Abstract
Severe noise exposure can induce heat shock proteins (Hsps), and exposure to moderate noise has been reported to confer protection against noise-induced damage to hearing. Whether there is any association of genetic variation in both constitutive and inducible hsp70 genes with noise-induced hearing loss (NIHL) is presently unknown. Using polymerase chain reaction-restriction fragment length polymorphism, we genotyped 3 polymorphisms (+190A/ B, +1267A/B, and +2437A/B) in the hsp70-1 (rs1043618), hsp70-2 (rs1061581), and hsp70-hom (rs2227956) genes, respectively, and investigated the associations of these polymorphisms with risk of developing NIHL in 194 automobile workers working in a similar noise environment as evaluated by audiological assessment. Multivariate logistic regression models were used to assess the associations with the risk genotypes, and Whap software was used to analyze their haplotypes. Our results showed that there was no statistically significant difference in the genotype and allele distributions of hsp70-1, hsp70-2, and hsp70-hom between the NIHL group and the normal group (P > 0.05) with and without adjustment for age, sex, smoking, history of explosive noise exposure, and cumulative noise exposure. However, haplotype analysis revealed that the Hap5 (ie, haplotype +190A/+1267B/+2437A) and Hap6 (ie, haplotype +190A/+1267B/+2437B) were significantly more frequent in the NIHL group than in the normal group (20/9, P = 0.022, and 7/0, P = 0.005, respectively). Compared with Hap1 (ie, +190A/+1267A/+2437A), Hap5 was associated with a nearly 3-fold increased risk of NIHL (adjusted odds ratio, 2.67; 95% confidence interval, 1.13–6.27). Seven of the NIHL patients had Hap6, but none of the controls had this haplotype. Our results suggest that some haplotypes of the hsp70 genes may be associated with a higher susceptibility to NIHL.
INTRODUCTION
Noise-induced hearing loss (NIHL), one of the most prevalent occupational hazards in modern industries, is considered a complex disease caused by a gene-environment interaction. Thus, some individuals are more susceptible to NIHL than others (Carlsson et al 2005), and therefore it is important to explain differences in susceptibility to NIHL in order to develop methods that can predict the risk. There are still limited data about genetic polymorphisms that may be involved in susceptibility to NIHL. Animal experiments suggested that the gene coding for otocadherin 23 (cdh23) and plasma membrane Ca2+-ATPase isoform 2 gene (PMCA2) might be involved in the susceptibility of NIHL (Kozel et al 1998; Holme et al 2004).
Heat shock proteins (Hsps), the phylogenically conserved proteins induced by numerous physical and physiological stresses, can also be induced by noise and ototoxic drugs (Lim et al 1993). Moreover, Hsps, when induced in response to moderate nontraumatic sound levels, can condition the ear to withstand effects of loud noise and protect the ear from hearing loss, although there is a noticeable individual variability (Yoshida et al 1999; Altschuler et al 2002). Hsps function as molecular chaperones and the 70-kDa heat shock proteins (HSP70) are well known to have functions related to stress tolerance.
The human hsp70 family consists of 3 main genes: hsp70-1, hsp70-2, and hsp70-hom (Milner and Campbell 1990). Hsp70-1 and hsp70-2 encode a similar heat-inducible protein Hsp70, but hsp70-1 is also constitutively expressed at a low level, whereas hsp70-hom encodes a non-heat-inducible protein that shares high homology with the protein products of hsp70-1/2. These genes are polymorphic, potentially accounting for variation in their functions and susceptibility to stress tolerance (Favatier et al 1997; Wu et al 2004). Some studies reported possible associations of single nucleotide polymorphisms (SNPs) in the hsp70 genes with Parkinson's disease (Wu et al 2004), abacavir hypersensitivity (Martin et al 2004), autoimmune diseases (Pugliese et al 1992; Favatier et al 1997; Fraile et al 1998; Vargas-Alarcon et al 2002), lung cancer (Rusin et al 2004), and acute high altitude illness (Zhou et al 2005).
