and devices (16). It has recently been applied to solving coupled Poisson-Nernst-Planck equations in the modeling of charge transport in ompF porin ion channels (17). In the current case, the interior of protein and membrane are not accessible to the electrolytes. Therefore, the nonlinear Poisson-Boltzmann equation is solved only in the solution, whereas the Poisson equation is solved inside the protein and membrane. The continuity of electrostatic potential is imposed at the solution/protein and solution/membrane interfaces. The simulations are carried out on uniform meshes of 2-Å resolution in each direction. The electrostatic potential thereby obtained, φ(r), is then used to evaluate the electrostatic free energy of the protein/membrane system as follows (14):
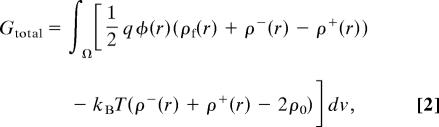 |
where the mobile cation density in the solution is given by ρ+(r) = ρ0 exp(−qφ(r)/kBT), and the mobile anion density is given by ρ−(r) = ρ0 exp(qφ(r)/kBT). The integration is over the volume of the entire system, Ω. The first term of the integrand accounts for both the electrostatic stress term, E⇑·D⇑/2 = qφ(ρf + ρ+ − ρ−)/2, and the entropy term, TΔS = −qφ(ρ+ − ρ−). The second term of the integrand is the excess osmotic pressure of the mobile ions. The electrostatic free energy of the protein (Gprotein) and the membrane (Gmembrane) are separately calculated by using the same approach for isolated protein and membrane, respectively. Consequently, the free energy of the electrostatic interaction is obtained as follows (6):
Orientation Flexibility.
Because of the Stokes drag force and dissipation of energy, an oscillating molecule in an electric field would ultimately settle in its equilibrium orientation of minimum potential energy. The molecule would move around this position because of thermal energy, kBT, or interaction with other molecules. In general, the ability of the protein to reorient with respect to the membrane/surface can be determined from the steepness of interaction free energy of the protein at its equilibrium orientation. For molecules with a steep minimum of interaction free energy, limited changes in their orientation may occur because of external effects, whereas for molecules with a shallow minimum, their orientation is prone to fluctuation because of thermal noise or environmental effects. For instance, in the presence of thermal noise, the probability that the protein in thermal equilibrium takes a given orientation with energy Ei is given by:
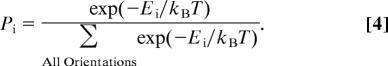 |
The orientation flexibility can be reduced by decreasing the ionic strength of the environment and increasing the charge density on the surface, both of which influence the electric field in the EDL at the interface.
Results
To test the agreement of our simulation method with experimental observations, we determined the equilibrium orientation of protein molecules on a surface through the analysis of electrostatic interactions occurring between the protein and the electric field in the EDL surrounding the charged interface. Of the examples involving direct attachment of soluble proteins to biomembranes, two cases have been reported with sufficient details and certainty. We applied our approach to these two proteins and determined the orientation of the entire protein structure. We found good agreement between our results and the experimental observations. Finally, we made a prediction concerning the orientation of cytochrome c.
Membrane Association of Type I Hexokinase.
