Abstract
Mammalian responses to bacterial lipopolysaccharide (LPS) from the outer membrane of Gram-negative bacteria can lead to an uncontrolled inflammatory reaction that can be deadly for the host. We checked whether heat shock protein 70 (Hsp70) protein is able to protect animals from the deleterious effects of bacterial LPS by monitoring the effect of exogenous Hsp70 injections before and after LPS administration. Our research with rats demonstrates for the first time that administration of exogeneous Hsp70 before and after LPS challenges can reduce mortality rates and modify several parameters of hemostasis and hemodynamics. Hsp70 isolated from bovine muscles showed significant protective effects against the impaired coagulation and fibrinolytic systems caused by LPS, and reduced the mortality caused by Escherichia coli and Salmonella typhimurium LPS injections significantly. Characteristically, Hsp70 preparations used in the experiments result in different effects when administered before and after an LPS challenge, and the effects of Hsp70 injections also differ significantly depending on the origin of the LPS (E coli vs S typhimurium). Based on our data, mammalian Hsp70 appears to be an attractive target in therapeutic strategies designed to stimulate endogenous protective mechanisms against many deleterious consequences of septic shock by accelerating the functional recovery of susceptible organs in humans.
INTRODUCTION
Gram-negative bacteria are responsible for 45–60% of sepsis caused by bacterial infection when mixed-organism infections are included. Lipopolysaccharide (LPS) or endotoxin plays a pivotal role in the initiation of a variety of host responses caused by Gram-negative bacterial infection. In spite of the fact that multiple clinical trials exploring various agents that antagonized LPS have been performed, none of them has so far been successful and hospital mortality from sepsis has ranged from 25% to 80% over the last few decades (Angus and Wax 2001). The lungs, kidneys, liver, cardiovascular system, central nervous, and coagulation system are commonly involved in multiple organ dysfunction syndrome (MODS) often observed after Gram-negative bacterial infection (Karima et al 1999).
There is abundant evidence that when in circulation bacterial endotoxins released from host cells activate a systematic inflammatory reaction, a complex of cellular and humoral responses in the host (Van Amersfoort et al 2003). It was also demonstrated that mortality of patients who have 3 or more types of organ failure is particularly high (Riedemann et al 2003), and, therefore, a search for agents that antagonized several sepsis-associated symptoms is needed.
Despite a growing understanding of the molecular mechanisms and pathogenesis of sepsis-induced damage, to the best of our knowledge the known antagonists of endotoxemia usually only partially suppress the manifestation of specific consequences of sepsis such as intravascular coagulation and cardiac dysfunction (Salkowski et al 1997; Karima et al 1999; Kutuzova et al 2001).
Heat-shock proteins (Hsp) are a family of highly conserved proteins that play a role in many physiological processes and protect cells and whole organisms from multiple stressors that would otherwise result in injury and death (Giffard et al 2004; Kiang 2004). Intracellular Hsp70 expression is induced by a wide variety of stimuli including heat, fever, hypoxia, oxygen radicals, endotoxins, cytokines, and heavy metal ions, and this protein shows strong immunotherapeutic potential (Hartl and Hayer-Hartl 2002; Todryk et al 2003; Kiang 2004).
The members of Hsp70 family of stress proteins are cytosolic and nuclear chaperones that prevent the aggregation of denatured or newly synthesized polypeptides and help them to adopt a native conformation. There are several chaperone mechanisms based on the inducible Hsp70 and constitutive Hsc70 members of Hsp70 family. Some of these machines serve for the correction of the structure of misfolded proteins, whereas others involving Hsp70 and Hsc70 with Bag-1 and CHIP proteins are focused at the proteolytic degradation of irreversely damaged polypeptides (Hartl and Hayer-Hartl 2002).
Another particular property of Hsp70 is its protective activity that was proven using numerous experimental systems both in vitro and in vivo. It is generally assumed that, once being induced, Hsp70 by interacting and inhibiting different regulatory pathways is able to protect both cells and the whole organism from a great variety of stressors (Todryk et al 2002; Novoselova et al 2005; Robinson et al 2005; Johnson and Fleshner 2006).
In addition to multiple intracellular functions of Hsp70 family members, there was considerable interest in their interaction with the immune system (Kumaraguru et al 2002; Tsan and Gao 2004). Recent evidence suggests that Hsps can stimulate cytokine production in immune cells by activating monocytes-macrophages similar to the effect of bacterial LPS (Asea et al 2000; Kol et al 2000; Flohe et al 2003). Thus, it was demonstrated that proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were activated by a very small quantity of Hsp70. There are, however, data suggesting that cytokine effects of Hsp70 may be due to contamination of the Hsp70 samples by LPS or/and LPS-associated proteins (Tsan and Gao 2004).
There are an increasing number of studies reporting the presence of Hsp70 in human serum after stress and under normal physiological conditions (Walsh et al 2001; Campisi et al 2003; Hunter-Lavin et al 2004). The evidence is accumulating showing that a marked increase in Hsp70 within peripheral circulation induced by an acute, relatively intense physical stressor or various other stimuli seems to facilitate recovery from bacterial challenge. Extensive studies in the past 10 years suggest that preinduction of Hsp70 may provide therapeutic strategies for Gram-negative sepsis-induced organ or tissue injury in various mammals including humans (Lau et 2000; Bruemmer-Smith et al 2001; Paidas et al 2002). Furthermore, there are data demonstrating Hsp70 induction after LPS administration (Zhang et al 1994; Heine et al 1999). It was also shown that LPS is able to stimulate the formation of Hsp70 and Hsp90 clusters, with Toll-like receptor 4 molecules being 1 of the 2 major LPS-binding receptors (Triantafilou and Triantafilou 2004).