We have previously observed that exposure to severe noise can induce antibodies against the inducible member of the Hsp70 family in steel industry workers (Wu et al 2001) and that the presence of anti-Hsp70 was associated with increased risk of high-frequency hearing loss and electrocardiography abnormalities in workers exposed to noise (Yang et al 2004; Yuan et al 2005). However, whether there is an association of polymorphisms of the hsp70 genes with susceptibility to NIHL remains unknown. We therefore analyzed the hsp70-1 +190A/B (rs1043618), hsp70-2 +1267A/B (rs1061581), and hsp70-hom 2437A/B (rs2227956) polymorphisms and investigated their associations with the risk of NIHL in 194 automobile workers exposed to noise.
SUBJECTS AND METHODS
Study subjects and environmental noise monitoring
A total of 194 autoworkers (132 men and 62 women) at the Dongfeng Motor Company (Shiyan, Hubei, China), who had been employed for at least 1 year, were included in this study. These subjects had a history of exposure only to occupational noise for at least 1 year but without exposure to other harmful factors, such as high temperature (>32°C) or known toxicants (eg, organic solvent or polycyclic aromatic hydrocarbons) in workplaces (Yang et al 2004). They had no history of fever or common infections such as influenza, diarrhea, pneumonia, or hepatitis within 1 month before medical examination. Furthermore, the subjects did not use any hearing protectors, such as earmuffs and disposable earplugs. Noise exposure levels at the selected workplaces were assessed with a sound pressure audiometer (BK-2231; Brüel & Kjaer Company, Naerum, Denmark) at 10 AM, 3 PM, and 5 PM for 3 consecutive days, twice per year, according to the Chinese national criterion for noise in the workplace (Liu and Cai 1995). To evaluate the actual noise exposure level of the worker, cumulative noise exposure (CNE) was calculated, based on the database of a 20-year noise exposure, according to monitoring data on A sound pressure level and employment time calculated as follows: Expc = Leq + 16.61 × log10(T/T0), dB(A) (Talbott et al 1999), where Expc is the cumulative noise exposure level; LeqA, the time-weighted average exposed sound pressure level A; T, the total adjusted time worked (in years); and T0, year 1.
Evaluation of health status
Health status was evaluated in all workers using a questionnaire and by clinical and laboratory examination. The questionnaire was used to obtain personal and family history including risk factors for hearing loss, such as age, employee years, lifestyle (smoking and drinking), history of explosive noise exposure, and history of diseases. The interview was performed by an industrial hygienist for each worker before the medical examination. The clinical laboratory examination included signs, weight, height, pulse, electrocardiogram, B-echography, blood pressure, blood routine, and hepatic function test. Venous blood was also collected in heparinized tubes to separate plasma and lymphocytes for the detection of hsp70 gene polymorphism.
Audiological assessment and definitions of hearing loss
Pure-tone audiometry was performed for both ears at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 8.0 kHz. All auditory tests were performed in a sound-attenuating booth by a trained technician. Audiometry was done using Swiss Midimate RT-150 Audiometer (Brüel & Kjaer Company) calibrated to ISO 389 (1985-E) for measurement of air conduction. Threshold value was defined as the lowest signal intensity that was detected in the subject at least 50% of the time, with a minimum of 3 tries. Masking was performed if the subject had a threshold value that differed by 40 dB or more between both ears. Otoscopic examination of the external acoustic meatus and tympanic membrane was done to exclude any ear diseases. Hearing loss can either be in the low-frequency range (0.5–2.0 kHz) or high-frequency range (4.0–8.0 kHz). We took the mean threshold of 0.5, 1.0, and 2.0 kHz (PTA512) as low-frequency hearing status and the mean threshold of 4.0, 6.0, and 8.0 kHz (PTA468) as high-frequency hearing status. Hearing threshold worse than 25 dB in either low frequency or high frequency was defined as hearing loss.