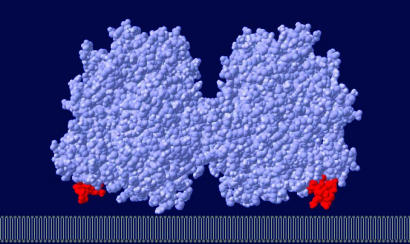
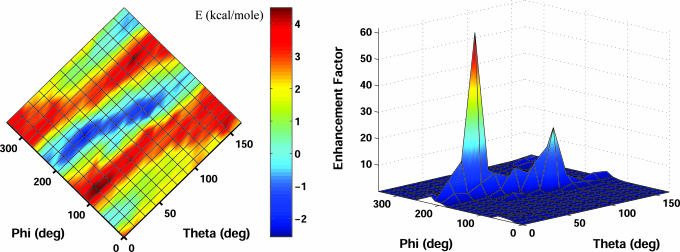
Substantial amounts of type I hexokinase, a key enzyme of the glucose metabolism, have been found associated with the outer mitochondrial membrane in several mammalian tissues (18–20). Mitochondrially bound enzyme is more active than the soluble enzyme because the enzyme binds with an orientation allowing it to use the ATP generated in mitochondria to phosphorylate glucose preferentially (21). In addition to electrostatic interactions, hydrophobic interactions were found to play an important role in possessing strong binding affinity. Attachment to the membrane appears to occur by a “binding domain” (8, 22), consisting of the N-terminal region of the molecule. Binding of the enzyme to mitochondria depends on a 15-residue hydrophobic sequence, which embeds into the hydrophobic core of the outer mitochondrial membrane. Maintenance of an intact hydrophobic sequence at the N terminus is critical to this interaction, loss of which by limited digestion with chymotrypsin generates a nonbinding enzyme (8). Moreover, only type I and type II hexokinases that contain the hydrophobic tail can bind mitochondria (23). Likewise, the tail was found essential for binding of the enzyme to an artificial matrix (24). Applying our simulation method, the protein was found to take up an orientation upon immobilization that allows an intimate association of the N-terminal segment with the membrane. As shown in Fig. 1, the N-terminal segments are targeted toward the charged interface, in accord with experimental observations (25). Electrostatic free energy of interaction for all possible angular orientations and the calculated orientation flexibility of the enzyme are shown in Fig. 2. Because of electrostatic interaction, the probability of taking the equilibrium orientation is enhanced by a factor of 50 as compared with random orientations.
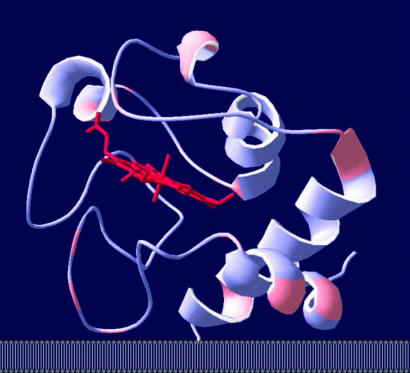
Fig. 1.
Predicted orientation of type I hexokinase adsorbed on the outer mitochondrial membrane. The two N-terminal membrane-binding peptides are shown in red. Targeting of the N-terminal segments toward the charged membrane surface is determined by the unique structural properties of type I hexokinase.
Fig. 2.
Electrostatic simualtion results for membrane association of type I hexokinase. (Left) The plot depicts the electrostatic free energy of interaction between type I hexokinase and the outer mitochondrial membrane occurring at 100 mM KCl. The charge density of the membrane is assumed to be ≈1 electron per 250 Å2. (Right) The enhancement factor is calculated as the ratio of the probability of orientations caused by the electrostatic interactions to that obtained from random orientations.
Membrane Association of Sarcomeric MtCK.
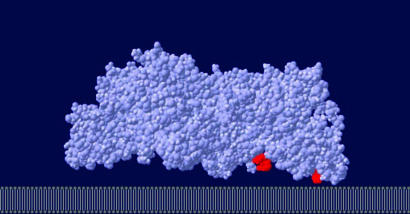
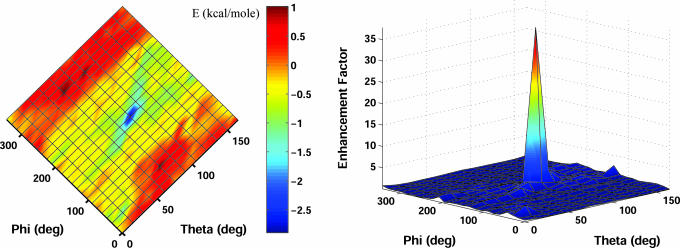
In view of the physiological function of creatine kinase, one of the most important properties of this enzyme is the distinct subcellular localization of its different isoforms (26). MtCK is a central enzyme in energy metabolism of tissues with high and fluctuating energy requirements. The enzyme is found exclusively in the mitochondrial intermembrane compartment, being attached as an octamer to the outer face of the inner mitochondrial membrane by electrostatic interactions between positively charged groups on the enzyme and negatively charged groups in the membrane (7). Its binding to the membrane is important in relation to its metabolite channeling function. Although its binding site on the membrane is not known, the phospholipid cardiolipin is thought to be part of this site, most likely through its negative charges (7, 27). The C terminus of vertebrate MtCK contains three basic residues that determine high-affinity MtCK/membrane interaction and protrude from the putative membrane binding face (7). These residues, which are largely conserved among vertebrate MtCK, correspond to Lys-369, Lys-379, and Lys-380 in human MtCK. Mutation of these lysines drastically reduces the capacity of MtCK to attach mitochondrial model membranes containing cardiolipin, providing further evidence that the three lysines largely determine membrane interaction of the octameric enzyme. Our simulation approach, when applied to the protein, results in an orientation for immobilization (Fig. 3), with specific spatial positioning of the three lysine residues close to the surface, known to be required for an efficient attachment. For MtCK, an enhancement factor of >30 was obtained at the equilibrium orientation (Fig. 4).