In spite of all of the scattered and sometimes contradictory facts and observations, it is clear that the nature of complex interaction between bacterial endotoxins and Hsps is far from being resolved. So far, clinical anti-LPS or anticytokine trials to block sepsis cascade have not been successful (Karima et al 1999). This might, in part, be the result of delays between the onset of sepsis and the beginning of treatment, and thus, new approaches targeting the mediators of the later stages of sepsis might be more effective.
In the experiments described herein, we have examined the protective effects of exogenous mammalian Hsp70 isolated from bovine muscles with various level of purification on endotoxin-induced damage by studying various clinical symptoms of sepsis induced in rats by intravenous injections of LPS originating from 2 bacteria species (Salmonella typhimurium and Esherichia coli). The data presented here show that Hsp70 with its protective power and ability to bind LPS can be a good candidate for the role of LPS antagonist when delivered to an organism affected by bacterial endotoxin.
MATERIALS AND METHODS
Animal use
Adult male Wistar rats weighing 300–350 g were used in all experiments. The animals were maintained in their home cages in a climate-controlled room with a 12:12 hour light-dark cycle and had free access to water and food. All procedures involving animals were reviewed and approved by the Animal Care and Use Committee of Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry.
Measurement of arterial blood pressure and mortality rate
Animals were anesthetized with chloral hydrate (400 mg/kg, intraperitoneally) and catheterized. Catheters were placed in the inferior vena cava (through the left femoral vein) for administration of drugs and in abdominal aorta (through the left femoral artery) for the monitoring of blood pressure. The experiments were started 24 hour after the implantation. Blood pressure was measured by SR-01 electro manometer. Mean arterial blood pressure (MAP) and heart rate (HR) were monitored before the experiments and during 5 hours after LPS and Hsp70 administration. Rats were randomly assigned to either LPS treatment groups (n = 10) or HSP70 and LPS treatment groups (n = 10). All reagents were dissolved in 0.9% NaCl.
In the first series of experiments, the effect of Hsp70 injection per se (unpurified and LPS-free samples) on various parameters of hemodynamics and hemostasis was monitored. HSP70 isolated from bovine muscle was administered (dose, 266 μg/kg). Animals injected with physiological solution were used as a control.
In the second series of experiments, the antagonizing and therapeutic role of unpurified and LPS-free HSP70 against endotoxemia caused by E coli LPS (Sigma, St Louis, MO, USA) intravenous injections (2 mg/kg) was monitored essentially as described above. HSP70 isolated from bovine muscle was administered 10 minutes before LPS injections (antagonizing role) or 10 minutes after LPS injections (therapeutic role). Animals injected with E coli LPS following physiological solution administration served as a control in this series of experiments. Blood samples were collected as described in order to get serum for endogenous Hsp70 level determination.
In the third series of experiments, in order to assess the antagonizing (preventive) role of unpurified and LPS-free Hsp70 against endotoxemia caused by Salmonella LPS injections, HSP70 isolated from bovine muscle was administered 10 minutes before LPS injections. S typhimurium LPS (Sigma) was administered to rats intravenously (4 mg/kg). Animals injected with Salmonella LPS following physiological solution administration served as a control in this series of experiments. In order to evaluate the possible therapeutic effect of Hsp70 preparation, the reagent (only LPS-free Hsp70 sample was used in this series) was administered 10 minutes after Salmonella LPS injections. The same doses of LPS were used (see above). Mortality of the animals after LPS administration was monitored during 24 hours after the injection.
Proteins isolation and measurements
Hsp70/Hsc70-containing samples used in this study were isolated from bovine red muscle, as described elsewhere, with a few modifications (Guzhova et al 1998). Briefly, extract from bovine muscle in a solution of 20 mM NaCl, 20 mM Tris-HCl, pH 7.5, 0.2 mM ethylenediamine-tetraacetic acid (EDTA), and 0.2 mM dithiothreitol was passed through a diethylaminoethyl-Sepharose column (Amersham, Uppsala, Sweden) followed by elution with 0.35 M NaCl; the eluate fraction was thereafter subjected to affinity chromatography on an adenosine triphosphate (ATP)-agarose gel (Sigma). After the elution, the protein was removed by the addition of 5 mM EDTA, followed by ammonium sulfate precipitation (65% of saturation). After resuspension, the protein was dialyzed against phosphate-buffered saline using Pierce Slide-a-Lyzer Cassettes (Pierce, Rockford, IL, USA). This non-detoxicated preparation was named “Hsp70-ND.” Removal of possible endotoxin contamination was further attained by using polymyxin B-agarose gel, and the resulting LPS-free preparation was named “Hsp70-DT.” Before use the protein solution was sterilized by ultrafiltration with the help of 0.2-μm pore microfilter (Sarstedt, Numbrecht, Germany). The purity of Hsp70/Hsc70 preparations from bovine muscle was confirmed by polyacrylamide gel electrophoresis followed by staining with Coomassie Blue and by the immunoblotting using monoclonal 3B5 anti-Hsp70 and N69 anti-Hsc70 antibodies (Guzhova et al 1997; Demidov et al 1999). Protein concentration was measured according to Bradford's protocol (Bradford 1976).
To measure the levels of Hsp70, blood samples were subjected to the analysis with the aid of a novel diagnostic developed by the authors (B.A.M., I.G.), Russian Patent N 2242764. It is based on the high affinity of Hsp70 to immobilized ATP. ATP was conjugated with the ovalbumin and the latter was immobilized on the surface of a 96-well enzyme immunoassay plate (Greiner, Microlon, Germany).
