Abstract
The Notch signaling pathway defines an evolutionarily conserved cell–cell interaction mechanism that throughout development controls the ability of precursor cells to respond to developmental signals. Here we show that Notch signaling regulates the expression of the master control genes eyeless, vestigial, and Distal-less, which in combination with homeotic genes induce the formation of eyes, wings, antennae, and legs. Therefore, Notch is involved in a common regulatory pathway for the determination of the various Drosophila appendages.
Keywords: appendage induction, eyeless, vestigial, Distal-less
How organ identity is determined is one of the fundamental questions in developmental biology. In Drosophila the imaginal discs, the primordia of the trunk, and the appendages of the adult fly provide a unique system to study the determination of organ identity. The imaginal disc cells maintain their disc-specific determination from the time they are established during embryogenesis until they finally differentiate during metamorphosis. Recently several Drosophila genes have been identified that are capable of inducing organogenesis when expressed ectopically. The most striking example being eyeless (ey), the homologue of Pax-6 in vertebrates, which is capable of inducing ectopic eyes on wings, legs, and antennae (1, 2). Vestigial (vg) when expressed ectopically can induce wings and halteres in other regions of the body plan than the thorax (3, 4). It therefore seems useful to consider these genes as master control genes for the morphogenesis of these organs (5, 6). To understand organogenesis, we have to elucidate the regulatory mechanisms of these master control genes.
The Notch signaling pathway defines an evolutionarily conserved cell–cell interaction mechanism, which throughout development controls the ability of precursor cells to respond to developmental signals (7–9). Given the fundamental role of the Notch pathway during development, we were interested to explore the role of Notch signaling in the determination of disc identity. In wing morphogenesis, Notch signaling triggers the expression of vg, a control gene for wing and haltere formation, along the dorso-ventral boundary of the wing disc (3, 10). In eye morphogenesis of Drosophila, Notch signaling participates in cell fate decisions during photoreceptor differentiation (11) and in dorso-ventral patterning (12, 13). A proneural function also has been suggested (14), but the function of Notch at earlier stages of eye development has not been examined. Here we show that Notch signaling regulates ey expression during eye induction. Significantly it also is involved in the determination of other Drosophila appendages, i.e. wings, antennae, and legs, and regulates the respective control genes vg (3) and Distal-less (Dll) (15). We conclude that Notch signaling is involved in a common regulatory pathway for the determination of the identity of the various Drosophila appendages.
Materials and Methods
Histochemistry and in Situ Hybridization.
For immunohistochemistry, staged larvae were dissected in cold PBS and fixed in PEM (100 mM Pipes, pH 6.9/2 mM MgSO4/1 mM EGTA/4% formaldehyde) for 25 min on ice. After washing with PBT (PBS containing 0.3% Triton X-100), blocking was performed in PBTB (PBS containing 0.3% Triton X-100 and 5% BSA) for 2 h at 4°C. Antibody staining was performed by using as primary antibodies mouse anti-β-galactosidase (Promega) at 1:1,000, rat anti-embryonic lethal abnormal visual system (ELAV) (16) at 1:20, rat anti-EY (17) at 1:300, Mouse anti-DLL (18) at 1:10, and rabbit anti-VG (19) at 1:200 overnight at 4°C. Immunofluorescent detection was performed by using dichlorotriazinyl aminofluorescein (DTAF) and Cy3-conjugated donkey anti-IgGs (Jackson ImmunoResearch). After washing with PBTB, discs were dissected in PBS and mounted in Vectashield (Vector Laboratories). The preparations were analyzed on a Zeiss Axiophot microscope equipped for epifluorescence.
β-galactosidase staining was performed as described (20). For cuticle preparations, adults were dissected in PBS, mounted in Faure's mounting medium. For in situ hybridization, the probes were labeled with digoxigenin-dUTP and detected by digoxigenin antibodies (Boehringer).
Clonal Analysis.
Su(H) mutant clones were induced by using the flippase (FLP/FRT) technique in larvae of the genotype w HSFlp; Su(H)SF8 FRT40A/N-myc FRT40A (21).
Results
Opposite Effects of Inhibition and Activation of Notch Signaling on Eye Morphogenesis.