Genotyping of hsp70 polymorphisms
DNA was isolated from lymphocytes of 3 mL of blood using a commercial DNA extraction kit according to the manufacturer's instructions (Gentra Corp., Minneapolis, MN, USA). Genotypes for polymorphisms +190A/B (rs1043618) in the hsp70-1 gene, +1267A/B (rs1061581) in the hsp70-2 gene, and +2437A/B (rs2227956) in the hsp70-hom gene were determined by previously described polymerase chain reaction (PCR)-based assays using the primers listed in Vargas-Alarcón et al (2002). Briefly, a PCR was carried out in a 25-μL volume containing 50 ng of genomic DNA, 200 μmol/L diethylnitrophenyl thiophosphates (dNTPs), 2 mM MgCl2, 1× Taq DNA polymerase buffer, 1 μmol/L each primer, and 1 unit of Taq DNA polymerase (Fermentas Inc., Hanover, MD, USA). The following PCR protocol was used for amplifying hsp70 genes: initial amplification by incubating the PCR mixture at 94°C for 5 minutes followed by 35 cycles of incubation at 94°C and corresponding anneal temperatures (57°C for hsp70-1, 56°C for hsp70-2 and hsp70-hom) for 1 minute each, 72°C for 1 minute, and a final incubation at 72°C for 10 minutes. For the detection of restriction fragment length polymorphism, the amplified PCR fragments of hsp70-1, hsp70-2, and hsp70-hom genes were digested with restriction enzymes BsrBI, PstI, and NcoI (Fermentas Inc.), respectively. Subsequently, the digested products of hsp70-1 gene were analyzed on 12% polyacrylamide gel and those of hsp70-2 and hsp70-hom genes were separated on 1.5% agarose gels. These gels were stained with ethidium bromide (0.5 μg/mL), and genotypes were determined by analyzing different bands described in Vargas-Alarcón et al (2002). All gel analyses were carried out blindly to the subject's disease status.
Statistical analysis
All data were entered into a computerized database. Further analysis was carried out by using the statistical analysis software SPSS package (SPSS Inc., Chicago, IL, USA). Measurements of continuous data were analyzed by univariate analysis of variance and Student's t-tests. Qualitative data were computed by the Pearson χ2 contingency tables. Genotype and allele frequencies for each polymorphic site were calculated, and the differences between the patients and controls were determined by the χ2 test. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were computed by multivariate logistic regression analysis to test the magnitude of associations between NIHL risk and the genotypes. The haplotype analyses of the 3 hsp70 SNP positions were performed using the program Whap (http://www.broad.mit.edu/personal/shaun/whap/) (Myers AJ et al 2005).
RESULTS
Characteristics of the subjects
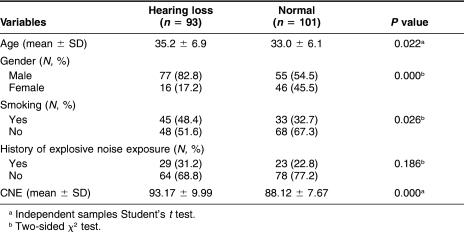
Table 1 lists potential risk factors for NIHL in the workers who had been exposed to occupational noise. The 93 subjects with hearing loss were compared with 101 subjects without hearing loss (considered to have normal hearing). Those who had hearing loss were more likely to be elders (P = 0.022), men (P = 0.000), and smokers (P = 0.026), and had a higher value of CNE (P = 0.000). However, there was no difference in history of exposure to explosive noise (P = 0.186).
Table 1.
Differences of factors in workers with NIHL and normal workers
Distribution of the hsp70 polymorphisms in NIHL and normal group
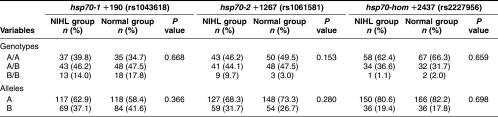
The distributions of the hsp70-1, hsp70-2, and hsp70-hom genotypes and alleles in NIHL and normal groups are listed in Table 2. There was no significant difference in the distributions of both variant genotypes (P = 0.668) and alleles (P = 0.366) of the hsp70-1 gene between the patients and controls. For the hsp70-2 gene, the frequency of the hsp70-2 +1267B/B genotype was slightly higher in the NIHL group (9.7%) than in the normal group (3.0%), but the difference in the distributions of variant genotypes was not significant (P = 0.153), nor were the allele frequency distributions (P = 0.280). For the hsp70-hom gene, the frequencies of the hsp70-hom genotypes and alleles were similar in the NIHL and normal groups (P = 0.659 and P = 0.698, respectively).