Fig. 3.
Predicted orientation of the sarcomeric MtCK adsorbed on membrane. Lysine side chains (residues 369, 379, and 380 indicated in color) have been proposed to participate in membrane binding (7). These basic residues interact with negatively charged cardiolipin, the main receptor for MtCK in the inner mitochondrial membrane.
Fig. 4.
Electrostatic simulation results for membrane association of sarcomeric MtCK. (Left) The plot depicts the electrostatic free energy of interaction between creatine kinase (MtCK) and the inner mitochondrial membrane occurring at 100 mM KCl. The charge density of the membrane is assumed to be ≈1 electron per 450 Å2. (Right) The enhancement factor caused by the electrostatic interaction.
Membrane Association of Cytochrome c.
Cytochrome c is a small, highly conserved protein and consists of a single polypeptide chain with a covalently attached heme as a redox-active cofactor, only one edge of which is accessible to the protein surface. Cytochrome c is often considered to represent a paradigm for electrostatically interacting peripheral membrane proteins because it is dissociated from lipid membranes by increasing ionic strength (10, 28, 29), while its orientation on membrane is not known with any degree of certainty (10–12, 29). Accordingly, we chose this protein to test our simulation approach in addition to the two examples discussed above, for which sufficient experimental work has been carried out and their orientation on membrane surface is known with confidence.
In the terminal step of the respiratory chain of aerobic organisms, the membrane-bound enzyme cytochrome c oxidase reduces oxygen to water, with cytochrome c delivering the required electrons. This process is accomplished by the docking of cytochrome c to the subunits of cytochrome bc1 (complex III) and cytochrome c oxidase (complex IV) that protrude from the inner membrane, to facilitate electron transfer (30). For cytochrome c to be an efficient electron conduit, the need for both rapid substrate complex formation and product complex dissociation would appear to be particularly stringent. In addition to these demanding docking requirements, the role of cytochrome c in signaling cell death (31) emphasizes the importance of defining its orientation on the membrane.
In horse cytochrome c, six or seven highly conserved lysine residues, including Lys-8, Lys-13, Lys-72, Lys-86, and Lys-87, surrounding the heme crevice of cytochrome c have been implicated in its binding to its electron transfer partners by chemical modification (32) and crystallographic studies (33). Therefore, a correct orientation of cytochrome c should allow for intimate contacts between its positively charged lysine residues with the negatively charged residues on the redox/oxidase enzyme systems. This requirement is in addition to allowing for efficient electron transfer between its heme group and those of its physiological partners on the inner mitochondrial membrane.
Applying our simulation approach to horse heart cytochrome c, the protein was found to take up an orientation as depicted in Fig. 5upon moving toward membrane. Although the exact orientation of cytochrome c on the inner mitochondrial membrane has not been demonstrated experimentally, presumably because of its small size and the dynamic nature of its binding, it appears logical that the overall orientation of the heme group should be horizontal and not vertical, as predicted in the present study (Fig. 5). This orientation is in accord with some speculations (e.g., ref. 11) and in discord with other speculations (e.g., ref. 29), based on results obtained with model phospholipid membranes. The lysine residues surrounding the heme, as shown in Fig. 5, are unbounded and available to interact with the negatively charged residues on the subunits of complex III/complex IV.
Fig. 5.
Predicted orientation of cytochrome c on the inner mitochondrial membrane at 100 mM KCl. The heme group is shown in red. Lysine residues shown in pink may interact with the negatively charged residues on the subunits of complex III/complex IV in addition to the head groups of cardiolipin. Further details are described in Results.
In the present simulation approach, the protein is assumed as a rigid structure but this could be an oversimplification because the protein is believed to undergo conformational changes upon binding to the membrane (34). Proposed structural changes in the protein molecule have gone so far as suggesting the possibility for the occurrence of a molten-globular state near the membrane surface (35).