The intracellular domain of the truncated Notch receptor reflects a constitutively activated state (Notch activated, Nact) and the extracellular domain of the truncated receptor mimics loss-of-function phenotypes representing a dominant negative form (Notch dominant negative, Ndn) (11, 22). To examine the role of Notch signaling in early eye development, we expressed these truncated forms in the early eye imaginal disc, using the GAL4 system (23) with the eye-specific enhancer of the ey gene (24). This eye-specific enhancer induces target gene expression in the eye primordia of the embryo and maintains expression throughout eye morphogenesis. In contrast to ey expression in the wild-type eye-antennal disc, the enhancer-driven reporter gene expression is not down-regulated in the differentiating cells posterior to the morphogenetic furrow but it extends all over the eye disc and into the area of the antennal disc where the rostral membrane is going to be formed (24). However, the expression in the antennal disc is quite variable from disc to disc. Consistent with previous loss of Notch function studies (25, 26), crossing ey enhancer-GAL4 (ey-GAL4) flies to a stock carrying Ndn under an upstream-activating sequence for GAL4 (UAS-Ndn) results in a strongly reduced eye phenotype in all transheterozygous flies similar to that of the ey2 mutant (Fig. 1b). Inhibition of Notch signaling by misexpression of Hairless (H) and dominant negative forms of Delta (Dl) and Serrate (Ser) also leads to a reduction or complete absence of the eye (data not shown).
Figure 1.
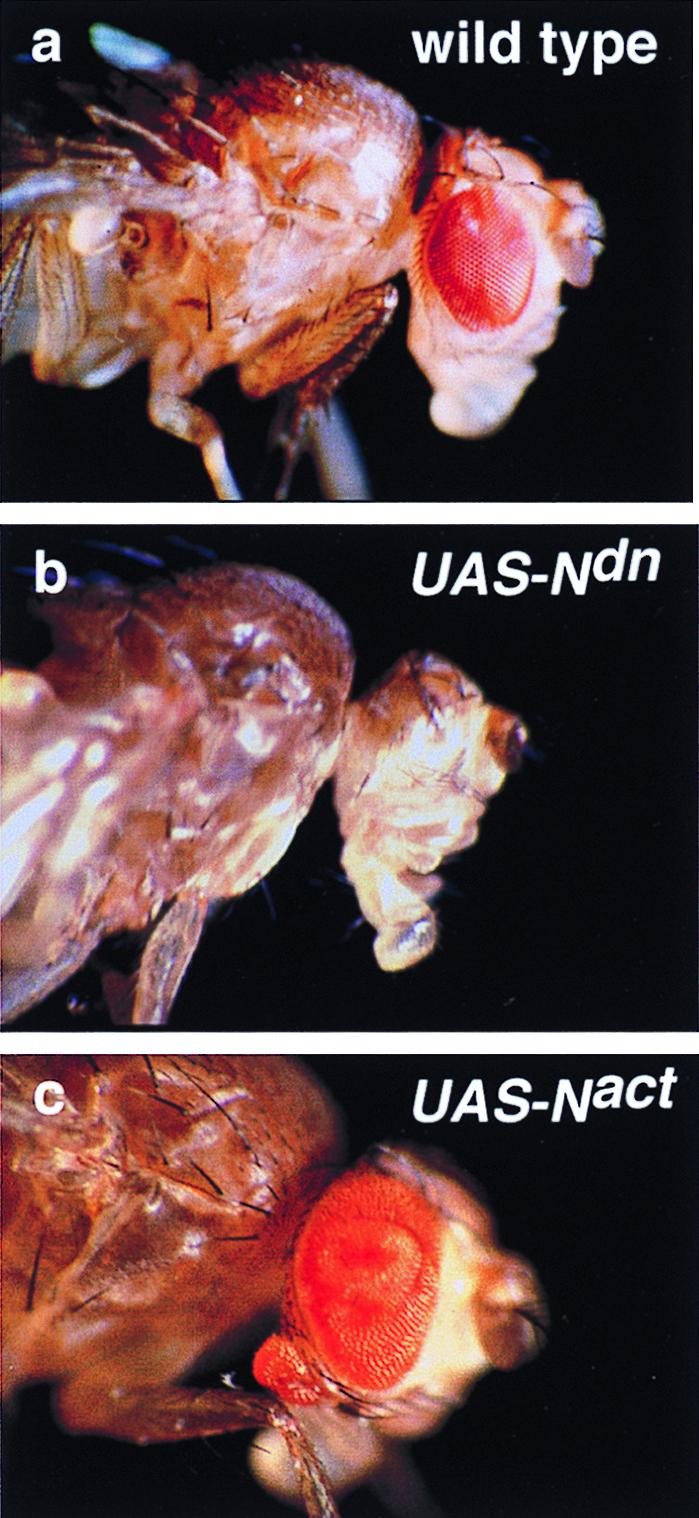
Eye reduction versus hyperplasia and ectopic eye induction by the inhibition and the activation of Notch signaling driven by ey-GAL4. (a) Wild-type fly head. (b) UAS-Ndn ey-GAL4 fly lacking eye. (c) UAS-Nact ey-GAL4 fly shows hyperplasia of the eye and an ectopic eye on the rostral membrane of the head.
