Abstract
Hsp70s are a family of ATP-dependent chaperones of relative molecular mass around 70 kDa. Immunization of mice with Hsp70 isolated from tumor tissues has been proved to elicit specific protective immunity against the original tumor challenge. In this work, we investigated whether Hsp70 can be used as vehicle to elicit immune response to its covalence-accompanying antigen. A recombinant protein expression vector was constructed that permitted the production of recombinant protein fusing tumor-associated antigen (eg, Mela) to the C terminus of Hsp70. We found that the Hsp70–Mela fusion protein can elicit strong cellular immune responses against murine tumor B16, which expresses protein Mela. The Hsp70 peptide-binding domain deletion mutant of the fusion protein was sufficient for inducing Mela-specific cytotoxic T lymphocyte but was not sufficient for engendering potent anti-tumor immunity against B16. We also found that host natural killer (NK) cells were stimulated in vivo by C-terminal domain of Hsp70. We thus presume that Hsp70 fusion proteins suppress tumor growth via at least 2 distinct pathways: one is covalence-accompanying antigen dependent; another is antigen independent. The C-terminal domain of Hsp70 seemed to be the crucial part in eliciting antigen-independent responses, including NK cell stimulation, against tumor challenges. Furthermore, we found that immunization with multiple Hsp70 fusion proteins resulted in a better anti-tumor effect.
INTRODUCTION
Mela, which contain gp70 and p15E (2 products of the env gene of an endogenous leukemia virus), is expressed in many tumors, including the murine melanoma B16 cell as a tumor-associated antigen (TAA; Hayashi et al 1992; Kershaw et al 2001; Bronte et al 2003), just like gp100/ Pmel17, TRP2, and MART-1 (Kawakami et al 1994; Wang et al 1996; Bloom et al 1997; Overwijk et al 1998). The finding of tumor-associated antigens was once regarded as a chance to develop an effective cancer therapeutic, but the results of immunotherapy with TAA were not satisfactory. TAAs need adjuvant to improve their immune system stimulatory ability.
Heat shock proteins (Hsps) are families of highly conserved proteins induced by a large variety of stresses, including heat shock (Lindquist 1986). Recent reports have shown the importance of Hsps, including Hsp70, Hsp90, Hsp110, glucose-regulated protein 170, and glucose-regulated protein 96 in immune reactions (Udono and Srivastava 1993; Udono and Srivastava 1994; Chandawarkar et al 1999; Asea et al 2000; Todryk et al 2003). Immunization of mice with Hsps preparations derived from tumor instead of normal cells has been shown to elicit specific protective immunity against the original tumor cells (Udono and Srivastava 1993; Wang et al 2001). This specific immunogenicity of tumor-derived Hsp preparations has been observed in a wide range of tumors, including hepatomas, fibrosarcomas, lung carcinoma, prostate cancer, spindle cell carcinoma, colon carcinoma, and melanoma in mice and rats (Srivastava and Das 1984; Srivastava et al 1986; Ullrich et al 1986; Palladino et al 1987; Udono et al 1994; Feldweg and Srivastava 1995). The anti-tumor effect of Hsp preparations was surprisingly potent against the original tumor cells. Moreover, treatment of mice with Hsps could also be used for therapy against the pre-existing primary tumor and its metastases (Tamura et al 1997; Parmiani et al 2001; Belli et al 2002; Mazzaferro et al 2003); in some cases, it can even eliminate tumor nodules that already exist (Udono and Srivastava 1993; Udono and Srivastava 1994; Chandawarkar et al 1999).
However, the mechanism in which Hsps protect hosts from tumor cell or virus challenge has not been fully understood up to now. Most immunotherapeutic approaches exploit the carrier function of Hsp for tumor-specific antigenic peptides. The noncovalent Hsp–peptide complex prepared by combining Hsps derived from normal cells with antigenic peptide in vitro also can induce peptide-specific immune response (Blachere et al 1997; Navaratnam et al 2001). Suzue et al (1997) succeeded in inducing ovalbumin (OVA)-specific CD8+ T lymphocyte immune response in mice via immunization with a covalently fused Hsp70–OVA construct. Most of the researchers attribute the Hsp anti-tumor function to the escorted peptides, which induce tumor-specific, CD8-positive T-cell responses.
These findings made us wonder whether a protein recombined covalently of a heat shock protein and a tumor-associated antigen could prevent tumor growth in vivo by eliciting an antigen-specific immune response and could be used as a tumor vaccine. It was suggested that potent tumor vaccines could be produced on large scales with the use of recombinant fusion proteins.
Here we constructed an hsp70 fusion protein having a fragment of Mela as fusion partner in the C terminus. The fusion protein exhibits strong anti-tumor activity as potent vaccines against B16 tumor challenges. It has recently been demonstrated that a certain portion of the ATPase domain of Hsp70 was another crucial part in eliciting peptide-specific immunity (Huang et al 2000; Udono et al 2001; Zhang et al 2006). A comparable Hsp70 C-terminal domain deletion mutant of the fusion protein was also constructed in the present work to investigate the function of the Hsp70 C-terminal domain in tumor suppression. We further investigated whether immunization with multiple Hsp70 fusion proteins (Hsp70–Mela, Hsp70–Pmel17, Hsp70–TRP2 and Hsp70–MART-1) would result in a better anti-tumor effect.
MATERIALS AND METHODS
Reagents and cell lines
Cloning T-vector pUCmT was purchased from Shanghai Biological Engineering Technology and Service CO., LTD. (Shanghai, China). Expression vector pET-28b was purchased from Novagen (Madison, WI, USA). Expression vector pGEX-5x-3 was purchased from Amersham Biosciences (Piscataway, NY, USA). M-MLV reverse transcriptase Taq DNA polymerase and restriction endonucleases were from TaKaRa (Tokyo, Japan). The lactate dehydrogenase kit was purchased from Shanghai KEHUA Bio-Engineering Co, Ltd (Shanghai, China).
The murine melanoma cell line B16 is a spontaneously arising melanoma of the C57BL/6 mouse; the Lewis lung carcinoma cell line LLC, which originated spontaneously in a C57/BL6 mouse, is a malignant tumor that produces spontaneous lung metastases; and CT26 is an undifferentiated colon adenocarcinoma cell line derived by intrarectal injections of N-nitroso-N-methylurethane in a BALB/c mouse. All 3 cell lines are gifts from Shanghai Institute of Medical Industry. The YAC-1 cell line (from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences), with noted sensitivity to natural killer (NK) cells, is a Moloney leukemia virus– induced mouse lymphoma.