Discussion
Stable immobilization of a protein with retention of biological activity is an important problem for the successful commercial development of biosensors, protein microarrays, or any other application involving the functional property of an immobilized protein. As for protein microarrays, the orientation of surface-bound proteins is recognized as an important factor related to its biological function and its availability to other proteins and ligands in solution. For a protein to become immobilized on a surface, its orientation plays an important role. The proper orientation would allow for interaction of those residues, which are not essential for its biological activity, with the corresponding sites on the surface. Ideally, this orientation would result in an immobilized preparation with the reactive components on the protein structure available for biological activity, minimum loss of flexibility, and strong associations, concomitantly. In approaching a charged surface, a protein molecule takes up an orientation determined by nonspecific and long-distance electrostatic interactions. However, a protein may assume alternate orientations because of short-range forces. In such cases, the equilibrium orientation can be directed through site-specific immobilization (36).
Among the studies related to interaction of proteins to solid interfaces, their binding to biological membranes and other subcellular structures have attracted the most attention because of their physiological significance and have provided useful information and guidelines for in vitro systems. In these studies, protein structure and the orientation with which it approaches the surface have been found crucial in determining the extent of interaction and its consequent biological function. Specific signaling domains have been identified that are ubiquitous in eukaryotic signal transduction and membrane trafficking. These domains regulate subcellular localization and protein function mainly by binding to the lipid components of biological membranes. The best known of these are the C1, C2, PH, and FYVE domains (see ref. 37 and references therein). The spatial distribution of the binding sites (domains) on the protein structure is crucial for intimate association with reactive components on the surface, necessitating the proper orientation of the protein molecule before its adsorption on the surface. The widespread ability of at least some glycolytic enzymes to bind to membranes or other cellular components is well documented (e.g., see ref. 38 and references therein). In addition, some studies have shown that charged small molecules alter interaction of the carboxyl-terminal C2 (Gla) domain of blood-clotting factor VIII and phosphatidyl serine of platelet membrane (39), a process that is essential for hemostasis. This finding, combined with the observation that certain mutants of blood-clotting factor VII show enhanced affinities for membranes (40), suggests how changes in the structure and polarity of a protein molecule can alter the affinity of binding.
This article describes a simulation procedure that predicts the orientation of a protein molecule related to its binding to a charged interface. The procedure was successfully tested by using reported experimental findings describing the association of two proteins with well understood structures and membrane-binding mechanisms. The agreement between calculation and experiments suggests the important role of electrostatic interaction in protein orientation and its adsorption to charged membranes. We propose that our simulation method may provide a useful and versatile tool for designing tailor-made protein orientations, by modifying charged residues on proteins using chemical modification or protein engineering. We hope that this method will enhance our ability to produce stable and fully functional immobilized proteins for numerous biological and biomedical applications.
Methods
Protein/Surface Representation.
For the model proteins presented here, the interacting surface (mitochondrial membranes) has been approximately modeled as a charged planar surface with charge density related to the density of polar heads of phospholipids in the membrane and the valence number of their deprotonated head groups. We assume that surface charge density of the outer mitochondrial membrane consisting of phosphatidyl choline, phosphatidyl ethanolamine, and phosphatidylinositol with an approximate percentage composition of 55%, 30%, and 15% (41), respectively, is one electron per 250 Å2. In the case of the inner membrane consisting of phosphatidyl choline, phosphatidyl ethanolamine, and cardiolipin with an approximate percentage composition of 40%, 45%, and 15% (42), respectively, the surface charge density is assumed to be ≈1 electron per 450 Å2. The environment is modeled as a homogeneous continuum dielectric medium containing 100 mM KCl. The atomic structure of the protein is defined by assigning each atom a radius, and the charge distribution inside the protein is represented by a collection of delta functions located at the nucleus of consisting atoms. Radius and partial charges for the protein atoms were assigned by using the PDB2PQR web server (43) based on the parameters of the AMBER99 force field (44). Finally, the protein/membrane model is then mapped onto a 3D lattice of points. We assume that the distance between the adsorbed protein and the membrane is 3 Å, because the interaction is most favorable at this distance (6, 45). At shorter distances, both the protein and membrane are increasingly desolvated, which is energetically unfavorable because of Born repulsion (5, 46, 47). The 3D simulation domain Ω is a 200 × 200 × 200 Å3, which extends several Debye lengths beyond the edge of the protein, ensuring that the system is electroneutral. The dielectric constants used for solution, membrane and protein are 80, 2, and 4, respectively.