Activation of Notch signaling by crossing ey-GAL4 flies to a UAS-Nact line leads to significant pupal lethality, but all transheterozygotes that escape lethality show hyperplasia of the eyes with a significant increase in the number of facets (Fig. 1c). The disc overgrowth is found in all eye discs of ey-GAL4 UAS-Nact larvae, consistent with a role of Notch signaling in growth control of the eye imaginal disc. Furthermore, about 16% of the escapers (19/119) formed ectopic eyes on the rostral membrane of the head, which is derived from the antennal disc (Fig. 1c).
Immunostaining of eye-antennal discs of ey-GAL4 UAS-lacZ UAS-Nact larvae using an ELAV antibody to identify the differentiating photoreceptor cells and a β-galactosidase antibody to monitor the Nact protein shows that both the strong hyperplasia of the eye disc and ectopic eye formation in the antennal disc correlate with the expression of Nact (Fig. 2 a and b). However, the time window for expression of the truncated receptors is critical. Transheterozygotes in which either Ndn or Nact were driven by the glass promoter GMR-GAL4, which drives expression in all cells posterior to the furrow only (27), showed only a mild phenotypic effect. As reported previously (11, 22), Ndn results in a roughening of the eye, whereas Nact produces a polished eye phenotype (data not shown). Therefore, the timing of Notch signaling is of crucial importance.
Figure 2.
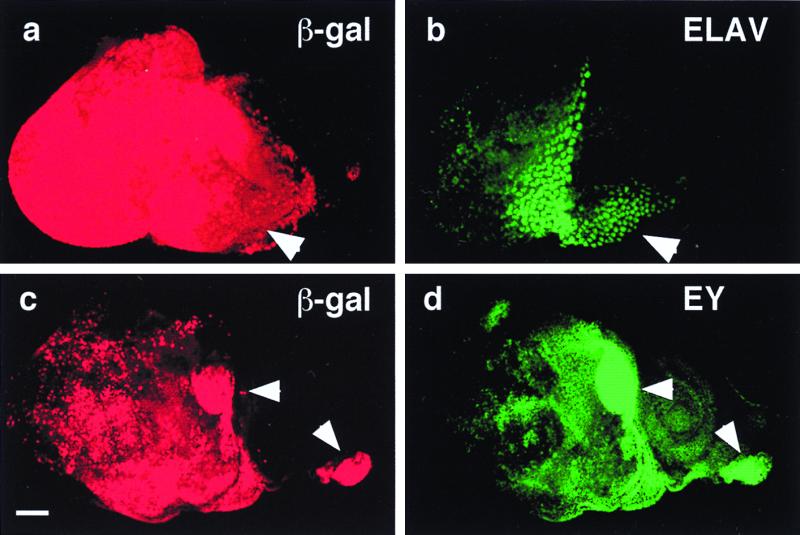
Induction of ectopic photoreceptor cells and eyeless by Notch signaling. (a) Anti-β-galactosidase (β-gal) antibody staining of a UAS-Nact UAS-lacZ ey-GAL4 eye-antennal disc. Activation of the lacZ reporter reflects the distribution of constitutively activated Notch protein. Arrowhead indicates hyperplastic portion. (b) Immunostaining of same disc as in a with antibody against the neuronal marker ELAV. In the hyperplastic portion (arrowhead) ectopically induced photoreceptors. (c) Anti-β-galactosidase antibody staining of a UAS-Nact UAS-lacZ ey-GAL4 eye antennal disc. Activation of the lacZ reporter reflects the distribution of Nact protein. Arrowheads indicate areas of strong lacZ expression. (Bar indicates 50 μm.) (d) Immunostaining of same disc as in c with antibody against EY. Ectopic EY expression is induced in the areas of strong lacZ expression (arrowheads).
Notch Signaling Regulates eyeless Expression.