Table 2.
hsp70-1, hsp70-2, and hsp70-hom genotype and allele frequencies (%) in NIHL group and normal group
Association of the genotypes with the risk of NIHL
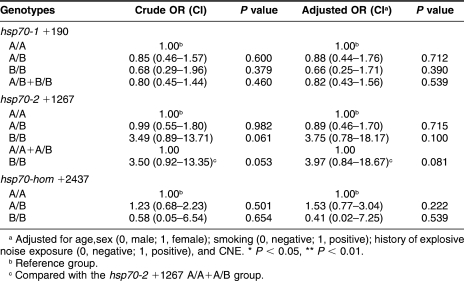
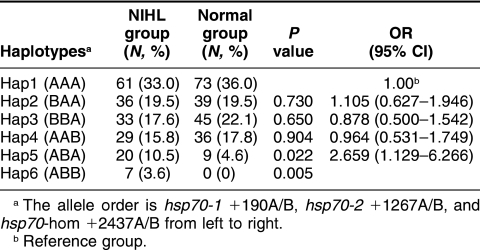
Crude and adjusted ORs for complex NIHL were used without and with adjustment for confounding factors. As shown in Table 3, after adjustment for age, sex (0, male; 1, female), smoking (0, negative; 1, positive), history of explosive noise exposure (0, negative; 1, positive), and CNE with multiple logistic regression analysis, no significant higher risk was found in any genotype of the 3 positions or the combinations of some genotypes (P > 0.05). However, the Hsp70-2 +1267BB genotype was associated with a borderline increased risk of NIHL (OR = 3.49; 95% CI = 0.89–13.71), compared with the Hsp70-2 +1267A/A genotype. When we used the Whap software to analyze the haplotypes based on the observed genotypes (Table 4), we found that the frequencies of haplotypes Hap5 (ie, +190A/+1267B and +2437A) and Hap6 (ie, +190A/+1267B/+2437B) were significantly higher in the NIHL group than in the normal one (20/9, P = 0.022, and 7/0, P = 0.005, respectively). Compared with Hap1 allele (ie, +190A/+1267A and +2437A), Hap5 allele was associated with a nearly 3-fold increased risk of NIHL (adjusted OR = 2.67 and 95% CI = 1.13–6.27). Of the NIHL patients, 7 had haplotype Hap6, whereas none of the normal controls had this haplotype. The association of haplotypes 5 and 6 with NIHL is very clear.
Table 3.
The ORs of different hsp70 genotypes for NIHL
TABLE 4.
hsp70 haplotype distribution and ORs in NIHL group and normal group
DISCUSSION
Excessive exposure to noise results in temporary or permanent changes in hearing ability in both humans and animals (Davis et al 1950; McFadden and Plattsmier 1982; Henry 1984). NIHL, previously known as industrial deafness, remains an important problem in occupational health. An interaction between environmental and genetic predisposing factors is thought to be involved in the etiology of NIHL. Studies in animal models suggest that genetic factors may influence individual susceptibility to noise; for example, certain mouse strains with age-related hearing loss have been reported to be more susceptible to noise (Erway et al 1996; Davis et al 2001). Mice in which genes like PMCA2 (Kozel et al 2002), cadherin 23 (Holme and Steel 2004), superoxide dismutase 1 (Ohlemiller et al 1999), or glutathione peroxidase 1 (Ohlemiller et al 2000) were knocked out have been reported to be more sensitive to NIHL. However, firm evidence for the involvement of genetic factors in human NIHL is still scarce. A possible association between the absence of glutathione-S-transferase M1 (GSTM1) and NIHL was reported (Rabinowitz et al 2002), but this was not confirmed in a recent study on 2 deletion polymorphisms in the GSTM1 and GSTT1 genes and the susceptibility to NIHL using susceptible and resistant NIHL in a Caucasian population from 3 distinct workplaces (Carlsson et al 2005). Another study suggested that SOD2 and paraoxonase polymorphisms could predispose to NIHL (Fortunato et al 2004)
The HSP70 family proteins may be the most predominant and particularly interesting group of proteins involved in the major histocompatibility complex in disease susceptibility (Favatier et al 1997). Several studies suggest that the polymorphisms of the hsp70 genes might be associated with many diseases; for example, individuals carrying P2/P2 genotype of the hsp70-2 gene have an increased risk of obesity in Tunisians (Chouchane et al 2001). The hsp70-2 gene polymorphic allele A may be a possible genetic marker of a less severe clinical phenotype associated with Crohn disease in Japanese patients (Esaki et al 1999). In ankylosing spondylitis, differences in hsp70-1 and hsp70-2 genotypes among ankylosing spondylitis patients and controls appear to be due to the linkage disequilibrium between HSP70 alleles and HLA-B*27 (Fraile et al 1998). We also found that individuals with hsp70-2 +1267B/B, hsp70-hom +2437A/B, +2437B/B genotypes are more susceptible to high-altitude illness, whereas those with hsp70-hom +2437A/B genotype are tolerant to low oxygen (Zhou et al 2005).