Simulations and Computational Resources.
Simulations were run on a Colfax AMD Dual Opteron server with RedHat Enterprise Linux (Sunnyvale, CA) with 4-GB memory. Runtime, i.e., iterations required for rotations of protein in a Java program and calculating electrostatic energy with PROPHET, varied from 1 to 3 days depending on protein size and complexity (hexokinase, 3 days; creatine kinase, 2 days; cytochrome c, 1 day).
Acknowledgments
We thank Prof. Michael Snyder and Dr. Steve W. Turner for invaluable comments and suggestions on the manuscript and Prof. Pehr A. B. Harbury and Mohsen Bayati for useful discussions. Y.L. was supported by the Network for Computational Nanotechnology (National Science Foundation Grant EEC-0228390). This research was supported by National Institutes of Health Grant P01 HG000205.
Abbreviations
- MtCK
mitochondrial creatine kinase
- EDL
electric double layer.
Footnotes
The authors declare no conflict of interest.
References
- 1.Essen L, Siegert R, Lehmann WD, Oesterhelt D. Proc Natl Acad Sci USA. 1998;95:11673–11678. doi: 10.1073/pnas.95.20.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoch GA, Yano JK, Wester MR, Griffin KJ, Stout CD, Johnson EF. J Biol Chem. 2004;279:9497–9503. doi: 10.1074/jbc.M312516200. [DOI] [PubMed] [Google Scholar]
- 3.Murray D, Arbuzova A, Honig B, McLaughlin S. Curr Top Membr. 2002;52:277–307. [Google Scholar]
- 4.Jakobsson E. Trends Biochem Sci. 1997;22:339–344. doi: 10.1016/s0968-0004(97)01096-7. [DOI] [PubMed] [Google Scholar]
- 5.Murray D, Hermida-Matsumoto L, Buser CA, Tsang J, Sigal CT, Ben-Tal N, Honig B, Resh MD, McLaughlin S. Biochemistry. 1998;37:2145–2159. doi: 10.1021/bi972012b. [DOI] [PubMed] [Google Scholar]
- 6.Diraviyam K, Stahelin RV, Cho W, Murray D. J Mol Biol. 2003;328:721–736. doi: 10.1016/s0022-2836(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 7.Schlattner U, Gehring F, Vernoux N, Tokarska-Schlattner M, Neumann D, Marcillat O, Vial C, Wallimann T. J Biol Chem. 2004;279:24334–24342. doi: 10.1074/jbc.M314158200. [DOI] [PubMed] [Google Scholar]
- 8.Polakis PG, Wilson JE. Arch Biochem Biophys. 1985;236:328–337. doi: 10.1016/0003-9861(85)90633-2. [DOI] [PubMed] [Google Scholar]
- 9.Smith AD, Wilson JE. Arch Biochem Biophys. 1991;287:359–366. doi: 10.1016/0003-9861(91)90490-a. [DOI] [PubMed] [Google Scholar]
- 10.Brown LR, Wüthrich K. Biochim Biophys Acta. 1977;468:389–410. doi: 10.1016/0005-2736(77)90290-5. [DOI] [PubMed] [Google Scholar]
- 11.Pachence JM, Amador S, Maniara G, Vanderkooi P, Dutton L, Blasie JK. Biophys J. 1990;58:379–389. doi: 10.1016/S0006-3495(90)82384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rytömaa M, Kinnunen PK. J Biol Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 13.Iverson SL, Enoksson M, Gogvadze V, Ott M, Orrenius S. J Biol Chem. 2004;279:1100–1107. doi: 10.1074/jbc.M305020200. [DOI] [PubMed] [Google Scholar]
- 14.Sharp KA, Honig B. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- 15.Pinto MR, Boulin DM, Rafferty CS, Smith RK, Coughran JWM, Kizilyalli IC, Thoma MJ. IEDM Technical Digest. NJ: IEEE, Piscataway; 1992. pp. 923–926. [Google Scholar]
- 16.Rafferty CS, Biegel B, Yu Z, Ancona MG, Bude J, Dutton RW. In: Simulation of Semiconductor Processes and Devices. De Meyer K, Biesemans S, editors. New York: Springer; 1998. pp. 137–140. [Google Scholar]
- 17.Van Der Straaten TA, Tang JM, Ravaioli U, Eisenberg RS, Aluru NR. J Comp Elec. 2003;2:29–47. [Google Scholar]