Immobilization was performed at 37°C for 1 hour in buffer T (20 mM Tris-HCl, pH 7.5, 140 mM NaCl, 10 mM MgCl2). Buffer T containing 0.2% Tween 20 (T-Tw) was used for all latter steps and for washes. After blocking of nonspecific binding with T-Tw, calibration standards of pure Hsp70 and cell extracts in T-Tw were applied to the wells. After 1-hour incubation, the wells were washed and anti-Hsp70 rabbit polyclonal antibodies R2 generated in the same laboratory were added, followed by goat anti-rabbit antibody conjugated with peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). o-Phenylenediamine (Sigma) served as the chromogen in reaction with hydrogen peroxide. The reaction was stopped by the addition of 0.2 M sulfuric acid and the optical density was measured at 450 nm using UNIPLAN AIFR-01 reader (ZAO Pikon, Moscow, Russia). Hsp70 quantities were determined using the calibration curve (0.50–250 ng/mL) obtained from the titrated standards.
Monitoring of hemostasis
Blood samples for hemostasis characteristics were taken from an arterial catheter before the experiment and 20 minutes and 5 hours after LPS administration. Blood for coagulometric analysis was collected into the tubes with Na-citrate in 9:1 ratio. The samples were centrifuged as described above to get the plasma and the following parameters were estimated: thromboplastin clotting time, prothrombin clotting time, plasma fibrinogen concentration, and time of fibrinolysis (using a single canal coagulometer, Thrombostat).
Statistics
All data were analyzed statistically using the Statistics for Windows program. Other analysis was carried out using the Wilcoxon test and analysis of variance (ANOVA 2) and the Dunkan range test. Statistical inference is based on levels of significance (P ≤ 0.05).
RESULTS
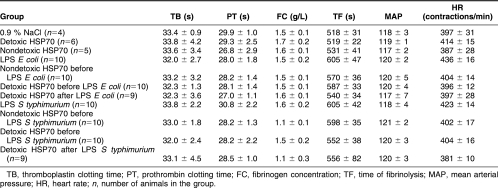
It is evident from Table 1 that animals from all of the experimental groups studied are characterized by similar background parameters of hemostasis and hemodynamics. The data presented in this table were used as a baseline in all comparative studies performed herein.
Table 1.
Background parameters of hemostasis and hemodynamics in Wistar rats used in the study
The effect of administration of various Hsp70 preparations on mortality rate and parameters of hemostasis and hemodynamics in rats
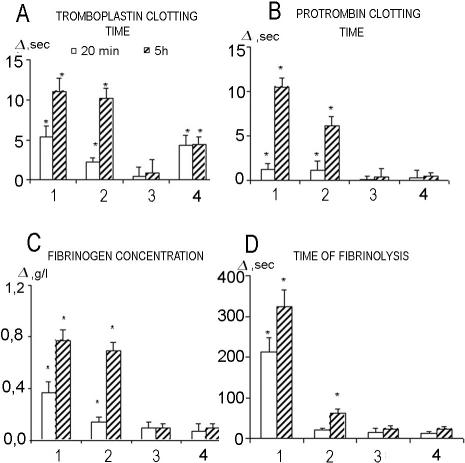
In the first series of experiments, the injection of LPS-free Hsp70-DT did not induce mortality in the animals, whereas unpurified by Polymyxin B Hsp70-ND resulted in the death of 2 animals of 6 tested (Table 2, Fig 1). Figure 2 shows that Hsp70-DT did not influence the hemostasis parameters studied, whereas administration of Hsp70-ND led to their significant increase. It was also shown that, although Hsp70-DT does not influence hemodynamics parameters, the injection of an unpurified sample of Hsp70 resulted in a significant decrease of MAP (hypotension) and increase in HR (Fig 3). Characteristically, in the latter case, a significant decrease in MAP was evident 2.5 hours after the Hsp70 administration, whereas HR increased 2 hours after injection.
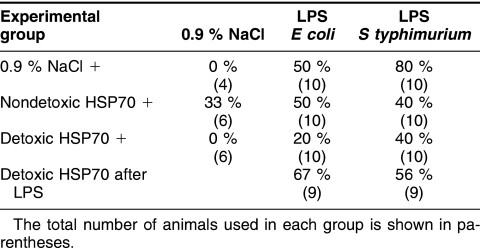
Table 2.
Mortality levels in (in percentage) in different experimental groups
Fig 1.
Effects of Hsp70 on LPS-induced mortality in rats. (A) The influence of Hsp70 preparations administration per se on mortality. 1, NaCl has been injected; 2, Hsp70-ND has been injected; 3, Hsp70-DT has been injected. (B) The influence of Hsp70 preparation administration on E coli LPS-induced mortality. 1, NaCl has been injected 10 minutes before LPS challenge; 2, Hsp70-ND has been injected 10 minutes before LPS challenge; 3, Hsp70-DT has been injected 10 minutes before LPS challenge; 4, Hsp70-DT has been injected 10 minutes after LPS challenge. (C) The influence of Hsp70 preparation administration on S typhimurium LPS-induced mortality. 1, NaCl has been injected 10 minutes before LPS challenge; 2, Hsp70-ND has been injected 10 minutes before LPS challenge; 3, Hsp70-DT has been injected 10 minutes before LPS challenge; 4, Hsp70-DT has been injected 10 minutes after LPS challenge
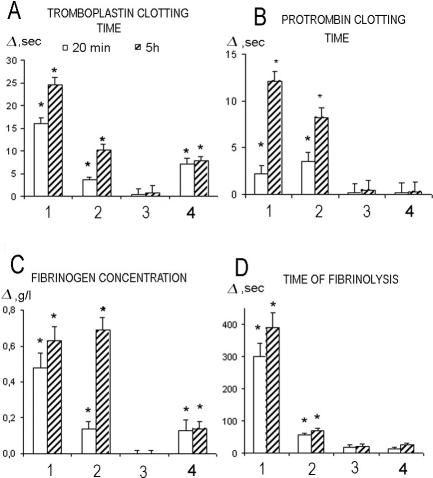
Fig 2.
