Abstract
The expression of defensive morphologies in prey often is correlated with predator abundance or diversity over a range of temporal and spatial scales. These patterns are assumed to reflect natural selection via differential predation on genetically determined, fixed phenotypes. Phenotypic variation, however, also can reflect within-generation developmental responses to environmental cues (phenotypic plasticity). For example, water-borne effluents from predators can induce the production of defensive morphologies in many prey taxa. This phenomenon, however, has been examined only on narrow scales. Here, we demonstrate adaptive phenotypic plasticity in prey from geographically separated populations that were reared in the presence of an introduced predator. Marine snails exposed to predatory crab effluent in the field increased shell thickness rapidly compared with controls. Induced changes were comparable to (i) historical transitions in thickness previously attributed to selection by the invading predator and (ii) present-day clinal variation predicted from water temperature differences. Thus, predator-induced phenotypic plasticity may explain broad-scale geographic and temporal phenotypic variation. If inducible defenses are heritable, then selection on the reaction norm may influence coevolution between predator and prey. Trade-offs may explain why inducible rather than constitutive defenses have evolved in several gastropod species.
Phenotypic plasticity, the capacity of an organism to produce different phenotypes in response to environmental cues, can be an important adaptive strategy in variable or changing environments (1, 2). Inducible defenses are a ubiquitous form of plasticity that involve the production of chemicals, morphologies, or behaviors by prey species in response to predator cues (3). These changes reduce prey vulnerability to inducing predators or herbivores. Inducible defenses occur in diverse taxa and examples include: production of chemical defenses in plants (4, 5), formation of spines in rotifers (6) and marine bryozoans (7) and neck teeth and helmets in cladocerans (8), diel vertical migration in marine (9) and freshwater zooplankton (10), and changes in body shape in fish (11) and shell shape and thickness in mollusks and barnacles (12–15).
Despite improved understanding of the cues inducing these defenses and their immediate adaptive value (3, 16), our understanding of how this phenomenon contributes to broader temporal and spatial patterns of phenotypic variation remains poor. To date, most studies have examined inducible defenses and their costs on very localized spatial scales (3–15). In doing so, there is limited consideration of environmental complexity and the interactive influences of others cues that are likely to occur across a broader scale. For example, many plant and animal species have wide altitudinal and latitudinal distributions where dramatic temperature gradients occur. Because temperature can profoundly influence developmental and metabolic rates (17, 18) and phenotypic plasticity is a developmental phenomenon, spatial or temporal variation in temperature may affect the speed, magnitude, and costs associated with induced defenses. Although a few geographic surveys of inducible defenses exist (19–21), there have been no direct tests of (i) how inducible defenses are expressed over broad spatial scales and (ii) whether such expression can explain observed broad-scale patterns of phenotypic variation. Attention to broader spatial scales may shed light on how natural selection shapes patterns of geographic variation (22) and arms races between predator and prey (23, 24).
Natural selection is thought to drive coevolution between predator and prey (23, 24), but the rate at which microevolutionary change occurs (25) and the extent to which selection operates on fixed vs. plastic phenotypes (2, 21, 26, 27) remain unclear. Rapid changes in prey defenses after contact with introduced predators (28, 29) have been cited as compelling evidence of the speed and intensity of natural selection (25). However, because inducible defenses occur rapidly, they may be responsible for what are viewed typically as examples of rapid evolution via natural selection. For example, it is generally assumed that predation by crushing predators (decapod crustaceans, fish) has selected for more robust, better-defended shell morphologies in molluskan prey (23, 24, 30). Recent work, however, has shown that gastropods and bivalves can alter shell form adaptively during ontogeny in response to predator effluent (12–15, 31). These induced responses appear ubiquitous; they have been found in multiple molluskan species, from different geographic regions, and in response to cues from several predator species. In addition, factors other than predator cues (e.g., wave exposure, water temperature) can modify molluskan shell form (32–35). In the case of water temperature, thinner shells are expected in colder waters because calcium carbonate saturation decreases and dissolution rates increase with decreasing water temperature (36, 37). Thus, broad-scale temporal and spatial variation in the expression of prey defenses could reflect differences in the concentration of predator cues or other cues that happen to covary with the observed gradient in predator abundance. Given that (i) predators often are distributed patchily in time and space and (ii) organisms frequently range over a latitudinal temperature gradient, the potential for plasticity to generate broad-scale phenotypic variation is great.