The reduced eye phenotype caused by expression of Ndn and the induction of ectopic eyes by the expression of Nact are similar not only to loss-and-gain mutants of ey but also resemble two other mutations acting downstream (17) in the ey developmental pathway, eyes absent (eya) (28) and dachshund (dac) (29). Furthermore, a second Pax-6 gene of Drosophila, twin of eyeless (toy), was found to be an upstream regulator of ey capable of inducing ectopic eyes by inducing ey (30). To determine the epistatic relationship of Notch to those genes, we studied the effect of Ndn on ectopic eye induction by ey and toy.
A dpp-enhancer GAL4 line (31) (dpp-GAL4) was crossed to flies carrying both UAS-Ndn and UAS-ey or alternatively to UAS-Ndn and UAS-toy. Transheterozygotes from both crosses exhibited ectopic eyes on legs and wings in all flies (data not shown). The size of the ectopic eyes were similar to those of the transheterozygous controls dpp-GAL4 UAS-ey and dpp-GAL4 UAS-toy, respectively, suggesting that Notch acts upstream of ey and toy. Double immunostaining of eye-antennal discs from transheterozygous ey-GAL4 UAS-Nact, UAS-lacZ using an anti-EY antibody to reveal EY protein and anti-β-galactosidase antibody to indirectly reveal Nact demonstrated that ey expression is induced in all eye discs by the activation of Notch signaling. Moreover, strong ectopic expression of EY protein was observed (Fig. 2d). The ectopic expression pattern of EY corresponds to that of lacZ reflecting the expression of Nact protein (Fig. 2c). Analysis of ey expression by in situ hybridization indicates that ey is induced at the transcriptional level (data not shown). Similarly, ectopic expression of toy also was induced in the antennal discs of ey-GAL4 UAS-Nact larvae (data not shown). Thus, activation of Notch signaling can induce toy and ey expression in antennal discs. Expression of Nact also correlates with the ectopic induction of photoreceptor cells as revealed by ELAV staining (Fig. 2 a and b).
Notch activation of ey and toy depends on the downstream effector of Notch, Suppressor of Hairless (32), because Su(H) mutant clones (21) generated anterior to the morphogenetic furrow in the eye fail to produce adult structures (data not shown), in agreement with a requirement of Notch signaling during eye morphogenesis.
Activation of Notch Signaling in an eyeless Mutant Background Induces Ectopic Antennae.
Consistent with the finding that ey acts downstream of Notch, the expression of Nact in an ey2 or eyR hypomorphic mutant background generates eyes of a reduced size. Approximately 72% (63/88) of the ey-GAL4 UAS-Nact; ey2 flies that survived were found to have reduced eyes and about 15% of these flies (13/88) had both a reduced original and a reduced ectopic eye. However, in addition to ectopic eyes Nact also induced ectopic antennae in 25% (22/88) of these flies on the side of the head that is derived from the eye disc. Many of the induced ectopic antennae were complete with all three antennal segments and the arista (Fig. 3a). Similar results also were obtained with eyR, the other hypomorphic allele (data not shown), but no ectopic antennae were found in ey-GAL4 UAS-Nact ey+ flies (Fig. 1c), indicating that Notch signaling induces antenna formation in a loss-of-function ey mutant background.
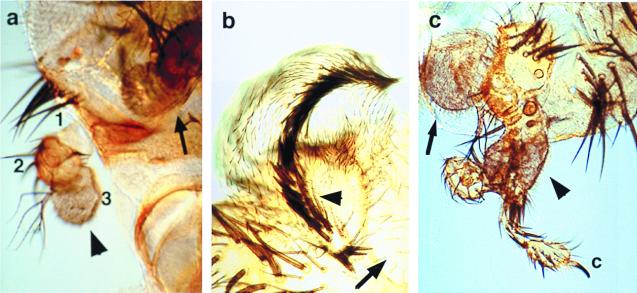
Figure 3.
Induction of ectopic antennae, wings, and legs in different genetic contexts. (a) Induction of an ectopic antenna (arrowhead) on the side of the head in UAS-Nact ey-GAL4 ey2 mutant fly. 1, 2, and 3: first, second, and third antennal segments, respectively. Arrow indicates normal antenna. (b) Induction of ectopic wing structures (arrowhead) in the eye region (arrow) by activation of Notch signaling and the simultaneous ectopic expression of Antennapedia (Antp). Wing blade and triple row of wing marginal bristles are induced in a UAS-Nact UAS-Antp ey-GAL4 fly replacing parts of the original eye. (c) Partial transformation of the ectopic antenna (arrowhead) into leg structures with a claw (c). Arrow indicates original antenna.