All 4 cell lines were cultured in medium RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 50 μg/ mL streptomycin, and 100 μg/mL benzyl-penicillin potassium (Sangon, Shanghai, China) at 37°C in a 5% CO2 incubator.
Antigen expression analysis
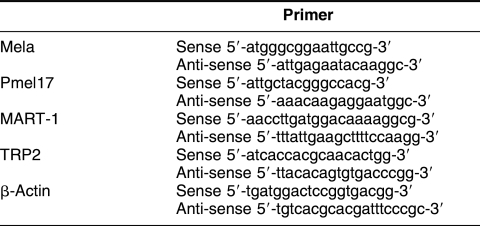
Total RNA was extracted from cultured cells with TRIzol (Invitrogen, Carlsbad, CA, USA), and reverse transcription was performed from total RNA by M-MLV reverse transcriptase (RT; RNase-free, TaKaRa). The mRNAs of Mela, Pmel17, TRP2, and MART-1 were analyzed via RT– polymerase chain reaction (PCR) with the use of specific primers (Table 1). After 5 minutes of initial denaturation, PCR was performed with recombinant Taq DNA polymerase in 30 amplification cycles (95°C for 30 seconds, 57°C for 30 seconds, 72°C for 30 seconds), with a final extension at 72°C for 7 minutes in a PTC-200 Peltier thermal cycler (MJ Research, Waltham, MA, USA). One-tenth of the reaction volume was run on a 2% agarose gel. As control for retrotranscription, PCR with specific primers for β-actin mRNA was also performed.
Table 1.
Primer sequences used to analyze antigen expression in B16, CT26, and LLC
Expression plasmid construct
DNA fragments of murine Hsp70 coding sequence and Mela (aa435–635) coding sequence were cloned into pUCmT from the total RNA of B16 melanoma cells according to the RT-PCR method (the primer sequences are listed in Table 2).
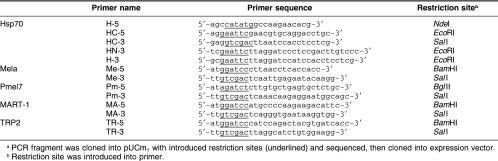
Table 2.
Primer sequences used to construct fusion protein expression plasmida
The DNA fragment containing the Hsp70 N-terminal ATPase domain (aa1–386) coding sequence (amplified with primers H-5 and HN-3) and the full-length Hsp70 coding sequence (amplified with primers H-5 and H-3) were inserted into pET-28b between the NdeI and EcoRI sites, respectively, forming plasmids named pET-28b– hsp70NTD and pET-28b–hsp70, respectively.
The DNA fragment containing the Mela (aa435–635) coding sequence (amplified with primers Me-5 and Me-3) was inserted into pET-28b–hsp70NTD and pET-28b– hsp70 between the BamHI and SalI sites, forming expression plasmids pET-28b–hsp70NTD–mela and pET-28b– hsp70–mela fusion proteins named Hsp70NTD–Mela and Hsp70–Mela, respectively.
To prepare Hsp70CTD (aa386–641), the coding sequence (amplified with primers HC-5 and HC-3) was inserted into the expression vector pGEX-5-3x between the EcoRI and SalI sites with correct open reading frames. We thus got a GST–Hsp70CTD recombinant protein expression plasmid, which is named pGEX-5-3x–hsp70CTD.
Similar to Mela, the DNA fragments of Pmel17, TRP2, and MART-1 were amplified from the total RNA of B16 melanoma cells by the RT-PCR method (the primer sequences are listed in Table 2). These DNA fragments were inserted into the plasmid pET-28b–hsp70 between the BamHI and SalI sites, respectively. Thus, we obtained Hsp70–Pmel17, Hsp70–TRP2, and Hsp70–MART-1 fusion protein expression plasmids.
All constructs were confirmed by DNA sequencing.
Fusion protein preparation
The expression plasmids obtained above were transformed into Escherichia coli strain BL21(DE3). The transformants were inoculated in Luria-Bertani broth and cultured with shaking (250 rpm) at 37°C overnight. The seed culture (10 mL) was transferred into 400 mL of fresh medium and cultured under the same conditions until an optical density at 600 nm (OD600) reached 1.0. Then isopropyl-beta-d-thiogalactopyranoside (final concentration 0.5 mM) was added for induction. The bacterial cells were cultured for another 3 hours and pelleted by centrifugation at 4000 × g for 15 minutes and washed once with phosphate-buffered saline (PBS).
All the 4 types of Hsp70 fusion proteins and Hsp70NTD–Mela were found located in pellets of cell lysate and dissolved with 8 M urea. They were purified with a Ni-NTA agarose column (Qiagen Inc, Hilden, Germany) according to the manufacturer's protocol. Refolding was achieved by applying the purified and denatured recombinant proteins to gel filtration chromatography with the use of Sephadex G25 (1.0 × 30 cm; Weisi, Shanghai, China). This column was equilibrated with 20 mM phosphate buffer containing 0.2 M arginine, pH 8.0, and the refolded proteins were obtained after elution. Meanwhile, GST–Hsp70CTD was purified as a soluble protein on a GST affinity column with glutathione-Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden).
The proteins were subsequently subjected to additional purification through diethylamino ethanol–anion exchange chromatography (Bio-Rad Biologic System, Hercules, CA, USA) to remove endotoxins in the protein preparations as previously described (Menoret 2004). The endotoxin contaminations were determined by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA).
Protein purity and concentration were verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The endotoxin contaminations were determined by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA).
Mice, immunization, and tumor protection assay
Male C57BL/6 mice and BALB/c mice at the ages of 6 to 8 weeks were purchased from Shanghai Institute of Medical Industry. All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Groups of mice were injected subcutaneously twice with 0.15 nmol of refolded protein or medium at a 1-week interval. In cytotoxicity assays, mice in each group were sacrificed 1 week after the boost, and spleens within groups were excised. In tumor protection assays, mice in each group received a tumor challenge. Tumor cell suspensions were injected subcutaneously into each mouse. Mice were monitored for evidence of tumor incidence by palpation and inspection every day until the tumors became palpable. Then tumor growth was recorded everyday with vernier calipers, measuring both the longitudinal and the transverse diameter. The volumes of tumor nodules were calculated by the formula
| Tumor volume = 0.5(length × width2), |
where length and width are measured in centimeters. In light of the uncertainty about tactual perception, we recorded the host mouse as a tumor-free mouse until a tumor nodule's volume reached 0.1 cm3.