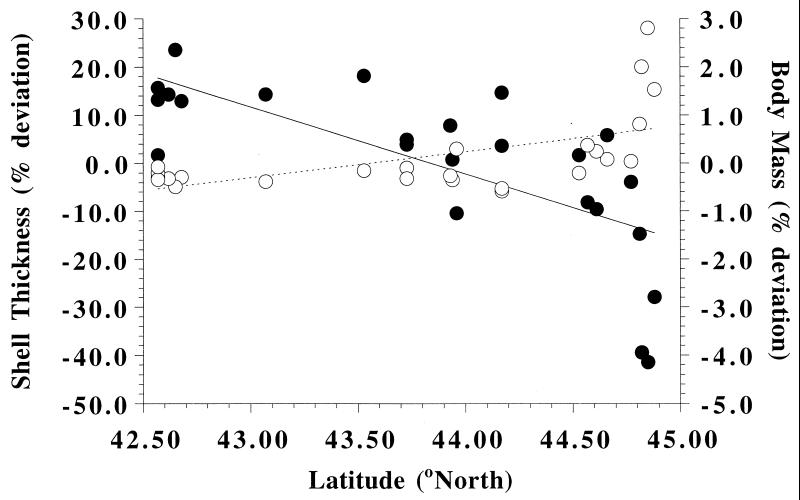
To test the potential for plasticity to influence prey phenotype across a broad spatial scale, we experimentally examined changes in shell morphology in intertidal herbivorous snail (Littorina obtusata) populations after exposure to an introduced molluskivorous crab (Carcinus maenas) in different temperature regimes in the northwestern Atlantic. Native to European waters, C. maenas invaded the Gulf of Maine from south of Cape Cod, MA, in the early 1900s and spread northward, reaching the Bay of Fundy by the 1950s (38). Presently, C. maenas is abundant on sheltered shores in the southern Gulf of Maine, but its populations are small and ephemeral in the northern Gulf (29, 39). After the C. maenas invasion, shell morphology in L. obtusata populations shifted rapidly (within 15–80 years) from more vulnerable, thin-shelled to better-defended, thick-shelled forms (29). This shift was interpreted as evidence of rapid, natural selection with C. maenas predation as the driving force (29). Presently, L. obtusata populations show striking phenotypic variation in the Gulf of Maine, with shell thickness decreasing and body mass increasing significantly with increased latitude (Fig. 1). Shell thickness in southern populations of L. obtusata is approximately 34% greater than in northern populations.
Figure 1.
Shell thickness (±SE; ●) and body mass (±SE; ○) for each of 25 L. obtusata populations as a function of latitude in the Gulf of Maine. Shell thickness and body mass of each snail were expressed as a deviation from a regression of (i) log shell thickness (Y) vs. log shell length (X) and (ii) log body mass (Y) vs. log shell mass (X), respectively, across all populations. Mean shell thickness and body mass were back-transformed for presentation and are expressed as a percent deviation from the appropriate regression. Each mean is based on a sample of 50 snails from each population. Shell thickness decreases with increasing latitude (Y = −13.89X + 608.87, R2 = 0.50, P < 0.0001), and body mass increases with increasing latitude (Y = 0.540X − 23.513, R2 = 0.31, P < 0.005). Error bars are smaller than symbols.
Three mechanisms could explain the patterns in shell thickness in L. obtusata. First, differential selection by C. maenas on thin-shelled morphs may have created both the historical shift and the geographic cline in snail shell thickness, because selection has acted longer and with greater intensity in the southern than in the northern Gulf of Maine. Alternatively, greater C. maenas abundance in the southern Gulf, both presently and historically, could have induced the formation of thicker shells. Third, latitudinal differences in water temperature in the Gulf of Maine may result in thinner shells at the colder northern sites. Water temperatures during the late spring and summer, when most snail growth occurs, averaged 6.8°C colder at our northern than our southern study site (35). Significantly, colder water temperatures also might limit the extent of inducible change in shell thickness for northern populations that come in contact with C. maenas.