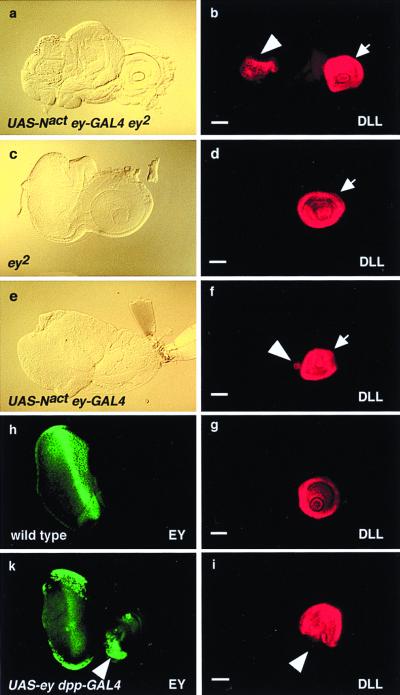
Because Distal-less in combination with extradenticle (exd) and homothorax (hth) specifies the antennae (33, 34), we monitored DLL expression in the eye discs that are capable of forming ectopic antennae. In wild-type larvae, DLL protein is expressed in the antennal but not in the eye disc (Fig. 4g). In all of the tested discs (30/30) in ey-GAL4 UAS-Nact ey2 animals that form ectopic antennae from the eye disc, significant DLL expression was detected ectopically (Fig. 4 a and b). By contrast, no ectopic expression of DLL was detected in the eye discs of ey2 control larvae (Fig. 4 c and d). In 14 of 30 ey-GAL4 UAS-Nact ey+ larvae additional ectopic expression of DLL in a few cells of the antennal disc (Fig. 4 e and f) was observed. This indicates that Notch signaling directly or indirectly induces ectopic expression of Dll in the eye-antennal disc, leading to the ectopic induction of antennae.
Figure 4.
Ectopic induction of Distal-less in eyeless2 mutant eye-antennal discs by the activation of Notch signaling driven by ey-GAL4. (a) Bright-field micrograph of an eye-antennal disc of UAS-Nact ey-GAL4 ey2larva. Note the hyperplasia of the eye disc. (b) Immunostaining of same disc as in a with antibody against DLL. Ectopic DLL expression is induced in the eye disc (arrowhead). Arrow indicates original DLL expression in the antennal disc. (c) Bright-field micrograph of an eye-antennal disc of a ey2 larva. The eye disc is reduced. (d) Immunostaining of the same disc as in c with antibody against DLL. DLL expression is confined to the antennal disc. (e) Bright-field micrograph of an eye-antennal disc of a UAS-Nact ey-GAL4 larva. (f) Immunostaining of the same disc as in e with antibody against DLL. Ectopic DLL expression is induced in a few cells of the antennal disc (arrowhead). Arrow indicates original DLL expression in the antennal disc. (g) Wild-type expression of DLL in eye-antennal disc. (h) Wild-type expression of EY in eye-antennal disc. (i) Immunostaining of same disc as in k with an antibody against DLL. Arrowhead indicates the repression of DLL in the region of ectopic EY expression. (k) Immunostaining of an eye-antennal disc of UAS-ey dpp-GAL4 larva with antibody against EY. Arrowhead indicates the ectopic expression of EY in the antennal disc. Posterior is to the left and dorsal is up. (Bars = 50 μm.)
Activation of Notch Signaling Combined with the Ectopic Expression of Antennapedia Induces Ectopic Wings and Legs on the Head.
The observation that Nact can induce both ectopic eyes and, in a specific genetic background, antennae led us to consider the possibility that Notch signaling also might induce the formation of other appendages in a different genetic context. To test this hypothesis, we combined the activation of Notch signaling with ectopic expression of Antennapedia (Antp). The latter is known to determine the identity of the second thoracic segment (T2) (35, 36), which on the dorsal side gives rise to a pair of wings and on the ventral side to a pair of second legs. For this purpose, transgenic flies of the constitution ey-GAL4 UAS-Nact UAS-Antp were generated. About 26% (17/65) of the flies escaping pupal lethality were found to have ectopic wings on the head (Fig. 3b). Almost all ectopic wing structures consisted of dorsal and ventral wing blades bordered by bristles of the wing margin (double and triple row), but lacking wing veins. In contrast, wing structures induced by the ectopic expression of vg, the wing margin is not formed (3), suggesting that Notch signaling and Antp are acting upstream of vg. Furthermore, about 17% (11/65) of these flies showed ectopic leg structures induced by secondary transformation of the ectopic antennal tissue into leg structures (e.g., arista into tarsus) (Fig. 3c). The ey-GAL4 UAS-Antp control flies did not show any ectopic wing structures, but they clearly exhibited reduced eyes, suggesting that the ectopic expression of Antp partially represses ey in the eye discs of these animals. An additional 10% (7/71) of these flies showed a transformation of the original antenna to leg structures. On the heads of ey-GAL4 UAS-Nact flies, no wing nor leg structures were found (Fig. 1c). Therefore, activation of Notch signaling when combined with the ectopic expression of Antp driven by ey-GAL4 is capable of inducing wing and leg structures on the head.