Evaluation of cytotoxic T lymphocyte activity and NK cell cytotoxicity
The percentage of lysed cells in the cytotoxicity assay was evaluated by lactate dehydrogenase (LDH) release assay, which has been used before (Zhang et al 2006), according to manufacturer's instructions. Spleens excised from the experimental mice were filtered through a 70-μm cell strainer (Falcon, Becton Dickinson Labware, San José, CA, USA), and the splenic lymphocytes were isolated from splenocytes with lymphocyte separation medium (LSM, ICN Biomedicals, Costa Mesa, CA, USA). In the cytotoxic T lymphocyte (CTL) assay, splenic lymphocytes (1 × 106 cells/mL) were cocultured with mitomycin C–treated B16 or LLC cells (1 × 106 cells/mL) for 5 days, then the nonadherent cells that acted as effector cells were collected and resuspended in the complete culture medium. In NK cell cytotoxicity assays, splenic lymphocytes acted as effector cells immediately.
Briefly, target cells (B16, LLC, or YAC-1 cells at a concentration of 5 × 105 cells/mL) were mixed with effector cells in different ratios and incubated in 96-well flat-bottom plates for 4 hours at 37°C in an atmosphere containing 5% CO2, followed by centrifugation at 500 × g for 5 minutes. The OD490s of the supernatants were measured with the Universal Microplate Reader. The percent cytotoxicity was calculated as
 |
where ODexperiment is LDH release activity resulting from cocultures of effector cells and target cells at different ratios; ODeffector spontaneous and ODtarget spontaneous are release activities from separate cultures of effector cells and target cells, respectively; and ODmaximum is LDH release activity from target cells lysed by 1% Nonidet P40 (NP 40, Fluka, Buchs, Switzerland).
Assay of splenic NK cell populations
Each spleen excised from the experimental mice was solely filtered through a 70-μm cell strainer (Falcon), and the splenic lymphocytes were isolated from splenocytes with LSM. Cells were then washed once with PBS and incubated with phycoerythrin (PE)–conjugated anti-NK1.1 antibody (eBioscience, San Diego, CA, USA). The stained cells were analyzed by flow cytometry with a Calibur FACScan (Becton Dickinson, Mountain View, CA, USA).
Statistical analysis
A Student's t-test was used to calculate the significance of statistical comparisons. Animal tumor-free survival is presented as Kaplan–Meier survival curves and was statistically analyzed by log-rank test. Values of P < 0.05 were considered to represent statistically significant differences. Results are presented as means ± standard deviations.
RESULTS
Antigen expression analysis
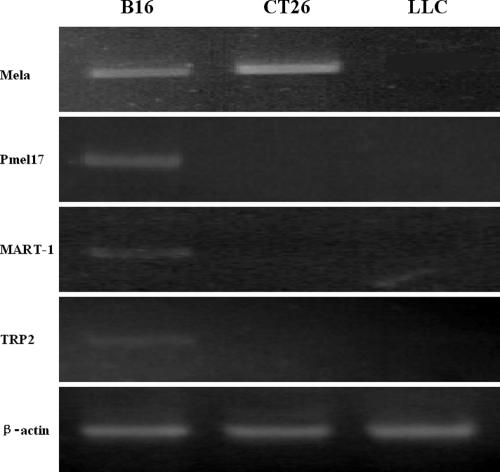
All 4 tumor-associated proteins (Mela, Pmel17, TRP2, and MART-1) were found to express in B16 by the RT-PCR method (Fig 1). Although LLC is a tumor cell line that originated spontaneously in the same host (a C57BL/6 mouse) as B16, none of the 4 antigen mRNAs were detected in LLC cells. Only the mRNAs of Mela was detected in CT26 cells.
Fig 1.
Antigen expression analysis. The mRNAs of Mela, Pmel17, TRP2, and MART-1 were detected in B16 cells by RT-PCR; none of the 4 antigen mRNAs were detected in LLC cells, and only Mela mRNA was detected in CT26 cells
Protein purification and refolding
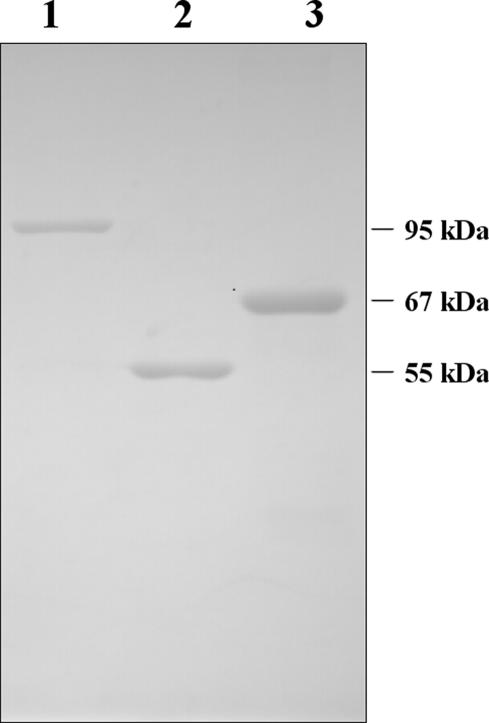
A recombinant system, developed to permit the production of fusion proteins in E. coli, was used to attach amino acids 435–635 of Mela, which contain 2 immunodominant epitopes (SPSYVYHQF, corresponding to Mela amino acids 454–462, and KSPWFTTL, corresponding to Mela amino acids 604–611; Bronte et al 2003), to the C terminus of full-length murine Hsp70. A comparable recombinant Hsp70NTD–Mela protein and a recombinant protein GST–Hsp70CTD were also produced. GST–Hsp70CTD was purified as a soluble protein with a GST affinity column. The other 2 recombinant proteins were expressed at high levels in E. coli but, unfortunately, were found mostly located in inclusion bodies. The latter 2 were denatured by 8 M urea and purified on a Ni-NTA agarose column. Refolded proteins were obtained through gel filtration chromatography. The purities of the recombinant proteins were assessed by SDS-PAGE (Fig 2), and the protein amounts were also determined.