Methods
In a field experiment, snails from two populations in the Gulf of Maine, one in Massachusetts and one in northern Maine (distance between sites, ≈400 km), were reciprocally transplanted between locations and reared either in the presence or absence of C. maenas effluent. In late April 1998, 144 juvenile L. obtusata snails (<6-mm shell length) were collected from two locations (Quoddy Head in Lubec, ME: 44°49.21′ N, 66°57.97′ W; and Lobster Cove in Manchester, MA: 42° 33.79′ N, 70° 46.19′ W). Individual snails were tagged with a small, color-coded dot of permanent ink that then was sealed with cyanoacrylate glue (34). Each snail was measured for initial shell length, shell thickness (14), shell mass, and body mass. To determine initial shell mass and body mass (defined by wet tissue mass) we used a nondestructive protocol (40). Using a Mettler PG503 analytical balance, we measured the mass (±0.001 g) of each snail while submerged in seawater (submerged mass) and then, after 30 min of drying, the total mass (±0.001 g) of each snail in air. Actual shell mass (Y) can be predicted accurately from submerged mass (X) by using regressions generated with a destructive sampling of snails from each population (both R2 for each population ≥ 0.99). To calculate body mass, we subtracted the estimate of actual shell mass from the total mass of snails when weighed in air. Because effluent treatment and rearing location may have affected these regressions, we generated a new set of regressions (all R2 ≥ 0.99) from destructive samples for each experimental group at the end of the experiment (i.e., 90 days growth in the field). Because this approach was not possible at the 45-day measurement period, we used the mean of the initial and final regression equations to estimate shell mass and body mass for this time period.
After initial measurements, snails were returned to appropriate field locations in early May 1998. Six snails (hereafter, response snails) from a single location and 60 g (wet mass) of brown algae (Ascophyllum nodosum) as food were placed in each of 48 replicate cylindrical containers (5-cm height × 10-cm diameter) that had plastic, mesh windows to permit water flow. Each container with response snails was secured beneath a similarly constructed container housing either (i) a single mature Carcinus maenas male (“Crab” treatment, +C; mean carapace width ± SE = 56.5 ± 6.4 mm) and 30 snails (hereafter, stimulus snails) or (ii) no crab (control, −C) and 30 conspecific stimulus snails. This design allowed crab effluent to drip directly onto the response snails in the crab treatments. Each pair of stimulus–response containers then was secured inside a larger cylindrical chamber (11-cm height × 28-cm diameter) that had mesh panels to permit water flow. At northern and southern locations, we anchored six replicates of each source population × effluent treatment combination in the midintertidal zone. All chambers were checked every 21 days to replace stimulus snails and the algal food supply in both stimulus and response containers. We measured snail shell length, shell thickness, and body mass after 45 and 90 days of growth in the field.
Data for each time period were analyzed with a three-factor analysis of covariance (ANCOVA) that treated source population, rearing location, and effluent treatment as fixed effects and replicate growth chambers as a random effect nested within each source population × rearing location × effluent treatment combination. For analysis of shell thickness, shell length was used as the covariate. We used shell mass as the covariate for our analysis of body mass because it provides an integrated measure of investment into shell material. ANCOVA was used to adjust for the potential effects of size on our response variables. Hence, least squares-adjusted means generated by ANCOVA are mean shell thickness and mean body mass for each treatment combination at the mean shell length and mean shell mass, respectively, of all snails in the analysis. Data conformed to all the assumptions of ANCOVA (41). A priori linear contrasts were conducted to determine whether exposure to C. maenas effluent significantly affected shell thickness and body mass of snails from each source population (North, N; South, S) raised at each location (N, S) (e.g., SS + C vs. SS − C, SN + C vs. SN − C in Fig. 2). Linear contrasts also were used to assess whether rearing location significantly affected shell thickness and body mass for snails from each source population exposed to each effluent treatment (e.g., SS + C vs. SN + C, SS − C vs. SN − C in Fig. 2).