In the present study, we did not find any statistically significant difference in the genotype and allele distributions of hsp70-1, hsp70-2, and hsp70-hom between the NIHL group and normal group, although it was suggestive that the hsp70-2 +1267B/B genotype might be associated with an increased risk of NIHL. These results suggested that the polymorphisms of the 3 SNPs of the hsp70 genes do not seem to play a major role in NIHL at least in the Chinese population, although larger studies are needed to confirm these findings. Although sometimes useful, single SNP may not be sufficiently informative in complex diseases. The haplotype-based linkage disequilibrium mapping has become a powerful and cost-effective method for performing genetic association studies, particularly in search of the genes causing complex diseases (Niu et al 2002; Salisbury et al 2003). In addition, haplotype information is useful for predicting the severity and prognosis of certain genetic diseases associated with a Mendelian inheritance (Cuppens 1998; Gambetti et al 2003).
Our results from haplotype analysis using the Whap software revealed that the frequencies of the Hap5 haplotype (ie, +190A/+1267B/+2437A) and Hap6 haplotype (ie, +190A/+1267B/+2437B) were significantly greater in the NIHL than in the normal group. The results suggest that individuals carrying Hap5 or Hap6 haplotypes are more prone to NIHL when exposed to noise. The hsp70-1 +190 polymorphism and the hsp70-2 +1267 polymorphism depend on silent change in the coding region; the hsp70-hom polymorphic site detected by NcoI is located on position +2437, which corresponds to a Met→Thr amino acid substitution at position 493 (Milner and Campbell 1992). Although individual SNP analysis did not show any association with NIHL, the combined information of these 3 SNPs, ie, the haplotypes, may play some role in the development of NIHL. However, the mechanisms by which these Hsp70 haplotypes are associated with the development of NIHL remain unknown but clearly warrant further investigations.
In summary, the present data suggest that genetic variation in the hsp70 genes may contribute to the susceptibility to NIHL. Further investigations with larger populations are warranted to confirm the significance of our findings and functionality of these observed haplotypes.
Acknowledgments
We are particularly grateful to all individuals who voluntarily participated in the study and to many members of the medical personnel of Dongfeng Motor Company (Shiyan, Hubei) for their generous help in the examination and sampling of subjects. This work was supported by research funds from the National Key Basic Research and Development Program (2002CB512905), the National Natural Science Foundation of China (NSFC) (to T.W.). T.W. and R.M.T. also acknowledge financial support from the NSFC of China and the Canadian Institute of Health Research (CIHR) for a research exchange program and an operating CIHR grant (to R.M.T.).