Changes of hemostasis parameters after administration of Hsp70-ND and Hsp70-DT preparations of Hsp70 (dose, 266 μg/kg). 1, NaCl (0.9%) (n = 4); 2, Hsp70-DT (n = 6); 3, Hsp70-ND (n = 6). *P < 0.05 using Wilcoxon test
Fig 3.
Changes in mean arterial pressure (MAP) (A) and heart rate (B) after administration of Hsp70-ND and Hsp70-DT preparations of Hsp70 (dose, 266 μg/kg). *Indicates statistically significant differences (using ANOVA 2 method) between groups 1 and 3. #Indicates statistically significant differences (using ANOVA 2 method) between groups 2 and 3
Effect of Hsp70 preparations on hemostasis and hemodynamics parameters under conditions of endotoxemia induced by E coli LPS
A second series of experiments showed that injection of E coli LPS led to 50% of mortality in the control (LPS only) group of animals (Table 2, Fig 1B). Prophylactic (preventive) administration of the Hsp70-DT before LPS injection decreases mortality rate to 20%, whereas Hsp70-ND (contaminated with LPS) has no apparent mortality suppression effect. Surprisingly, the administration of LPS-free Hsp70 after E coli LPS injection even slightly increased the mortality rate (up to 67%).
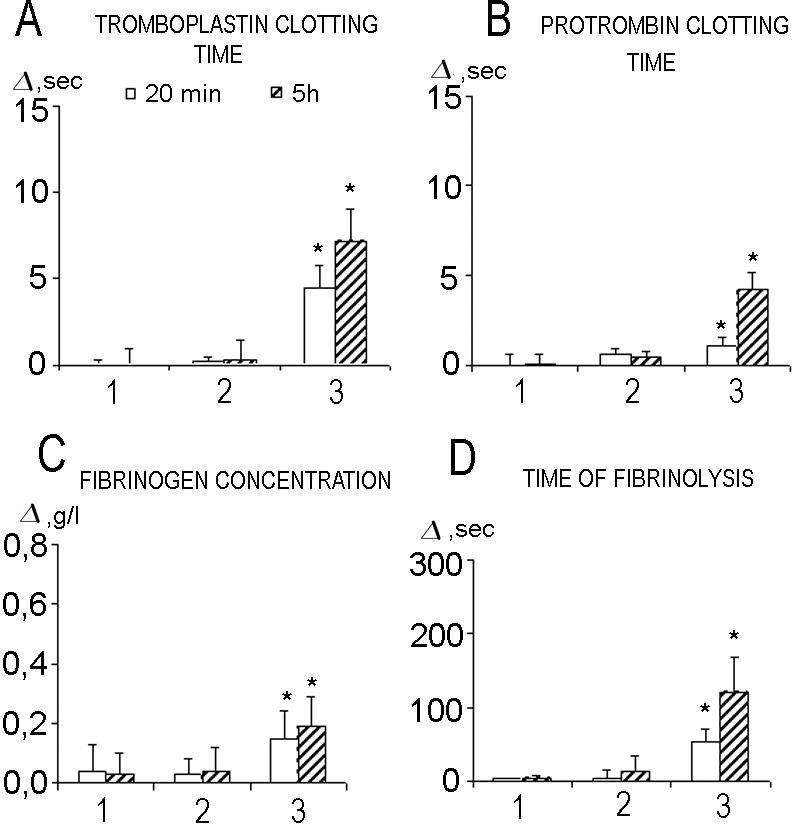
Monitoring hemostasis parameters after the administration of E coli LPS revealed significant increases in all of the parameters studied (Fig 4). Prophylactic administration of Hsp70-ND resulted in the decrease of fibrinolysis time and, to a lesser extent, prothrombin clotting time only. It was not effective in decreasing thromboplastin clotting time and fibrinogen concentration. On the other hand, prophylactic administration of Hsp70-DT effectively normalized (decreased) all hemostasis parameters otherwise increased due to E coli LPS administration (Fig 4). LPS-free Hsp70, injected after E coli LPS administration (therapeutic action), was also able to normalize most of the hemostasis parameters, thromboplastin clotting time being an exception. Thus, 20 minutes after E coli LPS injection, a significant increase in thromboplastin clotting time was evident independent of Hsp70-DT administration. The characteristic feature of thromboplastin clotting time kinetics in animals injected by Hsp70-DT after E coli LPS administration is the preservation of the same (increased) level of this parameter observed in 20 minutes after LPS administration for at least 5 hours (Fig 4). On the other hand, the increase of thromboplastin clotting time in the case when only E coli LPS was administrated was significantly higher at 5-hour point (Fig 4). The phenomenon observed implies hypocoaglutination, intravessel clotting time. This increase was still evident 5 hours after LPS administration.
Fig 4.