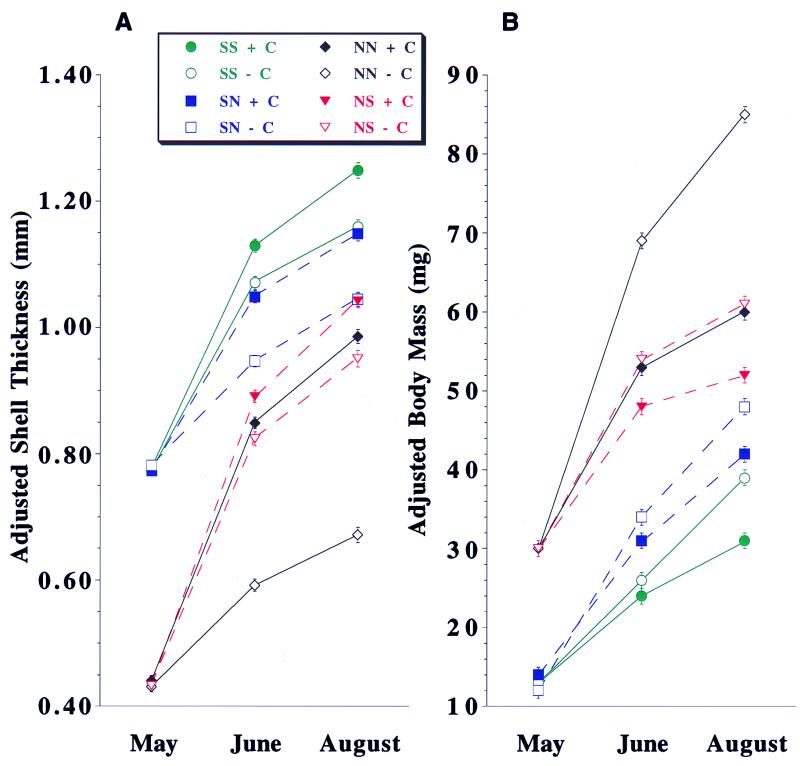
Figure 2.
Phenotypic plasticity in shell thickness (A) and body mass (B) for L. obtusata that were reciprocally transplanted between a southern (S; Manchester, MA) and northern (N; Lubec, ME) site and exposed to the presence (+C; solid symbols) or absence (−C; open symbols) of C. maenas effluent. Data are least squares-adjusted means (±SE) generated by ANCOVA (see Methods) for shell thickness (Y) vs. shell length (X) (A) and body mass (Y) vs. shell mass (X) (B). At each location, snails from each source population produced significantly thicker shells after 45 and 90 days (both P < 0.0001, ANCOVA) and significantly less body mass after 45 and 90 days (both P < 0.0001, ANCOVA) when raised with C. maenas. See Table 1 for linear contrasts. SS, South to South (green, solid line); SN, South to North (blue, dashed line); NN, North to North (black, solid line); NS, North to South (red, dashed line). May, initial phenotypic values; June, midpoint phenotypic values (45 days); August, final phenotypic values (90 days).
Results and Discussion
We found that exposure to predator effluent induced significant changes in shell thickness. At both locations, snails from each source population produced significantly thicker shells when raised with C. maenas than when raised in its absence (Fig. 2 and Table 1). This response was rapid with the majority of induced thickening occurring within 45 days. A significant trade-off in the form of reduced body mass was associated with thicker shells. At both locations, snails from each source population produced less body mass when raised with C. maenas than when raised in its absence (Fig. 2b and Table 1). Reductions in body mass were significant after 45 days but became more pronounced after 90 days. In three of four source population × rearing location combinations (i.e., SS, SN, NS), we observed similar increases in shell thickness (+8 to +10%) and similar decreases in body mass (−14 to −26%) between crab versus no-crab treatments after 90 days (Fig. 2 and Table 1). In contrast, northern snails raised at their native site with C. maenas (NN + C) produced dramatically thicker shells (+47%) and less body mass (−42%) than those raised without C. maenas (NN − C).