REFERENCES
- Altschuler RA, Fairfield D, Cho Y, Leonova E, Benjamin IJ, Miller JM, Lomax MI. Stress pathways in the rat cochlea and potential for protection from acquired deafness. Audiol Neurootol. 2002;7:152–156. doi: 10.1159/000058301.1420-3030(2002)007[0152:SPITRC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carlsson PI, Van Laer L, Borg E, Bondeson ML, Thys M, Fransen E, Van Camp G. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res. 2005;202:87–96. doi: 10.1016/j.heares.2004.09.005.0378-5955(2005)202[0087:TIOGVI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chouchane L, Danguir J, Beji C, Bouassida K, Camoin L, Sfar H, Gabbouj S, Strosberg AD. Genetic variation in the stress protein hsp70-2 gene is highly associated with obesity. Int J Obes Relat Metab Disord. 2001;25:462–466. doi: 10.1038/sj.ijo.0801545.0307-0565(2001)025[0462:GVITSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cuppens H, Lin W, and Jaspers M. et al. 1998 Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J Clin Invest. 101:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Morgan CT, Hawkins Jr JE, Galambos R, Smith FW. Temporary deafness following exposure to loud tones and noise. Acta Otolaryngol. 1950;88:1–56.0001-6489(1950)088[0001:TDFETL]2.0.CO;2 [PubMed] [Google Scholar]
- Davis RR, Newlander JK, Ling X, Cortopassi GA, Krieg EF, Erway LC. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7.0378-5955(2001)155[0082:GBFSTN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau YW, Davis RR, Krieg EF. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear Res. 1996;93:181–187. doi: 10.1016/0378-5955(95)00226-x.0378-5955(1996)093[0181:GOAHLI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Esaki M, Furuse M, Matsumoto T, Aoyagi K, Jo Y, Yamagata H, Nakano H, Fujishima M. Polymorphism of heat-shock protein gene HSP70-2 in Crohn disease: possible genetic marker for two forms of Crohn disease. Scand J Gastroenterol. 1999;34:703–707. doi: 10.1080/003655299750025912.0036-5521(1999)034[0703:POHPGH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Favatier F, Bornman L, Hightower LE, Günther E, Polla BS. Variation in hsp gene expression and Hsp polymorphism: do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones. 1997;2:141–155. doi: 10.1379/1466-1268(1997)002<0141:vihgea>2.3.co;2.1466-1268(1997)002[0141:VIHGEA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato G, Marciano E, and Zarrilli F. et al. 2004 Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 50:2012–2018. [DOI] [PubMed] [Google Scholar]
- Fraile A, Nieto A, Mataran L, Martin J. HSP70 gene polymorphisms in ankylosing spondylitis. Tissue Antigens. 1998;51:382–385. doi: 10.1111/j.1399-0039.1998.tb02977.x.0001-2815(1998)051[0382:HGPIAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterization. Br Med Bull. 2003;66:213–239. doi: 10.1093/bmb/66.1.213.0007-1420(2003)066[0213:SAFCCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Henry KR. Cochlear damage resulting from exposure to four different octave bands of noise at three ages. Behav Neurosci. 1984;98:107–117. doi: 10.1037//0735-7044.98.1.107.0735-7044(1984)098[0107:CDRFET]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2004;5:66–79. doi: 10.1007/s10162-003-4021-2.1525-3961(2004)005[0066:PHLAIS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel PJ, Davis RR, Krieg EF, Shull GE, Erway LC. Deficiency in plasma membrane calcium ATPase isoform 2 increases susceptibility to noise-induced hearing loss in mice. Hear Res. 2002;164:231–239. doi: 10.1016/s0378-5955(01)00420-8.0378-5955(2002)164[0231:DIPMCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, and Erway LC. et al. 1998 Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 273:18693–18696. [DOI] [PubMed] [Google Scholar]
- Lim HH, Jenkins OH, Myers MW, Miller JM, Altschuler RA. Detection of HSP72 synthesis after acoustic overstimulation in rat cochlea. Hear Res. 1993;69:146–150. doi: 10.1016/0378-5955(93)90102-7.0378-5955(1993)069[0146:DOHSAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Liu WK, Cai RT 1995 Noise. In: Physical Factors and Occupational Medicine, ed Liu WK, Cai RT. Science Publishing House, Beijing, China, 31–98. [Google Scholar]