- 18.Feik C, Benz R, Roos N, Brdiczka D. Biochim Biophys Acta. 1982;688:429–440. doi: 10.1016/0005-2736(82)90354-6. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JE. J Bioenerg Biomembr. 1997;29:97–102. doi: 10.1023/a:1022472124746. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Biochemistry. 1986;25:1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JE. Rev Physiol Biochem Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 22.Xie G, Wilson JE. Arch Biochem Biophys. 1988;267:803–810. doi: 10.1016/0003-9861(88)90090-2. [DOI] [PubMed] [Google Scholar]
- 23.Gelb BD, Adams V, Jones SN, Griffin LD, McGregor GR, McCabe ERB. Proc Natl Acad Sci USA. 1992;89:202–206. doi: 10.1073/pnas.89.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehsani-Zonouz A, Golestani A, Nemat-Grogani M. Mol Cell Biochem. 2001;223:81–87. doi: 10.1023/a:1017952827675. [DOI] [PubMed] [Google Scholar]
- 25.Mulichak AM, Wilson JE, Padmanabhan K, Garavito RM. Nat Struct Biol. 1998;5:555–560. doi: 10.1038/811. [DOI] [PubMed] [Google Scholar]
- 26.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheneval D, Carafoli E. Eur J Biochem. 1988;171:1–9. doi: 10.1111/j.1432-1033.1988.tb13750.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls P. Biochim Biophys Acta. 1974;346:261–310. doi: 10.1016/0304-4173(74)90003-2. [DOI] [PubMed] [Google Scholar]
- 29.Salemme FR, Freer ST, Xuong NH, Alden RA, Kraut J. J Biol Chem. 1973;248:3910–3921. doi: 10.2210/pdb1c2c/pdb. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson-Miller S, Brautigan DL, Margoliash E. J Biol Chem. 1976:1104–1115. [PubMed] [Google Scholar]
- 31.Yang J, Liu X, Bhalla K, Kim C, Ibrado A, Cai J, Peng T, Jones D, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson-Miller S, Brautigan DL, Margoliash E. J Biol Chem. 1978;253:149–159. [PubMed] [Google Scholar]
- 33.Pelletier H, Kraut J. Science. 1992;258:1748–1756. doi: 10.1126/science.1334573. [DOI] [PubMed] [Google Scholar]
- 34.Muga A, Mantsch HH, Surewicz WK. Biochemistry. 1991;30:7219–7224. doi: 10.1021/bi00243a025. [DOI] [PubMed] [Google Scholar]
- 35.Bychkova VE, Dujsekina AE, Klenin SI, Tiktopulo EI, Uversky VN, Ptitsyn OB. Biochemistry. 1996;35:6058–6063. doi: 10.1021/bi9522460. [DOI] [PubMed] [Google Scholar]
- 36.Domen PL, Nevens JR, Mallia AK, Hermanson GT, Klenk DC. J Chromatogr. 1990;510:293–302. doi: 10.1016/s0021-9673(01)93763-x. [DOI] [PubMed] [Google Scholar]
- 37.Hurley JH, Misra S. Annu Rev Biophys Biomol Struct. 2000;29:49–79. doi: 10.1146/annurev.biophys.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutowicz J, Terlecki G. Cell Mol Biol Lett. 2003;8:667–680. [PubMed] [Google Scholar]
- 39.Spiegel PC, Kaiser SM, Simon JA, Stoddard BL. Chem Biol. 2004;11:1413–1422. doi: 10.1016/j.chembiol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Harvey SB, Stone MD, Martinez MB, Nelsestuen GL. J Biol Chem. 2003;278:8363–8369. doi: 10.1074/jbc.M211629200. [DOI] [PubMed] [Google Scholar]
- 41.de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B. Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 42.Hovius H, Lambrechts H, Nicolay K, de Kruijff B. Biochim Biophys Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- 43.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. Nucleic Acids Res. 2004;32:665–667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang JM, Cieplak P, Kollman PA. J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- 45.Murray D, McLaughlin S, Honig B. J Biol Chem. 2001;276:45153–45159. doi: 10.1074/jbc.M101784200. [DOI] [PubMed] [Google Scholar]
- 46.Parsegian A. Nature. 1969;221:844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- 47.Ben-Tal N. J Phys Chem. 1995;99:9642–9645. [Google Scholar]