Table 1.
A priori linear contrasts on the effects of C. maenas effluent for each source population raised at each rearing location and rearing location for each source population in each effluent treatment
| Effect | Shell thickness
|
Body mass
|
||
|---|---|---|---|---|
| 45 Days | 90 Days | 45 Days | 90 Days | |
| Effluent effect | ||||
| SS + C vs. SS − C | 5.5%*** | 7.7%*** | −8.3%* | −25.8%*** |
| SN + C vs. SN − C | 10.8%*** | 10.0%*** | −9.7%* | −14.3%*** |
| NS + C vs. NS − C | 8.1%*** | 9.7%*** | −12.5%*** | −17.3%*** |
| NN + C vs. NN − C | 43.4%*** | 46.7%*** | −30.2%*** | −41.7%*** |
| Location effect | ||||
| SS + C vs. SN + C | 7.7%*** | 8.7%*** | −29.2%*** | −35.5%*** |
| SS − C vs. SN − C | 13.1%*** | 11.0%*** | −30.8%*** | −23.1%*** |
| NS + C vs. NN + C | 4.9%** | 5.8%*** | −10.4%*** | −15.4%*** |
| NS − C vs. NN − C | 39.2%*** | 41.5%*** | −27.8%*** | −39.3%*** |
Shown are the percent change in L. obtusata shell thickness and body mass. See legend of Fig. 2 for explanation of labels. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The location in which snails were raised also significantly affected both shell thickness and body mass. Controlling for source population and effluent treatment, snails raised at the warmer southern location produced thicker shells and less body mass than those raised at the colder northern location (e.g., SS + C vs. SN + C, Fig. 2; Table 1). A priori, we predicted that the location effects would reflect differences primarily in water temperature and that these differences would be similar in magnitude for both crab and no-crab treatments for a given source population. Although the location effect was consistent with that expected because of observed differences in water temperature (35), our data suggest that a more complex interaction exists between water temperature and naturally occurring (i.e., nonexperimental) crab effluent. For example, we found that the location effect on shell thickness was much greater for snails from the northern source population raised without (+41.5%; NS − C vs. NN − C) than with C. maenas (+5.8%; NS + C vs. NN + C) (Fig. 2a and Table 1). We hypothesize that northern “controls” raised in the south developed significantly thicker shells and less body mass than expected because they were responding to the effluent released by free-ranging crabs. This background effluent was largely absent for their counterparts raised in the north, where free-ranging C. maenas were rare. The location effect was less evident for the northern source population raised experimentally with crabs, because responses to the crab treatment likely masked responses to background effluent (Fig. 2 and Table 1). Finally, location effects on the southern source population were small for both crab (8.7%) and no-crab (11.0%) treatments. Two scenarios may explain these results. First, juvenile snails collected from the southern site for the experiment already may have started to thicken their shells in response to higher background levels of C. maenas effluent, which may have reduced their capability to respond to our experimental treatments. Second, there may be population-based ontogenetic constraints on plasticity. Selection by C. maenas may have shaped the reaction norms of southern snails to be less flexible. L. obtusata are direct developers with limited dispersal. Consequently, some localized adaptation in plasticity might be expected in southern populations given their longer historical contact with C. maenas and, thus, greater predictability of predation risk.