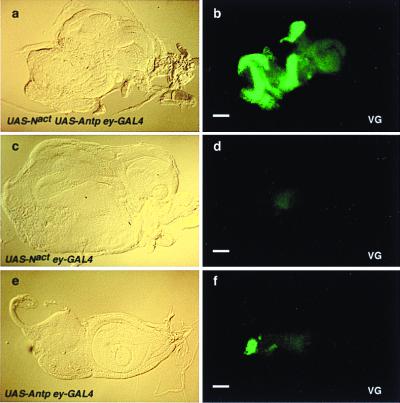
In wild-type larvae, the vg gene is expressed in the wing but not in the eye disc (19). By contrast, in ey-GAL4 UAS-Nact UAS-Antp animals in which ectopic wing structures are induced in the eye disc all of the tested eye discs (25/25) showed significant ectopic expression of VG protein (Fig. 5 a and b), whereas no ectopic expression of VG was detected in ey-GAL4 UAS-Nact control larvae (Fig. 5 c and d). However, ey-GAL4 UAS-Antp larvae showed VG expression in a small region of the eye discs in seven of 11 tested discs (Fig. 5 e and f), consistent with a synergistic effect of endogenous Notch activity with ectopic Antp expression on VG expression. It therefore appears that activation of Notch signaling in context of Antp expression induces vg expression in the eye discs and that there are synergistic effects between Notch signaling and Antp expression. Kim et al. (3) have shown that the Notch signaling pathway is used to specifically activate the boundary enhancer of the vg gene necessary for dorso-ventral wing formation. The same enhancer also may be used for ectopic formation of the wing, a point that has to be investigated further. A dorso-ventral boundary also is established by Notch in the eye disc that controls growth and polarity in the Drosophila eye (13).
Figure 5.
Ectopic induction of vestigial in the eye discs by the activation of Notch signaling and the simultaneous ectopic expression of Antennapedia driven by ey-GAL4. (a) Bright-field micrograph of an eye-antennal disc of a UAS-Nact UAS-Antp ey-GAL4 larva. Note hyperplasia of the eye disc. (b) Immunostaining of the same disc as in a with antibody against VG. Ectopic VG expression is induced in the eye disc. Note hyperplasia of the eye disc. (c) Bright-field micrograph of an eye-antennal disc of a UAS-Nact ey-GAL4 larva. (d) Immunostaining of the same disc as in c with antibody against VG. No VG expression is detected. (e) Bright-field micrograph of an eye-antennal disc of a UAS-Antp ey-GAL4 larva. (f) Immunostaining of the same disc as in e with antibody against VG. Ectopic VG expression is induced in a small region of the eye disc. Posterior is to the left and dorsal is up. (Bars = 50 μm.)
In ey-GAL4 UAS-Nact UAS-Antp ectopic legs also are induced on the head (Fig. 3c), which is accompanied by DLL expression in 21 of 21 tested eye discs (data not shown). In contrast, no DLL expression is detected in eye discs of ey-GAL4 UAS-Antp larvae (data not shown), which is in agreement with the adult phenotype of these animals.
In view of the above observations we propose that the effects of Notch signaling on the various appendages depend on the context provided by control genes such as ey and Antp. In the eye primordia, Notch signaling induces ey expression, which induces a cascade of downstream genes leading to eye morphogenesis. In conjunction with Antp, Notch signaling induces vg, leading to wing formation. At low levels of ey expression, Notch signaling induces Dll, leading to antenna morphogenesis. In the case of the leg, Notch also induces Dll expression that in conjunction with Antp leads to leg formation (15).
Discussion
Combinatorial Genetic Interactions Specify the Identity of the Various Appendages.