Fig 2.
Production and purification of recombinant proteins. Purified proteins were examined by SDS-PAGE and visualized by Coomassie staining. Lane 1, Hsp70–Mela; lane 2, GST– Hsp70CTD; lane 3, Hsp70NTD–Mela. Molecular mass markers are on the right side
The endotoxin contamination in protein preparation was assessed by the Limulus amebocyte lysate assay; all the preparations were contaminated with small amounts endotoxin (<50 EU/mg).
Three other Hsp70 fusion proteins, Hsp70–Pmel17, Hsp70–TRP2, and Hsp70–MART-1 were obtained by the similar method of preparing Hsp70–Mela (date not shown here). Hsp70–MART-1 contains full-length MART-1 at the C terminus of the fusion protein, Hsp70–Pmel17 amino acids 13–300 of Pmel17, and Hsp70–TRP2 amino acids 153–417 of TRP2.
Immunization with Hsp70–Mela but not Hsp70NTD– Mela suppresses B16 melanoma growth in vivo
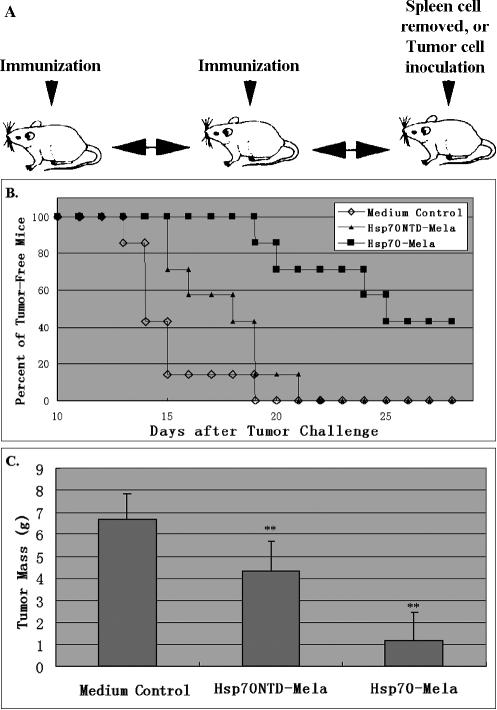
We first investigated the anti-tumor effect of the Hsp70– Mela fusion protein in vivo. As shown in Figure 3A, C57BL/6 mice were immunized with fusion protein twice at a 1-week interval then were challenged with 2 × 105 B16 melanoma cells by subcutaneous inoculation 1 week after the second immunization. Protection was observed in Hsp70–Mela-immunized mice, with a decrease in tumor incidence (Fig 3B). Kaplan–Meier curves were generated, showing that the tumor-free survival interval was 24.9 ± 1.2 days in the Hsp70–Mela-immunized group and 14.9 ± 0.7 days in the control group. The difference between the 2 groups was significant (P < 0.01; Fig 3B). As to the mass of tumor, immunization with Hsp70–Mela reduced tumor weight 82% by day 28 after tumor challenge (P < 0.01; Fig 3C). Hsp70–Mela exhibited potent anti-tumor activity against B16 melanoma challenge. However, the fusion protein Hsp70NTD–Mela had a weaker anti-tumor activity against B16 melanoma challenge compared with Hsp70–Mela. The group of mice immunized with Hsp70NTD–Mela showed a limited prolonged tumor-free survival interval (17.6 ± 0.9 days, 0.01 < P < 0.05), and tumor mass decreased only 36% in the Hsp70NTD–Mela-immunized group compared with the control group (P < 0.01).
Fig 3.
Suppressive effects against B16. Groups of mice (n = 7 per group) were immunized with Hsp70–Mela (filled squares), Hsp70NTD–Mela (filled triangles), or medium control (open diamonds) twice before tumor challenge by the injection schedule (A). Kaplan–Meier curves (B) showed that the tumor-free survival interval was 24.9 ± 1.2 days in the Hsp70–Mela immunized group, 17.6 ± 0.9 in the Hsp70NTD–Mela immunized group, and 14.9 ± 0.7 days in the control group. Comparison of animal survival curves was performed by log-rank test. Hsp70–Mela versus control (P = 0.0002), Hsp70NTD–Mela versus control (P = 0.043), Hsp70-Mela versus Hsp70NTD–Mela (P = 0.001). (C) Tumor tissue weights of the 3 groups of mice on day 28 after tumor challenge. Each mean tumor weight of a mouse injected with Hsp70N–Mela and Hsp70– Mela decreased significantly compared with the control group (P < 0.001). Results are expressed as the mean ± standard deviation (error bars). ** P < 0.01 versus the control group. Data represent 3 experiments
Hsp70–Mela and Hsp70NTD–Mela induce similar Mela-specific T-cell–mediated immune responses
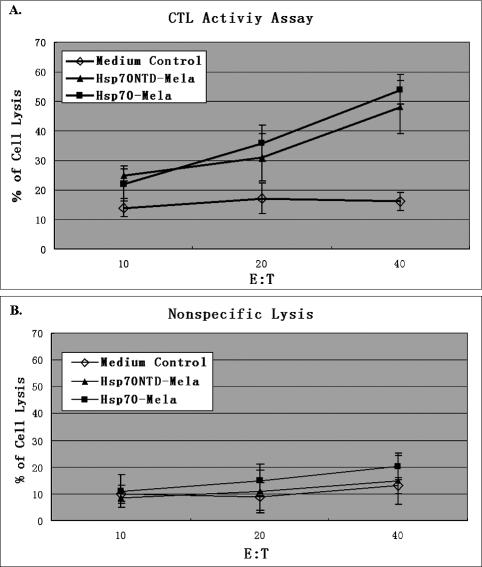
To find out the reason at cell level why Hsp70 fusion protein with Mela has incredibly reduced anti-tumor activity when its C-terminal domain is lost, a cytotoxicity assay was performed to determine the Mela-specific lysis of B16 by CTLs induced by immunization with Hsp70– Mela or Hsp70NTD–Mela. The splenic lymphocytes of immunized mice were pooled 1 week after a booster with recombinant protein, and the cytotoxicity on B16 was assayed after coculture of the splenic lymphocytes with B16 cells for 5 days. The lysis rate by Mela-specific CTLs from mice immunized with Hsp70–Mela was similar to that with Hsp70NTD–Mela; both were greater than that from mice immunized with medium control (P < 0.01; Fig 4A) and the 2 groups were not significantly different. The nonspecific lysis by the splenic lymphocytes was then assayed with LLC as the target cells because LLC did not express Mela (Fig 1) and has the same haplotype (H-2b) with B16. No significant nonspecific lysis was observed (Fig 4B). On the basis of these observations, we concluded that the Hsp70–Mela fusion protein vaccination elicited an in vivo Mela-specific T lymphocyte–mediated immune response, and the C-terminal domain deletion from the fusion protein did not wreck this function remarkably.