Our study illustrates the potential for inducible defenses to produce adaptive change over broad geographic and temporal scales. Previous studies attributed historical shifts in the shell form of two intertidal snail species in the Gulf of Maine to rapid selection after the introduction of C. maenas. After ≈84 years of contact with C. maenas, two L. obtusata populations showed 50–56% increases in shell thickness and a third showed an 82% increase (29). Shell thickness of the dogwhelk Nucella lapillus increased by 12% within 25–100 years (28). We found that exposure to C. maenas effluent can induce 8–47% increases in shell thickness in just 90 days (Table 1). Our experiments therefore suggest that predator-induced plasticity may play an important, and underappreciated, role in explaining the historical morphological changes described above and the present-day latitudinal variation in shell thickness (Fig. 1). Our data also suggest that, at the least, predator-induced responses are comparable in magnitude to any temperature-related latitudinal effects on shell form and can occur in colder regions, where shell production is more difficult. For example, were we to attribute all of the location effect to differences in water temperature (i.e., ignore location-specific differences in background crab effluent), the average location effect (14.6%) was remarkably similar to that observed in our crab treatments (15.6%).
Our data may aid in predicting responses by prey populations to anticipated changes in global climate and community composition. For example, warming trends in the Gulf of Maine over the last century are thought to have facilitated the northward expansion of C. maenas (38, 42). Temperature changes, in conjunction with increased rates of human-mediated biological invasions (43), will likely increase contact between prey populations and novel predators. The invasion history of Carcinus spp. is a case in point. Over the last two centuries, C. maenas and a sibling species, C. aestuarii, have invaded a number of regions around the globe (44), and their feeding activities are predicted to affect the composition of both hard- and soft-bottom benthic communities (45, 46). Our field experiment suggests that inducible defenses may ameliorate the short-term impact of these invaders on populations of molluskan prey and that these defenses can be expressed even in less favorable temperature regimes. That thin-shelled northern populations of L. obtusata possess the ability to modify shell form when confronted by C. maenas suggests that (i) predator-induced plasticity has evolved as a general defense against spatially or temporally variable predators and (ii) there are costs to increased shell thickness.
Trade-offs associated with predator-induced defenses are presumed to exist; otherwise, organisms should produce constitutive (i.e., fixed) rather than conditional phenotypes (2, 47). Indeed, trade-offs in growth rate (11, 48), size at maturity (49), and fecundity (50, 51) have been documented in a number of taxa. In our experiments, predator-induced increases in shell thickness were accompanied by reductions in body mass between 14.3 and 41.7% after 90 days (Table 1 and Fig. 2b). This trade-off likely reflects architectural constraints imposed by shell form rather than the relatively small energetic costs tied to the production of more shell material (52, 53). Because there is a maximum rate at which calcification can occur (53), the more material that is devoted to thickening the shell, the less that is available for linear shell growth. Reduced linear shell growth limits body mass because growth of soft-tissue cannot proceed ahead of the protective shell margin. In addition, thick-walled shells have less internal volume available for body growth than thin-walled shells of similar size and shape. Given that gastropod fecundity often is positively correlated with body size (54, 55), the existence of trade-offs in body mass may partly explain why inducible defenses in marine gastropod shell form have evolved.
Geographical and historical patterns of phenotypic variation are thought to reflect genetic differentiation produced by natural selection (22, 56). Undoubtedly, predator and prey populations have coevolved over a range of spatiotemporal scales (23, 24, 57). In many such cases, however, selection may have acted on genetic variation in plastic rather than constitutive traits. If so, adaptive phenotypic plasticity may be important to emerging patterns of geographic variation and could enhance speciation in allopatric populations (58). Indeed, the magnitude and spatial scale of the induced changes seen in our experiments suggest that recent or fossil transitions in molluskan shell form are not unequivocal evidence of rapid selection (25, 28, 29) or speciation (59). Moreover, the discovery of plastic increases in the claw size and crushing force of a crab in response to diet (60) indicates that adaptive plasticity may influence both sides of the evolutionary arms race. The ubiquity and impressive magnitude of predator-induced changes strongly suggests that phenotypic plasticity plays an important role in shaping ecological communities.
Acknowledgments
We thank the Marine Science Center in Nahant for use of the facilities and M. Bertness, R. Etter, P. Ewanchuk, S. Henschel, D. Lello, D. Rand, P. Schmidt, and J. Witman for constructive comments. This work was supported by a grant from the National Science Foundation (Integrative Biology and Neuroscience–Ecological and Evolutionary Physiology, 9817106) to G.C.T and L.D.S., a Mellon Foundation Postdoctoral Fellowship (G.C.T), and the Northeastern University Research and Scholarship Development Fund (L.D.S). This is contribution number 2287 from the School of Marine Science.