Segmental identity is specified by the homeotic genes that are active in a particular combination in each segment. Within a given segment, the appendages are specified by a different set of subsidiary control genes; the eyes are specified by ey, the wings and haltere by vg; the legs by Dll and the antennae by Dll in combination with extradenticle (exd) and homothorax (hth) (33, 34). Our results indicated that they all are regulated by Notch signaling, sharing the same cell signaling pathway, which raises the possibility that the appendage specificity is provided by a combinatorial interaction between Notch and the homeotic and the subsidiary control genes.
The repression of one control gene by the expression of another seems to be a widespread mechanism to ensure that the developmental pathways are mutually exclusive so that the formation of intermediary cell types is prevented. Similar to the repression of ey by Antp, we found that ey directly or indirectly represses Dll. In hypomorphic ey mutants, the activation of Notch signaling leads to ectopic expression of Dll in the eye disc, suggesting that ey might repress Dll in the wild-type eye disc. In dpp-GAL4 UAS-ey transheterozygous flies EY is expressed on the ventral side of the posterior half of the antennal discs under the control of the dpp-enhancer (Fig. 4k), whereas DLL is not detectable in this area (Fig. 4i). A similar mutually exclusive expression is found in the leg discs of these flies (data not shown), suggesting that ey represses Dll expression.
Based on these findings, we propose a model to explain the difference between the eye and antennal pathway starting from a common signaling mechanism. Notch signaling induces the expression of both ey and Dll. However, in the eye primordia ey represses Dll and induces eye morphogenesis. By contrast, in the antennal disc ey is repressed by a repressor, resulting in Dll expression that confers antennal (ventral appendage) specificity. Two of the possible candidates for the repressor are the homeobox genes exd and hth, because both exd− and hth− mutant clones in the rostral membrane region of the antennal disc can result in ectopic eye development, which presumably is caused by derepression of ey (37, 38). Both exd and hth also may function in conjunction with Dll serving as corepressors.
The Fundamental Role of Notch Signaling in Development and Evolution.
In this study, we have shown that Notch signaling regulates ey expression at the early stages of eye morphogenesis. By analogy to the Drosophila paradigm, it is therefore likely that the expression of Pax-6 may be regulated by Notch signaling, given the extraordinary conservation of Notch function from ascidians (39) to mammals (40). Notch signaling seems to participate in dorso-ventral patterning of the Drosophila wing (3, 10) and eye (12, 13) and also in the vertebrate limb (41, 42). Thus our study raises the possibility that Notch is involved in the control of both vertebrate and invertebrate appendage formation. In describing the developmental role of Notch (7), it has been proposed that Notch signaling modulates the ability of individual precursor cells to respond to developmental signals, whether differentiation, proliferation, or apoptotic cues. The present study extends the fundamental role of Notch by indicating that the implementation of entire developmental programs leading to appendage formation and organogenesis may be controlled by Notch activity.
Acknowledgments
We thank U. Walldorf for ey-GAL4 flies and EY antibody; S. Carroll and G. Halder for VG antibodies; B. Bello for UAS-Antp flies; S. Cohen for DLL antibodies; G. Morata and I. Guerrero for Dll-GAL4 flies; D. Ish-Horowicz, G. Halder, P. Callaerts, U. Walldorf, B. Bello, S. Flister, D. Resendez-Perez, M. Affolter, S. Cohen, and M. Seimiya for discussions; U. Kloter and T. Marty for technical assistance; and E. Marquardt-Wenger, R. Suter-Fritsche, and Céline Knecht for processing of the manuscript. This work was supported by the Kantons of Basel and the Swiss National Science Foundation. S.A.-T. is supported by the Howard Hughes Medical Institute and the Massachusetts General Hospital Cancer Center. The last stages of this work were supported by a Grant-in-Aid from the Ministry of Science, Culture and Education of Japan to S.K.