Changes of hemostasis parameters due to administration of E coli LPS (dose, 2 mg/kg) depending on concomitant introduction of Hsp70 preparations. 1, E coli LPS (n = 10); 2, Hsp70-ND plus E coli LPS (n = 10); 3, Hsp70-DT plus E coli LPS (n = 10); 4, E coli LPS plus Hsp70-DT (n = 9). *P < 0.05 using Wilcoxon test
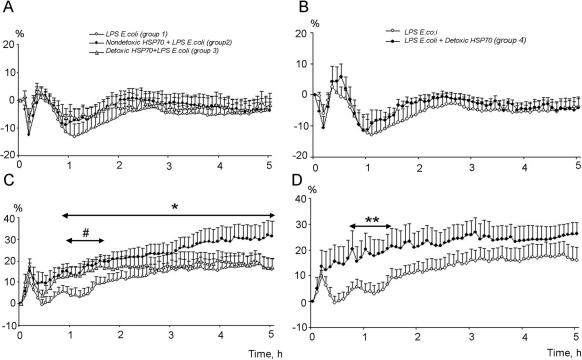
Intravenous E coli LPS injection resulted in a significant decrease (hypotension) of MAP with the following time course (Fig 5A): the original drop by 5.9% in comparison with the baseline value, with a tendency to increase after 20 minutes, followed by a second phase of hypotension occurring starting at 45 minutes and reaching maximal drop at 65 minutes (Fig 5A). Preventive administration of Hsp70-DT did not have a significant effect on MAP (Fig 5A).
Fig 5.
Patterns of mean arterial pressure (MAP) (A) and heart rate (B) after administration of E coli LPS only (group 1) and resulting from prophylactic action of Hsp70-ND (group 2) and Hsp70-DT (group 3); and patterns of MAP (C) and heart rate (D) after administration of E coli LPS (group 4) and after therapeutic administration of Hsp70-DT introduced after E coli LPS injection (group 5). *Indicates statistically significant differences (using ANOVA 2 method) between groups 1 and 2. #Indicates statistically significant differences (using ANOVA 2 method) between groups 1 and 3. **Indicates statistically significant differences (using ANOVA 2 method) between groups 4 and 5
It is evident from Figure 5, C and D, that E coli LPS injections resulted in a 2-phasic increase in HR. The first phase is comparatively short: the HR increased by 10 within the first 10 minutes and normalized at the 20-minute point. The normal level of HR is preserved till the 80-minute point, followed by another peak of increased HR, which lasts for 5 hours (Fig 5D).
Hsp70-DT introduced before E coli LPS significantly modified the HR pattern, and, to some extent, increased the tachycardia level (Fig 5C). On the other hand, preventive administration of this preparation does not influence tachycardia kinetics significantly (Fig 5B). Interestingly, in the experiments where therapeutic administration of Hsp70-DT (after LPS injection) was carried out, we failed to observe the 2-phase increase in HR evident in animals injected with E coli LPS only. Tachycardia in this series of experiments develops during the first 10 minutes and lasts during the whole period of registration (Fig 5D).
The effect of Hsp70 preparations on hemostasis and hemodynamics parameters in rats under conditions of endotoxemia induced by S typhimurium LPS
Four groups of animals were used in the experiments exploring Salmonella LPS. The first group was injected with LPS (dose = 4 mg/kg); in the second group the same dose of LPS was injected 10 minutes after administering Hsp70-ND (266 μg/kg); in the third group the LPS was injected 10 minutes after the administration of Hsp70-DT (266 μg/kg); finally, in the fourth group Hsp70-DT was injected 10 minutes after therapeutic LPS administration.
In the first control group where LPS of S typhimurium was used, an 80% mortality rate was detected (Fig 1, Table 2). However, the rate decreased and reached 40% after prophylactic Hsp70 injection of both forms of Hsp70 preparation (groups 2 and 3). Therapeutic injection of Hsp70 (group 4) also results in mortality rate decrease (up to 56%).
Intravenous administration of LPS isolated from Salmonella resulted in a significant increase in all hemostasis parameters monitored, similar to the pattern described above when E coli LPS was applied. The prophylactic administration of Hsp70-ND significantly reduced (normalized) all hemostasis values, with fibrinogen concentration being an exception (Fig 6). Characteristically, prophylactic administration of Hsp70-DT preparation resulted in complete prevention of all hemostasis parameters perturbations induced by Salmonella LPS injection (Fig 6). Therapeutic administration of this Hsp70 preparation also has complete protective effect in terms of prothrombin clotting time and time of fibrinolysis, and significantly reduced thromboplastin clotting time and fibrinogen concentration in comparison with group 1 (Fig 6).
Fig 6.
Changes of hemostasis parameters due to administration of S typhimurium LPS (dose, 4 mg/kg) depending on concomitant introduction of Hsp70 preparations. 1, S typhimurium LPS (n = 10); 2, Hsp70-ND plus S typhimurium LPS (n = 10); 3, Hsp70-DT plus S typhimurium LPS (n = 10); 4, S typhimurium LPS plus Hsp70-DT (n = 9). *P < 0.05 using Wilcoxon test
Administration of S typhimurium LPS per se characteristically changed the MAP pattern. The original rise of BP by 6.0 (=2%) in 20 minutes after the injection was followed by subsequent progressive drop of the average value and restoration of the original level (Fig 7A).
Fig 7.
Changes in mean arterial pressure (MAP) (A) and heart rate (B) after administration of S typhimurium LPS (group 1) and after preliminary administration of Hsp70-ND (group 2) and Hsp70-DT (group 3); and patterns of MAP (C) and heart rate (D) after administration of S typhimurium LPS (group 4) and after therapeutic administration of Hsp70-DT introduced 10 minutes after S typhimurium LPS injection (group 5). *Indicates statistically significant differences (using ANOVA 2 method) between groups 1 and 3. **Indicates statistically significant differences (using ANOVA 2 method) between groups 4 and 5
Prophylactic administration of Hsp70-ND does not influence the patterns of MAP observed after LPS injections, whereas prophylactic administration of LPS-free preparation surprisingly even led to higher degree of hypotension (Fig 7A).