Abbreviation
- ANCOVA
analysis of covariance
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040423397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040423397
References
- 1.Stearns S C. Bioscience. 1989;39:436–446. [Google Scholar]
- 2.Schlichting C D, Pigliucci M. Phenotypic Evolution: A Reaction Norm Perspective. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 3.Tollrian R, Harvell C D. The Ecology and Evolution of Inducible Defenses. Princeton: Princeton Univ. Press; 1999. [Google Scholar]
- 4.Berenbaum M R, Zangerl A R. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 10–32. [Google Scholar]
- 5.Dicke M. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 62–88. [Google Scholar]
- 6.Gilbert J J. Science. 1966;151:1234–1237. doi: 10.1126/science.151.3715.1234. [DOI] [PubMed] [Google Scholar]
- 7.Harvell C D. Science. 1984;224:1357–1359. doi: 10.1126/science.224.4655.1357. [DOI] [PubMed] [Google Scholar]
- 8.Dodson S I. Bioscience. 1989;39:447–453. [Google Scholar]
- 9.Bollens S M, Frost B W, Thoreson D S, Watts S J. Hydrobiologia. 1992;234:33–39. [Google Scholar]
- 10.Haney J F. Arch Hydrobiol Beih Ergebn Limnol. 1993;39:1–17. [Google Scholar]
- 11.Brönmark C, Miner J G. Science. 1992;258:1348–1350. doi: 10.1126/science.258.5086.1348. [DOI] [PubMed] [Google Scholar]
- 12.Lively C M. Evolution. 1986;40:232–242. doi: 10.1111/j.1558-5646.1986.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 13.Appleton R D, Palmer A R. Proc Natl Acad Sci USA. 1988;85:4387–4391. doi: 10.1073/pnas.85.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trussell G C. Evolution. 1996;50:448–454. doi: 10.1111/j.1558-5646.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 15.Leonard G H, Bertness M D, Yund P O. Ecology. 1999;80:1–14. [Google Scholar]
- 16.Havel J E. In: Predation: Direct and Indirect Impacts on Aquatic Communities. Kerfoot W C, Sih A, editors. Hanover: Univ. Press of New England; 1987. pp. 263–279. [Google Scholar]
- 17.Berven K A, Gill D E, Smith-Gill S J. Evolution. 1979;33:609–623. doi: 10.1111/j.1558-5646.1979.tb04714.x. [DOI] [PubMed] [Google Scholar]
- 18.Cossins A R, Bowler K. Temperature Biology of Animals. New York: Chapman and Hall; 1987. [Google Scholar]
- 19.Gliwicz M Z. Nature (London) 1986;320:746–748. [Google Scholar]
- 20.Harvell C D, Fenical W, Roussis V, Ruesink J L, Griggs C C, Greene C H. Mar Ecol Prog Ser. 1993;93:165–173. [Google Scholar]
- 21.Lively C M. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 245–258. [Google Scholar]
- 22.Endler J A. Geographic Variation, Speciation, and Clines. Princeton: Princeton Univ. Press; 1977. [PubMed] [Google Scholar]
- 23.Vermeij G J. Biogeography and Adaptation: Patterns of Marine Life. Princeton: Princeton Univ. Press; 1978. [Google Scholar]
- 24.Vermeij G J. Evolution and Escalation: An Ecological History of Life. Princeton: Princeton Univ. Press; 1987. [Google Scholar]
- 25.Thompson J N. Trends Ecol Evol. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. [DOI] [PubMed] [Google Scholar]
- 26.Thompson J D. Trends Ecol Evol. 1991;6:246–249. doi: 10.1016/0169-5347(91)90070-E. [DOI] [PubMed] [Google Scholar]
- 27.Harvell C D. Evolution. 1988;52:80–86. doi: 10.1111/j.1558-5646.1998.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 28.Vermeij G J. Nature (London) 1982;299:349–350. [Google Scholar]
- 29.Seeley R H. Proc Natl Acad Sci USA. 1986;83:6897–6901. doi: 10.1073/pnas.83.18.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer A R. Evolution. 1979;33:697–713. doi: 10.1111/j.1558-5646.1979.tb04722.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith, L. D. & Jennings, J. A. (2000) Marine Biology, in press.