Abbreviations
- EY
eyeless
- DLL
Distal-less
- VG
vestigial
- ELAV
embryonic lethal abnormal visual system
- UAS
upstream-activating sequence
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040556497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040556497
References
- 1.Quiring R, Walldorf U, Kloter U, Gehring W J. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 2.Halder G, Callaerts P, Gehring W J. Curr Opin Genet Dev. 1995;5:602–609. doi: 10.1016/0959-437x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Sebring A, Esch J J, Kraus M E, Vorwerk K, Magee J, Carroll S B. Nature (London) 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- 4.Weatherbee S D. Genes Dev. 1998;12:1474–1482. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehring W J. Genes Cells. 1996;1:11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- 6.Callaerts P, Halder G, Gehring W J. Annu Rev Neurosci. 1997;20:483–532. doi: 10.1146/annurev.neuro.20.1.483. [DOI] [PubMed] [Google Scholar]
- 7.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 8.Artavanis-Tsakonas S, Matsuno K, Fortini M E. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 9.Robey E. Curr Opin Genet Dev. 1997;7:551–557. doi: 10.1016/s0959-437x(97)80085-8. [DOI] [PubMed] [Google Scholar]
- 10.Neumann C J, Cohen S M. Development (Cambridge, UK) 1996;122:3477–3485. doi: 10.1242/dev.122.11.3477. [DOI] [PubMed] [Google Scholar]
- 11.Fortini M E, Rebay I, Caron L A, Artavanis-Tsakonas S. Nature (London) 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- 12.Papayannopoulos V, Tomlinson A, Panin V M, Rauskolb C, Irvine K D. Science. 1998;281:2031–2034. doi: 10.1126/science.281.5385.2031. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez M, de Celis J F. Nature (London) 1998;396:276–278. doi: 10.1038/24402. [DOI] [PubMed] [Google Scholar]
- 14.Baker N E, Yu S Y. Curr Biol. 1997;7:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- 15.Gorfinkiel N, Morata G, Guerrero I. Genes Dev. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinow S, White K. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- 17.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring W J. Development (Cambridge, UK) 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Benjumea F J, Cohen B, Cohen S M. Nature (London) 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 19.Williams J A, Bell J B, Carroll S B. Genes Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 21.Xu T, Rubin G M. Development (Cambridge, UK) 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 22.Rebay I, Fehon R G, Artavanis-Tsakonas S. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- 23.Brand A H, Perrimon N. Development (Cambridge, UK) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 24.Hauck B, Gehring W J, Walldorf U. Proc Natl Acad Sci USA. 1999;96:564–569. doi: 10.1073/pnas.96.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go M J, Eastman D S, Artavanis-Tsakonas S. Development (Cambridge, UK) 1998;124:2031–2040. doi: 10.1242/dev.125.11.2031. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Artavanis-Tsakonas S. Development (Cambridge, UK) 1997;124:3439–3448. doi: 10.1242/dev.124.17.3439. [DOI] [PubMed] [Google Scholar]
- 27.Ellis M C, O'Neill E M, Rubin G M. Development (Cambridge, UK) 1993;119:855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- 28.Bonini N M, Bui Q T, Gray-Board G L, Warrick J M. Development (Cambridge, UK) 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- 29.Shen W, Mardon G. Development (Cambridge, UK) 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- 30.Czerny T, Halder G, Callaerts P, Kloter U, Gehring W J, Busslinger M. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- 31.Staehling-Hampton K, Jackson P D, Clark M J, Brand A H, Hoffmann F M. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- 32.Fortini M E, Artavanis-Tsakonas S. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 33.Casares F, Mann R S. Nature (London) 1998;392:723–726. doi: 10.1038/33706. [DOI] [PubMed] [Google Scholar]
- 34.Gonzales-Crespo S, Abu-Shaar M, Torres M, Martinez C, Mann R S, Morata G. Nature (London) 1998;394:196–200. doi: 10.1038/28197. [DOI] [PubMed] [Google Scholar]
- 35.Schneuwly S, Klemenz R, Gehring W J. Nature (London) 1987;325:816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- 36.Struhl G. Proc Natl Acad Sci USA. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Crespo S, Morata G. Development (Cambridge, UK) 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- 38.Pai C Y, Kuo T S, Jaw T J, Kurant E, Chen C T, Bessarab D A, Salzberg A, Sun Y H. Genes Dev. 1998;12:435–446. doi: 10.1101/gad.12.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hori S, Saitoh T, Matsumoto M, Makabe K W, Nishida H. Dev Genes Evol. 1997;207:371–380. doi: 10.1007/s004270050126. [DOI] [PubMed] [Google Scholar]
- 40.Bao Z Z, Cepko C L. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Esteban C, Schwabe J W, De La Pena J, Foys B, Eshelman B, Belmonte J C. Nature (London) 1997;386:360–366. doi: 10.1038/386360a0. [DOI] [PubMed] [Google Scholar]
- 42.Sidow A, Bulotsky M S, Kerrebrock A W, Bronson R T, Daly M J, Reeve M P, Hawkins T L, Birren B W, Jaenisch R, Lander E S. Nature (London) 1997;389:722–725. doi: 10.1038/39587. [DOI] [PubMed] [Google Scholar]