Fig 4.
Cytotoxicity T-lymphocyte (CTL) assay. Groups of mice (n = 3) were immunized twice with Hsp70–Mela (filled squares), Hsp70NTD–Mela (filled triangles), or medium control (open diamonds). Mouse splenic lymphocytes were collected 1 week after the boost then stimulated for 5 days with mitomycin C–treated B16 cells. Cytotoxicity was measured by LDH release assay. B16 cells were used as target cells. Stimulated splenocytes were incubated with B16 cells at the indicated effector;th:;thtarget cell ratio (E/T) for 4 hours at 37°C. Similar specific lysis was observed in Hsp70–Mela and Hsp70NTD–Mela immunization groups, either of which is statistically significant compared with the control (A). The nonspecific lysis assay was also performed, in which the Mela-negative LLC acted as target cell. No significant nonspecific lysis was observed (B). Results are expressed as the mean ± standard deviation (error bars)
Hsp70CTD stimulates NK cell proliferation and enhances its tumor cell lysis activity in vivo
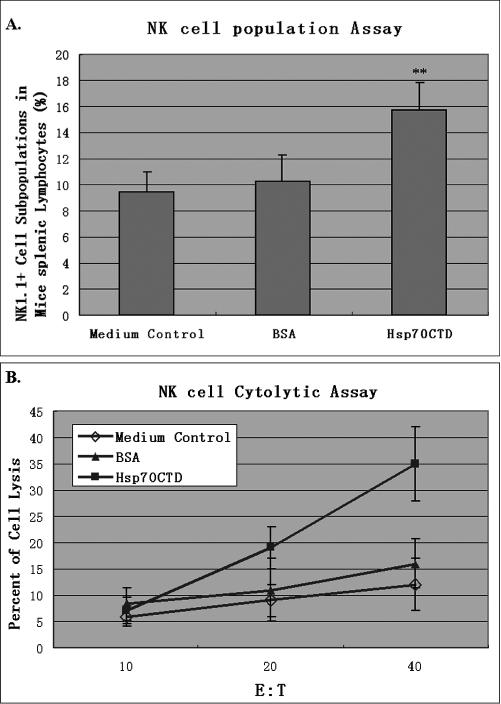
The Mela-specific T lymphocyte–mediated immune response of Hsp70–Mela and Hsp70NTD–Mela was not significantly different in vivo, so we focused on the NK cell, which is thought to provide the first line of defense against infection of microorganisms and the development of malignancy (Tarkkanen et al 1993). Recombinant Hsp70CTD or bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) at a dose of 0.15 nmol was injected into mice twice subcutaneously at a 1-week interval. One week after the boost, spleens were removed from the immunized mice, and NK cell populations in splenocytes were assayed by flow cytometry (the injection schedule seen in Fig 3A). The result showed that NK cell populations greatly increased after injection of Hsp70CTD but not BSA (Fig 5A), and a significantly enhanced tumor lysis activity was observed in a NK cell cytotoxicity assay in the group injected with Hsp70CTD (Fig 5B).
Fig 5.
NK cell stimulatory assay. Groups of C57BL/6 mice (n = 5 per group) were injected with 0.15 nmol of Hsp70CTD (filled squares), BSA (filled triangles), or PBS (open diamonds) subcutaneously twice at 1-week interval. Splenic lymphocytes were collected 1 week after the second injection and subsequently stained with PE-conjugated anti-mouse NK1.1 antibody; the NK cell populations were determined via flow cytometry (A). There is an increased splenic NK cell population in the mice immunized with Hsp70CTD (P < 0.01). NK cell cytotoxicities of the splenic lymphocytes were also determined (B) via LDH release assay, in which YAC-1 cells acted as target cells. The results indicated that splenic lymphocytes from the group of mice injected with Hsp70CTD but not with BSA had significantly increased NK cell cytotoxicities against YAC-1 (P < 0.01). Results are expressed as the mean ± standard deviation (error bars). ** P < 0.01 compared with the control group
Immunization with multiple Hsp70 fusion proteins have a remarkably enhanced anti-tumor effect
Because Hsp70–Mela exhibited a remarkable vaccine effect against B16, we wondered whether immunization with a mixture of multiple Hsp70 fusion proteins would have a better anti-tumor effect than a single Hsp70 fusion protein. Three other Hsp70 fusion proteins, Hsp70– Pmel17, Hsp70–MART-1, and Hsp70–TRP2, were obtained by a method similar to that for preparing Hsp70– Mela, and tumor protection assays were performed with the 4 proteins.
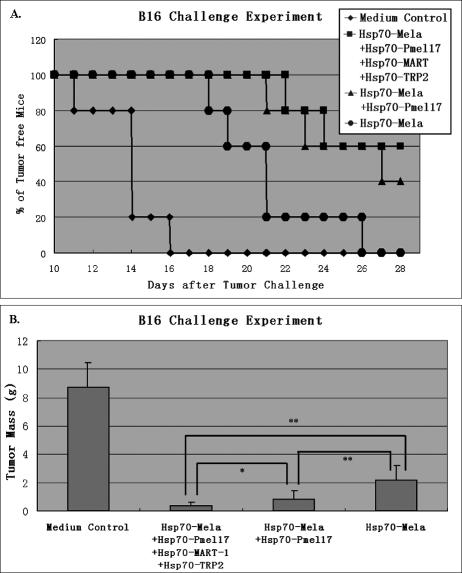
Groups of C57BL/6 mice (n = 5 per group) were immunized with Hsp70–Mela only, a mixture of Hsp70– Mela and Hsp70–Pmel17, or a mixture of 4 Hsp70 fusion proteins (Hsp70–Mela, Hsp70–Pmel17, Hsp70–MART-1, and Hsp70–TRP2) twice before inoculation of 2 × 106 B16 melanoma cells. Each dose of the 4 fusion proteins was 0.15 nmol, and a medium control group was set. Mice were monitored for evidence of tumor incidence every day. Kaplan–Meier curves were generated to analyze the tumor nodule incidence (Fig 6A). Compared with the single Hsp70 fusion protein group, each of the 4 Hsp70 fusion proteins and the 2 Hsp70 fusion proteins had a significantly prolonged tumor-free survival interval. However, the 2 latter groups were not significantly different.