- 32.Graus R R. Lethaia. 1974;7:303–314. [Google Scholar]
- 33.Kemp P, Bertness M D. Proc Natl Acad Sci USA. 1984;81:811–813. doi: 10.1073/pnas.81.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trussell G C. Ecology. 1997;78:1033–1048. [Google Scholar]
- 35.Trussell, G. C. (2000) Evolution, in press.
- 36.Clarke A. Oceanogr Mar Biol Ann Rev. 1983;21:341–453. [Google Scholar]
- 37.Vermeij G J. A Natural History of Shells. Princeton: Princeton Univ. Press; 1993. [Google Scholar]
- 38.Scattergood L W. Fisheries. Augusta, ME: Dept. of Sea and Shore Fish.; 1952. , Circular no. 8. [Google Scholar]
- 39.Seeley R H. Dissertation. New Haven, CT: Yale University; 1985. [Google Scholar]
- 40.Palmer A R. Malacologia. 1982;23:63–73. [Google Scholar]
- 41.Sokal R R, Rohlf F J. Biometry. 3rd ed. Stony Brook: State University of New York; 1995. [Google Scholar]
- 42.Welch W R, Churchill L U. Res. Ref. Doc.83/21. West Boothbay Harbor, ME: Maine Dept. of Marine Resources; 1983. [Google Scholar]
- 43.Cohen A N, Carlton J T. Science. 1998;279:555–558. doi: 10.1126/science.279.5350.555. [DOI] [PubMed] [Google Scholar]
- 44.Geller J B, Walton E D, Grosholz E D, Ruiz G M. Mol Ecol. 1997;6:901–906. doi: 10.1046/j.1365-294x.1997.00256.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen A N, Carlton J T, Fountain M C. Mar Biol. 1995;122:225–237. [Google Scholar]
- 46.Grosholz E D, Ruiz G M. Biol Conserv. 1996;78:59–66. [Google Scholar]
- 47.Stearns S C. The Evolution of Life Histories. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 48.Harvell C D. Am Nat. 1986;128:810–823. [Google Scholar]
- 49.Crowl T A, Covich A P. Science. 1990;247:949–951. doi: 10.1126/science.247.4945.949. [DOI] [PubMed] [Google Scholar]
- 50.Riessen H P. Limnol Oceanogr. 1984;29:1123–1127. [Google Scholar]
- 51.Lively C M. Ecology. 1986;67:858–864. [Google Scholar]
- 52.Palmer A R. Nature (London) 1981;292:150–152. [Google Scholar]
- 53.Palmer A R. Proc Natl Acad Sci USA. 1992;89:1379–1382. doi: 10.1073/pnas.89.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spight T M, Emlen J M. Ecology. 1976;57:1162–1178. [Google Scholar]
- 55.Palmer A R. J Exp Mar Biol Ecol. 1983;73:95–124. [Google Scholar]
- 56.Endler J A. Natural Selection in the Wild. Princeton: Princeton Univ. Press; 1986. [Google Scholar]
- 57.West K, Cohen A, Baron M. Evolution. 1991;45:589–607. doi: 10.1111/j.1558-5646.1991.tb04331.x. [DOI] [PubMed] [Google Scholar]
- 58.West-Eberhard M J. Annu Rev Ecol Syst. 1989;20:249–278. [Google Scholar]
- 59.Williamson P G. Nature (London) 1981;293:437–443. [Google Scholar]
- 60.Smith L D, Palmer A R. Science. 1994;264:710–712. doi: 10.1126/science.264.5159.710. [DOI] [PubMed] [Google Scholar]