- Martin AM, Nolan D, and Gaudieri S. et al. 2004 Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc Natl Acad Sci USA. 101:4180–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Plattsmier HS 1982 Exposure-induced loudness shifts and threshold shifts. In: New Perspectives on Noise-Induced Hearing Loss, ed Hamernik RP, Henderson D, Salvi R. Raven Press, New York, 363–373. [Google Scholar]
- Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095.0093-7711(1990)032[0242:SAEOTT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Polymorphic analysis of the three MHC-linked HSP70 genes. Immunogenetics. 1992;36:357–362. doi: 10.1007/BF00218042.0093-7711(1992)036[0357:PAOTTM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, and Marlowe L. et al. 2005 The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum Mol Genet. 14:2399–2404. [DOI] [PubMed] [Google Scholar]
- Niu T, Qin ZS, Xu X, Liu JS. Bayesian haplotype inference for multiple linked single-nucleotide polymorphisms. Am J Hum Genet. 2002;70:157–169. doi: 10.1086/338446.0002-9297(2002)070[0157:BHIFML]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, and Ding DL. et al. 1999 Targeted deletion of the cytosolic Cu/Zn superoxide dismutase gene (Sod1) increases susceptibility to noise induced hearing loss. Audiol Neurootol. 4:237–246. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043.1525-3961(2000)001[0243:TMOTGF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, Awdeh ZL, Galluzzo A, Yunis EJ, Alper CA, Eisenbarth GS. No independent association between HSP70 gene polymorphism and IDDM. Diabetes. 1992;41:788–791. doi: 10.2337/diab.41.7.788.0012-1797(1992)041[0788:NIABHG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Pierce Wise Sr J, Hur Mobo B, Antonucci PG, Powell C, Slade M. Antioxidant status and hearing function in noise-exposed workers. Hear Res. 2002;173:164–171. doi: 10.1016/s0378-5955(02)00350-7.0378-5955(2002)173[0164:ASAHFI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rusin M, Zientek H, and Krzesniak M. et al. 2004 Intronic polymorphism (1541–1542delGT) of the constitutive heat shock protein 70 gene has functional significance and shows evidence of association with lung cancer risk. Mol Carcinogen. 39:155–163. [DOI] [PubMed] [Google Scholar]
- Salisbury BA, Pungliya M, Choi JY, Jiang R, Sun XJ, Stephens JC. SNP and haplotype variation in the human genome. Mutat Res. 2003;526:53–61. doi: 10.1016/s0027-5107(03)00014-9.0027-5107(2003)526[0053:SAHVIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Talbott EO, Gibson LB, Burks A, Engberg R, MeHugh KP. Evidence for a dose-response relationship between occupational noise and blood pressure. Arch Environ Health. 1999;54:71–78. doi: 10.1080/00039899909602239.0003-9896(1999)054[0071:EFADRB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vargas-Alarcón G, Londoño JD, and Hernández-Pacheco G. et al. 2002 Heat shock protein 70 gene polymorphisms in Mexican patients with spondyloarthropathies. Ann Rheum Dis. 61:48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ma J, and Chen S. et al. 2001 Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones. 6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YR, Wang CK, and Chen CM. et al. 2004 Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson's disease. Hum Genet. 114:236–241. [DOI] [PubMed] [Google Scholar]
- Yang M, Zheng J, and Yang Q. et al. 2004 Frequency-specific association of antibodies against heat shock protein 60 and 70 with noise-induced hearing loss in Chinese workers. Cell Stress Chaperones. 9:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999;19:10116–10124. doi: 10.1523/JNEUROSCI.19-22-10116.1999.0270-6474(1999)019[10116:HSAPFP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yang M, and Yao H. et al. 2005 Plasma antibodies to heat shock protein 60 and heat shock protein 70 are associated with increased risk of electrocardiograph abnormalities in automobile workers exposed to noise. Cell Stress Chaperones. 10:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Wang F, Li F, Yuan J, Zeng H, Wei Q, Tanguay RM, Wu T. Association of polymorphisms of hsp70-2 and hsp-hom gene polymorphisms with risk of acute high-altitude illness in a Chinese young population. Cell Stress Chaperones. 2005;10:349–356. doi: 10.1379/CSC-156.1.1466-1268(2005)010[0349:AOPOHA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]