Fig 6.
Suppressive effect of multiple Hsp70 fusion proteins against B16. In the B16 challenge experiment, groups of C57BL/6 mice (n = 5 per group) were immunized with Hsp70–Mela only (filled cycles), a mixture of Hsp70–Mela and Hsp70–Pmel17 (filled triangles), or a mixture of 4 Hsp70 fusion proteins (Hsp70–Mela, Hsp70– Pmel17, Hsp70–MART-1, and Hsp70–TRP2; filled squares) twice before inoculation of 2 × 106 B16 melanoma cells. Each dose of the 4 fusion proteins is 0.15 nmol, and a medium control group (filled diamonds) was set. Kaplan–Meier curves (A) showed that the tumor-free survival interval was 26.0 ± 1.1 days in the 4 Hsp70 fusion protein immunization group, 25.4 ± 1.3 in the 2 Hsp70 fusion protein immunization group, 21.0 ± 1.4 in the Hsp70–Mela immunization group, and 13.8 ± 0.8 days in the control group. Comparison of animal survival curves was performed by log-rank test. The Hsp70– Mela group versus the 2 Hsp70 fusion proteins group (P = 0.033), Hsp70–Mela group versus the 4 Hsp70 fusion proteins group (P = 0.020), and the 2 Hsp70 fusion proteins group versus the 4 Hsp70 fusion proteins group (P = 0.57). At day 28 after tumor challenge, B16 melanoma nodules were excised away from the challenged mice and weighed. (B) Each of the 3 groups immunized with Hsp70 fusion proteins had a lower mean tumor weight than control group (P < 0.01). Among the 3 groups, the mean tumor weight of the group immunized with 4 Hsp70 fusion proteins at one time was significantly less than the group immunized with Hsp70–Mela only (P < 0.01) and the group immunized with Hsp70–Mela and Hsp70–Pmel17 (0.01 < P < 0.05), which was lighter than the group immunized with Hsp70–Mela only (P < 0.01). Results are expressed as the mean ± standard deviation (error bars). * P < 0.01, ** 0.01 < P < 0.05. Data are representative of 2 experiments
All the mice were sacrificed on day 28 after tumor challenge, and tumor tissues were excised and weighed (Fig 6B). As shown in Fig 6B, the group of mice immunized with 2 Hsp70 fusion proteins (Hsp70–Mela and Hsp70– Pmel17) had a lower mean tumor weight than the group of mice only immunized with Hsp70–Mela (P < 0.01), and the group of mice immunized with the 4 Hsp70 fusion proteins simultaneously had the lowest mean tumor weight among these groups (0.01 < P < 0.05, compared with the 2 Hsp70 fusion proteins group, P < 0.01, compared with the Hsp70–Mela group).
A mixture of multiple Hsp70 fusion proteins: a broad-spectrum tumor vaccine
We further investigated the anti-tumor effect of the multiple Hsp70 fusion proteins against tumor cell challenge other than B16 melanoma. A CT26 tumor protection assay was performed in BALB/c mice and LLC in C57BL/6.
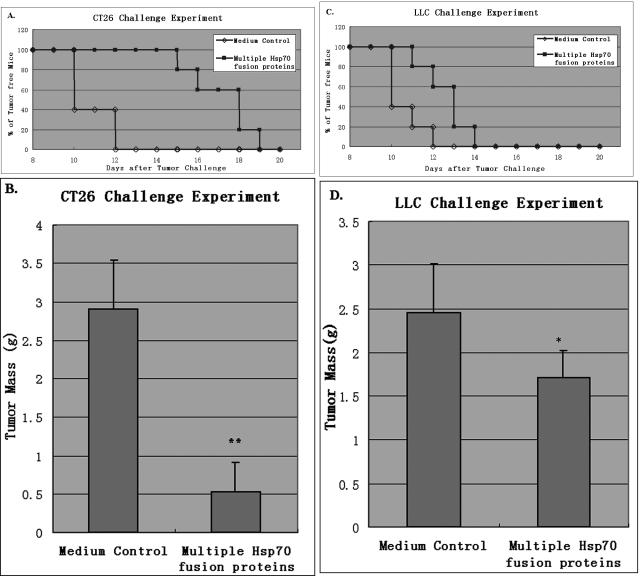
In the CT26 challenge experiment, groups of BABL/c mice (n = 5 per group) were immunized with the mixture of multiple Hsp70 fusion proteins (Hsp70–Mela, Hsp70–Pmel17, Hsp70–MART-1, and Hsp70–TRP2) or PBS twice before inoculation of 1 × 104 CT26 cells. Mice were monitored for evidence of tumor incidence every day. Kaplan–Meier curves were generated, and the log-rank test was used to analyze tumor nodule incidence; the tumor-free survival interval was 17.0 ± 0.6 days in the immunized group and 10.8 ± 0.5 days in the control group (Fig 7A). The difference between the 2 groups was significant (P = 0.002). These mice were sacrificed on day 20 after the tumor challenge, and tumor nodules were removed and weighed. As shown in Figure 7B, the CT26 tumor mass was reduced by 87% in the immunized group compared with the control group (P < 0.01).
Fig 7.