Significant increase in HR value after the injection of S typhimurium LPS was observed with the peak at 255 minutes in comparison with the original value. In general, administration of Hsp70-ND before LPS injection did not significantly affect MAP and HR parameters. On the other hand, Hsp70-DT introduced before LPS resulted in 2-phase pattern of tachycardia development and in general the level of tachycardia in these animals was lower in comparison with the group 1 where LPS only was injected (Fig 7B). Interestingly, Hsp70-DT administrated after LPS injections resulted in 30-minute delay in tachycardia development (Fig 7D).
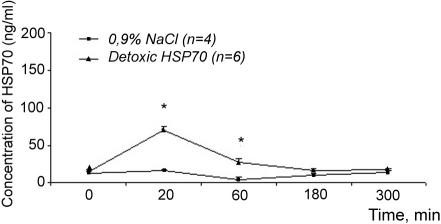
For the interpretation of our results, it was important to monitor the level of Hsp70 preparations injected into the experimental animals. The results of such analysis are depicted in Figure 8.
Fig 8.
Concentration of Hsp70 in the blood of experimental Wistar rats after administration of exogenous Hsp70 at the dose of 266 μg/ kg. *Significant differences between control (NaCl) and experimental groups
It is evident that concentration of endogenous Hsp70 in the blood of rats used in the analysis is very low and reaches only 10–15 ng/mL. Intravenous administration of Hsp70 preparations at the dose of 266 μg/kg into a rat with the weight of 300 g should increase the original concentration of Hsp70 up to 3000 ng/mL. However, in 20 minutes after injections, the concentration of hsp70 in the blood reached only 70 ng/mL (2% from theoretically calculated value) and in 60 minutes, only 30 ng/mL. Therefore, probably more than 90% of injected Hsp70 is eliminated from the blood within the first 20 minutes after injection. Recently, we (B.A.M., I.G.) have obtained similar data on Hsp70 kinetics in rats subjected to moderate hypoxia: Hsp70 appeared in blood flow within 30 minutes after the treatment and virtually disappeared during the next 15–20 minutes (personal communications). Rapid disappearance of Hsp70 from the blood flow has been also documented by other authors (eg, Fleshner and Johnson, 2005).
DISCUSSION
Injection of endotoxin (LPS) into mammals is a widely applied model of sepsis to study early host responses to acute systematic inflammation, resulting in the release of cytokines, which modify gene expression and induce fever, increase cardiac output, change arterial pressure, and alter various blood characteristics (Pajkrt et al 1997; Karima et al 1999).
In recent years, several investigations were performed to determine the role of intracellular Hsps in modifying both cellular and whole-body responses of various mammals to LPS injections (Koh et al 1999; Meldrum et al 1999; Ding et al 2001). Endogenous Hsp70 was proven to have inhibitory effects on cytokine release and modulate the fibrinolytic and coagulation systems during endotoxemia (Ding et al 2001; Suganuma et al 2002; Nakada et al 2005). The effect of (hsp) on the consequences of LPS administration has usually been studied by either induction of Hsps by heat treatment and other stimuli or by using inhibitors and inducers of certain Hsps (Ding et al 2001; Paidas et al 2002; Vega and De Maio 2003).
It is well known that cytokines released from monocytes and macrophages are major mediators of inflammation. It was shown that LPS-induced increases in the production of TNF-α, IL-1β, IL-10, and IL-12 in cells were significantly inhibited by the overexpression of Hsp70 (Ding et al 2001).
In the present study, we investigated various protective effects of exogenous Hsp70 injections from LPS challenge in a rat model. It is necessary to emphasize that there is a growing body of evidence suggesting that, under various conditions, Hsps are released from peripheral blood mononuclear cells (Guzhova et al 2001; Walsh et al 2001; Hunter-Lavin et al 2004; Johnson and Fleshner 2006). It was also shown that extacellular Hsps are important in the immune response, and cells can absorb and utilize Hsps from culture media (Guzhova et al 2001; Wallin et al 2002; Johnson and Fleshner 2006). Apparently, extracellular Hsp70 (eHsp70) acts as a danger signal to potentiate the nitric oxide (NO) response to bacterial challenge and facilitate recovery from bacterial inflammation (Campisi et al 2003). On the other hand, recent evidence suggests that the reported cytokine effects of Hsps may be due to the contaminating LPS and LPS-associated molecules (Tsan and Gao 2004).
In a way our data confirmed this conclusion, because unpurified Hsp70 preparation has essentially the same enhancing effect on all hemostasis parameters as administration of LPS per se isolated from both bacteria (Figs 2, 4, 6). However, in all cases the increase was significantly higher when LPS per se was used. We also found that in most cases preventive introduction of Hsp70-ND did not significantly modify the observed increase of hemostasis parameters, with time of fibrinolysis being an exception (Fig 2).