Suppressive effect of multiple Hsp70 fusion proteins against CT26 and LLC. Groups of BABL/c (or C57BL/6) mice (n = 5 per group) were immunized with the mixture of multiple Hsp70 fusion proteins (filled squares) or PBS (opened squares) twice before inoculation of 1 × 104 CT26 (or 5 × 104 LLC) cells. (A) Kaplan–Meier curves were generated to show the tumor-free survival interval in each group of the CT26 challenge experiment: 17.0 ± 0.6 days in the immunized group and 10.8 ± 0.5 days in the control group. The difference between the 2 groups was significant (P = 0.002). These mice were sacrificed on day 20 after the tumor challenge and tumor nodules were removed and weighed. (B) The CT26 tumor mass was reduced by 87% in the immunized group compared with the control group (P < 0.01). (C) Kaplan– Meier curves were generated to show the tumor-free survival interval of each group in the LLC challenge experiment: 12.6 ± 0.5 days in the immunized group and 10.6 ± 0.4 days in the control group. The difference between the 2 groups was significant (P = 0.002). These mice were sacrificed on day 20 after the tumor challenge, and tumor nodules were removed and weighed. (D) The LLC tumor mass was reduced by 29% in the immunized group compared with the control group (0.01 < P < 0.05). Tumor masses are expressed as the mean ± standard deviation (error bars). * P < 0.01, ** 0.01 < P < 0.05. Data are representative of 3 experiments
In the LLC challenge experiment, groups of C57BL/6 mice (n = 5 per group) were immunized with the mixture of multiple Hsp70 fusion proteins or PBS twice before inoculation of 5 × 104 LLC cells. Mice were monitored for evidence of tumor incidence every day. Kaplan– Meier curves were generated, and the log-rank test was used to analyze tumor nodule incidence; the tumor-free survival interval was 12.6 ± 0.5 days in the immunized group and 10.6 ± 0.4 days in the control group (Fig 7C). The difference between the 2 groups was significant (P = 0.02). These mice were sacrificed on day 20 after tumor challenge, and tumor nodules were removed and weighed. As shown in Figure 7D, LLC tumor mass was reduced by 29% in the immunized group compared with the control group (0.01 < P < 0.05).
DISCUSSION
Hsps are among the most highly conserved molecules of the biosphere (Kiang and Tsokos 1998). They are widely found in eukaryotes and prokaryotes, and their functions as molecular chaperones are essential for life. Hsps were recently found to play important roles in protective immunity (Wells and Malkovsky 2000; Srivastava and Amato 2001; Srivastava 2002). Because of the chaperone activities, Hsps are thought to participate in the presentation of antigen molecules, from which antigen-specific CTL responses are elicited. Recent studies have also shown that when TAA combines with Hsp70 covalently or noncovalently, its ability to elicit antigen-specific CTL in vivo could be dramatically enhanced (Blachere et al 1997; Huang et al 2000; Carlsson et al 2004). It has also been reported that a portion of the Hsp70 ATPase domain (aa281–385) was the critical region inducing CTL response (Huang et al 2000; Carlsson et al 2004).
To determine whether the Hsp70 ATPase domain is sufficient to act as a tumor vaccine when it is covalently combined with TAA, we constructed a recombinant protein fusing Mela to the C terminus of the Hsp70 N-terminal ATPase domain and a comparable recombinant protein fusing Mela to the C terminus of the full-length Hsp70. Each vaccine activity of the 2 fusion proteins was tested in this work. Hsp70NTD–Mela exhibited a relatively small anti-tumor effect against B16 melanoma challenge, whereas Hsp70–Mela acted as a potent vaccine. In the Mela-specific CTL assay, no significant difference was observed between the groups of Hsp70–Mela and Hsp70NTD–Mela. These results demonstrated that Hsps achieved their anti-tumor function not only via eliciting antigen-specific CTLs, but also via some other antigen-independent mechanism in which Hsp70CTD was indispensable.
Because the absence of the Hsp70 C-terminal domain resulted in the weakening of Hsp70 fusion protein anti-tumor activity, we were interested in the interaction between the Hsp70 C-terminal domain and the immunity system. In this work, we investigated Hsp70CTD for its effect on the NK cell, which is considered to play important roles in the immunologic control of infection (Multhoff 2002) and to have an ability to spontaneously lyse tumor cells (Karre et al 2005). Our result proved that injection of Hsp70CTD could increase NK cell population and enhance its cytolytic activity. Our result indicated that NK cell stimulation might be another important mechanism in which Hsps delay tumor incidence and suppress tumor growth. Hsps have also been shown to bind with cell surface receptors, stimulating cells to secrete cytokines (Binder et al 2004). It is possible that Hsp70CTD is critical in binding some of the receptors of which downstream signals are indispensable in tumor suppression. The mechanisms mentioned above can partly explain our observation that the absence of the Hsp70 C-terminal domain resulted in the weakening of Hsp70 fusion protein anti-tumor activity. However Hsp70CTD alone is not sufficient for suppressing tumor cell growth in vivo (data not show). We believed that Hsp70 fusion proteins elicited antigen-independent immune responses against tumor cells (Fig 7C); Hsp70CTD was essential but not sufficient to elicit them. NK cell stimulation was only one aspect of the responses. The anti-tumor mechanism of Hsp70 fusion proteins is more intricate than we supposed.
In this work, we also constructed 3 other Hsp70 fusion proteins containing TAAs (MART-1, Pmel17, and TRP2, each of which expresses in the B16 melanoma cell). Although single immunizations of Hsp70–Mela resulted in significant B16 melanoma growth delays, simultaneous immunization of the 4 Hsp70 fusion proteins further delayed the tumor nodule incidences and reduced tumor weights. It seems that the more Hsp70 fusion proteins used to immunize, the better the anti-tumor effect. Our observations confirm the prevalent hypothesis on the mechanism that it is a large and complex array of cancer-specific antigenic peptides bound to Hsps that leads to potent anti-tumor activity of Hsp preparations (Srivastava and Amato 2001).
When we applied the mixture of multiple Hsp70 fusion proteins to other tumor cell challenges (CT26 and LLC), the results were different between the 2 tumor challenge experiments. Although obvious tumor growth delays were observed in both tumor challenge experiments, the CT26 tumor mass was reduced by 82% in the immunized group compared with the control group, whereas the LLC tumor mass was reduced by only 29%. We had investigated the genotype of CT26 and LLC. None of the mRNA of the 4 TAAs were detected in LLC by the RT-PCR method, but the mRNA of Mela was detected in CT26 (Fig 1), which was consistent with some other reports (Bloom et al 1997; Bronte et al 2003). As a Mela expressing cell, the CT26 cell would be attacked by Mela-specific T lymphocytes, but vaccination with the multiple Hsp70 fusion proteins cannot induce any CTLs against LLC cells. Some antigen-independent mechanisms, including NK cell–mediated tumor cell lysis, must be stimulated by the Hsp70 fusion proteins. On the basis of our observations, we speculate that a single Hsp70 fusion protein with TAA could greatly delay the TAA-expressing tumor incidence, that a mixture of Hsp70 fusion proteins further the anti-tumor effect and broaden the spectrum, and that the more Hsp70 fusion proteins adopted, the better the effect and the broader the range of targets. Our work points out an approach to produce potent and broad-spectrum tumor vaccines on a large scale by biosynthesizing recombinant fusion proteins.
REFERENCES
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697.1078-8956(2000)006[0435:HSCPTA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Belli F, Testori A, and Rivoltini L. et al. 2002 Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 20:4169–4180. [DOI] [PubMed] [Google Scholar]
- Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x.0001-2815(2004)064[0442:THPRSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blachere NE, Li ZH, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315.0022-1007(1997)186[1315:HSPCRI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MB, PerryLalley D, Robbins PF, Li Y, ElGamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453.0022-1007(1997)185[0453:IOTPAA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Cingarlini S, and Apolloni E. et al. 2003 Effective genetic vaccination with a widely shared endogenous retroviral tumor antigen requires CD40 stimulation during tumor rejection phase. J Immunol. 171:6396–6405. [DOI] [PubMed] [Google Scholar]
- Carlsson B, Totterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigenTARP. Prostate. 2004;61:161–170. doi: 10.1002/pros.20091.0270-4137(2004)061[0161:GOCTLS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437.0022-1007(1999)189[1437:TDNOSI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldweg AM, Srivastava PK. Molecular heterogeneity of tumor rejection antigen heat-shock protein Gp96. Int J Cancer. 1995;63:310–314. doi: 10.1002/ijc.2910630227.0020-7136(1995)063[0310:MHOTRA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Matsubara H, and Yokota T. et al. 1992 Molecular-cloning and characterization of the gene encoding mouse melanoma antigen by cDNA library transfection. J Immunol. 149:1223–1229. [PubMed] [Google Scholar]
- Huang Q, Richmond JFL, Suzue K, Eisen HN, Young RA. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4(+) T cell independent. J Exp Med. 2000;191:403–408. doi: 10.1084/jem.191.2.403.0022-1007(2000)191[0403:IVCTLE]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy (Reprinted from Nature 1986; 319: 675–678) J Immunol. 2005;174:6566–6569. doi: 10.1038/319675a0.0022-1767(2005)174[6566:SROHLV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human-melanoma antigen recognized by the majority of HLA-A2–restricted tumor-infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347.0022-1007(1994)180[0347:IOTIPO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Hsu C, and Mondesire W. et al. 2001 Immunization against endogenous retroviral tumor-associated antigens. Cancer Res. 61:7920–7924. [PMC free article] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x.0163-7258(1998)080[0183:HSPKMB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443.0066-4154(1986)055[1151:THR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Coppa J, and Carrabba MG. et al. 2003 Vaccination with autologous tumor-derived heat-shock protein Gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 9:3235–3245. [PubMed] [Google Scholar]
- Menoret A. Purification of recombinant and endogenous Hsp70s. Methods. 2004;32:7–12. doi: 10.1016/s1046-2023(03)00180-4.1046-2023(2004)032[0007:PORAEH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperth. 2002;18:576–585. doi: 10.1080/0265673021000017109.0265-6736(2002)018[0576:AONKCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, and Irvine KR. et al. 1998 gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 188:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MA, Srivastava PK, Oettgen HF, Deleo AB. Expression of a shared tumor-specific antigen by 2 chemically-induced BALB/C sarcomas. Cancer Res. 1987;47:5074–5079.0008-5472(1987)047[5074:EOASTA]2.0.CO;2 [PubMed] [Google Scholar]
- Parmiani G, Belli F, and Testori A. et al. 2001 Vaccination with autologous heat-shock protein peptide complex-96 (HSPPC-96, oncophage (R)) in metastatic melanoma. Clin Cancer Res. 7:3814S–3814S. [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749.1474-1733(2002)002[0185:ROHPII]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/s0264-410x(00)00492-8.0264-410X(2001)019[2590:HSPTSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Das MR. The serologically unique cell-surface antigen of Zajdela ascitic hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984;33:417–422. doi: 10.1002/ijc.2910330321.0020-7136(1984)033[0417:TSUCAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava PK, Deleo AB, Old LJ. Tumor rejection antigens of chemically-induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407.0027-8424(1986)083[3407:TRAOCS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue K, Zhou XZ, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146.0027-8424(1997)094[13146:HSFPAV]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117.0193-4511(1997)278[0117:IOTWAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tarkkanen J, Kosunen TU, Saksela E. Contact of lymphocytes with Helicobacter-pylori augments natural-killer-cell activity and induces production of gamma-interferon. Infect Immun. 1993;61:3012–3016. doi: 10.1128/iai.61.7.3012-3016.1993.0019-9567(1993)061[3012:COLWHA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x.0019-2805(2003)110[0001:FOHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H, Levey DL, Srivastava PK. Cellular-requirements for tumor-specific immunity elicited by heat-shock proteins—tumor rejection antigen-gp96 primes CD8+ T-cells in-vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077.0027-8424(1994)091[3077:CFTIEB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Heat-shock protein-70 associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391.0022-1007(1993)178[1391:HPAPES]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, Hsp90, And Hsp70. J Immunol. 1994;152:5398–5403.0022-1767(1994)152[5398:COTIOS]2.0.CO;2 [PubMed] [Google Scholar]
- Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol. 2001;13:1233–1242. doi: 10.1093/intimm/13.10.1233.0953-8178(2001)013[1233:GOCTLB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ullrich SJ, Robinson EA, Law LW, Willingham M, Appella E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc Natl Acad Sci USA. 1986;83:3121–3125. doi: 10.1073/pnas.83.10.3121.0027-8424(1986)083[3121:AMTTAI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Appella E, Kawakami Y, Kang XQ, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207.0022-1007(1996)184[2207:IOTAAH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490.0022-1767(2001)166[0490:COHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wells AD, Malkovsky M. Heat shock proteins, tumor immunogenicity and antigen presentation: an integrated view. Immunol Today. 2000;21:129–132. doi: 10.1016/s0167-5699(99)01558-3.0167-5699(2000)021[0129:HSPTIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang W, Li Q, Huang W. Fusion protein of ATPase domain of Hsc70 with TRP2 acting as a tumor vaccine against B16 melanoma. Immunol Lett. 2006;105:167–173. doi: 10.1016/j.imlet.2006.02.004.0165-2478(2006)105[0167:FPOADO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]