On the other hand, all effects observed after injection of Hsp70-ND cannot be attributed to LPS contamination. Thus, such preparations of Hsp70 induced significant and consistent decrease in MAP level (hypotension) observed during at least 5 hours (Fig 3A), whereas administration of E coli LPS per se lead to the initial drop of this value (at 20-minute point) with subsequent restoration to the normal level (Fig 5A). In the group where S typhimurium LPS was used, a significant increase of this value at the same time point was observed in all variants (Fig 7A). Regarding heart rate, preventive administration of Hsp70-DT either had no visible effect or resulted in partial normalization of this parameter. On the other hand, therapeutic application of Hsp70-DT in the case of E coli LPS even led to a significant increase in tachycardia level (Fig 5D). Characteristically, therapeutic application of Hsp70-DT contrary to preventive use was not effective in decreasing mortality level and even slightly enhanced this parameter when E coli LPS was used. The therapeutic application of Hsp70 preparation might not be relevant in improving LPS-induced mortality, because the cascade of inflammatory mediators is already underway when intervention is started. These observations suggest that protective effect of Hsp70 preparations is apparently realized by mobilizing the innate immune effect (danger signal) rather than by simple absorption of LPS from the blood due to the high affinity of Hsp70 to LPS.
The data accumulated herein strongly support the conclusion that the effects observed were specific to exogenous Hsp70 and were not due to endotoxin contamination of the protein.
It is well known that LPS may induce hypotension and tachycardia in model organisms (Lin et al 1999; Van Amersfoort et al 2003). In our experiments, the reaction of rats to LPS of 2 bacteria compared in terms of hemodynamics parameters was not identical. Thus, E coli LPS injections resulted in hypotension and tachycardia, whereas S typhimurium LPS lead to the development of hypertension and tachycardia (Figs 5, 7). Furthermore, although the patterns of interaction of injected Hsp70 with both LPS was rather similar, a few characteristic differences were also detected (eg, Figs 4, 6). Along these lines, it was recently demonstrated that in vitro stimulation of rat splenocytes and macrophages with eHsp70 elevated NO, tumor necrosis factor-α, and various interleukins. Stimulation of cells with Hsp70 combined with LPS resulted in a greater NO and cytokine response than that observed after stimulation with eHsp70 or LPS alone. It was also demonstrated that stressor exposure elevates eHsp70, potentiates E coli–induced NO at the inflammatory site, and facilitates recovery from bacterial infection (Campisi et al 2003).
We cannot speculate at the present time about the major cause of death after LPS administration in our model or at what molecular level the protective action of Hsp70 is realized, primarily because sepsis affects multiple characters and launches a cascade of events at the cellular and organism level (Karima et al 1999; Angus and Wax 2001). Correlation analysis using all hemostasis and hemodynamics parameters and mortality revealed the best correlation between thromboplastin clotting time and mortality level (coefficient of correlation, 0.71). The data on therapeutic effect of LPS-free preparations also confirm this conclusion. Thus, it is evident that thromboplastin clotting time is the only parameter that was not normalized by therapeutic Hsp70 injections that were found ineffective in increasing survival rate after administering LPS of E coli. However, it is evident that other effects inflicted by administering LPS may also play an important role in increasing the mortality rate.
Although this is the first demonstration that administering exogeneous Hsp70 before and after an LPS challenge can reduce the mortality rate and modify several parameters of hemostasis and hemodynamics, the current studies did not address cellular and molecular signals responsible for the protective role of the Hsp70 preparations used. It is noteworthy that previously eHsp70 has been shown to be effective against various types of stress at the cellular and organism level (Flerov et al 2003; Novoselova et al 2005; Robinson et al 2005). Herein, we concentrated on the clinical manifestations of the sepsis, which were significantly modified due to Hsp70 administration.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research, Projects 06-04-48993, 06-04-48854, and 04-04-49237, and grants of the Russian Academy of Sciences (Molecular and Cellular Biology Program) to M.B.E., B.A.M., and V.L.K. We thank Prof. Martin G. Feder from the University of Chicago for critical reading of the manuscript.
REFERENCES
- Angus DC, Wax RC. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:109–116. doi: 10.1097/00003246-200107001-00035.0090-3493(2001)029[0109:EOSAU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft S, and Kurt-Jones EA. et al. 2000 HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 6:435–442. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999.0003-2697(1976)072[0248:ARASMF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bruemmer-Smith S, Stuber F, Schroeder S. Protective functions of intracellular heat-shock protein (HSP) 70-expression in patients with severe sepsis. Intensive Care Med. 2001;27:1835–1841. doi: 10.1007/s00134-001-1131-3.0342-4642(2001)027[1835:PFOIHP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TE, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2.1466-1268(2003)008[0272:SEHIAF]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidov ON, Tyrenko VV, Svistov AS, Komarova EY, Karpishenko AI, Margulis BA, Shevchenko YL. Heat shock proteins in cardiosurgery patients. Eur J Cardiothorac Surg. 1999;16:444–449. doi: 10.1016/s1010-7940(99)00291-2.1010-7940(1999)016[0444:HSPICP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ding XZ, Fernandez-Prada CM, Bhattacharjee AK, Hoover DL. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959.1043-4666(2001)016[0210:OOHIBL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Flerov MA, Ordyan NE, Margulis BA, Guzhova IV, V'yushina AV, Pivina SG, Gerasimova IA. The use of HSP70 for prevention of consequences of unavoidable stress in rats. Bull Exp Biol Med. 2003;136:120–122. doi: 10.1023/a:1026394218471.0007-4888(2003)136[0120:TUOHFP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signals and function. Int J Hyperthermia. 2005;5:457–471. doi: 10.1080/02656730500088211.0265-6736(2005)005[0457:EEHSPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Flohe SB, Bruggemann J, Lendemans S, Nikulina M, Meierhoff G, Flohe S, Kolb H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol. 2003;170:2340–2348. doi: 10.4049/jimmunol.170.5.2340.0022-1767(2003)170[2340:HHSPIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Giffard RG, Xu L, and Zhao H. et al. 2004 Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 207:3213–3220. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Arnholdt AC, and Darieva ZA. et al. 1998 Effects of exogenous stress protein 70 on the functional properties of human promomocytes through binding to cell surface and internalization. Cell Stress Chaperones. 3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-KB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2.1466-1268(1997)002[0132:MSPHIW]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova IV, Kislyakova KA, Moskaliova OS, Fridlanskaya II, Tytell M, Cheetham M, Margulis BA. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3.0006-8993(2001)914[0066:IVSSTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408.0193-4511(2002)295[1852:MCITCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heine H, Delude RL, Monks BG, Espevik T, Golenbock DT. Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J Biol Chem. 1999;274:21049–21055. doi: 10.1074/jbc.274.30.21049.0021-9258(1999)274[21049:BLIEOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davis EL, Bacelar M, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. BBRC. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075.0006-291X(2004)324[0511:HRFPBM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukocyte Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523.0741-5400(2006)079[0425:RSSPAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endogenous shock and organ failure. Mol Med Today. 1999;5:123–132. doi: 10.1016/s1357-4310(98)01430-0.1357-4310(1999)005[0123:TMPOES]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kiang JG. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell Res. 2004;14:450–459. doi: 10.1038/sj.cr.7290247.1001-0602(2004)014[0450:IHSPKA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Koh Y, Lim C, and Kim M. et al. 1999 Heat shock response decreased endotoxin-induced acute lung injury in rats. Respirology. 4:325–330. [DOI] [PubMed] [Google Scholar]
- Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13.0022-1767(2000)164[0013:CEHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kumaraguru U, Gierynska M, Norman S, Bruce BD, Rouse BT. Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J Virol. 2002;76:136–141. doi: 10.1128/JVI.76.1.136-141.2002.1098-5514(2002)076[0136:IWCCIL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, Albrecht RM, Erickson CM, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides blocks the binding and internalization of lipopolysaccharide in RAW. J Immunol. 2001;167:482–489. doi: 10.4049/jimmunol.167.1.482.0022-1767(2001)167[0482:DLAFRS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. 2000. Am J Physiol. 2000;278:H1439–H1445. doi: 10.1152/ajpheart.2000.278.5.H1439.0002-9513(2000)278[H1439:PAEBHI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lin HC, Wan FJ, Kang BH, Wu CC, Tseng CJ. Systemic administration of lipopolysaccharide induces release of nitric oxide and glutamate and c-fos expression in the nucleus tractus solitarii of rats. Hypertension. 1999;33:1218–24. doi: 10.1161/01.hyp.33.5.1218.0194-911X(1999)033[1218:SAOLIR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Meng X, Shames BD, Pomerantz B, Donnahoo KK, Banerjee A, Harken AN. Liposomal delivery of heat-shock protein 72 into the heart prevents endotoxin-induced myocardial contractile dysfunction. Surgery. 1999;126:135–141.0039-6060(1999)126[0135:LDOHPI]2.0.CO;2 [PubMed] [Google Scholar]
- Nakada J, Matsura T, and Okazaki N. et al. 2005 Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and heat shock protein 70 induction. Shock. 24:482–487. [DOI] [PubMed] [Google Scholar]
- Novoselova TV, Margulis BA, Novoselov SS, Sapozhnikov AM, van der Spuy J, Cheetham ME, Guzhova IV. Treatment with extracellular HSP70/HSC70 protein can reduce polyglutamine toxicity and aggregation. J Neurochem. 2005;94:597–606. doi: 10.1111/j.1471-4159.2005.03119.x.0022-3042(2005)094[0597:TWEHPC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Paidas CN, Mooney ML, Nicholas GT, De Maio A. Accelerated recovery after endotoxic challenge in heat shock-pretreated mice. Am J Physiol. 2002;282:R1374–R1381. doi: 10.1152/ajpregu.00280.2001.0002-9513(2002)282[R1374:ARAECI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pajkrt D, Lerch PG, and Van der Poll T. et al. 1997 Differential effects of reconstituted high-density lipoprotein on coagulation, fibrinolysis and platelet activation during human endotoxemia. Thromb Haemost. 77:303–307. [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523.0021-9738(2003)112[0460:TEOS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005.0270-6474(2005)025[9735:EHSPAC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;8:239–3247. doi: 10.1128/iai.65.8.3239-3247.1997.0019-9567(1997)008[0239:LAMLAD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma T, Irie K, Fujii E, Yoshioka T, Muraki T. Effect of heat stress on lipopolysaccharide-induced vascular permeability change in mice. J Pharmacol Exp Ther. 2002;303:656–663. doi: 10.1124/jpet.102.035758.0022-3565(2002)303[0656:EOHSOL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x.0019-2805(2003)110[0001:FOHSPS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32:636–639. doi: 10.1042/BST0320636.0300-5127(2004)032[0636:HPAHPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003.0002-9513(2004)286[C739:CFOHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Van Amersfoort ES, Van Berkel TJC, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;3:379–414. doi: 10.1128/CMR.16.3.379-414.2003.1098-6618(2003)003[0379:RMAMII]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega VL, De Maio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14:764–773. doi: 10.1091/mbc.E02-08-0498.1059-1524(2003)014[0764:GTATRT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin RP, Lundqvist A, More SH, Von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/s1471-4906(01)02168-8.1471-4906(2002)023[0130:HPAAOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2.1466-1268(2001)006[0386:EISHIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Takahashi K, Jiang GZ, Zhang XM, Kawai M, Fukada M, Yokochi T. In vivo production of heat shock protein in mouse peritoneal macrophages by administration of lipopolysaccharide. Infect Immun. 1994;62:4140–4144. doi: 10.1128/iai.62.10.4140-4144.1994.0019-9567(1994)062[4140:IVPOHS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]